An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Ethics


Defining ethical challenge(s) in healthcare research: a rapid review
Guy schofield.
1 Centre for Ethics in Medicine, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, BS8 2PS UK
3 Palliative and End of Life Care Research Group, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, BS8 2PS UK
Mariana Dittborn
2 Paediatric Bioethics Centre, Great Ormond Street Hospital, London, WC1N 3JH UK
Lucy Ellen Selman
Richard huxtable, associated data.
All data is presented in this manuscript.
Despite its ubiquity in academic research, the phrase ‘ethical challenge(s)’ appears to lack an agreed definition. A lack of a definition risks introducing confusion or avoidable bias. Conceptual clarity is a key component of research, both theoretical and empirical. Using a rapid review methodology, we sought to review definitions of ‘ethical challenge(s)’ and closely related terms as used in current healthcare research literature.
Rapid review to identify peer-reviewed reports examining ‘ethical challenge(s)’ in any context, extracting data on definitions of ‘ethical challenge(s)’ in use, and synonymous use of closely related terms in the general manuscript text. Data were analysed using content analysis. Four databases (MEDLINE, Philosopher’s Index, EMBASE, CINAHL) were searched from April 2016 to April 2021.
393 records were screened, with 72 studies eligible and included: 53 empirical studies, 17 structured reviews and 2 review protocols. 12/72 (17%) contained an explicit definition of ‘ethical challenge(s), two of which were shared, resulting in 11 unique definitions. Within these 11 definitions, four approaches were identified: definition through concepts; reference to moral conflict, moral uncertainty or difficult choices; definition by participants; and challenges linked to emotional or moral distress. Each definition contained one or more of these approaches, but none contained all four. 68/72 (94%) included studies used terms closely related to synonymously refer to ‘ethical challenge(s)’ within their manuscript text, with 32 different terms identified and between one and eight different terms mentioned per study.
Conclusions
Only 12/72 studies contained an explicit definition of ‘ethical challenge(s)’, with significant variety in scope and complexity. This variation risks confusion and biasing data analysis and results, reducing confidence in research findings. Further work on establishing acceptable definitional content is needed to inform future bioethics research.
Methodological rigour within research is a cornerstone in the production of high-quality findings and recommendations. Across the range of empirical methodologies, a broad collection of protocol development tools, methodology guidelines, and reporting guidelines have been developed and evidence of their use is increasingly required by journals [ 1 – 6 ]. Within both empirical bioethics and descriptive ethics, there has been an accompanying increase in the acknowledgment of the importance of methodological rigour in the empirical elements, including within the recent consensus statement on quality standards in empirical bioethics research by Ives et al. [ 7 – 9 ]. Aligned with this aim for rigour, definitional clarity of key terms used within a research project is a component of research quality [ 10 , 11 ]. Improving the quality of empirical bioethics is also itself an ethical imperative [ 9 ].
We recently conducted a systematic review examining ‘ethical challenges’ as reported by specialist palliative care practitioners [ 12 ]. Our review, alongside our initial scoping search findings and reading of the literature, suggested that, although many authors use the term ‘ethical challenge(s)’ in empirical ethics research, there appeared to be no commonly described or accepted definition. Furthermore, papers retrieved rarely defined ‘ethical challenge(s)’ explicitly , which has also been noted by other researchers examining other topic areas [ 13 – 15 ]. Our review further suggested that authors frequently use terms closely related to ‘ethical challenge(s)’—such as ‘moral dilemmas’ or ‘ethical issues’—interchangeably with ‘ethical challenge(s)’ throughout manuscripts, rather than staying with the original term. Research shows that non-philosophers may understand these related terms in heterogeneous ways which may additionally affect understanding of texts across different readerships [ 16 , 17 ].
Without a clear definition of an ethical challenge, each researcher must use individual judgement to ascertain whether they have identified an instance of one within their dataset. This potentially generates an unnecessary source of bias, particularly if multiple researchers are involved in data collection, extraction, or analysis. This risks generating misleading ethical analyses, evaluations, or recommendations. Additionally, and more broadly, if primary studies do not define the term, then work based on these—such as systematic reviews of individual studies or those undertaking secondary data analysis—may unknowingly compare different phenomena without a mechanism for mitigating the effects this introduces.
In the hope of prompting a debate on this topic, we therefore undertook a rapid review, which aimed to explore existing definitions of “ethical challenge(s)” and the use of other closely related terms within recent empirical healthcare ethics literature.
We conducted a rapid review examining the usage of the term ‘ethical challenge(s)’ over the last 5 years in published research articles, in order to identify and summarise if, and how, the term was defined. As a secondary aim, we examined authors’ uses of closely related alternative terms within the included article texts separate to their use within any explicit definitions that may be present.
Rapid reviews use abridged systematic review methodology to understand the evidence base on a particular topic in a time and resource efficient manner [ 18 – 22 ]. Comparative reviews of topics in which both a rapid review and a systematic review had been undertaken demonstrated that the overall conclusions were similar, although rapid reviews were less likely to contain social and economic data, and systematic reviews contained more detailed recommendations [ 18 – 20 , 23 , 24 ]. The Cochrane Rapid Review Methods Group has recently released interim methodological guidelines for undertaking rapid reviews [ 6 ], advising authors to describe where their protocol deviates from a systematic review and detail any biases that these deviations may introduce [ 18 , 19 , 21 ]. We have followed the Cochrane recommended methodology [ 6 ]. A rapid review reporting guideline is currently under development [ 25 ] and this review is therefore reported based on the PRISMA 2020 statement for systematic reviews, with justifications provided where our approach deviated [ 26 ].
Prospective review protocol registration on the PROSPERO database is the current gold standard, but, at the time of writing, PROSPERO does not accept records for rapid reviews [ 27 ]. The protocol was therefore not published in advance.
Eligibility criteria
The inclusion and exclusion criteria are summarised in Table Table1. 1 . We used Strech et al.’s Methodology, Issues, Participants (MIP) structure for our eligibility criteria, which is recommended for systematic reviews in ‘empirical bioethics’ [ 28 ]. The criteria reflect three assumptions. First, that the inclusion of ‘ethical challenge(s)’ in the title would increase the likelihood that this was the authors’ preferred term for the concept under investigation, and therefore increase the probability of a definition being provided. Second, that studies aiming to describe empirical data and identify ethical challenges in real-world contexts are most likely to contain a definition to guide researchers in identifying these challenges as they collect and analyse data. Third, that structured reviews of studies of ethical challenges are likely to include a definition to allow researchers to reliably recognise an ethical challenge in retrieved records. We used a 5-year timeframe as a date restriction. This reflected a balance between adequately covering recent use of the term and time and resource restrictions of the rapid review.
Inclusion and exclusion criteria
Information sources
The search strategy was as follows:
‘ethical challenge’.ti OR ‘ethical challenges’.ti.
We searched Medline (Ovid interface), Philosopher’s Index (OVID interface), EMBASE (OVID interface), and CINAHL (Cumulative Index to Nursing and Allied Health Literature, EBSCO interface) for studies indexed over a five-year period between April 2016 and April 2021. These resources cover the breadth of healthcare research. Including Philosopher’s Index increased coverage of the bioethics literature. We did not search the grey literature [ 6 ]. The search strategy was tested by successfully retrieving three sentinel studies known to the research team.
Study selection
Retrieved studies were imported into Endnote X9.2 [ 29 ]. Records unavailable through institutional subscriptions were requested from corresponding authors. If unavailable 14 days after the request, the record was excluded. A random sample of 20% of records were dual screened at the title/abstract level by GS/MD. After discussion, the remainder were screened by GS. At full-text screening, a further 20% were dual screened by GS/MD and, again after discussion, the remaining studies were screened by GS.
Data extraction and analysis
Data extraction was undertaken using a pre-piloted form, with the first 5 records dually extracted by GS and MD. Data from the remaining included studies was then extracted by GS, with correctness and completeness checked by MD. We collected data on date of publication, authors, journal, country (for primary studies), methodology, definition of ‘ethical challenge(s)’ (present (yes/no)) and (where offered) the definition provided, and any closely related terms used, with counts of all terms used in each article. For closely related terms, data was extracted from the authors’ text, but not from direct quotations from qualitative research. Where definitions of ‘ethical challenge(s)’ were offered and/or related terms were identified, these were categorised and counted following the principles of summative content analysis [ 30 ]. Summative content analysis combines both the quantitative counting of specific content or words/terms with latent content analysis to identify and categorise their meanings. We identified keywords (‘ethical challenge(s)’ and closely related terms) deployed by the authors of the included papers, both prior to and during data analysis, and analysed the retrieved definitions. This approach allowed for exploration of both the content of definitions and development of insights into the use of related terms.
Risk of bias assessment
The focus of the rapid review was the definition of the term ‘ethical challenge(s)’ within retrieved records. We therefore did not undertake quality assessment for the included studies and reviews.
831 records were retrieved, reduced to 393 after de-duplication. 238 records were excluded after reviewing the title and/or abstract. 157 records were identified for full text screening, with 3 unavailable [ 31 – 33 ]. 82 records were excluded at full text stage and 72 records were included for analysis. See Fig. 1 for the PRISMA flowchart.

PRISMA flow diagram of record identification
Record characteristics
Of the 72 included records, 53 were empirical studies [ 34 – 86 ], 10 non-systematic reviews [ 87 – 96 ], 7 systematic reviews [ 12 – 14 , 97 – 100 ], 1 systematic review protocol [ 101 ], and 1 non-systematic review protocol [ 102 ]. Of the 53 empirical studies, 42 (79%) were qualitative studies [ 34 – 36 , 38 – 44 , 47 , 48 , 50 – 52 , 54 – 58 , 60 , 62 – 67 , 69 , 71 – 77 , 79 – 81 , 83 – 86 ], 6 (12%) used a mixed methods approach [ 45 , 46 , 53 , 59 , 61 , 68 ], and 5 (10%) were quantitative [ 37 , 49 , 70 , 78 , 82 ]. 7/56 empirical studies, all qualitative interview studies, recruited participants internationally with no specific location stated [ 40 , 54 , 55 , 58 , 60 , 63 , 73 ]. Of the remaining studies, all but one were single-country studies: Botswana [ 75 ], Canada [ 41 , 65 ], China [ 57 ], Denmark [ 39 , 43 ], Dominican Republic [ 44 ], Germany [ 51 , 84 ], India [ 61 ], Iran [ 38 , 46 , 49 , 68 , 70 – 72 , 78 , 82 , 98 ], Italy [ 45 ], Mexico [ 87 ], the Netherlands [ 76 ], New Zealand [ 47 ], Norway [ 42 , 52 , 56 , 64 , 80 , 81 , 83 ], Saudi Arabia [ 34 – 37 ], Tanzania [ 69 , 74 ], Uganda [ 67 ], UK [ 86 ], and USA [ 50 , 53 , 59 , 62 , 66 , 77 , 79 , 85 , 85 ]. The remaining study was undertaken in both Sierra Leone and the UK [ 48 ]. See Table Table2 2 for a summary.
Included study details
12/72 (17%) of retrieved studies offered an explicit definition for ‘ethical challenge(s)’ [ 12 – 14 , 48 , 50 , 56 , 57 , 66 , 69 , 81 , 98 , 101 ]. Definitions were more likely to be found in more recent publications, with 4/12 included studies published in 2016–2018 [ 14 , 48 , 56 , 81 ], and 8/12 published in 2019–2021 [ 12 , 13 , 50 , 57 , 66 , 69 , 98 , 101 ]. The included study locations were evenly distributed, matching the overall pattern of retrieved studies, with studies from high- [ 48 , 50 , 56 , 66 , 81 ], middle- [ 57 , 98 ], and low-income settings [ 48 , 69 ]. The identified studies included eight qualitative studies [ 48 , 50 , 56 , 57 , 66 , 69 , 81 , 98 ], 3 systematic reviews [ 12 – 14 ], and 1 systematic review protocol [ 101 ]. Two of these records were the systematic review protocol and the report from our group, which accordingly contained the same definition [ 12 , 101 ], leaving 11 unique definitions. Definitions of ‘ethical challenge(s)’ identified in included studies are provided in Table Table3. 3 . Additionally, 68/72 (94%) reports used closely related terms synonymously in place of ‘ethical challenge(s)’ throughout their manuscript text, with between 1 and 8 different terms used within each report, and 32 different terms were identified. This occurred in both those reports that contained a definition and those that did not. See Table Table4 4 for terms and frequencies.
Details of studies that contained an explicit definition of ‘ethical challenges’
Use of terms closely related to ‘ethical challenge’
Those records that offered explicit definitions used four approaches: (1) definition through concepts [ 12 , 57 , 66 ]; (2) reference to moral conflict, moral uncertainty or difficult choices [ 13 , 14 , 48 , 57 , 69 , 98 ]; (3) definition by study participants [ 12 , 48 , 50 , 56 ]; or (4) challenges as linked to their ability to generate emotional or moral distress within healthcare practitioners [ 14 , 14 , 66 , 81 ]. Each definition was associated with one or more of the identified elements, although none covered all four approaches. We describe these approaches below.
Approach 1: definition through concepts
This approach involves primarily defining ‘ethical challenge(s)’ in terms of related concepts. All three definitions using this approach defined ‘ethical challenge(s)’ as a summative collection of related concepts, including ‘ethical dilemmas’, ‘moral dilemmas’, ‘moral challenges’, ‘ethical issues’, and ‘ethical conflicts’ [ 12 , 57 , 66 ], for example:
‘The expression “ethical challenges” mainly refers to ethical dilemmas and ethical conflicts as well as other scenarios where difficult choices have to be made’ [ 57 ] p34
Only one went on to define the other concepts they utilised, ‘ethical dilemmas’ and ‘ethical conflicts’:
‘Ethical dilemmas are described as situations that cannot be solved; decisions made between two options may be morally plausible but are equally problematic due to the circumstances. Ethical conflicts, on the contrary, arise when one is aware of the necessity of proper actions but he or she may have trouble exercising these actions because of certain internal or external factors.’ [ 57 ] p34
Approach 2: moral conflict, moral uncertainty or difficult choices
This approach anchors an ethical challenge to the requirement for an agent to make a (difficult) choice in a situation where moral principles conflict, or there is moral uncertainty as to the ‘right’ way forward.
‘In this context, ethical challenge refers to the situation whereby every alternative is morally wrong and still one has to make a choice’ [ 69 ] p676 ‘An ethical challenge occurs when one does not know how to behave and act in the best way…’ [ 14 ] p93
Approach 3: definition by study participants
Four of the definitions involved research participants themselves defining something as an ‘ethical challenge’ [ 12 , 48 , 50 , 56 ], with three studies explicitly stating that participants would lead this definitional work [ 48 , 50 , 56 ]. Draper & Jenkins offer a starting definition, adopted from Schwartz et al. [ 103 ] with which to prime participants, while Forbes and Phillips [ 50 ] and Jakobsen and Sørlie [ 56 ] left the definition fully with their participants (Table (Table3). 3 ). Finally, Schofield et al. proposed a very broad definition (Table (Table3), 3 ), alongside the specific statement that either participants or researchers could nominate something as an ‘ethical challenge’ [ 12 ].
Approach 4: emotional or moral distress
This final approach was to tie ethical challenges to situations where participants feel ‘discomfort’, emotional distress or more specifically moral distress or moral residue [ 14 , 66 , 81 ]. Larkin et al. are clear that this distress must be tied to moral causes, but Hem et al. and Storaker et al. also refer more broadly to ‘discomfort’ [ 14 ] and ‘emotional stress’ [ 81 ] respectively. For example:
‘In this article, ethical challenges refer to values that entail emotional and moral stress in healthcare personnel.’ [ 81 ] p557
To the authors’ knowledge, this is the first rapid review to examine the use of the term ‘ethical challenge(s)’ in empirical healthcare research literature. Notably, only 12/72 (17%) of included studies published in the last 5 years contained a definition for ‘ethical challenge(s)’, despite this being the focus of the research being reported. The definitions identified were found in qualitative studies and systematic reviews and were evenly distributed geographically across high-, middle- and low-income settings. Definitions contained one or more of the identified approaches, although none contained elements from all four. Taken together, these findings suggest that a clear definition of ‘ethical challenge(s)’, and consistent use thereof, is currently lacking.
The four approaches indicate the diverse approaches to understanding ‘ethical challenge(s)’. Approaches 1 and 2 explore the concept from opposite viewpoints, with approach 1 looking from the conceptual perspective, through terms such as ‘dilemmas’ and ‘conflict’, and approach 2 from a participant perspective, specifically in those situations in which someone is trying to make a decision in circumstances where the preferred option is not possible or when they perceive there to be clash in values they feel are important. Within the concept-led definitions (approach 1), the use of a plurality of terms highlights a potential risk of bias, as different readers may interpret these differently. For example, some terms, such as ‘moral dilemma’, have relatively well understood specific meanings for some readers, particularly those with philosophical training [ 104 – 106 ]. The presence in the literature of specific and multiple meanings for some related terms highlights the importance of empirical studies providing a definition of these additional terms alongside their primary definition for ‘ethical challenge(s)’. This is more likely to be relevant where an a priori definition is used, but may be relevant to any prompting text for studies using a participant-led process, as in the study by Draper and Jenkins [ 48 ]. This clarity is important for both readers and future researchers who may undertake a secondary analysis of the data.
Approach 3 involves facilitating participants to nominate something as an ethical challenge [ 12 , 48 , 50 , 56 ]. This speaks to an important question about who, in a research context, is permitted to define or describe the object of interest, in this case ‘ethical challenge(s)’. Restricting the identification of ‘ethical challenge(s)’ to researchers alone may introduce bias by excluding input from those without bioethical ‘expertise’, but with important lived experience of the context under investigation. There is evidence that although clinicians can be sensitive to major ethical dilemmas, they can be less sensitive to small everyday ethical elements in clinical practice, and that ethical awareness varies between individuals [ 107 , 108 ]. Additionally, there is evidence in healthcare ethics research that patients and carers identify ethical challenges in situations that healthcare workers do not [ 109 ]. Therefore, relying entirely on a particular stakeholders’ perspectives (such as clinicians’) may risk missing important ethical challenges present in a scenario (assuming, of course, that we can settle what counts as an ‘ethical challenge(s)’).
In Approach 4, ethical challenges were linked to situations in which participants felt discomfort [ 14 ], emotional stress [ 81 ], moral distress or moral residue [ 66 ]. These concepts are themselves defined in quite varied ways (see, for example, definitions of ‘moral distress’ in a systematic review by Morley et al. [ 110 ]), potentially leading to additional conceptual confusion. Identifying triggers for moral distress is important, as high levels of moral distress are known to have negative impacts on work environments and lead to increased levels of compassion fatigue, increased staff turnover rates and poorer patient outcomes [ 110 – 112 ]. However, it is also possible that the requirement that, to be identified as an ethical challenge, the situation must invoke stress or distress might result in the under-identification of ethical challenges. We anticipate that many practitioners will daily manage multiple low-level ethical challenges, many of which will not generate moral distress or leave a moral residue. As such, the presence of moral distress may not be sufficient or even necessary in order to label a moral event an ‘ethical challenge’. However, the relationship between ‘ethical challenge(s)’ and moral distress is complex, and some might argue that the latter has an important relationship to the former. For example, moral distress, as conceived by Jameton and others [ 110 , 113 , 114 ], is linked to the after-effects of having to handle ethical challenge(s), so some researchers might view the generation of moral distress as relevant to identifying ethical challenges.
Although our review revealed these four approaches, the wider literature indicates there may be alternative approaches available. For example, other potential approaches would define ethical challenges as events that interact with moral principles, such as autonomy, beneficence, non-maleficence or justice, as proposed by Beauchamp and Childress [ 115 ], or as events in which those principles clash, for example as used by Klingler et al. in their research focusing on ethical issues in health surveillance [ 116 ]. However, these approaches were not seen amongst our included papers.
Returning to our included papers, the high rates of use of closely related terms within included manuscript texts may add to difficulties in understanding the exact object of interest if these terms are being used as synonyms for ‘ethical challenge(s)’. This may be particularly the case if terms used include those such as ‘moral dilemma’, which (as shown above) will have specific meanings for some readers. Interchangeable, undefined usage of these terms by study authors within study texts risks further exacerbating the problems caused by a lack of definitional clarity.
Strengths and limitations
This rapid review is the first systematic attempt to describe the definitions of ‘ethical challenge(s)’ available within the recent published literature.
There are, however, five limitations to note. First, the review only includes results from the past 5 years, which inevitably means that older publications, which may have contained further definitions of ‘ethical challenge(s)’, were excluded. The focus on the previous 5 years does, however, allow for an assessment of the term’s use(s) within a reasonable period of time and was felt to be appropriate given the aims and resources available to this project.
Second, our three assumptions listed in the methodology section may have excluded some records that contained a relevant definition. However, these assumptions, and the resulting focus on two search terms, allowed for a balance between retrieved record numbers and team resources.
Third, the four databases searched were chosen for their focus on the healthcare ethics literature; we may therefore may have missed relevant usage in other fields or disciplines. Similarly, we did not search the grey literature, which might have excluded relevant research.
Fourth, for resource reasons, the assessment as to whether a related term was being used interchangeably in the text was undertaken by a single researcher (GS). This subjective assessment risks miscalculating both the number of interchangeable terms identified and the frequency counts.
Finally, we did not review the theoretical literature for conceptual definitions of ‘ethical challenge(s)’, hence the definitions we identified might not match completely conceptual understandings of the term. However, our review shows how the term is currently being used in the research literature. Indeed, if there are strong conceptual definitions within the theoretical literature, then it is clear that they are currently not reaching the researchers whose work was identified by our review.
This review is the first, to our knowledge, to identify and describe definitions (and uses) of the widely-utilised concept of ‘ethical challenge(s)’ within healthcare research. Only 17% (12/72) of retrieved papers presented an explicit definition of ‘ethical challenge(s)’ before beginning to investigate this concept in context. The definitions found contained one or more of four identified approaches, with significant cross-reference to related terms and concepts which themselves have variation in their accepted meanings. We recommend that researchers define the phenomenon of interest—in this case, ‘ethical challenge(s)’—to help ensure clarity. This should either be a priori, or, if using an approach that includes participant participation in the generation of the definition, reporting their final working definition a posteriori. The choice of definition should be justified, including the decision as to whether to include participants in this process. Additionally, if a definition references other conceptual terms, then consideration should be given to defining these as well.
The results of this rapid review suggest that a common conceptual understanding of the term ‘ethical challenge(s)’ is lacking within empirical bioethical research and that there is a need for researchers in this area to consider what conceptual formulations might be most useful. Again, failure to use definitions of crucial research concepts within empirical bioethics research potentially generates confusion and avoidable bias within research outputs, risking misleading ethical analyses, evaluations, and resulting recommendations. We therefore hope this review will help stimulate debate amongst empirical bioethics researchers on possible definitional content for such a commonly used term and prompt further discussion and research. Additionally, given the central role of patient and public partnership and involvement in research, further thought should be given to who should be involved in nominating something as a challenge worthy of study.
Following on from this work, there would be value in conducting an empirical bioethical project combining a full systematic review of definitions of ‘ethical challenge(s)’ (and related terms) integrated with an exploration of the conceptual literature to generate recommendations for approaches towards the content of potential definitions, perhaps related to the identified approaches above. Such a project could also ask authors who currently use the term ‘ethical challenge(s)’ in their research how they conceptualise this. Furthermore, work to better understand the benefits of including study participants in the definition process is also important. Finally, whilst researchers should justify whatever approach they choose to take, there may be merit in examining whether anything is lost if studies lack a robust or agreed definition, or whether doing so affords a flexibility and openness that allows for a broader range of ethical challenges to be identified.
Acknowledgements
Not applicable.
Authors' contributions
GS, MD and RH conceived of the idea for the review; LES, GS, MD and RH designed the review protocol; GS and MD conducted the literature searching, screening, data extraction and led on data interpretation but all authors were involved; GS led on drafting the manuscript; all authors critically revised the manuscript for content and approved the version to be published. All authors read and approved the final manuscript.
GS is supported by a Wellcome Trust Research Award for Health Professionals (208129/Z/17/Z). LES is funded by a Career Development Fellowship from the National Institute for Health Research. RH is part-funded by the Wellcome Trust (209841/Z/17/Z) and the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. He serves on various local, regional, and national ethics committees and related groups. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health, or any of the other organisations with and for whom the authors work.
Availability of data and materials
Declarations.
The authors declare that there are no conflicts of interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Medical Research Ethics: Challenges in the 21st Century
- © 2023
- Tomas Zima 0 ,
- David N. Weisstub 1
Charles University, Prague, Czech Republic
You can also search for this editor in PubMed Google Scholar
International Academy of Law and Mental Health, Montréal, Canada
- Provides a current review of medical research ethics on a global basis
- Aims to promote a discussion about controversial and foundational aspects in the field
- Concentrates on key areas of medical research where there are core ethical issues
Part of the book series: Philosophy and Medicine (PHME, volume 132)
10k Accesses
3 Citations
16 Altmetric
- Table of contents
About this book
Editors and affiliations, about the editors, bibliographic information.
- Publish with us
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents(26 chapters)
Front matter, philosophical foundations, embryo research ethics.
- Robert George, Christopher Tollefsen
The Ethics of Medical Research
- David Novak
Genopolitics: Biotechnology Norms and the Liberal International Order
- Jonathan Moreno
Vulnerability
Persons and groups: protection of research participants with vulnerabilities as a process.
- Paweł Łuków
Centring the Human Subject: Catalyzing Change in Ethics and Dementia Research
- Gloria Puurveen, Jim Mann, Susan Cox
Unproven Stem Cell-Based Interventions: Addressing Patients’ Unmet Needs or Causing Patient Harms?
- Kirstin R. W. Matthews
Genetic Privacy in the Age of Consumer and Forensic DNA Applications
- Sheldon Krimsky
Neuroscience
Ethical issues in neuroscience research.
- Walter Glannon
Should Prisoners’ Participation in Neuroscientific Research Always Be Disregarded When Making Decisions About Early Release?
- Elizabeth Shaw
Applying Neuroscience Research: The Bioethical Problems of Predicting and Explaining Behavior
- David Freedman
Direct Benefit, Equipoise, and Research on the Non-consenting
- Stephen Napier
The Ethics of Surgical Research and Innovation
- Wendy A. Rogers, Katrina Hutchison
Palliative Care
Opening death’s door: psilocybin and existential suffering in palliative care.
- Duff R. Waring
- 21st Century Bioethics
- Medical Ethics in the 21st Century
- Global Bioethics
- Medical Jurisprudence
- Medical Research Ethics
- Bioethics in the 21st Century
This book provides a current review of Medical Research Ethics on a global basis. The book contains chapters that are historically and philosophically reflective and aimed to promote a discussion about controversial and foundational aspects in the field. An elaborate group of chapters concentrates on key areas of medical research where there are core ethical issues that arise both in theory and practice: genetics, neuroscience, surgery, palliative care, diagnostics, risk and prediction, security, pandemic threats, finances, technology, and public policy.This book is suitable for use from the most basic introductory courses to the highest levels of expertise in multidisciplinary contexts. The insights and research by this group of top scholars in the field of bioethics is an indispensable read for medical students in bioethics seminars and courses as well as for philosophy of bioethics classes in departments of philosophy, nursing faculties, law schools where bioethics is linked to medical law, experts in comparative law and public health, international human rights, and is equally useful for policy planning in pharmaceutical companies.
David N. Weisstub
Professor Tomas Zima, Head of the Institute of Medical Biochemistry and Laboratory Medicine First Faculty of Medicine Charles University in Prague, served as Dean of the First Faculty of Medicine and Rector Magnificus of Charles University. Professor Zima is author of 470 articles which have been cited over 4500 times in the Science Citation Index. He has also written nine books and is Author of over 75 additional chapters. He has lectured globally at 160 universities and served in many leadership roles including as Member of the European Commission’s Scientific Panel for Health (SPH). He is Chair of the Advisory Board for the International Academy of Medical Ethics and Public Health.
Book Title : Medical Research Ethics: Challenges in the 21st Century
Editors : Tomas Zima, David N. Weisstub
Series Title : Philosophy and Medicine
DOI : https://doi.org/10.1007/978-3-031-12692-5
Publisher : Springer Cham
eBook Packages : Religion and Philosophy , Philosophy and Religion (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
Hardcover ISBN : 978-3-031-12691-8 Published: 03 January 2023
Softcover ISBN : 978-3-031-12694-9 Published: 04 January 2024
eBook ISBN : 978-3-031-12692-5 Published: 01 January 2023
Series ISSN : 0376-7418
Series E-ISSN : 2215-0080
Edition Number : 1
Number of Pages : XVI, 499
Number of Illustrations : 3 b/w illustrations, 3 illustrations in colour
Topics : Bioethics
Policies and ethics
- Find a journal
- Track your research

- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents
Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
The Ethics of Clinical Research
Clinical research attempts to address a relatively straightforward, and important challenge: how do we determine whether one medical intervention is better than another, whether it offers greater clinical benefit and/or poses fewer risks? Clinicians may one day be able to answer these questions by relying on computer models, thereby avoiding reliance on clinical research and the ethical concerns it raises. Until that day, clinical researchers begin by testing potential new medical interventions in the laboratory, and often in animals. While these methods can provide valuable information and, in the case of animal research, raise important ethical issues of their own, potential new interventions eventually must be tested in human beings. Interventions which work miracles in test tubes and in mice, often leave humans untouched, or worse off.
Testing medical interventions in humans typically poses some risks to the participants, no matter how many laboratory and animal tests precede it. In this way, the process of collecting data through clinical trials to improve health and well-being inevitably exposes research participants to some risks for the benefit of future patients. This brings us to the central ethical challenge posed by clinical research: When is it ethically permissible to expose research participants to risks of harm for the benefit of others? The present entry focuses on this concern, and canvasses the most prominent attempts to address it. The present entry largely brackets the range of interesting and important ethical challenges that arise in the course of conducting clinical research: How should it be reviewed? Who may conduct it? What must potential participants understand to give valid consent? May it be conducted in countries that will not be able to afford the intervention being tested? Do investigators have any obligations to treat unrelated medical conditions in participants that they uncover during the course of their research?
One might attempt to address the central ethical challenge by limiting clinical research to the medical setting, offering experimental interventions to patients who want to try them. This approach, which has the virtue of evaluating interventions in the process of trying to help individual patients, makes sense for comparisons of two or more interventions that are widely accepted and already in clinical use. In contrast, this approach poses enormous scientific and practical problems with respect to testing new interventions. On the practical side, who would be willing to manufacture a new intervention without knowing whether it works? What dose should be used? How often should the new drug be taken? More importantly, this approach might not yield reliable information as to whether the new treatment is useful or harmful until hundreds, perhaps thousands of people have received it. Clinical research is designed to address these concerns by systematically assessing potential new treatments in a small number of individuals, including very sick ones, before offering them more widely. As we go about our daily lives, driving cars, flushing our waste down the drain, exhaling, getting a dog, we inevitably expose others to risks of harm. Despite the fact that these practices pervade our lives, there has been surprisingly little philosophical analysis of the conditions under which they are acceptable (Hayenhjelm and Wolff 2012). In addition to being of value in its own right, evaluation of the central ethical challenge posed by clinical research thus provides an opportunity to consider one of the more fundamental concerns in moral theory: when is it acceptable to expose some individuals to risks of harm?
1. What is Clinical Research?
2. early clinical research, 3. abuses and guidelines, 4. clinical research and clinical care, 5. a libertarian analysis, 6. contract theory, 7. minimal risks, 8. goals and interests, 9. industry sponsored research, 10. learning health care, other internet resources, related entries.
Human subjects research is research which studies humans, as opposed to animals, atoms, or asteroids. Assessment of whether humans prefer 100 dollars or a 1% chance of 10,000 dollars constitutes human subjects research. Clinical research refers to the subset of human subjects research that evaluates the impact interventions have on human beings with the goal of assessing whether they might help to improve human health and well-being. The present analysis focuses on research that is designed to improve human health and well-being by identifying better methods to treat, cure or prevent illness or disease. This focus is intended to bracket the question of whether research on enhancements that might increase our well-being by making us better than normal, allowing us to remember more or worry less, without treating, curing, or preventing illness or disease qualifies as clinical research.
We shall also bracket the question of whether quality improvement and quality assurance projects qualify as clinical research. To briefly consider the type of research at the heart of this debate, consider a hospital which proposes to evaluate the impact of checklists on the quality of patient care. Half the nurses in the hospital are told to continue to provide care as usual; the other half are provided with a checklist and instructed to mechanically check off each item as they complete it when caring for their patients. The question of whether this activity constitutes clinical research is of theoretical interest as a means to clarifying the precise boundaries of the concept. Should we say that this is not clinical research because the checklist is used by the nurses, not administered to the patients? Or should we say this is clinical research because it involves the systematic testing of a hypothesis which is answered by collecting data on patient outcomes? The answer has significant practical implications, determining whether these activities must satisfy existing regulations for clinical research, including whether the clinicians need to obtain patients’ informed consent before the nurses taking care of them can use the checklist.
While clinical medicine is enormously better than it was 100 or even 50 years ago, there remain many diseases against which current clinical medicine offers an inadequate response. To name just a few, malaria kills over a million people, mostly children, every year; chronic diseases, chief among them heart disease and stroke, kill millions each year, and there still are no effective treatments for Alzheimer disease. The social value of clinical research lies in its ability to collect information that might be useful for identifying improved methods to address these conditions. Yet, it is the rare study which definitively establishes that a particular method is effective and safe for treating, curing or preventing some illness. The success of specific research studies more commonly lies in the gathering of information that, together with the results of many other studies, may yield these improvements. For example, prior to establishing the efficacy of a new treatment for a given condition, researchers typically need to identify the cause of the condition, possible mechanisms for treating it, a safe and effective dose, and ways of testing whether the drug alters the course of the disease.
The process of testing potential new treatments can take 10–15 years, and is standardly divided into phases. Formalized phase 0 studies are a relatively recent phenomenon involving the testing of interventions and methods which might be used in later phase studies. A phase 0 study might be designed to determine the mechanism of action of a particular drug and evaluate different ways to administer it. Phase 1 studies are the earliest tests of a new intervention and are conducted in small numbers of individuals. Phase 1 studies are designed to evaluate the pharmacokinetics and pharmacodynamics of new treatments, essentially evaluating how the drug influences the human body and how the human body influences the drug. Phase 1 studies also evaluate the risks of the treatment and attempt to identify an appropriate dose to be used in subsequent phase 2 studies. Phase 1 studies pose risks and frequently offer little if any potential for clinical benefit to participants. As a result, a significant amount of the ethical concern over clinical research focuses on phase 1 studies.
If phase 1 testing is successful, potential new treatments go on to larger phase 2 studies which are designed to further assess risks and also to evaluate whether there is any evidence that the treatment might be beneficial. Successful phase 2 studies are followed by phase 3 studies which involve hundreds, sometimes thousands of patients. Phase 3 studies are designed to provide a rigorous test of the efficacy of a treatment and frequently involve randomization of participants to the new treatment or a control, which might be the current treatment or a placebo. Finally, post-marketing or phase 4 studies evaluate the use of interventions in clinical practice.
The first three phases of clinical trials typically include purely research procedures, such as blood draws, imaging scans, or biopsies, that are included in the study to collect data regarding the treatment under study. Analysis of the central ethical challenge posed by clinical research thus focuses on three related risk-benefit profiles: (a) the risk-benefit profile of the interventions(s) under study; (b) the risk-benefit profile of the included research procedures; and (c) the risk-benefit profile of the study as a whole.
In some cases, receipt of potential new treatments is in the ex ante interests of research participants. For example, the risks posed by an experimental cancer treatment might be justified by the possibility that it will extend participants’ lives. Moreover, the risk/benefit profile of the treatment might be as favorable for participants as the risk/benefit profile of the available alternatives. In these cases, receipt of the experimental intervention ex ante promotes participants’ interests. In other cases, participation in research poses ‘net’ risks, that is, risks of harm which are not, or not entirely, justified by potential clinical benefits to individual participants. A first in human trial of an experimental treatment might involve a single dose to see whether humans can tolerate it. And it might occur in healthy individuals who have no need of treatment. These studies pose risks to participants and offer essentially no chance for clinical benefit. The qualifier to ‘essentially’ no chance of clinical benefit is intended to capture the fact that the research procedures included in clinical trials may inadvertently end up providing some clinical benefit to some participants. For example, a biopsy that is used to collect research data may disclose a previously unidentified and treatable condition. The chance for such benefit, albeit real, is typically so remote that it is not sufficient to compensate for the risks of the procedure. Whether a study as a whole poses net risks depends on whether the potential benefits of the experimental intervention compensate for its risks plus the net risks of the research procedures included in the study.
Clinical research which poses net risks raises important ethical concern. Net-risk studies raise concern that participants are being used as mere means to collect information to benefit future patients. Research procedures that pose net risks may seem to raise less concern when they are embedded within a study which offers a favorable risk-benefit profile overall. Yet, since these procedures pose net risks, and since the investigators could provide participants with the new potential treatment alone, they require justification. An investigator who is about to insert a needle into a research participant to obtain some blood purely for laboratory purposes faces the question of whether doing so is ethically justified, even when the procedure is included in a study that offers participants the potential for important medical benefit. The goal of ethical analyses of clinical research is to provide an answer.
Clinical research poses three types of net risks: absolute, relative, and indirect (Rid and Wendler 2011). Absolute net risks arise when the risks of an intervention or procedure are not justified by its potential clinical benefits. Most commentators focus on this possibility with respect to research procedures which pose some risks and offer (essentially) no chance of clinical benefit, such as blood draws to obtain cells for laboratory studies. Research with healthy volunteers is another example which frequently offers no chance for clinical benefit. Clinical research also poses absolute net risks when it offers a chance for clinical benefit which is not sufficient to justify the risks participants face. A kidney biopsy to obtain tissue from presumed healthy volunteers may offer some very low chance of identifying an unrecognized and treatable pathology. This intervention nonetheless poses net risks if the chance for clinical benefit for the participants is not sufficient to justify the risks of their undergoing the biopsy.
Relative net risks arise when the risks of a research intervention are justified by its potential clinical benefits, but the intervention’s risk-benefit profile is less favorable than the risk-benefit profile of one or more available alternatives. Imagine that investigators propose a randomized, controlled trial to compare an inexpensive drug against an expensive and somewhat more effective drug. Such trials make sense when, in the absence of a direct comparison, it is unclear whether the increased effectiveness of the more expensive drug justifies its costs. In this case, receipt of the cheaper drug would be contrary to participants’ interest in comparison to receiving the more expensive drug. The trial thus poses relative net risks to participants.
Indirect net risks arise when a research intervention has a favorable risk-benefit profile, but the intervention diminishes the risk-benefit profile of other interventions provided as part of or in parallel to the study. For example, an experimental drug for cancer might undermine the effectiveness of other drugs individuals are taking for their condition. The risks of research participation can be compounded if the indicated response to the harm in question poses additional risks. Kidney damage suffered as the result of research participation might lead to the need for short-term dialysis which poses additional risks to the individual; a participant who experiences a postlumbar puncture headache might need a ‘blood patch’ which poses some risk of blood entering the epidural space which would call for a further response which carries additional risks. While commentators tend to focus on the risks of physical harm, participation in clinical research can pose other types of risks as well, including psychological, economic, and social risks. Depending on the study and the circumstances, individuals who are injured as the result of participating in research might incur significant expenses. Most guidelines and regulations stipulate that evaluation of the acceptability of clinical research studies should take into account all the different risks to which participants are exposed.
To assess the ethics of exposing research participants to risks, one needs an account of why exposing others to risks raises ethical concern in the first place. Being exposed to risks obviously raises concern to the extent that the potential harm to which the risk refers is realized: the chance of a headache turns into an actual headache. Being exposed to risks also can lead to negative consequences as a result of the recognition that one is at risk of harm. Individuals who recognize that they face a risk may become frightened; they also may take costly or burdensome measures to protect themselves. In contrast, the literature on the ethics of clinical research implicitly assumes that being exposed to risks is not per se harmful. The mere fact that participants are exposed to risks is not regarded as necessarily contrary to their interests. It depends on whether the risk is realized in an actual harm.
It is one thing to expose a consenting adult to risks to save the health or life of an identified and present other, particularly when the two individuals are first degree relatives. It is another thing, or seems to many to be another thing, to expose consenting individuals to risks to help unknown and unidentified, and possibly future others. Almost no one objects to operating on a healthy, consenting adult to obtain a kidney that might save a present and ailing sibling, even though the operation poses some risk of serious harm and offers the donor no potential for clinical benefit. Attempts to obtain a kidney from a healthy, consenting adult and give it to an unidentified individual are met with greater ethical concern. The extent of the concern increases as the path from risk exposure to benefit becomes longer and more tenuous. Many clinical research studies expose participants to risks in order to collect generalizable information which, if combined with the results of other, as yet non-existent studies, may eventually benefit future patients through the identification of a new intervention, assuming the appropriate regulatory authorities approve it, some company or group chooses to manufacture it, and patients can afford to purchase it. The potential benefits of clinical research may thus be realized someday, but the risks and burdens are clear and present.
Increasingly, researchers obtain and store human biological samples for use in future research studies. These studies raise important questions regarding what might be called ‘contribution’ and ‘information’ risks. The former question concerns the conditions under which it is acceptable to ask individuals to contribute to answering the scientific question posed by a given study (Jonas 1969). The fact that this question has been ignored by many commentators and regulations may trace to an overly narrow understanding of individuals’ interests. Individuals undoubtedly have an interest in avoiding the kinds of physical harms, pain, infection, loss of organ function, that they face in clinical research. This leaves the question of whether individuals’ interests are implicated, and whether they can be set back, by contributing to particular projects, activities and goals?
Consider the routine practice of storing leftover samples of participants’ blood for use in future research projects. For the purposes of protecting participants’ interests, does it matter what goals these future studies attempt to advance? Can individuals be harmed if their samples are used to promote goals which conflict with their fundamental values? For example, are the interests of individuals who fundamentally oppose cloning set back if their samples are used in a study that successfully identifies improved methods to clone human beings?
The possibility of information risks garnered significant attention when investigators used DNA samples obtained from members of the Havasupai tribe in Arizona to study “theories of the tribe’s geographical origins.” The study’s conclusion that early members of the tribe had migrated from Asia across the Bering Strait contradicted the tribe’s own views that they originated in the Grand Canyon (Harmon 2010). Can learning the truth, in this case the origins of one’s tribal group, harm research participants?
Attempts to determine when it is acceptable to expose participant to risks for the benefit of others, including future others who do not yet exist, have been significantly influenced by its history, by how it has been conducted and, in particular, by how it has been misconducted (Lederer 1995; Beecher 1966). Thus, to understand the current state of the ethics of clinical research, it is useful to know something of its past.
Modern clinical research may have begun on the 20 th of May, 1747, aboard the HMS Salisbury. James Lind, the ship’s surgeon, was concerned with the costs scurvy was exacting on British sailors, and was skeptical of some of the interventions, cider, elixir of vitriol, vinegar, sea-water, being used at the time to treat it. Unlike other clinicians of his day, Lind did not simply assume that he was correct and treat his patients accordingly. He designed a study. He chose 12 sailors from among the 30 or so Salisbury’s crew members who were suffering from scurvy, and divided them into six groups of 2 sailors each. Lind assigned a different intervention to each of the groups, including two sailors turned research participants who received 2 oranges and 1 lemon each day. Within a week these two were sailors again; the others remained patients, and several were dying.
The ethics of clinical research begins by asking how we should think about the fate of these latter sailors. Did Lind treat them appropriately? Do they have a moral claim against Lind? It is widely assumed that physicians should do what they think is best for the patient in front of them. Lind, despite being a physician, did not follow this maxim. He felt strongly that giving sea water to individuals with scurvy was a bad idea, but he gave sea water to 2 of the sailors in his study to test whether he, or others, were right. To put the fundamental concern raised by clinical research in its simplest form: Did Lind sacrifice these two sailors, patients under his care, for the benefit of others?
Lind’s experiments represent perhaps the first modern clinical trial because he attempted to address one of the primary challenges facing those who set out to evaluate medical treatments. How does one show that any differences in the outcomes of the treatments under study are a result of the treatments themselves, and not a result of the patients who received them, or other differences in the patients’ environment or diet? How could Lind be confident that the improvements in the two sailors were the result of the oranges and lemons, and not a result of the fact that he happened to give them to the two patients who occupied the most salutary rooms on the ship? Lind tried to address the challenge of confounding variables by beginning with patients who were as similar as possible. He carefully chose the 12 subjects for his experiment from a much larger pool of ailing sailors; he also tried to ensure that all 12 received the same rations each day, apart from the treatments provided as part of his study. It is also worth noting that Lind’s dramatic results were largely ignored for decades, leading to uncounted and unnecessary deaths, and highlighting the importance of combining clinical research with effective promulgation and implementation. The Royal Navy did not adopt citrus rations for another 50 years (Sutton 2003), at which point scurvy essentially disappeared from the Royal Navy.
Lind’s experiments, despite controlling for a number of factors, did not exclude the possibility that his own choices of which sailors got which treatment influenced the results. More recent experiments, including the first modern randomized, placebo controlled trial of Streptomycin for TB in 1948 (D’Arcy Hart 1999), attempt to address this concern by assigning treatments to patients using a random selection process. By randomly assigning patients to treatment groups these studies ushered in the modern era of controlled, clinical trials. And, by taking the choice of which treatment a given patient receives out of the hands of the treating clinician, these trials underscore and, some argue, exacerbate the ethical concerns raised by clinical research (Hellman and Hellman 1991). A foundational principle of clinical medicine is the importance of individual judgment. A physician who decides which treatments her patients receive by flipping a coin is guilty of malpractice. A clinical investigator who relies on the same methods receives awards and gets published in elite journals. One might conclude that sacrifice of the interests of some, often sick patients, for the benefit of future patients, is essentially mandated by the scientific method (Miller & Weijer 2006; Rothman 2000). The history of clinical research seems to provide tragic support for this view.
The history of clinical research is littered with abuses. Indeed, one account maintains that the history of pediatric research is “largely one of child abuse” (Lederer and Grodin 1994, 19; also see Lederer 2003). This history has had a significant influence on how research ethicists understand the concerns raised by clinical research and on how policy makers attempt to address them. In particular, policy makers have responded to previous abuses by developing guidelines intended to prevent their recurrence.
The most influential abuses in this regard were the horrific experiments conducted by Nazi physicians during WW II (abuses perpetrated by Japanese physicians were also horrific, but have received significantly less attention). Response to these abuses led to the Nuremberg Code (Grodin & Annas 1996; Shuster 1997), which is frequently regarded as the first set of formal guidelines for clinical research, an ironic claim on two counts. First, there is some debate over whether the Nuremberg Code was intended to apply generally to clinical research or whether, as a legal ruling in a specific trial, it was intended to address only the cases before the court (Katz 1996). Second, the Germans themselves had developed systematic research guidelines as early as 1931 (Vollmann & Winau 1996). These guidelines were still legally in force at the time of the Nazi atrocities and clearly prohibited a great deal of what the Nazi doctors did.
Wide consensus developed by the end of the 1950s that the Nuremberg Code was inadequate to the ethics of clinical research. Specifically, the Nuremberg Code did not include a requirement that clinical research receive independent ethics review and approval. In addition, the first and longest principle in the Nuremberg Code states that informed consent is “essential” to ethical clinical research (Nuremberg Military Tribunal 1947). This requirement provides a powerful safeguard against the abuse of research participants. It also appears to preclude clinical research with individuals who cannot consent.
One could simply insist that the informed consent of participants is necessary to ethical clinical research and accept the opportunity costs thus incurred. Representatives of the World Medical Association, who hoped to avoid these costs, began meeting in the early 1960s to develop guidelines, which would become the Declaration of Helsinki, to address the perceived shortcomings of the Nuremberg Code (Goodyear, Krleza-Jeric, and Lemmens 2007). They recognized that insisting on informed consent as a necessary condition for clinical research would preclude a good deal of research designed to find better ways to treat dementia and conditions affecting children, as well as research in emergency situations. Regarding consent as necessary precludes such research even when it poses only minimal risks or offers participants a compensating potential for important clinical benefit. The challenge, still facing us today, is to identify protections for research participants which are sufficient to protect them without being so strict as to preclude appropriate research designed to benefit the groups to which they belong.
The Declaration of Helsinki (World Medical Organization 1996) allows individuals who cannot consent to be enrolled in clinical research based on the permission of the participant’s representative. The U.S. federal regulations governing clinical research take a similar approach. These regulations are not laws in the strict sense of being passed by Congress and applying to all research conducted on U.S. soil. Instead, the regulations represent administrative laws which effectively attach to clinical research at the beginning and the end. Research conducted using U.S. federal monies, for instance, research funded by the NIH, or research involving NIH researchers, must follow the U.S. regulations (Department of Health and Human Services 2005). Research that is included as part of an application for approval from the U.S. FDA also must have been conducted according to FDA regulations which, except for a few exceptions, are essentially the same. Although many countries now have their own national regulations (Brody 1998), the U.S. regulations continue to exert enormous influence around the world because so much clinical research is conducted using U.S. federal money and U.S. federal investigators, and the developers of medical treatments often want to obtain approval for the U.S. market.
The abuses perpetrated as part of the infamous Tuskegee syphilis study were made public in 1972, 40 years after the study was initiated. The resulting outcry led to the formation of the U.S. National Commission, which was charged with evaluating the ethics of clinical research with humans and developing recommendations for appropriate safeguards. These deliberations resulted in a series of recommendations for the conduct of clinical research, which became the framework for existing U.S. regulations. The U.S. regulations, like many regulations, place no clear limits on the risks to which competent and consenting adults may be exposed. In contrast, strict limits are placed on the level of research risks to which those unable to consent may be exposed, particularly children. In the case of pediatric research, the standard process for review and approval is limited to studies that offer a ‘prospect of direct’ benefit and research that poses minimal risk or a minor increase over minimal risk. Studies that cannot be approved in one of these categories must be reviewed by an expert panel and approved by a high government official. While this 4th category offers important flexibility, it implies that, at least in principle, U.S. regulations do not mandate a ceiling on the risks to which pediatric research participants may be exposed for the benefit of others. This reinforces the importance of considering how we might justify exposing participants to research risks, both minimal and greater than minimal, for the benefit of others.
Lind’s experiments on scurvy exemplify the fact that clinical research is often conducted by clinicians and often is conducted on patients. Many commentators have thus assumed that clinical research should be governed by the ethics of clinical care, and the methods of research should not diverge from the methods that are acceptable in clinical care. On this approach, participants should not be denied any beneficial treatments available in the clinical setting and they should not be exposed to any risks not present in the clinical setting.
Some proponents (Rothman 2000) argue that this approach is implied by the kind of treatment that patients, understood as individuals who have a condition or illness needing treatment, are owed. Such individuals are owed treatment that promotes, or at least is consistent with their medical interests. Others (Miller & Weijer 2006) argue that the norms of clinical research derive largely from the obligations that bear on clinicians. These commentators argue that it is unacceptable for a physician to participate in, or even support the participation of her patients in a clinical trial unless that trial is consistent with the patients’ medical interests. To do less is to provide substandard medical treatment and to violate one’s obligations as a clinician.
The claim that the treatment of research participants should be consistent with the norms which govern clinical care has been applied most prominently to the ethics of randomized clinical trials (Hellman & Hellman 1991). Randomized trials determine which treatment a given research participant receives based on a random process, not based on clinical judgment of which treatment would be best for that patient. Lind assigned the different existing treatments for scurvy to the sailors in his study based not on what he thought was best for them, but based on what he thought would yield an effective comparative test. Lind did not give each intervention to the same number of sailors because he thought that all the interventions had an equal chance of being effective. To the contrary, he did this because he was confident that several of the interventions were harmful and this design was the best way to show it. Contemporary clinical researchers go even further, assigning participants to treatments randomly. Because this aspect of clinical research represents a clear departure from the practice of clinical medicine it appears to sacrifice the interests of participants in order to collect valid data.
One of the most influential responses to this concern (Freedman 1987) argues that randomization is acceptable when the study in question satisfies what has come to be known as ‘clinical equipoise.’ Clinical equipoise obtains when, for the population of patients from which participants will be selected, the available clinical evidence does not favor one of the treatments being used over the others. In addition, it must be the case that there are no treatments available outside the trial that are better than those used in the trial. Satisfaction of these conditions seems to imply that the interests of research participants will not be undermined in the service of collecting scientific information. If the available data do not favor any of the treatments being used, randomizing participants seems as good a process as any other for choosing which treatment they receive.
Proponents of clinical equipoise as an ethical requirement for clinical research determine whether equipoise obtains not by appeal to the belief states of individual clinicians, but based on whether there is consensus among the community of experts regarding which treatment is best. Lind believed that sea water was ineffective for the treatment of scurvy. Yet, in the absence of agreement among the community of experts, this view essentially constituted an individual preference rather than a clinical norm. This suggests that it was acceptable for Lind to randomly assign sailors under his care to the prevailing treatments in order to test, in essence, whose preferred treatment was the best. In this way, the existence of uncertainty within the community of experts seems to offer a way to reconcile the methods of clinical research with the norms of clinical medicine.
Critics respond that even when clinical equipoise obtains for the population of patients, the specific circumstances of individual patients within that population might imply that one of the treatments under investigation is better for them (Gifford 2007). A specific patient may have reduced liver function which places her at greater risk of harm if she receives a treatment metabolized by the liver. And some patients may have personal preferences which incline them toward one treatment over another (e.g., they may prefer a one-time riskier procedure to multiple, lower risk procedures which pose the same collective risk). Current debate focuses on whether randomized clinical trials can take these possibilities into account in a way that is consistent with the norms of clinical medicine.
Even if the existence of clinical equipoise can justify some randomized trials, a significant problem remains, namely, many studies and procedures which are crucial to the identification and development of improved methods for protecting and advancing health and well-being are inconsistent with participants’ medical interests. This concern arises for many phase 1 studies which offer essentially no chance for medical benefit and pose at least some risks, and to that extent are inconsistent with the participants’ medical interests.
Phase 3 studies which randomize participants to a potential new treatment or existing standard treatment, and satisfy clinical equipoise, typically include purely research procedures, such as additional blood draws, to evaluate the drugs being tested. Enrollment in these studies may be consistent with participants’ medical interests in the sense that the overall risk-benefit ratio is at least as favorable as the available alternatives. Yet, evaluation of the overall risk-benefit profile of the study masks the fact that it includes individual procedures which are contrary to participants’ medical interests, and contrary to the norms of clinical medicine.
The attempt to protect research participants by appeal to the obligations clinicians have to promote the medical interests of their patients also seems to leave healthy volunteers unprotected. Alternatively, proponents might characterize this position in terms of clinicians’ obligations to others in general: clinicians should not perform procedures on others unless doing so promotes the individual’s clinical interests. This approach seems to preclude essentially all research with healthy volunteers. For example, many phase 1 studies are conducted in healthy volunteers to determine a safe dose of the drug under study. These studies, vital to drug development, are inconsistent with the principle that clinicians should expose individuals to risks only when doing so is consistent with their clinical interests. It follows that appeal to clinical equipoise alone cannot render clinical research consistent with the norms of clinical practice.
Commentators sometimes attempt to justify net-risk procedures that are included within studies, and studies that overall pose net risks by distinguishing between ‘therapeutic’ and ‘non-therapeutic’ research (Miller and Weijer 2006). The claim here is that the demand of consistency with participants’ medical interests applies only to therapeutic research; non-therapeutic research studies and procedures may diverge from these norms to a certain extent, provided participants’ medical interests are not significantly compromised. The distinction between therapeutic and non-therapeutic research is sometimes based on the design of the studies in question, and sometimes based on the intentions of the investigators. Studies designed to benefit participants, or investigators who intend to benefit participants are conducting therapeutic studies. Those designed to collect generalizable knowledge or in which the investigators intend to do so constitute non-therapeutic research.
The problem with the distinction between therapeutic and non-therapeutic research so defined is that research itself often is defined as a practice designed to collect generalizable knowledge and conducted by investigators who intend to achieve this end (Levine 1988). On this definition, all research qualifies as non-therapeutic. Conversely, most investigators intend to benefit their participants in some way or other. Perhaps they design the study in a way that provides participants with clinically useful findings, or they provide minor care not required for research purposes, or referrals to colleagues. Even if proponents can make good on the distinction between therapeutic and non-therapeutic research in theory, these practices appear to render it irrelevant to the practice of clinical research. More importantly, it is not clear why investigators’ responsibilities to patients, or patients’ claims on investigators, should vary as a function of this distinction. Why think that investigators are allowed to expose patients to some risks for the benefit of others, but only in the context of research that is not designed to benefit the participants? To apply this proposed resolution to pediatric research, why might it be acceptable to expose infants to risks for the benefit of others, but only in the context of studies which offer the infants no chance for personal benefit?
To take one possibility, it is not clear that this view can be defended by appeal to physicians’ role responsibilities. A prima facie plausible view holds that physicians’ role responsibilities apply to all encounters between physicians and patients who need medical treatment. This view would imply that physicians may not compromise patients’ medical interests when conducting therapeutic studies, but also seems to prohibit non-therapeutic research procedures with patients. Alternatively, one might argue that physicians’ role responsibilities apply only in the context of clinical care and so do not apply in the context of clinical research at all. This articulation yields a more plausible view, but does not support the use of the therapeutic/ non-therapeutic distinction. It provides no reason to think that physicians’ obligations differ based on the type of research in question.
Critics argue that these problems highlight the fundamental confusion that results when one attempts to evaluate clinical research based on norms appropriate to clinical medicine. They instead distinguish between the ethics of clinical research and the ethics of clinical care, arguing that it is inappropriate to assume that investigators are subject to the claims and obligations which apply to physicians, despite the fact that the individuals who conduct clinical research often are physicians (Miller and Brody 2007).
The claim that clinical research should satisfy the norms of clinical medicine has this strong virtue: it provides a clear method to protect individual research participants and reassure the public that they are being so protected. If research participants are treated consistent with their medical interests, we can be reasonably confident that improvements in clinical medicine will not be won at the expense of exploiting them. Most accounts of the ethics of clinical research now recognize the limitations of this approach and search for ways to ensure that research participants are not exposed to excessive risks without assuming that the claims of clinical medicine apply to clinical researchers (Emanuel, Wendler, and Grady 2000; CIOMS 2002). Dismissal of the distinction between therapeutic and non-therapeutic research thus yields an increase in both conceptual clarity and concern regarding the potential for abuse of research participants.
Clinicians, first trained as physicians taught to act in the best interests of the patient in front of them, often struggle with the process of exposing some patients to risky procedures for the benefit of others. It is one thing for philosophers to insist, no matter how accurately, that research participants are not patients and need not be treated according to the norms of clinical medicine. It is another thing for clinical researchers to regard research participants who are suffering from disease and illness as anything other than patients. These clinical instincts, while understandable and laudable, have the potential to obscure the true nature of clinical research, as investigators and participants alike try to convince themselves that clinical research involves nothing more than the provision of clinical care. One way to try to address this collective and often willful confusion would be to identify a justification for exposing research participants to net risks for the benefit of others.
It is often said that those working in bioethics are obsessed with the principle of respect for individual autonomy. Advocates of this view of bioethicists cite the high esteem accorded in the field to the requirement of obtaining individual informed consent and the frequent attempts to resolve bioethical challenges by citing its satisfaction. One might assume that this view within bioethics traces to implicit endorsement of a libertarian analysis according to which it is permissible for competent and informed individuals to do whatever they prefer, provided those with whom they interact are competent, informed and in agreement. In the words of Mill, investigators should be permitted to conduct research and expose participants to risks provided they obtain their “free, voluntary, and undeceived consent and participation” (On Liberty, page 11). Setting aside the question of whether this view accurately characterizes bioethics and bioethicists generally, it does not apply to the vast majority of work done on the ethics of clinical research. Almost no one in the field argues that it is permissible for investigators to conduct any research they want provided they obtain the free and informed consent of those they enroll.
Current research ethics does place significant weight on informed consent and many regulations and guidelines devote much of their length to articulating the requirement for informed consent. Yet, as exemplified by the response to the Nuremberg Code, almost no one regards informed consent as necessary and sufficient for ethical research. Most regulations and guidelines, beginning with the Declaration of Helsinki, first adopted in 1964 (World Medical Organization 1996), allow investigators to conduct research on humans only when it has been approved by an independent group charged with ensuring that the study is ethically acceptable. Most regulations further place limits on the types of research that independent ethics committees may approve. They must find that the research has important social value and the risks have been minimized, thereby restricting the types of research to which even competent adults may consent. Are these requirements justified, or are they inappropriate infringements on the free actions of competent individuals? The importance of answering this question goes beyond its relevance to debates over Libertarianism. Presumably, the requirements placed on clinical research have the effect of reducing to some extent the number of research studies that get conducted. The fact that at least some of the prohibited studies likely would have important social value, helping to identify better ways to promote health and well-being, provides a normative reason to eliminate the restrictions, unless there is some compelling reason to retain them.
The libertarian claim that valid informed consent is necessary and sufficient to justify exposing research participants to risks for the benefit of others seems to imply, consistent with the first principle of the Nuremberg Code, that research with individuals who cannot consent is unethical. This plausible and tempting claim commits one to the view that research with children, research in many emergency situations, and research with the demented elderly all are ethically impermissible. One could consistently maintain such a view but the social costs of adopting it would be great. It is estimated, for example, that approximately 70% of medications provided to children have not been tested in children, even for basic safety and efficacy (Roberts, Rodriquez, Murphy, Crescenzi 2003; Field & Behrman 2004; Caldwell, Murphy, Butow, and Craig 2004). Absent clinical research with children, pediatricians will be forced to continue to provide frequently inappropriate treatment, leading to significant harms that could have been avoided by pursuing clinical research to identify better approaches.
One response would be to argue that the Libertarian analysis is not intended as an analysis of the conditions under which clinical research is acceptable. Instead, the claim might be that it provides an analysis of the conditions under which it is acceptable to conduct clinical research with competent adults. Informed consent is necessary and sufficient for enrolling competent adults in research. While this view does not imply that research with participants who cannot consent is impermissible, it faces the not insignificant challenge of providing an account for why such research might be acceptable.
Bracketing the question of individuals who cannot consent, many of the limitations on clinical research apply to research with competent adults. How might these limitations be justified? One approach would be to essentially grant the Libertarian analysis on theoretical grounds, but then argue that the conditions for its implementation are rarely realized in practice. In particular, there are good reasons, and significant empirical data, to question how often clinical research actually involves participants who are sufficiently informed to provide valid consent. Even otherwise competent adults often fail to understand clinical research sufficiently to make their own informed decisions regarding whether to enroll (Flory and Emanuel 2004).
To consider an example which is much discussed in the research ethics literature, it is commonly assumed that valid consent for randomized clinical trials requires individuals to understand randomization. It requires individuals to understand that the treatment they will receive, if they enroll in the study, will be determined by a process which does not take into account which of the treatments is better for them (Kupst 2003). There is an impressive wealth of data which suggests that many, perhaps most individuals who participate in clinical research do not understand this (Snowden 1997; Featherstone and Donovan 2002; Appelbaum 2004). The data also suggest that these failures of understanding often are resistant to educational interventions.
With this in mind, one might regard the limitations on research with competent adults as betraying the paternalism embedded in most approaches to the ethics of clinical research (Miller and Wertheimer 2007). Although the charge of paternalism often carries with it some degree of condemnation, there is a strong history of what is regarded as appropriate paternalism in the context of clinical research. This too may have evolved from clinical medicine. Clinicians are charged with protecting and promoting the interests of the patient “in front of them”. Clinician researchers, who frequently begin their careers as clinicians, may regard themselves as similarly charged. Paternalism involves interfering with the liberty of agents for their own benefit (Feinberg 1986; see also entry on paternalism ). As the terms are used in the present debate, ‘soft’ paternalism involves interfering with the liberty of an individual in order to promote their interests on the grounds that the action being interfered with is the result of impaired decision-making: “A freedom-restricting intervention is based on soft paternalism only when the target’s decision-making is substantially impaired, when the agent lacks (or we have reason to suspect that he lacks) the information or capacity to protect his own interests—as when A prevents B from drinking the liquid in a glass because A knows it contains poison but B does not” (Miller & Wertheimer 2007). ‘Hard’ paternalism, in contrast, involves interfering with the liberty of an individual in order to promote their interests, despite the fact that the action being interfered with is the result of an informed and voluntary choice by a competent individual.
If the myriad restrictions on clinical research were justified on the basis of hard paternalism they would represent restrictions on individuals’ autonomous actions. However, the data on the extent to which otherwise competent adults fail to understand what they need to understand to provide valid consent suggests that the limitations can instead be justified on the grounds of soft paternalism. This suggests that while the restrictions may limit the liberty of adult research participants, they do not limit their autonomy. In this way, one may regard many of the regulations on clinical research not as inconsistent with the libertarian ideal, but instead as starting from that ideal and recognizing that otherwise competent adults often fail to attain it.
Even if most research participants had sufficient understanding to provide valid consent, it would not follow that there should be no limitations on research with competent adults. The conditions on what one individual may do to another are not exhausted by what the second individual consents to. Perhaps some individuals may choose for themselves to be treated with a lack of respect, even tortured. It does not follow that it is acceptable for me or you to treat them accordingly. As independent moral agents we need sufficient reason to believe that our actions, especially the ways in which we treat others, are appropriate, and this evaluation concerns, in typical cases, more than just the fact that the affected individuals consented to them.
Understood in this way, many of the limitations on the kinds of research to which competent adults may consent are not justified, or at least not solely justified, on paternalistic grounds. Instead, these limitations point to a crucial and often overlooked concern in research ethics. The regulations for clinical research often are characterized as protecting the participants of research from harm. Although this undoubtedly is an important and perhaps primary function of the regulations, they also have an important role in limiting the extent to which investigators harm research participants, and limiting the extent to which society supports and benefits from a process which inappropriately harms others. It is not just that research participants should not be exposed to risk of harm without compelling reason. Investigators should not expose them to such risks without compelling reason, and society should not support and benefit from their doing so.
This aspect of the ethics of clinical research has strong connections with the view that the obligations of clinicians restrict what sort of clinical research they may conduct. On that view, it is the fact that one is a physician and is obligated to promote the best interests of those with whom one interacts professionally which determines what one is allowed to do to participants. This connection highlights the pressing questions that arise once we attempt to move beyond the view that clinical research is subject to the norms of clinical medicine. There is a certain plausibility to the claim that a researcher is not acting as a clinician and so may not be subject to the obligations that bear on clinicians. Or perhaps we might say that the researcher/subject dyad is distinct from the physician/patient dyad and is not necessarily subject to the same norms. But, once we conclude that we need an account of the ethics of clinical research, distinct from the ethics of clinical care, one is left with the question of which limitations apply to what researchers may do to research participants.
It seems clear that researchers may not expose research participants to risks without sufficient justification, and also clear that this claim applies even to those who provide free and informed consent. The current challenge then is to develop an analysis of the conditions under which it is acceptable for investigators to expose participants to risks and determine to what extent current regulations need to be modified to reflect this analysis. To consider briefly the extent of this challenge, and to underscore and clarify the claim that the ethics of clinical research go beyond the protection of research participants to include the independent consideration of what constitutes appropriate behavior on the part of investigators, consider an example.
Physical and emotional abuse cause enormous suffering, and a good deal of research is designed to study various methods to reduce instances of abuse and also to help victims recover from being abused. Imagine that a team of investigators establishes a laboratory to promote the latter line of research. The investigators will enroll consenting adults and, to mimic the experience of extended periods of abuse in real life, they will abuse their participants emotionally and physically for a week. The abused participants will then be used in studies to evaluate the efficacy of different methods for helping victims to cope with the effects of abuse.
The proper response to this proposal is not to claim that the study is acceptable because the participants are competent and they gave informed consent. The proper response is to point out that, while these considerations are undoubtedly important, they do not establish that the study is ethically acceptable. One needs to consider many other things. Is the experiment sufficiently similar to real life abuse that its results will have external validity? Are there less risky ways to obtain the same results? Finally, even if these questions are answered in a way that supports the research, the question remains whether investigators may ethically treat their participants in this way. The fact that essentially everyone working in research ethics would hold that this study is unethical—investigators are not permitted to treat participants in this way—suggests that research ethics, both in terms of how it is practiced and possibly how it should be practiced, goes beyond respect for individual autonomy to include independent standards on investigator behavior. Defining those standards represents one of the more important challenges for research ethics.
As exemplified by Lind’s experiments on treatments for scurvy, clinical research studies were first conducted by clinicians wondering whether the methods they were using were effective. To answer this question, the clinicians altered the ways in which they treated their patients in order to yield information that would allow them to assess their methods. In this way, clinical research studies initially were part of, but an exception to standard clinical practice. As a result, clinical research came to be seen as an essentially unique activity. And widespread recognition of clinical research’s scandals and abuses led to the view that this activity needed its own extensive regulations.
More recently, some commentators have come to question the view that clinical research is a unique human activity, as well as the regulations and guidelines which result from this view. In particular, it has been argued that this view has led to overly restrictive requirements on clinical research, requirements that hinder scientists’ ability to improve medical care for future patients, and also fail to respect the liberty of potential research participants. This view is often described in terms of the claim that many regulations and guidelines for clinical research are based on an unjustified ‘research exceptionalism’ (Wertheimer 2010).
The central ethical concern raised by clinical research involves the practice of exposing participants to risks for the benefit of others. Yet, as noted previously, we are constantly exposing individuals to risks for our benefit and the benefit of others. When you drive to the store, you expose your neighbors to some increased risk of pollution for the benefits you derive from shopping; speeding ambulances expose pedestrians to risks for the benefit of the patients they carry; factories expose their workers to risks for the benefit of their customers; charities expose volunteers to risks for the benefit of recipients. Despite this similarity, clinical research is widely regarded as ethically problematic and is subject to significantly greater regulation, review, and oversight (Wilson and Hunter 2010). Almost no one regards driving, ambulances, charities, or factories as inherently problematic. Even those who are not great supporters of a given charity do not argue that it treats its volunteers as guinea pigs, despite exposing them to risks for the benefit of others. And no one argues that charitable activities should satisfy the requirements that are routinely applied to clinical research, such as the requirements for independent review and written consent based on an exhaustive description of the risks and potential benefits of the activity, its purpose, duration, scope, and procedures.
Given that many activities expose some to risks for the benefit of others, yet are not subject to such extensive regulation, some commentators conclude that many of the requirements for clinical research are unjustified (Sachs 2010, Stewart et al. 2008, and Sullivan 2008). This work is based on the assumption that, when it comes to regulation and ethical analysis, we should treat clinical research as we treat other activities in daily life which involve exposing some to risks for the benefit of others. This assumption leads to a straightforward solution to the central ethical problem posed by clinical research.
Exposing factory workers to risks for the benefit of others is deemed ethically acceptable when they agree to do the work and are paid a fair wage. The solution suggested for the ethical concern of non-beneficial research is to obtain consent and pay research participants a fair wage for their efforts. This view is much less restrictive than current regulations for clinical research, but seems to be less permissive than a Libertarian analysis. The latter difference is evident in claims that research studies should treat participants fairly and not exploit them, even if individuals consent to being so treated.
The gap between this approach and the traditional view of research ethics is evident in the fact that advocates of the traditional view tend to regard payment of research participants as exacerbating rather than resolving its ethical concerns, raising, among others, worries of undue inducement and commodification. Those who are concerned about research exceptionalism, in contrast, tend to regard payment as it is regarded in most other contexts in daily life: some is good and more is better.
The claims of research exceptionalism have led to valuable discussion of the extent to which clinical research differs from other activities which pose risks to participants for the benefit of others and whether any of the differences justify the extensive regulations and guidelines standardly applied to clinical research. Proponents of research exceptionalism who regard many of the existing regulations as unjustified face the challenge of articulating an appropriate set of regulations for clinical research. While comparisons to factory work provide a useful lens for thinking about the ethics of clinical research, it is not immediately obvious what positive recommendations follow from this perspective. After all, it is not as if there is general consensus regarding the regulations to which industry should be subject. Some endorse minimum wage laws; others oppose them. There are further arguments over whether workers should be able to unionize; whether governments should set safety standards for industry; whether there should be rules protecting workers against discrimination.
A few commentators (Caplan 1984; Harris 2005; Heyd 1996) try to justify exposing research participants to risks for the benefit of others by citing an obligation to participate in clinical research. At least all individuals who have access to medical care have benefited from the efforts of previous research participants in the form of effective vaccines and better medical treatments. One might try to argue that these benefits obligate us to participate in clinical research when its our turn.
Current participation in clinical research typically benefits future patients. However, if we incur an obligation for the benefits that are due to previous research studies, we presumably are obligated to the patients who participated in those studies, an obligation we cannot discharge by participating in current studies. This approach also does not provide a way to justify the very first clinical trials, such as Lind’s, which of necessity enrolled participants who had never benefited from previous clinical research.
Alternatively, one might argue that the obligation to participate does not trace to the benefits we receive from the efforts of previous research participants. Rather, the obligation is to the overall social system of which clinical research is a part (Brock 1994). For example, one might argue that individuals acquire this obligation as the result of being raised in the context of a cooperative scheme or society. We are obligated to do our part because of the many benefits we have enjoyed as a result of living within such a scheme.
The first challenge for this view is to explain why the mere enjoyment of benefits, without some prospective agreement to respond in kind, obligates individuals to help others. Presumably, your doing a nice thing for me yesterday, without my knowledge or invitation, does not obligate me to do you a good turn today. This concern seems even greater with respect to pediatric research. Children certainly benefit from previous research studies, but typically do so unknowingly and often with vigorous opposition. The example of pediatric research makes the further point that justification of clinical research on straightforward contractualist grounds will be difficult at best. Contract theories have difficulties with those groups, such as children, who do not accept in any meaningful way the benefits of the social system under which they live (Gauthier 1990).
In a Rawlsian vein, one might try to establish an obligation to participate in clinical research based on the choices individuals would make regarding the structure of society from a position of ignorance regarding their own place within that society, from behind a veil of ignorance (Rawls 1999). To make this argument, one would have to modify the Rawlsian argument in several respects. The knowledge that one is currently living could well bias one’s decision against the conduct of clinical research. Those who know they are alive at the time the decision is being made have already reaped many of the benefits they will receive from the conduct of clinical research.
To avoid these biases, we might stretch the veil of ignorance to obscure the generation to which one belongs—past, present or future (Brock 1994). Under a veil of ignorance so stretched, individuals might choose to participate in clinical research as long as the benefits of the practice exceed its overall burdens. One could then argue that justice as fairness gives all individuals an obligation to participate in clinical research when their turn comes. This approach seems to have the advantage of explaining why we can expose even children to some risks for the benefit of others, and why parents can give permission for their children to participate in such research. This argument also seems to imply not simply that clinical research is acceptable, but that, in a range of cases, individuals have an obligation to participate in it. It implies that adults whose turn has come are obligated to participate in clinical research, although for practical reasons we might refrain from forcing them to do so.
This justification for clinical research faces several challenges. First, Rawlsian arguments typically are used to determine the basic structure of society, that is, to determine a fair arrangement of the basic institutions within the society (Rawls 1999). If the structure of society meets these basic conditions, members of the society cannot argue that the resulting distribution of benefits and burdens is unfair. Yet, even when the structure of society meets the conditions for fairness, it does not follow that individuals are obligated to participate in the society so structured. Competent adults can decide to leave a society that meets these conditions rather than enjoy its benefits (whether they have any better places to go is another question). The right of exit suggests that the fairness of the system does not generate an obligation to participate, but rather defends the system against those who would argue that it is unfair to some of the participants over others. At most, then, the present argument can show that it is not unfair to enroll a given individual in a research study, that this is a reasonable thing for all individuals, including those who are unable to consent.
Second, it is important to ask on what grounds individuals behind the veil of ignorance make their decisions. In particular: are these decisions constrained or guided by moral considerations? (Dworkin 1989; Stark 2000). It seems plausible to think that they would be. After all, we are seeking the ethical approach or policy with respect to clinical research. The problem, then, is that the answer we get in this case may depend significantly on which ethical constraints are built into the system, rendering the approach question begging. Most importantly, we are considering whether it is ethical to expose individuals who cannot consent to risks for the benefit of others. If it isn’t, then it seems that this should be a limitation on the choices individuals can make from behind the veil of ignorance, in which case appeal to those choices will not be able to justify pediatric research, nor research with incompetent adults. And if this research is ethical it is unclear why we need this mechanism to justify it.
Proponents might avoid this dilemma by assuming that individuals behind the veil of ignorance will make decisions based purely on self-interest, unconstrained by moral limits or considerations. Presumably, many different systems would satisfy this requirement. In particular, the system that produces the greatest amount of benefits overall may well be one that we regard as unethical. Many endorse the view that clinical research studies which offer no potential benefit to participants and pose a high chance of serious risk, such as death, are unethical, independent of the magnitude of the social value to be gained. For example, almost all research ethicists would regard as unethical a study which intentionally infects a few participants with the HIV virus, even when the study offers the potential to identify a cure for AIDS. Yet, individuals behind the veil of ignorance who make decisions based solely on self-interest might well allow this study on the grounds that it offers a positive cost-benefit ratio overall: the high risks to a few participants are clearly outweighed by the potential to save the lives of millions.
The question here is not whether a reasonable person would choose to make those who are badly off even worse off in order to elevate the status of those more privileged. Rather, both options involve some individuals being in unfortunate circumstances, namely, infected with the HIV virus. The difference is that the one option (not conducting the study) involves many more individuals becoming infected over time, whereas the other option involves significantly fewer individuals being infected, but some as the result of being injected in the process of identifying a cure. Since the least desirable circumstances (being infected with HIV) are the same in both cases, the reasonable choice, even if one endorses the maximin strategy, seems to be whichever option reduces the total number of individuals who are in those circumstances, revealing that, in the present case at least, the Rawlsian approach seems not to take into account the way in which individuals end up in the positions they occupy.
Limits on risks are a central part of almost all current research regulations and guidelines. With respect to those who can consent, there is an essentially implicit agreement that the risks should not be too high (as noted earlier, some argue that there should not be any net risks to even competent adults in the context of so-called therapeutic research). However, there is no consensus regarding how to determine which risks are acceptable in this context. With respect to those who cannot consent, many commentators argue that clinical research is acceptable when the net risks are very low. The challenge, currently faced by many in clinical research, is to identify a standard, and find a reliable way to implement it, for what constitutes a sufficiently low risk in this context. An interesting and important question in this regard is whether the level of acceptable risks varies depending on the particular class of individuals who cannot consent. Is the level of acceptable risks the same for individuals who were once competent, such as previously competent adults with Alzheimer disease, individuals who are not now but are expected to become competent, such as healthy children, and individuals who are not now and likely never will be competent, such as individuals born with severe cognitive disabilities?
Some argue that the risks of clinical research qualify as sufficiently low when they are ‘negligible’, understood as risks that do not pose any chance of serious harm (Nicholson 1986). Researchers who ask children a few questions for research purposes may expose them to risks no more worrisome than that of being mildly upset for a few minutes. Exposing participants to a risk of minor harm for the benefit of others does not seem to raise ethical concern. Or one might argue that the ethical concerns it raises do not merit serious ethical concern. Despite the plausibility of these views, very few studies satisfy the negligible risk standard. Even routine procedures that are widely accepted in pediatric research, such as single blood draws, pose some, typically very low risk of more than negligible harm.
Others (Kopelman 2000; Resnik 2005) define risks as sufficiently low or ‘minimal’ when they do not exceed the risks individuals face during the performance of routine examinations. This standard provides a clear and quantifiable threshold for acceptable risks. Yet, the risks of routine medical procedures for healthy individuals are so low that this standard seems to prohibit intuitively acceptable research. This approach faces the additional problem that, as the techniques of clinical medicine become safer and less invasive, increasing numbers of procedures used in clinical research would move from acceptable to unacceptable. And, at a theoretical level, one might wonder why we should think that the risks we currently happen to accept in the context of clinical care for healthy children should define the level of risk that is acceptable in clinical research. Why think that the ethical acceptability of a blood draw in pediatric research depends on whether clinicians still use blood draws as part of clinical screening for healthy children?
Many guidelines (U.S. Department of Health and Human Services 2005; Australian National Health and Medical Research Council 1999) and commentators take the view that clinical research is ethically acceptable as long as the net risks do not exceed the risks individuals face in daily life. Many of those involved in clinical research implicitly assume that this minimal risk standard is essentially equivalent to the negligible risk standard. If the risks of research are no greater than the risks individuals face in daily life, then the research does not pose risk of any serious harm. As an attitude toward many of the risks we face in daily life, this view makes sense. We could not get through the day if we were conscious of all the risks we face. Crossing the street poses more risks than one can catalog, much less process readily. When these risks are sufficiently low, psychologically healthy individuals place them in the cognitive background, ignoring them unless the circumstances provide reason for special concern (e.g. one hears a siren, or sees a large gap in the sidewalk).
Paul Ramsey reports that members of the US National Commission often used the terms ‘minimal’ and ‘negligible’ in a way which seemed to imply that they were willing to allow minimal risk research, even with children, on the grounds that it poses no chance of serious harm (Ramsey 1978). The members then went on to argue that an additional ethical requirement for such research is a guarantee of compensation for any serious research injuries. This approach to minimal risk pediatric research highlights nicely the somewhat confused attitudes we often have toward risks, especially those of daily life.
We go about our daily lives as though harms with very low probability are not going to occur, effectively treating low probability harms as zero probability events. To this extent, we are not Bayesians about the risks of daily life. We treat some possible harms as impossible for the purposes of getting through the day. This attitude, crucial to living our lives, does not imply that there are no serious risks in daily life. The fact that our attitude toward the risks of everyday life is justified by its ability to help us to get through the day undermines its ability to provide an ethical justification for exposing research participants to the same risks in the context of non-beneficial research (Ross & Nelson 2006).
First, the extent to which we ignore the risks of daily life is not a fully rational process. In many cases, our attitude regarding risks is a function of features of the situation that are not correlated directly with the risk level, such as our perceived level of control and our familiarity with the activity (Tversky, Kahneman 1974; Tversky, Kahneman 1981; Slovic 1987; Weinstein 1989). Second, to the extent that the process of ignoring some risks is rational, we are involved in a process of determining which risks are worth paying attention to. Some risks are so low that they are not worth paying attention to. Consideration of them would be more harmful (would cost us more) than the expected value of being aware of them in the first place.
To some extent, then, our attitudes in this regard are based on a rational cost/benefit analysis. To that extent, these attitudes do not provide an ethical argument for exposing research participants to risks for the benefit of others. The fact that the costs to an individual of paying attention to a given risk in daily life are greater than the benefits to that individual does not seem to have any relevance for what risks we may expose them to for the benefit of others. Finally, there is a chance of serious harm from many of the activities of daily life. This reveals that the ‘risks of daily life’ standard does not preclude the chance of some participants experiencing serious harm. Indeed, one could put the point in a much stronger way. Probabilities being what they are, the risks of daily life standard implies that if we conduct enough minimal risk research eventually a few participants will die and scores will suffer permanent disability.
As suggested above, a more plausible line of argument would be to defend clinical research that poses minimal risk on the grounds that it does not increase the risks to which participants are exposed. It seems plausible to assume that at any given time an individual will either be participating in research or involved in the activities of daily life. But, by assumption, the risks of the two activities are essentially equivalent, implying that enrollment in the study, as opposed to allowing the participant to continue to participate in the activities of daily life does not increase the risks to which he is exposed.
The problem with this argument is that the risks of research often are additive rather than substitutive. For example, participation in a study might require the participant to drive to the clinic for a research visit. The present defense succeeds to the extent that this trip replaces another trip in the car, or some similarly risky activity in which the participant would have been otherwise involved. In practice, this often is not the case. The participant instead may simply put off the trip to the mall until after the research visit. In that case, the participant’s risk of serious injury from a car trip may be doubled as a result of her participation in research. Moreover, we accept many risks in daily life because the relevant activities offer those who pursue them a chance of personal benefit. We allow children to take the bus because we assume that the benefits of receiving an education justify the risks. The fact that we accept these risks given the potential benefits provides no reason to think that the same risks or even the same level of risk would be acceptable in the context of an activity which offers no chance of medical benefit. Finally, applied strictly, this justification seems to imply that investigators should evaluate what risks individuals would face if they did not enroll in the research, and enroll only those who would otherwise face similar or greater levels of risk.
In one of the most influential papers in the history of research ethics, Hans Jonas (1969) argues that the progress clinical research offers is normatively optional, whereas the need to protect individuals from the harms to which clinical research exposes them is mandatory. He writes:
… unless the present state is intolerable, the melioristic goal [of biomedical research] is in a sense gratuitous, and this is not only from the vantage point of the present. Our descendants have a right to be left an unplundered planet; they do not have a right to new miracle cures. We have sinned against them if by our doing, we have destroyed their inheritance not if by the time they come around arthritis has not yet been conquered (unless by sheer neglect). (Jonas 1969, 230–231)
Jonas’s view does not imply that clinical research is necessarily unethical, but the conditions on when it may be conducted are very strict. This argument may seem plausible to the extent that one regards, as Jonas does, the benefits of clinical research to be ones that make an acceptable state in life even better. The example of arthritis cited by Jonas characterizes this view. Curing arthritis, like curing dyspepsia, baldness, and the minor aches and pains of living and aging, may be nice, but may be thought to address no profound problem in our lives. If this were all that clinical research had to offer, we might be reluctant to accept many risks in order to achieve its goals. We should not, in particular, take much chance of wronging individuals, or exploiting them to realize these goals.
This argument makes sense to the extent that one regards the status quo as acceptable. Yet, without further argument, it is not clear why one should accept this view; it seems almost certain that those suffering from serious illness that might be addressed by future research will not accept it. Judgments regarding the present state of society concern very general level considerations and a determination that society overall is doing fairly well is consistent with many individuals suffering terrible diseases. Presumably, the suffering of these individuals provides some reason to conduct clinical research. In response, one might understand Jonas to be arguing that the present state of affairs involves sufficiently good medicine and adequately flourishing lives such that the needs which could now be addressed by additional clinical research are not of sufficient importance to justify the risks raised by conducting it. It might have been the case, at some point in the past, that life was sufficiently nasty, brutish and short to justify running the risk of exploiting research participants in the process of identifying ways to improve the human lot. But, we have advanced, in part thanks to clinical research, well beyond that point. This reading need not interpret Jonas as ignoring the fact that there remain serious ills to be cured. Instead, he might be arguing that these ills, while real and unfortunate, are not of sufficient gravity, or perhaps prevalence to justify the risks of conducting clinical research.
This view implicitly expands the ethical concerns raised by clinical research. We have been focusing on the importance of protecting individual research participants. However, Jonas assumes that clinical research also threatens society in some sense. There are at least two possibilities here. First, it might be thought that the conduct of unethical research reaches beyond individual investigators to taint society as a whole. This does not seem unreasonable given that clinical research typically is conducted in the name of and often for the benefit of society. Second, one might be concerned that allowing investigators to expose research participants to some risks for the benefit of others might put us on a slippery slope that ends with serious abuses throughout society.
An alternative reading would be to interpret Jonas as arguing from a version of the active-passive distinction. It is often claimed that there is a profound moral difference between actively causing harm versus merely allowing harm to occur, between killing someone versus allowing them to die, for example. Jonas often seems to appeal to this distinction when evaluating the ethics of clinical research. The idea is that conducting clinical research involves investigators actively exposing individuals to risks of harm and, when those harms are realized, it involves investigators actively harming them. The investigator who injects a participant with an experimental medication actively exposes the individual to risks for the benefit of others and actively harms, perhaps even kills those who suffer harm as a result. And, to the extent that clinical research is conducted in the name of and for the benefit of society in general, one can say without too much difficulty that society is complicit in these harms. Not conducting clinical research, in contrast, involves our allowing individuals to be subject to diseases that we might otherwise have been able to avoid or cure. And this situation, albeit tragic and unfortunate, has the virtue of not involving clear moral wrongdoing.
The problem with at least this version of the argument is that the benefits of clinical research often involve finding safer ways to treat disease. The benefits of this type of clinical research, to the extent they are realized, involve clinicians being able to provide less harmful, less toxic medications to patients. Put differently, many types of clinical research offer the potential to identify medical treatments which harm patients less than current ones. This not an idle goal. One study found that the incidence of serious adverse events from the appropriate use of clinical medications (i.e. excluding such things as errors in drug administration, noncompliance, overdose, and drug abuse) in hospitalized patients was 6.7%. The same study, using data from 1994, concludes that the approved and properly prescribed use of medications is likely the 5 th leading cause of death in the US (Lazarou, Pomeranz, and Corey 1998).
These data suggest that the normative calculus is significantly more complicated than the present reading of Jonas suggests. The question is not whether it is permissible to risk harming some individuals in order to make other individuals slightly better off. Instead, we have to decide how to trade off the possibility of clinicians exposing patients to greater risks of harm (albeit with a still favorable risk-benefit ratio) in the process of treating them versus clinical researchers exposing participants to risk of harm in the process of trying to identify improved methods to treat others. This is not to say that there is no normative difference between these two activities, only that that difference is not accurately described as the difference between harming individuals versus improving their lot beyond some already acceptable status quo. It is not even a difference between harming some individuals versus allowing other individuals to suffer harms. The argument that needs to be made is that harming individuals in the process of conducting clinical research potentially involves a significant moral wrong not present when clinicians harm patients in the process of treating them.
The primary concern here is that, by exposing participants to risks of harm, the process of conducting clinical research involves the threat of exploitation of a particular kind. It runs the risk of investigators treating persons as things, devoid of any interests of their own. The worry here is not so much that investigators and participants enter together into the shared activity of clinical research with different, perhaps even conflicting goals. The concern is rather that, in the process of conducting clinical research, investigators treat participants as if they had no goals at all or, perhaps, that any goals they might have are normatively irrelevant.
Jonas argues that this concern can be addressed, and the process of experimenting on some to benefit others made ethically acceptable, only when the research participants share the goals of the research study. Ethically appropriate research, on Jonas’s view, is marked by: “appropriation of the research purpose into the person’s own scheme of ends” (Jonas 1969, 236). And assuming that it is in one’s interests to achieve one’s, at least, proper goals, it follows that, by participating in research, participants will be acting in their own interests, despite the fact that they are thereby being exposed to risky procedures which are performed to collect information to benefit others.
Jonas claims in some passages that research participants, at least those with an illness, can share the goals of a clinical research study only when they have the condition or illness under study (Jonas 1969). These passages reveal something of the account of human interests on which Jonas’s arguments rely. On standard preference satisfaction accounts of human interests, what is in a given individual’s interests depends on what the individual happens to want or prefer, or the goals the individual happens to endorse, or the goals the individual would endorse in some idealized state scrubbed clean of the delusions, misconceptions and confusion which inform their actual preferences (Griffin 1986). On this view, participation in clinical research would promote an individual’s interests as long as she was well informed and wanted to participate. This would be so whether or not she had the condition being studied. Jonas’s view, in contrast, seems to be that there are objective conditions under which individuals can share the goals of a given research study. They can endorse the cause of curing or at least finding treatments for Alzheimer disease only if they suffer from the disease themselves.
One possible objection would be to argue that there are many reasons why an individual might endorse the goals of a given study, apart from having the disease themselves. One might have family members with the disease, or co-religionists, or have adopted improved treatment of the disease as an important personal goal. The larger question is whether participants endorsing the goals of a clinical research study is a necessary condition on its acceptability. Recent commentators and guidelines rarely, if ever, endorse this condition, although at least some of them might be assuming that the requirement to obtain free and informed consent will ensure its satisfaction. It might be assumed, that is, that competent, informed, and free individuals will enroll in research only when they share the goals of the study in question.
Jonas was cognizant of the extent to which the normative concerns raised by clinical research are not exhausted by the risks to which participants are exposed, but also include the extent to which investigators and by implication society are the agents of their exposure to risks. For this reason, he recognized that the libertarian response is inadequate, even with respect to competent adults who truly understand. Finally, to the extent Jonas’s claims rely on an objective account of human interests, one may wonder whether he adopts an overly restrictive one. Why should we think, on an objective account, that individuals will have an interest in contributing to the goals of a given study only when they have the disease it addresses? Moreover, although we will not pursue the point here, appeal to an objective account of human interests raises the possibility of justifying the process of exposing research participants to risks for the benefit of others on the grounds that contributing to valuable projects, including presumably some clinical research studies, promotes (most) individuals’ interests (Wendler 2010).
The fundamental ethical challenge posed by clinical research is whether it is acceptable to expose some to research risks for the benefit of others. In the standard formulation, the one we have been considering to this point, the benefits that others enjoy as the result of participants’ participation in clinical research are medical and health benefits, better treatments for disease, better methods to prevent disease.
Industry funded research introduces the potential for a very different sort of benefit and thereby potentially alters, in a fundamental way, the moral concerns raised by clinical research. Pharmaceutical companies typically focus on generating profit and increasing stock price and market share. Indeed, it is sometimes argued that corporations have an obligation to their shareholders to pursue increased market share and share price (Friedman 1970). This approach may well lead companies to pursue new medical treatments which have little or no potential to improve overall health and well-being (Huskamp 2006; Croghan and Pittman 2004). “Me-too” drugs are the classic example here. These are drugs identical in all clinically relevant respects to approved drugs already in use. The development of a me-too drug offers the potential to redistribute market share without increasing overall health and well-being.
There is considerable debate regarding how many me-too drugs there really are and what is required for a drug to qualify as effectively identical (Garattini 1997). For example, if the existing treatment needs to be taken with meals, but a new treatment need not, is that a clinically relevant advance? Bracketing these questions, a drug company may well be interested in a drug which clearly qualifies as a me-too drug. The company may be able, by relying on a savvy marketing department, to convince physicians to prescribe, and consumers to request the new one, thus increasing profit for the company without advancing health and well-being.
The majority of clinical research was once conducted by governmental agencies. For example, the US NIH is likely the largest governmental sponsor of clinical research in the world. However, its research budget has declined over the past 20 years (Mervis 2004, 2008), and it is estimated that a majority, perhaps a significant majority of clinical research studies are now conducted by industry: “as recently as 1991 eighty per cent of industry-sponsored trials were conducted in academic health centers…Impatient with the slow pace of academic bureaucracies, pharmaceutical companies have moved trials to the private sector, where more than seventy per cent of them are now conducted” (Elliott 2008, Angell 2008, Miller and Brody 2005).
In addition to transforming the fundamental ethical challenge posed by clinical research, industry sponsored research has the potential to transform the way that many of the specific ethical concerns are addressed within that context. For example, the possibility that investigators and funders may earn significant amounts of money from their participation in clinical research might, it is thought, warp their judgment in ways that conflict with appropriate protection of research participants (Fontanarosa, Flanagin, and DeAngelis 2005). When applied to investigators and funders this concern calls into question the very significant percentage of research funded by and often conducted by for-profit organizations. Skeptics might wonder whether the goal of making money has any greater potential to influence judgment inappropriately compared to many other motivations that are widely accepted, even esteemed in the context of clinical research, such as gaining tenure and fame, impressing one’s colleagues, or winning the Nobel Prize.
Financial conflicts of interest in clinical research point to a tension between relying on profits to motivate research versus insulating drug development and testing from the profit motive as a way of protecting research participants and future patients (Psaty and Kronmal 2008). Finally, if a company can make billions of dollars a year from a single drug, one wonders what constitutes an appropriate response to the participants who were vital to its development. On a standard definition, whether a given transaction is fair depends on the risks and burdens that each party to the transaction bears and the extent to which others benefit from the party’s participation in the transaction (see entry on exploitation ). A series of clinical research studies can result in a company earning tens of billions of dollars in profits. Recognizing that a fair level of benefit is a complex function of participants’ inputs compared to the inputs of others, and the extent to which third parties benefit from those inputs, it is difficult to see how one might fill in the details of this scenario to show that the typically minimal, or non-existent compensation offered to research participants is fair.
At the same time, addressing this potential for exploitation by offering substantial payments to research participants who contribute to especially lucrative studies would introduce its own set of ethical concerns: is payment an appropriate response to the kind of contribution made by research participants; might payment constitute an undue inducement to participate; will payment undermine other participants’ altruistic motivations; to what extent does payment encourage research participants to provide misleading or false information to investigators in order to enroll and remain in research studies?
Like most early clinical research, James Lind’s experiments on treatments for scurvy took place in the clinical setting, with a physician evaluating different possible treatments in his patients. This practice eventually led to concern that it did not offer sufficient protection for patients who were frequently unaware that they were involved in research. And this concern led to separating clinical research from clinical care and subjecting it to significantly more extensive regulations, including independent review and extensive consent. This approach offered greater protections for research participants and likely led to more sophisticated clinical trials as the result of being conducted by dedicated researchers rather than clinicians in their spare time.
This segregation of clinical research from clinical care has also had significant drawbacks. It makes clinical research much more expensive and much more difficult to conduct. Given that studies are conducted in specialized environments, this approach has also undermined to some extent the relevance that the findings of clinical trials have for clinical care. For example, clinical trials assessing possible new treatments for hypertension are conducted in individuals who are aware that the trials exist and take the time to find out what is required to enroll. These studies frequently have trouble enrolling sufficient numbers of participants and take years to complete. At the same time, on the other side of the clinical research/clinical care divide, millions of patients with hypertension who might be eligible for a clinical trial are obtaining care from their doctors. Moreover, the countless data points generated by these encounters, what dose did the patient receive, did they experience any side effects, how long did they last, end up gathering dust in the patients’ medical records rather than being systematically collected and used to inform future practice.
In response, commentators have called for developing learning health care systems. According to one definition, a learning health care system is one in which “science, informatics, incentives, and culture are aligned for continuous improvement and innovation, with best practices seamlessly embedded in the care process, patients and families active participants in all elements, and new knowledge captured as an integral by-product of the care experience” (Committee on Learning 2013). The important point for present purposes is that these commentators are calling for the desegregation of clinical research and clinical care, with clinical research once again being conducted in the context of clinical care. This approach offers what seems a potentially appealing response to the central challenge of justifying the risks to which research participants are exposed. Put simply, this practice would be justified by the fact that the practice of exposing individuals to research risks is an essential component of a learning health care system which continuously evaluates methods of providing clinical care and passes the benefits of the improvements on to its members (Faden et al 2013).
The segregated model of clinical research was designed to protect research participants from exploitation. The primary drawbacks are that it is inefficient and it raises concerns over free riders, allowing patients to realize the benefits of improved clinical care without having to accept any of the risks associated with its generation. Learning health care systems are intended to address the problem of inefficiency. Given that all the members of learning health care systems are possible research participants and also beneficiaries of research, they may, in the process, address concerns over free riders.
The ethical challenge learning health care systems face is whether it is possible to do away with the segregated model, along with its regulations and practices, without reintroducing the potential for participant exploitation. For example, should individuals in a learning health care system be told that their data might be used for research purposes? Should they be notified when it is? To what extent can patients in learning health care systems be exposed to added risks for the purposes of research? Should they be permitted to decline being so exposed? In the end, then, on-going attempts to address the concerns raised by clinical research raise new ethical concerns and, thereby, offer opportunities for philosophers and others looking for interesting, not to mention practically important issues in need of analysis and resolution.
- Angell, M., 2008. “Industry sponsored clinical research: a broken system,” Journal of the American Medical Association , 80: 899–904.
- Appelbaum, P.S., with C.W. Lidz and T. Grisso, 2004. “Therapeutic misconception in clinical research: frequency and risk factors,” IRB: Ethics and Human Research , 26: 1–8.
- Australian Government, National Health and Medical Research Council, 1999. National statement on ethical conduct in research involving humans . Ch 2.1: 92. Commonwealth of Australia, 1999.
- Beecher, H. K., 1966. “Ethics and clinical research,” N Engl J Med , 274: 1354–60.
- Brock, D. W., 1994. “Ethical issues in exposing children to risks in research,” Chapter 3 (pp. 81–101) of Grodin and Glantz (eds.), Children as Research Subjects , New York: Oxford University Press.
- Brody, B.A., 1998. The Ethics of Biomedical Research: An International Perspective , Oxford: Oxford University Press.
- Caldwell, P.H.Y., with S.B. Murphy, P.H. Butow, and J.C. Craig, 2004. “Clinical trials in children,” Lancet , 364: 803–11.
- Caplan, A., 1984. “Is there a duty to serve as a subject in biomedical research?” IRB: Ethics and Human Research , 6: 1–5.
- Committee on the Learning Health Care System in America; Institute of Medicine; Smith M, Saunders R, Stuckhardt L, et al. (eds.), Best Care at Lower Cost: The Path to Continuously Learning Health Care in America , Washington, D.C.: National Academies Press; 2013 May 10.
- Council for International Organizations of Medical Sciences, 2002. International ethical guidelines for biomedical research involving human subjects . Geneva: CIOMS.
- Croghan, T.W., and P.M. Pittman, 2004. “The medicine cabinet: What’s in it, why, and can we change the contents?” Health Affairs , 23: 23–33.
- D’Arcy Hart, P., 1999. “A change in scientific approach: From alternation to randomised allocation in clinical trials in the 1940s,” BMJ , 319: 572–3.
- Department of Health and Human Services, 2005. Code of Federal Regulations : Title 45 (Public Welfare). Part 46: protection of human subjects (45 CFR 46). US Government Printing Office.
- Dworkin, R., 1989, “The original position,” in Reading Rawls , Norman Daniels (ed.), Stanford: Stanford University Press, 16–53.
- Dworkin, G., 2005. “Paternalism,” in Stanford Encyclopedia of Philosophy (Winter 2005 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/win2005/entries/paternalism/ >.
- Elliott, C., 2008, “Guinea-pigging,” The New Yorker , January 7, 2008, p. 36.
- Emanuel, E.J., with D. Wendler and C. Grady 2000. “What makes clinical research ethical?,” Journal of the American Medical Association , 283: 2701–11.
- Faden, R.R., and T.L. Beauchamp, 1986. A History and Theory of Informed Consent , New York: Oxford University Press, pp. 200–232.
- Faden, R.R., N.E. Kass, S.N. Goodman, P. Pronovost, S. Tunis, and T.L Beauchamp. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics , Hastings Center Report 2013; Spec No: S16-27.
- Featherstone, K., and J.L. Donovan, 2002. “Why don’t they just tell me straight, why allocate it? The struggle to make sense of participating in a randomised controlled trial,” Social Science and Medicine , 55: 709–19.
- Feinberg, J., 1986. Harm to Self , Oxford: Oxford University Press, pp. 3–26.
- Field, M.J., and R.E. Behrman, 2004. The Ethical Conduct of Clinical Research Involving Children , Washington DC: National Academies Press, Ch. 2.
- Flory, J., and E. Emanuel, 2004. “Interventions to improve research participants’ understanding in informed consent for research: A systematic review,” Journal of the American Medical Association , 292: 1593–1601.
- Fontanarosa P.B., with A. Flanagin A. and C.D. DeAngelis, 2005. “Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies,” Journal of the American Medical Association , 294: 110–11.
- Freedman, B., 1987. “Equipoise and the ethics of clinical research,” The New England Journal of Medicine , 317: 141–45.
- Friedman M., 1970, “The social responsibility of business is to increase its profits,” The New York Times Magazine , September 13, 1970.
- Garattini S., 1997. “Are me-too drugs justified?,” J Nephrol , 10: 283–94.
- Gauthier, D., 1990. Morals by Agreement , Oxford: Clarendon Press.
- Gifford, F., 2007. “Pulling the plug on clinical equipoise: A critique of Miller and Weijer,” Kennedy Institute of Ethics Journal , 17: 203–26.
- Goodyear, M.D., with K. Krleza-Jeric and T. Lemmens, 2007. “The Declaration of Helsinki,” BMJ , 335: 624–5.
- Grady, C., 2005. “Payment of clinical research subjects,” J Clin Invest , 115: 1681–7.
- Griffin, J., 1986. Well-being: Its Meaning, Measurement and Moral Importance , Oxford: Clarendon.
- Grodin, M.A., and G.J. Annas, 1996. “Legacies of Nuremberg: Medical ethics and human rights,” Journal of the American Medical Association , 276: 1682–83.
- Harmon, A., 2010. “Indian Tribe Wins Fight to Limit Research of Its DNA,” New York Times , 21 April 2010.
- Harris, J., 2005. “Scientific research is a moral duty,” Journal of Medical Ethics , 31: 242–48.
- Hayenhjelm, M., and J. Wolff, 2012. “The moral problem of risk impositions: A survey of the literature,” European Journal of Philosophy , 20 (Supplement S1): E26–E51.
- Hellman, S., and D.S. Hellman, 1991. “Of mice but not men: Problems of the randomized clinical trial,” The New England Journal of Medicine , 324: 1585–89.
- Heyd, D., 1996. “Experimentation on trial: Why should one take part in medical research?”, Jahrbuch fur Recht und Ethik [Annual Review of Law and Ethics] , 4: 189–204.
- Huskamp, H.A., 2006. “Prices, profits, and innovation: Examining criticisms of new psychotropic drugs’ value,” Health Affairs , 25: 635–46.
- Jonas, H., 1969. “Philosophical reflections on experimenting with human subjects”, Daedalus , 98: 219–247.
- Katz, J., 1996. “The Nuremberg Code and the Nuremberg trial. A reappraisal,” Journal of the American Medical Association 276: 1662–6.
- Kopelman, L.M., 2000. “Children as research subjects: A dilemma,” Journal of Medicine and Philosophy , 25: 745–64.
- Kupst, M.J., with A.F. Patenaude, G.A. Walco, and C. Sterling, 2003. “Clinical trials in pediatric cancer: Parental perspectives on informed consent,” Journal of Pediatric Hematology and Oncology , 25: 787–90.
- Lazarou, J., with B.H. Pomeranz and P.N. Corey, 1998. “Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies,” Journal of the American Medical Association ; 279: 1200–05.
- Lederer, S.E., 1995. Subjected to Science: Human Experimentation in America before the Second World War . Baltimore: Johns Hopkins University Press.
- –––, 2003, “Children as guinea pigs: Historical perspective,” Accountability in Research , 10(1): 1–16.
- Lederer, S.E., and M.A. Grodin, “Historical overview: Pediatric experimentation,” in M.A. Grodin and L.H. Glantz (eds.), Children as Research Subjects: Science, Ethics and Law , New York: Oxford University Press, 1994.
- Levine, R.J., 1988. Ethics and Regulation of Clinical Research . 2nd ed. New Haven, Conn: Yale University Press.
- Macklin, R., 1981. “Due and undue inducements: On paying money to research subjects,” IRB: A Review of Human Subjects Research , 3: 1–6.
- Mervis, J., 2004. “U.S. Science budget: Caught in a squeeze between tax cuts and military spending,” Science , 30: 587.
- –––, 2008. “U.S. Budget: Promising year ends badly after fiscal showdown squeezes science,” Science , 319: 18–9.
- Mill, John Stuart, 1869, On Liberty . Page reference to On Liberty and Other Writings , Stefan Collini (ed.), Cambridge, Cambridge University Press, 2005, 12th edition.
- Miller, F.G., and H. Brody, 2007. “Clinical equipoise and the incoherence of research ethics,” Journal of Medicine and Philosophy , 32: 151–65.
- Miller, F.G., and A. Wertheimer, 2007. “Facing up to paternalism in research ethics,” Hastings Center Report , 37: 24–34.
- Miller, P.B., and C. Weijer, 2006. “Trust based obligations of the state and physician-researchers to patient-subjects,” Journal of Medical Ethics , 32: 542–47.
- National Bioethics Advisory Commission (NBAC), 2001. Ethical and Policy Issues in Research Involving Human Participants . Washington, DC: NBAC.
- Nicholson, R.H., 1986. Medical Research with Children: Ethics, Law, Practice . Oxford: Oxford University Press. Pages 87–100.
- Nuremberg Code, 1947, in Trials of war criminals before the Nuremberg Military Tribunals under Control Council Law No. 10 , Vol. 2, Washington, D.C.: U.S. Government Printing Office, 1949, pp. 181–182. Reprinted in Journal of the American Medical Association , 276: 1961.
- Psaty, B.M., and R.A. Kronmal, 2008. “Reporting mortality findings in trials of rofecoxib for Alzheimer disease or cognitive impairment: A case study based on documents from rofecoxib litigation,” Journal of the American Medical Association , 299: 1813–7.
- Ramsey, P., 1978. “Ethical dimensions of experimental research on children”, in Research on Children: Medical Imperatives, Ethical Quandaries, and Legal Constraints , J. van Eys (ed.), Baltimore: University Park Press, p. 61.
- Rawls, J., 1999. A Theory of Justice . Cambridge, Mass: Belknap Press of Harvard University Press.
- Resnik, D.B., 2005. “Eliminating the daily life risks standard from the definition of minimal risk,” Journal of Medical Ethics , 31: 35–8.
- Rid, A., and D. Wendler, 2011. “A framework for risk-benefit evaluations in biomedical research,” Kennedy Institute of Ethics Journal , 21(2): 141–179.
- Roberts, R., with W. Rodriquez, D. Murphy, and T. Crescenzi, 2003. “Pediatric drug labeling: Improving the safety and efficacy of pediatric therapies,” Journal of the American Medical Association , 290: 905–11.
- Ross, L.F., and R.M. Nelson, 2006. “Pediatric research and the federal minimal risk standard,” Journal of the American Medical Association , 295: 759.
- Rothman, D.J., 2000. “The shame of medical research”, The New York Review of Books , 47 (19): 60–64.
- Sachs, Ben, 2010. “The exceptional ethics of the investigator-subject relationship,” Journal of Medicine and Philosophy , 35: 64–80.
- Shuster, E., 1997. “Fifty years later: The significance of the Nuremberg Code,” The New England Journal of Medicine , 337: 1436–40.
- Slovic, P., 1987. “Perception of risk,” Science , 236: 280–85.
- Snowdon, C., with J. Garcia, and D. Elbourne, 1997. “Making sense of randomization: Responses of parents of critically ill babies to random allocation of treatment in a clinical trial,” Social Science and Medicine , 45: 1337–55.
- Spilker, B., 1991. Guide to clinical trials , Philadelphia: Lippincott, Williams and Wilkins.
- Stark, C., 2000. “Hypothetical consent and justification,” Journal of Philosophy , 97: 313–34.
- Stewart, Paul M., with Anna Stears, Jeremy W. Tomlinson, and Morris J. Brown, 2008. “Regulation—the real threat to clinical research,” British Medical Journal , 337: 1085–1087.
- Sullivan, Richard, 2008. “The good, the bad and the ugly: Effect of regulation on cancer research,” Lancet Oncology , 9: 2–3.
- Sutton, G., 2003. “Putrid gums and ‘dead men’s cloaths’: James Lind aboard the Salisbury,” Journal of the Royal Society of Medicine , 96: 605–8.
- Tversky, A., and D. Kahneman, 1974. “Judgments under uncertainty: Heuristics and biases,” Science , 185: 1124–31.
- –––, 1981, “The framing of decisions and the rationality of choice,” Science , 211: 453–8.
- Vollmann J., and R. Winau, 1996. “Informed consent in human experimentation before the Nuremberg code,” British Medical Journal , 313: 1445–7.
- Weinstein, N., 1989. “Optimistic biases about personal risks,” Science , 246: 1232–3.
- Wenar, L., 2008. “John Rawls”, The Stanford Encyclopedia of Philosophy (Summer 2008 Edition) , Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/sum2008/entries/rawls/ >.
- Wendler, D., 2010, The Ethics of Pediatric Research , Oxford: Oxford University Press.
- Wertheimer, A., 2008. “Exploitation”, The Stanford Encyclopedia of Philosophy (Fall 2008 Edition), Edward N. Zalta (ed.), URL = < https://plato.stanford.edu/archives/fall2008/entries/exploitation/ >.
- –––, 2010, Rethinking the Ethics of Clinical Research: Widening the Lens , Oxford: Oxford University Press.
- Wilson, James, and David Hunter, 2010. “Research exceptionalism,” American Journal of Bioethics , 10: 45–54.
- World Medical Organization, 1996. Declaration of Helsinki , British Medical Journal, 313 (7070): 1448–1449.
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
- Bioethics.net , organized by the editors of the American Journal of Bioethics.
- Bioethics Literature Database , German site for conducting searches
- National Reference Center for Bioethics Literature , organized by the research library at the Kennedy Institute of Ethics
- Nuffield Council on Bioethics , organized by the Nuffield council, the preeminent organization on ethics in Britain
- Office for Human Research Protections (OHRP), Department of Health and Human Services, website of the office that oversees U.S. research regulations.
- PRIM&R , Public Responsibility in Medicine and Research
- SARETI , The South African Research Ethics Training Initiative
cloning | contract law, philosophy of | decision-making capacity | exploitation | health | informed consent | original position | paternalism | Rawls, John | risk
Copyright © 2021 by David Wendler < dwendler @ nih . gov >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
- Open access
- Published: 19 March 2024
Ethical challenges in global research on health system responses to violence against women: a qualitative study of policy and professional perspectives
- Natalia V. Lewis 1 na1 ,
- Beatriz Kalichman 2 na1 ,
- Yuri Nishijima Azeredo 2 ,
- Loraine J. Bacchus 3 &
- Ana Flavia d’Oliveira 2
BMC Medical Ethics volume 25 , Article number: 32 ( 2024 ) Cite this article
321 Accesses
4 Altmetric
Metrics details
Studying global health problems requires international multidisciplinary teams. Such multidisciplinarity and multiculturalism create challenges in adhering to a set of ethical principles across different country contexts. Our group on health system responses to violence against women (VAW) included two universities in a European high-income country (HIC) and four universities in low-and middle-income countries (LMICs). This study aimed to investigate professional and policy perspectives on the types, causes of, and solutions to ethical challenges specific to the ethics approval stage of the global research projects on health system responses to VAW.
We used the Network of Ethical Relationships model, framework method, and READ approach to analyse qualitative semi-structured interviews ( n = 18) and policy documents ( n = 27). In March-July 2021, we recruited a purposive sample of researchers and members of Research Ethics Committees (RECs) from the five partner countries. Interviewees signposted policies and guidelines on research ethics, including VAW.
We developed three themes with eight subthemes summarising ethical challenges across three contextual factors. The global nature of the group contributed towards power and resource imbalance between HIC and LMICs and differing RECs’ rules. Location of the primary studies within health services highlighted differing rules between university RECs and health authorities. There were diverse conceptualisations of VAW and vulnerability of research participants between countries and limited methodological and topic expertise in some LMIC RECs. These factors threatened the timely delivery of studies and had a negative impact on researchers and their relationships with RECs and HIC funders. Most researchers felt frustrated and demotivated by the bureaucratised, uncoordinated, and lengthy approval process. Participants suggested redistributing power and resources between HICs and LMICs, involving LMIC representatives in developing funding agendas, better coordination between RECs and health authorities and capacity strengthening on ethics in VAW research.
Conclusions
The process of ethics approval for global research on health system responses to VAW should be more coordinated across partners, with equal power distribution between HICs and LMICs, researchers and RECs. While some of these objectives can be achieved through education for RECs and researchers, the power imbalance and differing rules should be addressed at the institutional, national, and international levels. Three of the authors were also research participants, which had potential to introduce bias into the findings. However, rigorous reflexivity practices mitigated against this. This insider perspective was also a strength, as it allowed us to access and contribute to more nuanced understandings to enhance the credibility of the findings. It also helped to mitigate against unequal power dynamics.
Peer Review reports
Introduction
Violence against women (VAW) is a global public health and clinical problem leading to increased mortality and morbidity among women and their children [ 1 ]. Globally, 27% of ever-partnered women aged 15–49 years have experienced physical and/or sexual intimate partner violence in their lifetime, with 13% experiencing it in the past year. Low-income countries reported higher prevalence compared with high-income countries [ 2 ]. Health systems have a crucial role in a multisector response to VAW through identifying and supporting people who have experienced violence [ 3 ]. Prior research identified considerable system-, organisation-, and individual- level barriers to health system responses to VAW, especially in low-income and middle-income countries (LMICs) [ 4 ] and proposed a framework for improving health system readiness to address VAW [ 5 ]. In the past decade, governments and other funders in high-income countries (HIC) made substantial investments in global research addressing the Sustainable Development Goals, including elimination of VAW [ 6 ].
Studying VAW as a global public health and clinical problem requires collaboration between researchers from different disciplines and countries. Such multidisciplinary and multiculturalism create challenges in adhering to a single set of ethical standards applied across differing country-specific contexts characterised by power and resource inequalities. Research activities happen in the contexts which reflect both global and local cultural and social dynamics, with research ethics regulations varying not only across countries but also across fields of knowledge which means that multidisciplinary multicounty research is bound to face specific challenges. Members of global research groups are embedded within their teams and organisations which have differing resources, structures, cultures and politics. The organisations are influenced by the differing economic, social, and political environments. The provision of funding and research capacity from HICs to LMICs exacerbates existing power imbalances. Which ethical standards hold precedence – those developed by the international community, the HIC funder and grant holding institution, the LMICs where the research is taking place, or all the above?
Studies on VAW fall into the category of sensitive topics because they impose additional emotional burden and threat to physical and social self of participants and researchers. The sensitivity of the VAW research is also determined by the exploration of culturally and politically rooted issues of social control, coercion and domination, interests of powerful people, the ‘sacred’ concepts of family relations and power, and the lived realities of people who have experienced or used violence [ 7 ]. The increased sensitivity surrounding the topic of VAW gives rise to additional ethical dilemmas concerning the principles of respect for persons, confidentiality, justice, beneficence, and nonmaleficence [ 8 ]. Global groups studying health system response to VAW should resolve these dilemmas while applying international-, funder-, and country-specific ethical requirements to the sociocultural and economic context, VAW services and health systems in LMICs. How can LMIC researchers adhere to all the ethical requirements while protecting their cultural diversity and the safety of their research participants, communities, and researchers?
Previous studies have acknowledged ethical challenges in global research [ 9 ] including studies on VAW [ 8 , 10 ]. Ethical and methodological challenges in global research on VAW are interlinked and both can undermine the quality of the data and findings [ 11 , 12 ]. Recent theoretical developments equipped researchers with tools for exploring and addressing ethical challenges in global research. Reid et al. [ 13 ] created the ‘4Ps’ model: Place, People, Principle and Precedent—for analysing and developing solutions to ethical conflicts in global research. Morrison et al. [ 14 ] developed the Network of Ethical Relationships (NER) model in the context of global population health research. NER identified relational challenges within research teams, with Research Ethics Committees (RECs), funders, and participants which were embedded in the complex and conflicting normative framework regarding HIC and LMIC legal rules, societal norms, moral values, and institutional rules. The ethical relationship challenges were explained by differing cultural backgrounds, REC requirements and participant values, conflicting requirements between HIC and LMIC RECs and funding procedures. However, to our knowledge, no studies have addressed ethical challenges in global research on health system response to VAW. This study aimed to investigate professional and policy perspectives on the types, causes of, and solutions to ethical challenges specific to the REC approval stage of a global research programme on health system responses to VAW.
Study context: the global health research group
This paper draws on our experience as a global research group on health system responses to VAW in LMICs. The partnership included two universities in a European HIC and four LMIC universities (one South American, one in the Middle-Eastern region, and two in different South Asian countries). The group, funded by the government agency in the European HIC, aimed to: (i) develop and pilot-test LMIC-specific interventions in sexual and reproductive health services addressing VAW, (ii) strengthen the research capacity of HIC and LMICs universities, (iii) evaluate capacity strengthening activities (Fig. 1 ).
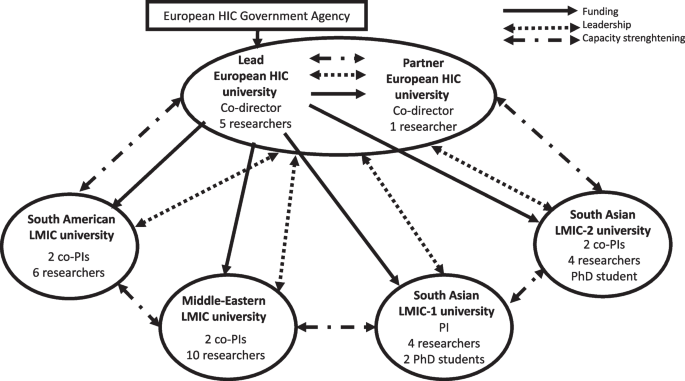
Global research group on health system responses to violence against women in low- and middle-income countries
The countries were diverse amongst themselves, with different health systems and research infrastructures, which shaped all aspects of the research, including ethics approval. At macro level, the HIC funding agency dictated the financial and management structure of the global research group. The funder created conditions for maintaining power at the lead contracting university in the HIC, which held the grant and distributed funds quarterly to the five partner universities. Two group co-directors were also based at the HIC universities. Each LMIC university had a local principal investigator or co-principal investigators with a team of researchers and PhD students responsible for country-specific primary studies. HIC researchers supported the study designs and methodological development, were involved in the capacity strengthening workstream, and led on syntheses of findings from LMIC primary studies.
At meso level, the composition of the group also created conditions for maintaining power at HIC universities because their researchers had more methodological expertise and experience. However, researchers in both HIC universities and two LMIC universities brought together extensive expertise and experience in the VAW topic. We proactively explored and addressed potential power imbalances through meetings and research capacity strengthening activities. As described in a separate publication, we carried out a baseline evaluation and mapping exercise of the research capacities within and across all country teams [ 15 ]. The evaluation showed that while the HIC teams included more mid-career and senior researchers with extensive methodological expertise in health system responses to VAW, their knowledge of the health systems and socio-cultural-historical contexts in the partner LMICs was limited. In contrast, LMICs teams had a greater proportion of early career researchers and less expertise in some research methods. However, they were well embedded within the local communities and health care systems where the primary research was conducted. They also possessed a high degree of local knowledge regarding power dynamics between different stakeholders, processes for engaging with them, and political and cultural sensitivity. It is important to acknowledge that the power imbalance was partly influenced by the nature of the work being done by the different teams. The LMICs partners were primarily responsible for fieldwork, a role typically assigned to early career researchers, whereas the HIC partners focussed more on supporting instrument development, data analysis, and capacity strengthening workpackage requiring researchers with greater experience.
To reflect on the power imbalances between the teams, we organised a participatory workshop with researchers from all country teams [ 15 ]. We agreed on shared values, identified barriers, and planned capacity strengthening activities. The shared values included: mutual learning, respect, fair opportunity, clear boundaries, honesty, and transparency. LMIC researchers identified barriers related to limited methodological expertise, access to training courses, information technologies and English-language skills for academic writing which we targeted through the capacity strengthening activities. We mapped areas of methodological expertise within countries and identified opportunities for mentoring and mutual learning across partners. For example, the South American team which was involved in developing ethical principles for the WHO Multi-Country Study on Women’s Health and Domestic Violence Against Women [ 16 ] delivered ethics training to the whole group. Researchers from the Middle-Eastern and European teams co-delivered a workshop on measurement and routinely collected data. Researchers from the South American and South Asian teams co-led a workshop on how we use feminist principles and theory in our research on VAW. Mutual learning took place through joint development of protocols and research tools for primary studies, early career researchers virtual peer support and education group, virtual monthly team meetings, and annual face-to-face and hybrid workshops in partner countries.
During one of the group meetings, an LMIC researcher raised concerns about the group adopting terminology used by HIC policy makers and funders which perpetuated the existing power imbalance. They argued that term “capacity building” implied a lack of research capacity in LMICs which they found disempowering. This conflicted with the shared values of respect and mutual learning within our group, as well as the extensive experience in VAW research present in the South American and South Asian partner universities. As a result, we revised our terminology and replaced the term ‘capacity building’ with ‘capacity strengthening’.
From the start, the group made efforts to carry out equitable work despite inequitable conditions, whilst navigating variations in institutional research ethics review requirements, and adhering to diverse regulations imposed by academic and health system institutions. To conduct primary studies, we had to obtain ethics approvals from two HIC and four LMIC universities, as well as additional approvals from the health authorities in all LMICs. This process highlighted power dynamics between the different countries and institutions involved. Tensions arose because of the disparate policies and practices. We encountered challenges that were not previously reported in literature. The two HIC university RECs had conflicting requirements regarding the sequence of ethics approvals among group partners. The REC at the lead HIC university encouraged a local ethics review where possible because the local REC would possess the most relevant expertise to assess the ethics application for research undertaken in the country concerned. This approach aimed to prevent contradictory responses from two separate REC decisions. In contrast, the second HIC university insisted that their REC would require reviewing all studies involving their staff, irrespective of the country involved and whether a local review was already being conducted. They required the local ethics approvals to be provided for their final decision. The conflicting requirements had repercussions on the timely execution of the primary studies in LMICs. The lead HIC REC advised that if the LMIC teams have undergone a local research ethics review and received a favourable ethical opinion, they could commence the research activities specified in their ethics applications and favourable opinions. In contrast, the other HIC REC insisted that the project should not commence until full ethical approval had been obtained from their university, alongside local ethical approval.
Another challenge at the meso level arose from the differing policies and practices for research data management. The HIC funder and two HIC university RECs requested detailed data management plans compliant with the European Union General Data Protection Regulation. However, partners in LMIC were unable to fully comply with the same standards due to different legal requirements in their respective countries and varying policies and processes for research data management within their universities. As a temporary solution, the lead HIC university granted all LMIC principal investigators/co-investigators and their researchers an honorary status enabling them to access secure departmental file storage. The partners signed Data Sharing Agreement and Data Repository Agreement for using Research Data Repository at the lead HIC university for storing and sharing research data which underpinned outputs from the primary studies.
Study design
The international team of researchers with backgrounds in psychology (NVL, YNA), policy (BK), medicine (AFDO, NVL), and social science (LJB) conducted a qualitative study comprising of semi-structured interviews and a document review of ethics policies and guidelines. Our positionality in the critical realism ontology [ 17 ] and feminist epistemologies and methodologies [ 18 ] influenced the choice of a qualitative research design to explore the contextual factors and processes shaping researchers’ and REC members’ experiences during the ethics approval phase of global research projects on health system responses to VAW. Our approach was also informed by discourses on decolonising global health research [ 19 ] and epistemic injustice in academic global health [ 20 ]. We recognised the existence of international and institutional hierarchies, that (post)colonial legacies shape the field of global research on VAW and that systemic changes are needed to shift the hierarchies of power [ 19 ]. We believed that the experiences and views of the HIC and LMIC researchers and participants were equally credible. We acknowledged that researchers and participants would impact on each other, and that the researchers’ backgrounds would influence data production and analysis. We challenged epistemic injustice through fostering co-creation of knowledge by researchers and study participants with similar experiences. The authors who conducted interviews (NVL, BK) were members of the same global health group; three authors (NVL, LJB, AFDO) were also interviewed as research participants. These authors were not involved in the analysis of their transcripts.
We followed the READ (ready your material, extract data, analyse data, distil your findings) approach for document review [ 21 ] and the framework method [ 22 ] for data analysis. While interviews explored individual experiences of ethics approval for global research in health system responses to VAW, review of policies and guidelines allowed to contextualise these experiences. Concepts within the NER model were used as sensitising devices which informed the analysis [ 14 ].
Data collection
We conducted online semi-structured qualitative interviews in March-July 2021. Data set size for interviews was informed by the model and concept of information power [ 23 ]. We assumed that our study would need less participants because of the narrow aim, high specificity of participants for the study aim, established NER model, strong interview dialog, and cross-case analysis.
We used purposive sampling strategy to recruit researchers and REC members with rich and diverse experience of ethics approvals for global research, representing five partner countries in our global health research group. The study was advertised via an email sent to the group mailing list and snowballed via professional networks. Interested individuals emailed study researchers who confirmed eligibility, provided further information, and arranged interviews on Zoom/Teams. Interviews were conducted in the language of choice of the interviewees. Participants provided verbal informed consent. The topic guides explored experiences of applying ethics policies and guidelines in practice, following REC processes, obtaining ethics approvals, challenges faced, and proposed causes and solutions (Additional file 1 ). Interviews were audio recorded, professionally transcribed, checked, and anonymised.
We identified policies and guidelines on research ethics through interview participants, electronic searches, and reference checking. Two researchers (NVL, BK) searched websites of the HIC and LMIC universities and RECs involved in the research, as well as of HIC funders and think tanks using terms “violence” “women”, “ethic*”, “guideline”. We retrieved, screened, and selected documents meeting our inclusion criteria: international, national, and institutional policies and guidelines from the five partner countries that discussed global research and/or research on VAW.
We started data analysis while conducting interviews to refine topic guides for further interviews and to identify additional documents. For the document review, we customised an Excel proforma [ 21 ] to extract data on title, author, year, source, target audience, key messages, data relevant to global research and studies on VAW. During data extraction, we made notes about how each document addressed ethical issues in global research and/or research on VAW. Interview transcripts and documents were imported into NVIVO 12 for data management and coding. The analysis was conducted using a combination of inductive and deductive approaches. Researchers (NVL, BK, AFDO) worked on their subsets of transcripts and documents in English and local language. These researchers read and re-read two interview transcripts and independently manually coded text relevant to the research questions. The researchers compared initial codes and developed a ‘working analytical framework’ which they then applied to their subsets of transcripts and documents in NVIVO [ 22 ]. The framework was refined through four cycles of revisions during coding process. We then grouped our codes into candidate themes, mapped them on the constructs of the NER model in an Excel framework matrix in English, and developed final analytical themes. Researchers (NVL, BK) wrote descriptive accounts of the analytical themes with illustrative quotes. The study team met regularly to discuss the codes, themes, matrix, and descriptive accounts paying attention to the similarities and differences within and between interviews and documents, countries, and institutions.
We conducted 18 interviews with researchers ( n = 11) and REC members ( n = 7) representing all five partner countries and a wide range of professional experience (2–25 years) (Table 1 ).
Interviews in English ( n = 15) and local language ( n = 3) lasted between 27 and 80 min (mean 46 min). Despite support from local researchers, we could not recruit REC members from one South-Asian LMIC. When approached, REC members declined participation explaining that the study was not supported by their institutional and national REC and that it is difficult to speak about issues and challenges which may be against their government. In addition, they felt that this study should have been conducted in collaboration with their RECs and some of the members as co-authors (email correspondence). In contrast, REC at the lead HIC university, transferred our ethics application to a different faculty to prevent conflict of interest.
We analysed 27 documents (4 international, 17 national, 6 institutional), that were categorised into educational material, guidelines, legal documents, policy documents, reports, standard operational procedures, and statements (Table 2 ).
The reports by HIC funders produced in partnership with researchers from different countries included analysis of global inequalities and consent. The HIC was the only country that wrote documents detailing how to operate as an international research funder, although LMIC had documents detailing international partnerships. Two LMICs had national ethics regulations while all other partners had institutional documents.
Interview participants signposted the same high-level policies and guidelines on ethics in global research: the Helsinki declaration [ 24 ], the Council for International Organizations of Medical Sciences’ (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Subjects [ 25 ] and Nuffield Council’s guidelines [ 26 ]. National and institutional research ethics policies and guidelines were built on the international principles and standards which were tailored to the local context. All guidelines for global research stipulated compliance with international and national laws and regulations and required ethics approvals in countries where research activities took place and in the country funding the study. Documents from HICs and LMICs highlighted the importance of respecting local societal norms, conducting research that benefits local communities and strengthens local capacities.
Our framework analysis generated 20 thematic codes, 12 candidate themes, and 3 final analytical themes summarising ethical challenges at the approval stage resulting from the global nature of the group, location of primary studies within health systems, and VAW topic. Within each theme, we reported perspectives on causes, impact, and solutions across the interviews and documents (Table 3 , Fig. 2 ).
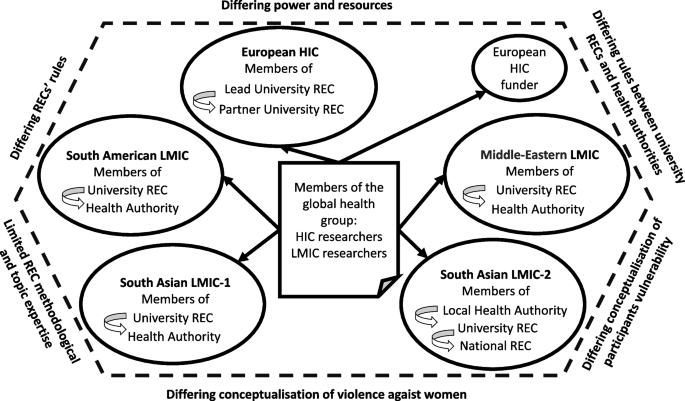
NER model for global research on health system responses to violence against women, research ethics committees’ approval stage
Challenges resulting from the global nature of the group
Location of research collaborators in HIC and several LMICs contributed to the challenges caused by factors in the following areas.

Differing power and resources
Documentary and interview data suggested that hierarchical power imbalance between HIC and LMIC countries could be a contextual factor at the macro and meso levels. The power imbalance contributed towards ambiguity and frustration among researchers and REC members, and tensions between HIC and LMIC partners and between researchers, RECs and funders. The Nuffield report emphasised issues of power imbalance and the possible differences and conflicts between ethics committees in different countries [ 26 ]. In contrast, major European HIC funders of global research imposed ethics standards and processes that were based on their national laws as a global benchmark:
“[HIC funder name] is governed by [HIC name] law. The legislation supporting this policy relates to work carried out in the [HIC name]. We expect researchers to use similar standards and principles for any research outside the [HIC name]." ([HIC funder] Policy 2021).
Most interview participants perceived power imbalance between HICs (i.e., funder) and LMICs as a macro-level barrier. Some researchers and REC members felt that HIC policy makers and funders imposed their research agenda on LMICs, used funding as a mechanism for compliance, and set ethics regulations that did not suit the local context or were difficult to comply with because of limited research capacity and differing structures and resources in LMICs:
“It is much more likely when the project is managed by a general PI [principal investigator] of an institution from the global north, with funding from the global north, [that] countries from the global south that often participate with research participants and less with the thinking people, have much more difficulty to establish the limits and characteristics, the local peculiarities. So, I think it's more of a question of politics and power within research than the questions of Ethics Committees.” Interviewee 7, University REC and Hospital REC member, South American LMIC.
Some interview participants perceived the setting of funding priorities for global research by HICs without engaging with LMIC researchers and policy makers as a way of recolonising their research agenda. It was proposed that the solution was to engage local communities in research priority setting with funders:
“The only research funding is from international aid. Agencies become bureaucracies. In countries like our government doesn’t dictate policy, doesn’t set policy, it’s international aid that does. They dictate. Environment, this, that, that, what environment for god’s sake? We have so many wars here, every time we rebuild it gets destroyed. They set it in relation to their own priorities. It didn’t used to be like that. It used to be that agencies came, discussed, etc. and we together did things. Now, everything is on website, take it or leave it. The ethics is part and parcel of this approach of trying to say, “Wait a minute, do not colonise us that way too.” Interviewee 11, University REC member, Middle-Eastern LMIC.
While HIC funders mandated "that research performed in partner countries is conducted in accordance with regulations and to a standard no less stringent than those applicable in the [European HIC]" ([HIC funder] Policy 2021), some interviewees thought it was problematic due to the lack of consideration for the LMIC context in which research is being conducted. For example, LMIC and HIC researchers agreed that some LMIC universities did not have policies, processes, and resources for implementing stringent HIC requirements for data management. While their local RECs scrutinised the application sections about study design, methods, funding, they did not request the detailed data management plan which was an essential part of the HIC ethics applications.
Differing RECs rules
The international and local policies and guidelines required ethics approvals from RECs in the funder country and in the countries where research activities took place. At meso level, the power was in the hands of multiple RECs in HIC and LMICs. Each REC interpreted and applied the universal ethics principles and international policies and guidelines differently. This resulted in the challenge of ‘differing RECs rules’ with varied requirements, processes, and timeframes which lacked coordination and challenged timely delivery of primary studies across LMICs. Researchers had to ‘problem-solve’ ethical approval conundrums themselves because RECs did not talk to each other and exercised power through standardisation of their approval process which many researchers described as highly bureaucratised, predominantly biomedical, and severely outdated. The power was in the RECs hands and researchers had to abide by the RECs rules with which they often did not agree. To mitigate these tensions, researchers used informal support from more experienced colleagues in their teams and partner countries, adapted study documentation previously approved by their RECs, and submitted ethics applications to the RECs “where you know people to make it easy” (Interviewee 13, University researcher, South-Asian LMIC). REC members followed their in-house standard operating procedures and best practice examples of previously approved research projects and felt that they adequately supported researchers throughout the application process. REC members thought that they treated local and global projects equally. They also thought that the international studies were more trustworthy because they had a higher level of funder scrutiny and the ability to recruit the best local experts.
Interviewees suggested measures at the macro level for mitigating power imbalances between HICs and LMICs. They proposed proactively lobbying HIC funders about LMIC priorities "to rethink research ethics in a way that is compatible with our local and regional [context]" (Interviewee 11, University REC member, Middle-Eastern LMIC). At the meso level, interviewees highlighted the importance of mutual learning and respecting country-specific contexts, transparent communication, and agreed partnership-wide practices for country-specific informed consent, safeguarding, data management, and helping each other with developing ethics applications and responding to RECs queries:
“Particularly in a global context, learning from others, because there is often a perception that it might be what people may consider the gold standard. It may not be, there may be more innovative ways to manage ethics and other regulatory approval processes from our global partners”. Interviewee 3, University REC member, European HIC.
To comply with the HIC funder’s requirements and improve their data safety, LMIC researchers wanted local policies for data management, additional funding to buy encrypted equipment and secure data storage, and education for local RECs and researchers on data management plans:
“…lobby and advocate for a strict data governance section within the [ethics application] pro forma that the ethical committee has." Interviewee 18, University researcher, South-Asian LMIC.
Challenge resulted from the location of primary studies within health system
Location of primary studies in LMIC health care services was a contextual factor at the meso level which contributed to the challenge of reconciling the differing requirements and rules of both the university REC and health authority REC.
Differing rules between university RECs and health authorities
Some LMIC researchers identified differing rules between university RECs and health authorities as a barrier which caused ambiguity and delays in local ethics approvals. To conduct research with health care professionals and patients, it was necessary to obtain ethics approvals from academic REC and ethics and/or regulatory approvals from the relevant health authority (e.g., Ministry of Health, Municipal health authority, healthcare setting). In two LMICs, this parallel process created an extra challenge for researchers. They experienced confusion, frustration, and delays because the two bodies had differing perspectives on the same issues and their approval processes were not coordinated:
“A problem that I always have, the university has a standard informed consent, and they understand that informed consent starts with a lot of data on the interviewee. When they send it to the municipality, the municipality says to me, "Oh, this is no good, this informed consent, we don't like it. You can't ask for all of this information," and I agree. Then I have to do something in between, because I have to negotiate with the two agencies, they ask for different things.” Interviewee 5, University researcher, South-American LMIC.
The conflicting rules could be explained by the varying perceived roles and responsibilities among research and health care approval bodies. Although REC members from universities and health authorities felt that they were responsible for the safety of research participants, the latter thought that they had better knowledge of their services and therefore an additional responsibility for the research participants as service users:
“Our concern is with protection of the users of our healthcare system within our jurisdiction, this is our chief concern. Even for very simple research the most important thing is how the municipality treat its healthcare service users. What guides us is mainly what the healthcare system means here in our city, because that is what we work with, the healthcare system users as research participants. So what guides us is the healthcare service, its logic, it’s dynamic, a research project can’t muddle with the services' dynamic or the work of the healthcare professionals.” Interviewee 6, Municipal REC member, South American LMIC.
Interviewees wanted more coordination between university and health system RECs which would harmonise and expedite the two approval processes.
" a coordination between the ethics board and the ministries itself. Like some kind of internal platform between the [cabinet work] and the policy level. I wish there was something like that so that the process would be a bit easier. Or some person from the ethics itself would be more cooperative and would help us to coordinate with them somehow so that the bureaucratic process is shortened." Interviewee 17, University researcher, South-Asian LMIC.
Challenges resulted from the VAW topic
VAW as research topic was another factor at the macro and meso levels which required additional labour, time and resources for obtaining ethics approvals in LMICs. Documents and interview data identified additional VAW-specific challenges in the following areas.
Differing conceptualisation of VAW
Sometimes the funder’s conceptualisation of VAW as a research topic differed from the local REC and researchers view due to the country-specific societal norms and political situation, resulting in ambiguity and frustration for researchers and REC members. One REC member from the LMIC experiencing protracted armed conflict thought that the conceptualisation of VAW in their country had been shaped primarily through the views of international aid agencies/research funders, with gender often being a substitute word for women and VAW being researched as an interpersonal problem in isolation from the chronic violence at the community and society levels. Such narrow conceptualisation could influence the choice of the research tools and produce biased findings. A researcher from the same LMIC explained that their country specific political and social context required researchers to defend their choice of international partners to get ethics approval for VAW research:
“VAW is perceived as a problem that should be treated on a local level. It is a sensitive topic rooted in the culture and religion. In our culture, religion, values, and traditions are strongly expressed and strongly engaged even within administration and research. You need to defend your research not only from an ethical point of view, but also from intention of why you’re doing this research with international partners.” Interviewee 10, University researcher, Middle-Eastern LMIC.
Differing conceptualisation of vulnerability
While most documents and interviewees acknowledged that certain groups of research participants were more vulnerable than others and needed extra protection, only VAW-specific ethics guideline [ 29 ] and some experienced VAW researchers acknowledged vulnerabilities and protection for the researchers. According to REC members, they treated VAW like any sensitive topic and required proof of safeguarding and support resources for research participants. We found divergent views on the concept of vulnerability when applied in the VAW research context. In generic research ethics documents, vulnerability of research participants was defined as impairing their capacity to consent. Vulnerability meant that certain groups had limited ability to understand the nature of research and make informed decision about taking part, the possibility of being exploited and harmed by research. Several documents referred to vulnerable groups generically without providing specific definitions of clarifications regarding the types of individuals or conditions they encompassed. Other sources listed vulnerable groups, all of which were at risk of experiencing VAW – i.e., victims of traumatic events and sexual abuse, pregnant/lactating women, all women, women from orthodox communities, individuals disadvantaged by gender.
In contrast, VAW-specific ethics document [ 29 ], and some researchers from HIC and South Asian LMIC-2 recognised women who have experienced violence as capable of participating in research. The primary concern regarding women’s vulnerability in relation to participating in VAW research stemmed from the potential risk of experiencing further violence. Therefore, the protection measures ensured safety, confidentiality, and signposting to specialist VAW services. Researchers from two partner countries emphasised that all women who have experienced violence have agency and some of them are empowered by their lived experience. Therefore, that they should not be regarded as incapable of providing informed consent to participate in research. One researcher highlighted differing HIC and LMIC societal norms regarding vulnerability of research participants with the former fostering power among participants:
“I really like that the European context is stricter because it really ensures safety, security, privacy of the women. Especially when we are working with vulnerable groups. I think it comes out of respect for participants. Because I think in the [South Asian LMIC-2] context, vulnerable groups are sympathised and not empathised, maybe. Because out of sympathy you only feel pity for these women, and you don’t respect them as humans. When you respect a person then you would definitely think about how the researchers protect these women from being harmed or revictimized.” Interviewee 18, University researcher, South-Asian LMIC.
High-level guidelines and all interviewees acknowledged the need for additional time and effort for addressing issues of vulnerability in ethics applications through ensuring confidentiality, safety, and provision of referral/signposting to specialist VAW services. They highlighted the importance of the adequate time allocation for lengthy ethics approval processes which were sometimes delayed the commencement of the primary studies. Interviewees suggested allocating at least six months for obtaining ethics approvals.
"The special nature of this research topic [VAW] demands that safety concerns be considered from the very beginning of a study through its implementation and dissemination. This means that violence research will likely require a longer timeframe and a greater investment of resources to ensure these issues are fully addressed." (WHO Recommendations 2001 [ 29 ]).
From the perspective of healthcare REC, the established policies and care pathways should ensure the safety and appropriate care of patients affected by violence identified through studies on VAW and health:
“We have to read it [ethics application] carefully and make sure that if the researcher discovers that the woman is in fact experiencing violence it is notified in the national database. We have to be mindful of those things since they are health policies, the research project cannot go against our health policy, our care policy. We have a violence department here in the municipality, people that work solely with this, so a project like this has to know this exists and have a dialog with this area. We ask “what are you going to do when you see the person is experiencing violence? What care will you offer? How will you do it? What are you going to offer this person?”. We have to see if everything was thought of, otherwise this person will come here, do the research, get the data and just leave.” Interviewee 6, Municipal REC member, South American LMIC.
Limited REC methodological and topic expertise
Researchers from the two South Asian LMICs felt frustrated with the limited VAW methodological and subject expertise of their RECs who dismissed qualitative and mixed methods, verbal informed consent, and remote data collection. They highlighted the importance of educating RECs and researchers on the specifics of the ethics applications for VAW research. For instance, one LMIC interviewee produced a resource sheet on VAW research for her institutional REC to support their ethics application and strengthen REC capacity. They noticed that their institutional REC application process improved over time with more VAW projects being undertaken. REC members also talked about continuing training and dialogue between RECs, RECs and researchers to strengthen capacity for ethical conduct of research. The interviewees agreed that the changes should occur at the institutional level:
“Simultaneously making sure ethics boards are having proper policies, guidelines, regulations, and qualified people who are able to review the ethics and provide substantial feedback to applicants. Not depending on who you know within the community and the ethics committee to push your application through.” Interviewee 14, University researcher, South-Asian LMIC.
This qualitative study of professional and policy perspectives generated three themes summarising and explaining challenges in global research on VAW and health at the ethics approval stage. The global nature of the research contributed towards differing power dynamics and resource distribution between HIC and LMICs and discrepant RECs rules across countries and institutions. HIC and LMIC researchers tried to mitigate the conflicting RECs rules by collaborating and supporting each other during the ethics application process. However, they lacked autonomy and capacity to shift the power from HIC or harmonise rules across RECs. Location of the primary studies in LMIC healthcare services contributed towards divergent institutional rules across academic RECs and health authorities that researchers tried to conciliate by negotiating the differences. The VAW topic contributed towards differing conceptualisations of VAW and participants vulnerability and limited methodological and topic expertise in some LMIC RECs which researchers addressed through helping REC to develop capacity.
These contextual factors had a negative impact on researchers and teams' morale, and the relationships between researchers, RECs, and HIC funders. Furthermore, they posed a substantial risk to the timely completion of studies. Most researchers felt frustrated and demotivated by the hierarchical, bureaucratised, uncoordinated, and lengthy approval processes. Participants suggested several strategies to address the power imbalances and challenges identified in the study. This included advocating for the involvement of LMIC representatives in shaping HIC funding agendas for global health research, prompting a redistribution of power between the HIC and LMICs at the macro- and meso- levels, fostering coordination between academic RECs and health authorities and between HIC and LMICs RECs, and prioritising capacity strengthening on ethics in VAW research. While these issues were present in all countries, their manifestations varied in terms of forms and degree due to the disparities in research infrastructure and healthcare systems.
Our analysis was informed by the NER model for global population health research [ 30 ] which we applied to the topic of global research on health system responses to VAW. Our study confirmed findings on ethical challenges in global health research reported in prior literature [ 14 , 31 ] and discovered new challenges specific to the REC approval stage of studies on VAW as part of the global health agenda. These challenges were multifactorial and resulted from the global nature of the research group (disparities in power and resources, divergent RECs’ rules), location of primary study within LMIC health system (differing rules between university RECs and health authorities), and the topic of VAW (differing conceptualisation of VAW and vulnerability, limited methodological and topic expertise).
Our finding on the power asymmetry between HICs and LMICs as the major systemic driver of ethical challenges supports current discourse on decolonising research agendas and building equitable global health research partnerships [ 32 ]. While all interviewees and most high-level policies acknowledged power imbalance and advocated for equitable partnerships, researchers and REC members felt that HIC funders continued to dictate global health research agendas and impose their own institutional rules and societal norms on LMIC partners. Indeed, our interviewees perceived the agendas and rules prescribed by HIC funders and policy makers as a form of recolonisation which reinforced inequalities between HICs and LMICs at the macro level and jeopardised research integrity. Our global health research group tried to redress power imbalances through reflexivity about positionality during the research process which helped to establish and maintain equitable relationships within and between HIC and LMIC teams. However, our efforts at the meso (group) level could not change power asymmetry between HIC funder/RECs and LMIC researchers/RECs. As suggested by our findings and prior literature, rebalancing power requires interventions at the level of HIC policy makers and funders and HIC and LMIC RECs [ 10 , 33 ].
Our finding on the challenge of disjointed academic RECs and health system authorities which imposed differing rules and lacked communication with each other is consistent with prior literature that found highly bureaucratised, disjointed, and lengthy ethics approval processes across HICs and LMICs [ 14 ]. Our interviewees’ suggestions for improving consistency and joined-up working amongst HIC/LMIC and academic RECs/health authorities support recommendations for more collaborative capacity strengthening and harmonisation across RECs in global research projects [ 34 ].
Our finding on differing conceptualisations of VAW reflects previous research that reported a lack of consensus regarding the definition of VAW and terminology used by researchers, practitioners, and research participants [ 35 ]. In the context of global research, the differences in definitions of VAW used by HIC funders, LMIC researchers and REC members were rooted in country-specific socio-political contexts. Some LMIC interviewees felt that HIC governments and funders imposed research agendas which defined VAW as a relationship problem and did not recognise the intersecting systemic violence and lived experience of people in a war torn LMIC. Nor do they acknowledge the ways in which political conflict can exacerbate different forms of gender-based violence. In contrast, LMIC participants living in countries affected by armed conflict acknowledged the complex interplay between individual, relationship, community, and societal factors that put people at risk of experiencing and using violence. This finding supports recommendations for inclusive agenda setting for global research, emphasising the importance of involving HIC funders, LMIC governments and researchers in setting priorities and co-designing research programmes that address the unique needs of LMICs and align with their socio-political contexts [ 33 ].
Our finding on the differing conceptualisations of vulnerability of research participants in global research on VAW could be explained by cultural variation regarding the concept of gender roles in different societies and the feminist ethos of VAW research. As highlighted by our interview participants, such conceptual differences have implications for the choice of research methodology and advocacy for participants. Feminist theories and approaches widely used and accepted in HICs might not offer a useful framework for transforming the realities of women experiencing violence in LMICs. Generalising their validity without contextual tailoring to LMIC-specific political and cultural contexts might hamper the very efforts to end violence [ 36 ]. Similarly, when applying methods and advocacy tools that have been developed in HICs to different LMICs, global research groups should consider the distinct context factors and actively seek the input of those who have local expertise and knowledge, to ensure the best recruitment, experiences of research participants and data generated [ 37 ].
The ethical debates surrounding the inclusion of women who experience violence as research participants revolve around ensuring their protection while also avoiding their undue exclusion from participating [ 38 ]. It is acknowledged that women who experience violence are not a homogeneous group, and therefore, considerations must be made to ensure their diverse experiences and perspectives are represented in research [ 39 , 40 ].
Prior research has produced convincing evidence regarding the challenges in global research during ethics approval stage [ 34 ]. Future research should identify and evaluate policies and interventions that aim to address the causes of these challenges.
Strengths and limitations
This study combined findings from qualitative interviews and complementary documentary analysis on ethics in global research on VAW and health. The use of qualitative methodology matched the objective of illuminating and contextualising the subjective experiences of researchers and REC members regarding obtaining ethics approval for global research projects. We added credibility to our findings by integrating results of interview and document analyses, involvement of three researchers from HIC and LMIC in data coding, whole team discussions of candidate and final analytical themes, and providing supporting quotes. We contributed towards transferability of our findings to similar contexts and participant groups through drawing a geographically diverse sample from one HIC and four LMICs across Europe, South America, Middle-Eastern region, and South Asia and through reporting socio-demographic characteristics of the participants. Table 1 shows that our purposive sampling strategy produced a maximum variation participant group in terms of countries, roles, years of relevant experience. However, the transferability has been limited by recruiting from the five partner countries within one global health research group. The sub-group from one South Asian LMIC did not include REC members.
Throughout the study, we critically examined and reflected on our own roles and influences of our values, assumptions, and experiences on the data we generated and analysis we produced. Our dual role as qualitative researchers and members of the global health group with direct experience of obtaining research ethics approvals allowed us to provided valuable insights, interpretations, and perspectives that contributed to the depth and richness of the findings. However, we acknowledge that the dual roles of author and research participant among three of the authors could also be seen as a limitation. It has the potential to introduce bias, as our perspectives may have influenced the interpretation of the findings. To mitigate this, we employed rigorous reflexivity practices, continuously interrogating our biases, and the impact of our involvement on the outcomes. Simultaneously, this insider perspective constituted a strength, enabling us to access and contribute to more nuanced understandings and ensure that researchers’ voices are accurately represented. In turn, this strengthened the credibility and relevance of the findings. It also helped to address potential prejudices and power imbalances because of the shared decision making in the interpretation and writing of the paper.
Global research on health system responses to VAW generated additional challenges during application for ethics approvals across HIC and LMIC partners. These challenges were driven by power and resource asymmetries between HICs and LMICs, differing rules between RECs and between academic RECs and health authorities, varying conceptualisations of VAW and participant vulnerability, limited methodological and topic expertise in some LMIC RECs. The challenges had a negative impact on researchers’ relationships with RECs and funders. They imposed additional emotional labour on researchers and threatened timely delivery of the programme of the research. The process of ethics approval for global research on health system responses to VAW requires greater flexibility to accommodate country-specific contexts, with equal power distribution between HICs and LMICs, researchers and RECs. While some of these objectives can be achieved through educating individual RECs members, researchers, and funders, the power asymmetry and differing rules and contextualisation should be addressed at the meso (institutional) and macro (country) levels.
It is very important to conduct global research on health system responses to VAW to develop evidence-based interventions. Although a higher level of scrutiny during ethics approval stage might be justified, this should not hinder research on this topic, since findings are important to identify gaps in service provision and inform development of evidence-based interventions. By upholding high ethical standards in global research on health system responses to VAW, we ensure the opportunity for a comprehensive and evidence-based approach to addressing the issues. This, in turn, enhances the outcomes and results for women who have experienced violence.
Availability of data and materials
Due to the sensitivity of the data involved, these data are published as a controlled dataset at the University of Bristol Research Data Repository data.bris , at https://doi.org/ https://doi.org/10.5523/bris.3qs252vyomger219n9f5fcv5u3 [ 41 ]. The metadata record published openly by the repository at this location clearly states how data can be accessed by bona fide researchers. Requests for access will be considered by the University of Bristol Data Access Committee, who will assess the motives of potential data re-users before deciding to grant access to the data. No authentic request for access will be refused and re-users will not be charged for any part of this process.
Abbreviations
High-income country
Low- and middle-income country
Research Ethics Committee
- Violence against women
World Health Organisation
Butchart A, Mikton C, Dahlberg LL, Krug EG. Global status report on violence prevention 2014. Inj Prev. 2015;21(3):213. https://doi.org/10.1136/injuryprev-2015-041640 .
Sardinha L, Maheu-Giroux M, Stockl H, Meyer SR, Garcia-Moreno C. Global, regional, and national prevalence estimates of physical or sexual, or both, intimate partner violence against women in 2018. Lancet. 2022;399(10327):803–13. https://doi.org/10.1016/S0140-6736(21)02664-7 .
Article PubMed PubMed Central Google Scholar
Garcia-Moreno C, Hegarty K, d’Oliveira AF, Koziol-McLain J, Colombini M, Feder G. The health-systems response to violence against women. Lancet. 2015;385(9977):1567–79. https://doi.org/10.1016/S0140-6736(14)61837-7 .
Article PubMed Google Scholar
Colombini M, Dockerty C, Mayhew SH. Barriers and facilitators to integrating health service responses to intimate partner violence in low- and middle-income countries: a comparative health systems and service analysis. Stud Fam Plann. 2017;48(2):179–200. https://doi.org/10.1111/sifp.12021 .
Colombini M, Mayhew SH, Garcia-Moreno C, d’Oliveira AF, Feder G, Bacchus LJ. Improving health system readiness to address violence against women and girls: a conceptual framework. BMC Health Serv Res. 2022;22(1):1429. https://doi.org/10.1186/s12913-022-08826-1 .
OECD. Global outlook on financing for sustainable development 2023: no sustainability without equity. Paris: OECD Publishing; 2022.
Book Google Scholar
Schraiber LB, D’Oliveira AF, Portella AP, Menicucci E. Gender-based violence in public health: challenges and achievements. Cien Saude Colet. 2009;14(4):1019–27. https://doi.org/10.1590/s1413-81232009000400009 .
Fontes LA. Ethics in violence against women research: the sensitive, the dangerous, and the overlooked. Ethics Behav. 2004;14(2):141–74. https://doi.org/10.1207/s15327019eb1402_4 .
Steinert JI, Nyarige DA, Jacobi M, Kuhnt J, Kaplan L. A systematic review on ethical challenges of ‘field’ research in low-income and middle-income countries: respect, justice and beneficence for research staff? BMJ glob. 2021;6(7):e005380. https://doi.org/10.1136/bmjgh-2021-005380 .
Article Google Scholar
Weber S, Hardiman M, Kanja W, Thomas S, Robinson-Edwards N, Bradbury-Jones C. Towards ethical international research partnerships in gender-based violence research: insights from research partners in Kenya. Viol Against Women. 2022;28(11):2909–31. https://doi.org/10.1177/10778012211035798 .
Ellsberg M, Heise L, Peña R, Agurto S, Winkvist A. Researching domestic violence against women: methodological and ethical considerations. Stud Fam Plann. 2001;32(1):1–16. https://doi.org/10.1111/j.1728-4465.2001.00001.x .
Article CAS PubMed Google Scholar
Kelmendi K. Violence against women: methodological and ethical issues. Psychology. 2013;4:559–65. https://doi.org/10.4236/psych.2013.47080 .
Reid C, Calia C, Guerra C, Grant L, Anderson M, Chibwana K, et al. Ethics in global research: Creating a toolkit to support integrity and ethical action throughout the research journey. Res Ethics. 2021;17(3):359–74. https://doi.org/10.1177/1747016121997522 .
Morrison K, Tomsons S, Gomez A, Forde M. Network of ethical relationships model for global North-South population health research. Glob Public Health. 2018;13(7):819–42. https://doi.org/10.1080/17441692.2016.1276948 .
Hawcroft C, Rossi E, Tilouche N, d’Oliveira AF, Bacchus LJ. Engaging early career researchers in a global health research capacity-strengthening programme: a qualitative study. Health Res Policy Syst. 2023;21(1):19. https://doi.org/10.1186/s12961-022-00949-5 .
García-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts C. WHO multi-country study on women’s health and domestic violence against women. Initial results on prevalence, health outcomes and women’s responses. Geneva, Switzerland: World Health Organization [WHO]; 2005.
Google Scholar
Alderson P. Critical realism for health and illness research. A practical introduction. Bristol, UK: Policy Press; 2021.
Wigginton B, Lafrance MN. Learning critical feminist research: A brief introduction to feminist epistemologies and methodologies. Feminism & Psychology. 0(0):0959353519866058. https://doi.org/10.1177/0959353519866058 .
Khan M, Abimbola S, Aloudat T, Capobianco E, Hawkes S, Rahman-Shepherd A. Decolonising global health in 2021: a roadmap to move from rhetoric to reform. BMJ Glob Health. 2021;6(3):e005604. https://doi.org/10.1136/bmjgh-2021-005604 .
Bhakuni H, Abimbola S. Epistemic injustice in academic global health. Lancet Glob Health. 2021;9(10):e1465–70. https://doi.org/10.1016/S2214-109X(21)00301-6 .
Dalglish SL, Khalid H, McMahon SA. Document analysis in health policy research: the READ approach. Health Pol Plan. 2020;35(10):1424–31. https://doi.org/10.1093/heapol/czaa064 .
Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117. https://doi.org/10.1186/1471-2288-13-117 .
Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753–60. https://doi.org/10.1177/1049732315617444 .
World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053 .
Article CAS Google Scholar
CIOMS. International ethical guidelines for health-related research involving humans. fourth edition. Geneva: Council for International Organizations of Medical Sciences; 2016.
Nuffield Council on Bioethics. The ethics of research related to healthcare in developing countries. London: Nuffield Council on Bioethics; 2002.
Nuffield Council on Bioethics. The ethics of research related to healthcare in developing countries: a follow-up discussion paper. London: Nuffield Council on Bioethics; 2005.
Nuffield Council on Bioethics. Research in global health emergencies: ethical issues. London: Nuffield Council on Bioethics; 2020.
World Health Organisation. Putting women first: Ethical and safety recommendations for research on domestic violence against women. Geneva: WHO; 2001.
Tomsons S, Morrison K, Gomez A, Forde M. Ethical issues facing North- South research teams. Glob Popul Health Res. Final report. 2013. https://doi.org/10.13140/RG.2.1.4337.9920 .
Shanks K, Paulson J. Ethical research landscapes in fragile and conflict-affected contexts: understanding the challenges. Res Ethics. 2022;18(3):169–92. https://doi.org/10.1177/17470161221094134 .
Kumar M, Atwoli L, Burgess RA, Gaddour N, Huang KY, Kola L, et al. What should equity in global health research look like? Lancet. 2022;400(10347):145–7. https://doi.org/10.1016/S0140-6736(22)00888-1 .
Dodson J, UKCDS. Building partnerships of equals. The role of funders in equitable and effective international development collaborations: UK Collaborative on Development Sciences 2017.
Ng LC, Hanlon C, Yimer G, Henderson DC, Fekadu A. Ethics in global health research: the need for balance. Lancet Glob Health. 2015;3(9):e516–7. https://doi.org/10.1016/S2214-109X(15)00095-9 .
McGarry J, Ali P. Researching domestic violence and abuse in healthcare settings: Challenges and issues. J Res Nurs. 2016;21(5–6):465–76. https://doi.org/10.1177/1744987116650923 .
Reverter S. Epistemologies of violence against women. A proposal from the South. Cogent Soc Sci. 2022;8(1):2038356. https://doi.org/10.1080/23311886.2022.2038356 .
Adams V, Biehl J. The work of evidence in critical global health. Med Anthropol Theory. 2016;3(2):100.
Cook E, Markham S, Parker J, John A, Barnicot K, McManus S. Risk, responsibility, and choice in research ethics. Lancet Psychiatry. 2022;9(1):5–6. https://doi.org/10.1016/S2215-0366(21)00434-X .
Garcia-Moreno C, Zimmerman C, Morris-Gehring A, Heise L, Amin A, Abrahams N, et al. Addressing violence against women: a call to action. Lancet. 2015;385(9978):1685–95. https://doi.org/10.1016/S0140-6736(14)61830-4 .
World Health O. Researching violence against women : practical guidelines for researchers and activists / Mery Ellsberg, Lori Heise. Geneva: World Health Organization; 2005.
N Lewis G Feder 2023 Ethical challenges in global health research on violence against women: a qualitative study of policy and professional perspectives https://doi.org/10.5523/bris.3qs252vyomger219n9f5fcv5u3
Download references
Acknowledgements
Research Fellow Sandi Dheensa, Bristol Medical School, UK for conducting qualitative interview. Professor Jonathan Ives, Bristol Medical School, UK for advice on study design, policy documents, target journal.
This research was funded by the NIHR (17/63/125) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK government.
Author information
Natalia V. Lewis and Beatriz Kalichman joint first author.
Authors and Affiliations
Centre for Academic Primary Care, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK
Natalia V. Lewis
Department of Preventive Medicine, Medical School, University of São Paulo, São Paulo, Brazil
Beatriz Kalichman, Yuri Nishijima Azeredo & Ana Flavia d’Oliveira
Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, UK
Loraine J. Bacchus
You can also search for this author in PubMed Google Scholar
Contributions
NVL and LJB designed the study. NVL and AFDO supervised the study. NVL and BK collected data. NVL, BK, AFDO analysed data. NVL, BK, YNA, LJB, AFDO contributed to interpretation of study findings. NVL and BK wrote first draft. All authors contributed to three revisions and read and approved the final manuscript.
Authors’ information
NVL, PhD in Health Psychology, Senior Research Fellow in Primary Care, is a mixed methods health services researcher with medical clinical background and specialism in health system responses to VAW. BK, MSc in Urban and Reginal Planning exploring the importation of theory of gentrification from HICs, PhD candidate in Collective Health investigating how power relations between partners shape global health research partnerships, with special focus on the knowledge produced. YNA, MSc on violence perpetrated by professionals in health services, PhD in Collective Health studying the relations between the development of technology and clinical expertise. LJB, BSc MA PhD, Professor of Global Health, is a social scientist whose research focuses on the development and evaluation of complex interventions within health systems and services that address violence against women and against men in same sex relationships. AFDO, PhD in Preventive Medicine, has been studying VAW for the last 25 years. She participated in the development and revisions of the WHO ethical guidelines in VAW.
Corresponding author
Correspondence to Natalia V. Lewis .
Ethics declarations
Ethics approval and consent to participate.
This study has been conducted according to the principles in the Declaration of Helsinki. To prevent conflict of interest in interviews with our own Faculty REC members, this ethics application was reviewed by the Faculty of Social Sciences and Law Research Ethics Committee at University of Bristol, UK (22 February 2021 (ref 116510)). Participants provided verbal informed consent before interview started. Documents for policy review were publicly available online. We anonymised interviewees and did not reference national and institutional documents to protect the anonymity of the study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lewis, N.V., Kalichman, B., Azeredo, Y.N. et al. Ethical challenges in global research on health system responses to violence against women: a qualitative study of policy and professional perspectives. BMC Med Ethics 25 , 32 (2024). https://doi.org/10.1186/s12910-024-01034-y
Download citation
Received : 14 July 2023
Accepted : 08 March 2024
Published : 19 March 2024
DOI : https://doi.org/10.1186/s12910-024-01034-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health research
- International collaboration
- Global health
- Research ethics
- Ethical issues
- Qualitative study
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
- Share full article
Advertisement
Supported by
Biden Signs Executive Order to Expand Research on Women’s Health
The president said that improving women’s health was crucial to ensuring a healthy, stable economy.
Biden Signs Executive Order to Boost Women’s Health Research
The executive order is aimed at addressing the underrepresentation of women in health research..
We’ve launched the first ever White House initiative on women’s health research to pioneer the next generation of scientific research and discovery in women’s health. Think of all the breakthroughs we’ve made in medicine across the board, but women have not been the focus. And today — [applause] today, we’re jumpstarting that investment by dedicating $200 million in the National Institutes of Health to tackle some of the most pressing health problems facing women today. With the executive order I’m about to sign, I’m directing the most comprehensive set of executive actions ever taken to improve women’s health — ever taken. And I’m going to ensure that women’s health is integrated and prioritized across the entire federal government. It’s not just in women’s health, not just at N.I.H., the National Science Foundation or the Defense Department, the Environmental Protection Agency. I mean, across the board. This is really serious.

By Zolan Kanno-Youngs
Reporting from Washington
President Biden on Monday signed an executive order to expand the federal government’s research into women’s health, including midlife conditions like menopause, arthritis and heart disease, as well as issues specifically affecting women in the military.
In what the White House described as the “most comprehensive” action by a president on women’s health research, Mr. Biden directed federal agencies to ensure that they are using federal funds to research health conditions and diseases that disproportionately affect women.
Standing alongside the first lady, Jill Biden, and Vice President Kamala Harris, Mr. Biden said improving women’s health was crucial to guaranteeing a healthy, stable economy.
“There’s not a damn thing a man can do a woman can’t do,” Mr. Biden said. “To state the obvious, if you want to have the strongest economy in the world, you can’t leave half of the country behind.”
Carolyn M. Mazure, a psychologist and a professor at the Yale School of Medicine, who is the chairwoman of the White House initiative on Women’s Health Research, told reporters on Sunday night that health conditions like heart disease, Alzheimer’s, menopause and fibroids would be a focus of the expanded research effort.
“I’m not even a betting woman,” said Maria Shriver, the former first lady of California, who also attended the event, “but I’ll bet today that this is the first time a president of the United States has ever signed an executive order that mentions the words ‘menopause’ and ‘women’s midlife health’ in it.”
After the U.S. Supreme Court overturned Roe v. Wade in 2022 and the Alabama Supreme Court ruled last month that frozen embryos should be considered children , threatening in vitro fertilization, the Biden campaign has increasingly accused Republicans of undermining women’s health. During his State of the Union address this month, Mr. Biden said such decisions would motivate women to vote in the November election, while also saying his White House would commit to investing in women’s health in the year ahead.
“You can’t lead America with old ideas and take us backwards,” Mr. Biden said, adding, “To lead the land of possibilities, you need a vision for the future laying out what we can and should do and what we’re going to do.”
Mr. Biden’s executive order will require agencies to report annually their investments in women’s health research and to study ways that artificial intelligence can be used to advance such research. The National Institutes of Health will increase by 50 percent investments in small businesses focused on women’s health. The Defense Department also plans to invest $10 million to learn more about cancers and mental health issues affecting women in active military service.
The White House has called on Congress to pass a plan to invest $12 billion to create a new fund for women’s health research at the National Institutes of Health. In the meantime, the executive order signed on Monday directed the N.I.H. to spend $200 million on women’s health research. Dr. Biden traveled to Cambridge, Mass., last month to announce the first step of the women’s health initiative: $100 million to support women’s health researchers and start-up companies.
Zolan Kanno-Youngs is a White House correspondent, covering President Biden and his administration. More about Zolan Kanno-Youngs
- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
Research Roundup: How the Pandemic Changed Management
- Mark C. Bolino,
- Jacob M. Whitney,
- Sarah E. Henry

Lessons from 69 articles published in top management and applied psychology journals.
Researchers recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic that were published between March 2020 and July 2023 in top journals in management and applied psychology. The review highlights the numerous ways in which employees, teams, leaders, organizations, and societies were impacted and offers lessons for managing through future pandemics or other events of mass disruption.
The recent pandemic disrupted life as we know it, including for employees and organizations around the world. To understand such changes, we recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic. These papers were published between March 2020 and July 2023 in top journals in management and applied psychology.
- Mark C. Bolino is the David L. Boren Professor and the Michael F. Price Chair in International Business at the University of Oklahoma’s Price College of Business. His research focuses on understanding how an organization can inspire its employees to go the extra mile without compromising their personal well-being.
- JW Jacob M. Whitney is a doctoral candidate in management at the University of Oklahoma’s Price College of Business and an incoming assistant professor at Kennesaw State University. His research interests include leadership, teams, and organizational citizenship behavior.
- SH Sarah E. Henry is a doctoral candidate in management at the University of Oklahoma’s Price College of Business and an incoming assistant professor at the University of South Florida. Her research interests include organizational citizenship behaviors, workplace interpersonal dynamics, and international management.
Partner Center
Language selection
- Français fr
Government of Canada makes significant investment in the future of applied public health research
From: Canadian Institutes of Health Research
News release
Today, the Honourable Mark Holland, Minister of Health, announced the 2024 cohort of Applied Public Health Chairs, the program’s fourth cohort, with an investment of $13.8 million from the Canadian Institutes of Health Research and the Public Health Agency of Canada. The Chairs will lead high-quality research programs to tackle pressing public health challenges, work with decision makers from various sectors and communities, and support evidence-informed decisions that improve health and health equity.
March 26, 2024 – Ottawa, Ontario – Canadian Institutes of Health Research
Responding to the importance of public health to Canadians, the Applied Public Health Chairs program was established in 2008 to support creating public health solutions informed by the best available scientific evidence. The 2024 Chairs will continue that critical work on the most critical areas of public health in Canada. With the knowledge they gain through their research, the Chairs and their teams will work with decision makers, both within and outside of the public health sector, to contribute to the development of solutions to some of Canada’s biggest public health challenges.
“Public health leaders cannot tackle the challenges facing our country without the valuable knowledge gained through scientific research. By working with key decision makers, the Chairs will provide critical, applied, evidence-based information that will shape the programs and initiatives that support the health of all people living in Canada.” The Honourable Mark Holland Minister of Health
“This is the fourth cohort of APHCs that CIHR and PHAC have supported since 2008, led by the CIHR Institute of Population and Public Health. The 2024 Chairs cover a remarkable diversity of topics and approaches to study many of today’s most pressing population and public health problems in Canada. By working with decision-makers throughout their research program, mentoring the next generation of researchers, and contributing to evidence-informed decision-making and the uptake of evidence in public health and other sectors, the work these Chairs do will benefit Canada for years to come.” Dr. Katherine Frohlich Scientific Director, CIHR Institute of Population and Public Health
“The important public health challenges we face, from the impacts of climate change to more frequent pandemics, require innovative solutions informed by the best available evidence using collaborative and integrated approaches. By investing in applied research, we are helping to generate evidence that is most useful to public health decision-making, in order to improve outcomes for all people living in Canada.” Dr. Sarah Viehbeck Chief Science Officer, Public Health Agency of Canada
Quick facts
The Applied Public Health Chair program was launched in 2008 to strengthen the skills and capacity of public health researchers, and since its inception has supported more than 45 leading public health researchers.
With this investment, the Government of Canada, through CIHR and PHAC, is providing $13.8 million to support 12 research projects over six years.
Related products
- 2024 Applied Public Health Chairs
Associated links
- Applied Public Health Chair Program
Christopher Aoun Press Secretary Office of the Honourable Mark Holland Minister of Health 613-957-0200
Media Relations Canadian Institutes of Health Research [email protected]
At the Canadian Institutes of Health Research (CIHR) we know that research has the power to change lives. As Canada's health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
Page details
When you have eliminated the JavaScript, whatever remains must be an empty page.
Sorry, but you must enable JavaScript to view the Keele website.
- Undergraduate 2025
- Undergraduate 2024
- Postgraduate
- New Students
- Student Home
- Welcome new students
- Cost-of-living support
- Health and wellbeing
- Student KLE
- Staff Directory
- Hong Kong SAR
- Saudi Arabia
- UK International
- Rest of the world
- Faculty of Medicine and Health Sciences
- Faculty news
Researchers to study challenges faced by deprived communities in accessing musculoskeletal care

Keele researchers are embarking on an important new study to investigate the challenges deprived communities face when accessing musculoskeletal (MSK) care.
The ‘Way-in to MSK’ study, led by the Royal National Orthopaedic Hospital, has been funded by the National Institute for Health and Care Research (NIHR) School of Primary Care Research (SPCR) and initially aims to improve the delivery of MSK services to communities in two specific areas - Enfield, north London, and Stoke-on-Trent - before applying learnings more widely.
The new study focuses on communities that experience economic and social deprivation in each of the two research areas. Feedback from the SPCR identified this research study to be an important project with the potential to have enormous impact on patients and carers as well as primary and secondary care services.
Timely access to musculoskeletal services in primary care is important because early intervention can prevent long-term disability in patients. Such conditions not only have a significantly detrimental impact on the patients and their families, but also increase cost and resource pressures on the NHS.
The researchers will analyse the challenges these communities face when accessing primary MSK care through in-depth interviews. The knowledge gained from this will be mobilised across local health services with the aim of developing a comprehensive care model to effectively address the challenges identified. This new model will be co-designed by stakeholders and local communities and lead to future research and transformation of local health services.
Alice Moult, Research Fellow in Keele’s School of Medicine, is one of the researchers involved and said: “We are looking forward to working with our local community to understand how we can support access to these much needed services; the community voice will be at the heart of this study.”
Anthony Gilbert, a post-doctoral research physiotherapist at RNOH who is leading the study, said: “We have seen first-hand how difficult it is for people to access MSK care, particularly when they have competing priorities like work, juggling scarce finances or accessing food banks to feed their families. This research will help to understand what patients require to in order to access the musculoskeletal care they need.”
- Construction officially begins on new Stoke-on-Trent and Staffordshire Institute of Technology
- Keele establishes doctoral scholarships programme to tackle issues facing rural communities
- Second-year criminology student becomes published author
- Keele University to mark 75th anniversary by giving 750 volunteer days to the community
- Keele researcher receives major grant from renowned US charity
Contact us Andy Cain, Media Relations Manager +44 1782 733857 Abby Swift, Senior Communications Officer +44 1782 734925 Adam Blakeman, Press Officer +44 7775 033274 Strategic Communications and Brand [email protected] .
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
Research Identifies Characteristics of Cities That Would Support Young People’s Mental Health
Survey responses from global panel that included young people provide insights into what would make cities mental health-friendly for youth
As cities around the world continue to draw young people for work, education, and social opportunities, a new study identifies characteristics that would support young urban dwellers’ mental health. The findings, based on survey responses from a global panel that included adolescents and young adults, provide a set of priorities that city planners can adopt to build urban environments that are safe, equitable, and inclusive.
To determine city characteristics that could bolster youth mental health, researchers administered an initial survey to a panel of more than 400, including young people and a multidisciplinary group of researchers, practitioners, and advocates. Through two subsequent surveys, participants prioritized six characteristics that would support young city dwellers’ mental health: opportunities to build life skills; age-friendly environments that accept young people’s feelings and values; free and safe public spaces where young people can connect; employment and job security; interventions that address the social determinants of health; and urban design with youth input and priorities in mind.
The paper was published online February 21 in Nature .
The study’s lead author is Pamela Collins, MD, MPH, chair of the Johns Hopkins Bloomberg School of Public Health’s Department of Mental Health. The study was conducted while Collins was on the faculty at the University of Washington. The paper was written by an international, interdisciplinary team, including citiesRISE, a global nonprofit that works to transform mental health policy and practice in cities, especially for young people.
Cities have long been a draw for young people. Research by UNICEF projects that cities will be home to 70 percent of the world’s children by 2050. Although urban environments influence a broad range of health outcomes, both positive and negative, their impacts manifest unequally. Mental disorders are the leading causes of disability among 10- to 24-year-olds globally. Exposure to urban inequality, violence, lack of green space, and fear of displacement disproportionately affects marginalized groups, increasing risk for poor mental health among urban youth.
“Right now, we are living with the largest population of adolescents in the world’s history, so this is an incredibly important group of people for global attention,” says Collins. “Investing in young people is an investment in their present well-being and future potential, and it’s an investment in the next generation—the children they will bear.”
Data collection for the study began in April 2020 at the start of the COVID-19 pandemic. To capture its possible impacts, researchers added an open-ended survey question asking panelists how the pandemic influenced their perceptions of youth mental health in cities. The panelists reported that the pandemic either shed new light on the inequality and uneven distribution of resources experienced by marginalized communities in urban areas, or confirmed their preconceptions of how social vulnerability exacerbates health outcomes.
For their study, the researchers recruited a panel of more than 400 individuals from 53 countries, including 327 young people ages 14 to 25, from a cross-section of fields, including education, advocacy, adolescent health, mental health and substance use, urban planning and development, data and technology, housing, and criminal justice. The researchers administered three sequential surveys to panelists beginning in April 2020 that asked panelists to identify elements of urban life that would support mental health for young people.
The top 37 characteristics were then grouped into six domains: intrapersonal, interpersonal, community, organizational, policy, and environment. Within these domains, panelists ranked characteristics based on immediacy of impact on youth mental health, ability to help youth thrive, and ease or feasibility of implementation.
Taken together, the characteristics identified in the study provide a comprehensive set of priorities that policymakers and urban planners can use as a guide to improve young city dwellers' mental health. Among them: Youth-focused mental health and educational services could support young people’s emotional development and self-efficacy. Investment in spaces that facilitate social connection may help alleviate young people’s experiences of isolation and support their need for healthy, trusting relationships. Creating employment opportunities and job security could undo the economic losses that young people and their families experienced during the pandemic and help cities retain residents after a COVID-era exodus from urban centers.
The findings suggest that creating a mental health-friendly city for young people requires investments across multiple interconnected sectors like transportation, housing, employment, health, and urban planning, with a central focus on social and economic equity. They also require urban planning policy approaches that commit to systemic and sustained collaboration, without magnifying existing privileges through initiatives like gentrification and developing green spaces at the expense of marginalized communities in need of affordable housing.
The authors say this framework underscores that responses by cities should include young people in the planning and design of interventions that directly impact their mental health and well-being.
“ Making cities mental health friendly for adolescents and young adults ” was co-authored by an international, interdisciplinary team of 31 researchers led by the University of Washington Consortium for Global Mental Health, Urban@UW, the University of Melbourne, and citiesRISE. Author funding is listed in the Acknowledgements section of the paper.
Related Content

Student Spotlight: Glendedora Dolce

Study Estimates Nearly 70 Percent of Children Under Six in Chicago May Be Exposed to Lead-Contaminated Tap Water

Hidden Food Insecurity: The Adolescents Who Aren’t Getting Enough to Eat

A Zero-Emissions Transition Would Save Kids’ Lives

Child Diarrhea Has a Cheap and Easy Fix—Why Isn’t It Reaching Patients?
Mobile Menu Overlay
The White House 1600 Pennsylvania Ave NW Washington, DC 20500
FACT SHEET: President Biden Issues Executive Order and Announces New Actions to Advance Women’s Health Research and Innovation
In his State of the Union address, President Biden laid out his vision for transforming women’s health research and improving women’s lives all across America. The President called on Congress to make a bold, transformative investment of $12 billion in new funding for women’s health research. This investment would be used to create a Fund for Women’s Health Research at the National Institutes of Health (NIH) to advance a cutting-edge, interdisciplinary research agenda and to establish a new nationwide network of research centers of excellence and innovation in women’s health—which would serve as a national gold standard for women’s health research across the lifespan.
It is long past time to ensure women get the answers they need when it comes to their health—from cardiovascular disease to autoimmune diseases to menopause-related conditions. To pioneer the next generation of discoveries, the President and the First Lady launched the first-ever White House Initiative on Women’s Health Research , which aims to fundamentally change how we approach and fund women’s health research in the United States.
Today, President Biden is signing a new Executive Order that will direct the most comprehensive set of executive actions ever taken to expand and improve research on women’s health. These directives will ensure women’s health is integrated and prioritized across the federal research portfolio and budget, and will galvanize new research on a wide range of topics, including women’s midlife health.
The President and First Lady are also announcing more than twenty new actions and commitments by federal agencies, including through the U.S. Department of Health and Human Services (HHS), the Department of Defense (DoD), the Department of Veterans Affairs (VA), and the National Science Foundation (NSF). This includes the launch of a new NIH-wide effort that will direct key investments of $200 million in Fiscal Year 2025 to fund new, interdisciplinary women’s health research—a first step towards the transformative central Fund on Women’s Health that the President has called on Congress to invest in. These actions also build on the First Lady’s announcement last month of the Advanced Research Projects Agency for Health (ARPA-H) Sprint for Women’s Health , which committed $100 million towards transformative research and development in women’s health.
Today, the President is issuing an Executive Order that will:
- Integrate Women’s Health Across the Federal Research Portfolio . The Executive Order directs the Initiative’s constituent agencies to develop and strengthen research and data standards on women’s health across all relevant research and funding opportunities, with the goal of helping ensure that the Administration is better leveraging every dollar of federal funding for health research to improve women’s health. These actions will build on the NIH’s current policy to ensure that research it funds considers women’s health in the development of study design and in data collection and analysis. Agencies will take action to ensure women’s health is being considered at every step in the research process—from the applications that prospective grantees submit to the way that they report on grant implementation.
- Prioritize Investments in Women’s Health Research . The Executive Order directs the Initiative’s constituent agencies to prioritize funding for women’s health research and encourage innovation in women’s health, including through ARPA-H and multi-agency initiatives such as the Small Business Innovation Research Program and the Small Business Technology Transfer Program. These entities are dedicated to high-impact research and innovation, including through the support of early-stage small businesses and entrepreneurs engaged in research and innovation. The Executive Order further directs HHS and NSF to study ways to leverage artificial intelligence to advance women’s health research. These additional investments—across a wide range of agencies—will support innovation and open new doors to breakthroughs in women’s health.
- Galvanize New Research on Women’s Midlife Health . To narrow research gaps on diseases and conditions associated with women’s midlife health or that are more likely to occur after menopause, such as rheumatoid arthritis, heart attack, and osteoporosis, the President is directing HHS to: expand data collection efforts related to women’s midlife health; launch a comprehensive research agenda that will guide future investments in menopause-related research; identify ways to improve management of menopause-related issues and the clinical care that women receive; and develop new resources to help women better understand their options for menopause-related symptoms prevention and treatment. The Executive Order also directs the DoD and VA to study and take steps to improve the treatment of, and research related to, menopause for Service women and women veterans.
- Assess Unmet Needs to Support Women’s Health Research . The Executive Order directs the Office of Management and Budget and the Gender Policy Council to lead a robust effort to assess gaps in federal funding for women’s health research and identify changes—whether statutory, regulatory, or budgetary—that are needed to maximally support the broad scope of women’s health research across the federal government. Agencies will also be required to report annually on their investments in women’s health research, as well as progress towards their efforts to improve women’s health.
Today, agencies are also announcing new actions they are taking to promote women’s health research , as part of their ongoing efforts through the White House Initiative on Women’s Health Research. Agencies are announcing actions to:
Prioritize and Increase Investments in Women’s Health Research
- Launch an NIH-Cross Cutting Effort to Transform Women’s Health Throughout the Lifespan. NIH is launching an NIH-wide effort to close gaps in women’s health research across the lifespan. This effort—which will initially be supported by $200 million from NIH beginning in FY 2025—will allow NIH to catalyze interdisciplinary research, particularly on issues that cut across the traditional mandates of the institutes and centers at NIH. It will also allow NIH to launch ambitious, multi-faceted research projects such as research on the impact of perimenopause and menopause on heart health, brain health and bone health. In addition, the President’s FY25 Budget Request would double current funding for the NIH Office of Research on Women’s Health to support new and existing initiatives that emphasize women’s health research.
This coordinated, NIH-wide effort will be co-chaired by the NIH Office of the Director, the Office of Research on Women’s Health, and the institute directors from the National Institute on Aging; the National Heart, Lung, and Blood Institute; the National Institute on Drug Abuse; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Institute on Arthritis, Musculoskeletal and Skin Diseases.
- Invest in Research on a Wide Range of Women’s Health Issues. The bipartisan Congressionally Directed Medical Research Program (CDMRP), led out of DoD, funds research on women’s health encompassing a range of diseases and conditions that affect women uniquely, disproportionately, or differently from men. While the programs and topic areas directed by Congress differ each year, CDMRP has consistently funded research to advance women’s health since its creation in 1993. In Fiscal Year 2022, DoD implemented nearly $490 million in CDMRP investments towards women’s health research projects ranging from breast and ovarian cancer to lupus to orthotics and prosthetics in women. In Fiscal Year 2023, DoD anticipates implementing approximately $500 million in CDMRP funding for women’s health research, including in endometriosis, rheumatoid arthritis, and chronic fatigue.
- Call for New Proposals on Emerging Women’s Health Issues . Today, NSF is calling for new research and education proposals to advance discoveries and innovations related to women’s health. To promote multidisciplinary solutions to women’s health disparities, NSF invites applications that would improve women’s health through a wide range of disciplines—from computational research to engineering biomechanics. This is the first time that NSF has broadly called for novel and transformative research that is focused entirely on women’s health topics, and proposals will be considered on an ongoing basis.
- Increase Research on How Environmental Factors Affect Women’s Health. The Environmental Protection Agency (EPA) is updating its grant solicitations and contracts to ensure that applicants prioritize, as appropriate, the consideration of women’s exposures and health outcomes. These changes will help ensure that women’s health is better accounted for across EPA’s research portfolio and increase our knowledge of women’s environmental health—from endocrine disruption to toxic exposure.
- Create a Dedicated, One-Stop Shop for NIH Funding Opportunities on Women’s Health. Researchers are often unaware of existing opportunities to apply for federal funding. To help close this gap, NIH is issuing a new Notice of Special Interest that identifies current, open funding opportunities related to women’s health research across a wide range of health conditions and all Institutes, Centers, and Offices. The NIH Office of Research on Women’s Health will build on this new Notice by creating a dedicated one-stop shop on open funding opportunities related to women’s health research. This will make it easier for researchers and institutions to find and apply for funding—instead of having to search across each of NIH’s 27 institutes for funding opportunities.
Foster Innovation and Discovery in Women’s Health
- Accelerate Transformative Research and Development in Women’s Health. ARPA-H’s Sprint for Women’s Health launched in February 2024 commits $100 million to transformative research and development in women’s health. ARPA-H is soliciting ideas for novel groundbreaking research and development to address women’s health, as well as opportunities to accelerate and scale tools, products, and platforms with the potential for commercialization to improve women’s health outcomes.
- Support Private Sector Innovation Through Additional Federal Investments in Women’s Health Research. The NIH’s competitive Small Business Innovation Research Program and the Small Business Technology Transfer Program is committing to further increasing—by 50 percent—its investments in supporting innovators and early-stage small businesses engaged in research and development on women’s health. These programs will solicit new proposals on promising women’s health innovation and make evidence-based investments that bridge the gap between performance of basic science and commercialization of resulting innovations. This commitment for additional funds builds on the investments the Administration has already made to increase innovation in women’s health through small businesses, including by increasing investments by sevenfold between Fiscal Year 2021 and Fiscal Year 2023.
- Advance Initiatives to Protect and Promote the Health of Women. The Food and Drug Administration (FDA) seeks to advance efforts to help address gaps in research and availability of products for diseases and conditions that primarily impact women, or for which scientific considerations may be different for women, and is committed to research and regulatory initiatives that facilitate the development of safe and effective medical products for women. FDA also plans to issue guidance for industry that relates to the inclusion of women in clinical trials and conduct outreach to stakeholders to discuss opportunities to advance women’s health across the lifespan. And FDA’s Office of Women’s Health will update FDA’s framework for women’s health research and seek to fund research with an emphasis on bridging gaps in knowledge on important women’s health topics, including sex differences and conditions that uniquely or disproportionately impact women.
- Use Biomarkers to Improve the Health of Women Through Early Detection and Treatment of Conditions, such as Endometriosis. NIH will launch a new initiative dedicated to research on biomarker discovery and validation to help improve our ability to prevent, diagnose, and treat conditions that affect women uniquely, including endometriosis. This NIH initiative will accelerate our ability to identify new pathways for diagnosis and treatment by encouraging multi-sector collaboration and synergistic research that will speed the transfer of knowledge from bench to bedside.
- Leverage Engineering Research to Improve Women’s Health . The NSF Engineering Research Visioning Alliance (ERVA) is convening national experts to identify high-impact research opportunities in engineering that can improve women’s health. ERVA’s Transforming Women’s Health Outcomes Through Engineering visioning event will be held in June 2024, and will bring together experts from across engineering—including those in microfluidics, computational modeling, artificial intelligence/imaging, and diagnostic technologies and devices—to evaluate the landscape for new applications in women’s health. Following this event, ERVA will issue a report and roadmap on critical areas where engineering research can impact women’s health across the lifespan.
- Drive Engineering Innovations in Women’s Health Discovery . NSF awardees at Texas A&M University will hold a conference in summer 2024 to collectively identify challenges and opportunities in improving women’s health through engineering. Biomedical engineers and scientists will explore and identify how various types of engineering tools, including biomechanics and immuno-engineering, can be applied to women’s health and spark promising new research directions.
Expand and Leverage Data Collection and Analysis Related to Women’s Health
- Help Standardize Data to Support Research on Women’s Health. NIH is launching an effort to identify and develop new common data elements related to women’s health that will help researchers share and combine datasets, promote interoperability, and improve the accuracy of datasets when it comes to women’s health. NIH will initiate this process by convening data and scientific experts across the federal government to solicit feedback on the need to develop new NIH-endorsed common data elements—which are widely used in both research and clinical settings. By advancing new tools to capture more data about women’s health, NIH will give researchers and clinicians the tools they need to enable more meaningful data collection, analysis, and reporting and comprehensively improve our knowledge of women’s health.
- Reflect Women’s Health Needs in National Coverage Determinations. The Centers for Medicare & Medicaid Services (CMS) will strengthen its review process, including through Coverage with Evidence Development guidance, to ensure that new medical services and technologies work well in women, as applicable, before being covered nationally through the Medicare program. This will help ensure that Medicare funds are used for treatments with a sufficient evidence base to show that they actually work in women, who make up more than half of the Medicare population.
- Leverage Data and Quality Measures to Advance Women’s Health Research. The Centers for Disease Control and Prevention (CDC) and the Health Resources and Services Administration (HRSA) are building on existing datasets to improve the collection, analysis, and reporting of information on women’s health. The CDC is expanding the collection of key quality measures across a woman’s lifespan, including to understand the link between pregnancy and post-partum hypertension and heart disease, and plans to release the Million Hearts Hypertension in Pregnancy Change Package. This resource will feature a menu of evidence-informed strategies by which clinicians can change care processes. Each strategy includes tested tools and resources to support related clinical quality improvement. HRSA is modernizing its Uniform Data System in ways that will improve the ability to assess how women are being served through HRSA-funded health centers. By improving the ability to analyze data on key clinical quality measures, CDC and HRSA can help close gaps in women’s health care access and identify new opportunities for high-impact research.
Strengthen Coordination, Infrastructure, and Training to Support Women’s Health Research
- Launch New Joint Collaborative to Improve Women’s Health Research for Service Members and Veterans. DoD and VA are launching a new Women’s Health Research collaborative to explore opportunities that further promote joint efforts to advance women’s health research and improve evidence-based care for Service members and veterans. The collaborative will increase coordination with the goal of helping improve care across the lifespan for women in the military and women veterans. The Departments will further advance research on key women’s health issues and develop a roadmap to close pressing research gaps, including those specifically affecting Service women and women veterans.
- Coordinate Research to Advance the Health of Women in the Military. DoD will invest $10 million, contingent on available funds, in the Military Women’s Health Research Partnership. This Partnership is led by the Uniformed Services University and advances and coordinates women’s health research across the Department. The Partnership is supporting research in a wide range of health issues affecting women in the military, including cancers, mental and behavioral health, and the unique health care needs of Active Duty Service Women. In addition, the Uniformed Services University established a dedicated Director of Military Women’s Health Research Program, a role that is responsible for identifying research gaps, fostering collaboration, and coordinating and aligning a unified approach to address the evolving needs of Active Duty Service Women.
- Support EPA-Wide Research and Dissemination of Data on Women’s Health. EPA is establishing a Women’s Health Community of Practice to coordinate research and data dissemination. EPA also plans to direct the Board of Scientific Counselors to identify ways to advance EPA’s research with specific consideration of the intersection of environmental factors and women’s health, including maternal health.
- Expand Fellowship Training in Women’s Health Research. CDC, in collaboration with the CDC Foundation and American Board of Obstetrics and Gynecology, is expanding training in women’s health research and public health surveillance to OBGYNs, nurses and advanced practice nurses. Through fellowships and public health experiences with CDC, these clinicians will gain public health research skills to improve the health of women and children exposed to or affected by infectious diseases, mental health and substance use disorders. CDC will invite early career clinicians to train in public health and policy to become future leaders in women’s health research.
Improve Women’s Health Across the Lifespan
- Create a Comprehensive Research Agenda on Menopause. To help women get the answers they need about menopause, NIH will launch its first-ever Pathways to Prevention series on menopause and the treatment of menopausal symptoms. Pathways to Prevention is an independent, evidence-based process to synthesize the current state of the evidence, identify gaps in existing research, and develop a roadmap that can be used to help guide the field forward. The report, once completed, will help guide innovation and investments in menopause-related research and care across the federal government and research community.
- Improve Primary Care and Preventive Services for Women . The Agency for Healthcare Research and Quality (AHRQ) will issue a Notice of Intent to publish a funding opportunity announcement for research to advance the science of primary care, which will include a focus on women’s health. Through this funding opportunity, AHRQ will build evidence about key elements of primary care that influence patient outcomes and advance health equity—focusing on women of color—such as care coordination, continuity of care, comprehensiveness of care, person-centered care, and trust. The results from the funding opportunity will shed light on vital targets for improvements in the delivery of primary healthcare across a woman’s lifespan, including women’s health preventive services, prevention and management of multiple chronic diseases, perinatal care, transition from pediatric to adult care, sexual and reproductive health, and care of older adults.
- Promote the Health of American Indian and Alaska Native Women. The Indian Health Service is launching a series of engagements, including focus groups, to better understand tribal beliefs related to menopause in American Indian and Alaska Native Women. This series will inform new opportunities to expand culturally informed patient care and research as well as the development of new resources and educational materials.
- Connect Research to Real-World Outcomes to Improve Women’s Mental and Behavioral Health. The Substance Abuse and Mental Health Services Administration (SAMHSA) is supporting a range of health care providers to address the unique needs of women with or at risk for mental health and substance use disorders. Building on its current efforts to provide technical assistance through various initiatives , SAMHSA intends, contingent on available funds, to launch a new comprehensive Women’s Behavioral Health Technical Assistance Center. This center will identify and improve the implementation of best practices in women’s behavioral health across the life span; identify and fill critical gaps in knowledge of and resources for women’s behavioral health; and provide learning opportunities, training, and technical assistance for healthcare providers.
- Support Research on Maternal Health Outcomes. USDA will fund research to help recognize early warning signs of maternal morbidity and mortality in recipients of Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and anticipates awarding up to $5 million in Fiscal Year 2023 to support maternal health research through WIC. In addition, research being conducted through the Agricultural Research Service’s Human Nutrition Research Centers is focusing on women’s health across the lifespan, including the nutritional needs of pregnant and breastfeeding women and older adults.
Stay Connected
We'll be in touch with the latest information on how President Biden and his administration are working for the American people, as well as ways you can get involved and help our country build back better.
Opt in to send and receive text messages from President Biden.

IMAGES
VIDEO
COMMENTS
To the authors' knowledge, this is the first rapid review to examine the use of the term 'ethical challenge (s)' in empirical healthcare research literature. Notably, only 12/72 (17%) of included studies published in the last 5 years contained a definition for 'ethical challenge (s)', despite this being the focus of the research being ...
Despite its ubiquity in academic research, the phrase 'ethical challenge(s)' appears to lack an agreed definition. A lack of a definition risks introducing confusion or avoidable bias. Conceptual clarity is a key component of research, both theoretical and empirical. Using a rapid review methodology, we sought to review definitions of 'ethical challenge(s)' and closely related terms as ...
Because addressing issues in medical ethics often requires multidisciplinary expertise — in philosophy, biomedical research, clinical practice, law, policy, and communication, among other fields ...
Tomas Zima, David N. Weisstub. Provides a current review of medical research ethics on a global basis. Aims to promote a discussion about controversial and foundational aspects in the field. Concentrates on key areas of medical research where there are core ethical issues. Part of the book series: Philosophy and Medicine (PHME, volume 132)
The top 10 most-read medical ethics articles in 2021. Dec 29, 2021 . 3 MIN READ. By. Kevin B. O'Reilly , Senior News Editor. Print Page. Each month, the AMA Journal of Ethics® ( @JournalofEthics) gathers insights from physicians and other experts to explore issues in medical ethics that are highly relevant to doctors in practice and the future ...
Research is a duty for health professionals and in the best interest of patients in times of a pandemic: Empirical exploration and ethical implications of the Research Ethics in Times of Pandemic ...
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other. ... Tuskegee syphilis study The Tuskegee syphilis study was an American public health study that violated research ethics throughout its 40-year run from 1932 to 1972. In this study, ...
The FDA allows some critically ill patients to access unapproved therapies. The AMA Code of Medical Ethics has guidance for shared decision-making. Ethics of Medical Research & Innovation discusses the responsibilities in clinical research. Learn more about the ethics of medical research on the AMA.
Ethical concerns relating to paying clinical research participants center on the undue influence and coercive effect of offering money in exchange for accepting the risk of contracting a disease or otherwise being harmed by the research process. BMC Medical Ethics cites the example of research conducted in Malawi that paid participants US$10 ...
Types of Ethical Challenges. Studies related to medical education need to follow basic ethical principles of autonomy, beneficence, nonmaleficence, and justice. These studies thus need to consider key elements, such as informed consent, fair selection of participants, equipoise, potential benefits, minimization of risks, and attention to social ...
Research ethics govern the standards of conduct for scientific researchers. It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. ... The HIV epidemic has raised many ethical challenges for public health officials, researchers and clinicians, reaching from macro-level ...
The two comprehensive and pioneering documents about research ethical issues are considered to be the Nuremberg Code and the Declaration of Helsinki. 2.1. Nuremberg Code (1947) ... Ethics of medical research on human subjects must be clinically justified and scientifically sound. Informed consent is a mandatory component of any clinical research.
Defining those standards represents one of the more important challenges for research ethics. As exemplified by Lind's experiments on treatments for scurvy, clinical research studies were first conducted by clinicians wondering whether the methods they were using were effective. ... Medical Research with Children: Ethics, Law, Practice ...
In order to work with unexpected issues that arise in the research field, with ethical conflicting situations, it is necessary to take action with reflexivity, that is, critically analyze the own research process, be constantly attentive to what occurs in the empirical context of the study, and how this affects the participant, the researcher ...
The ethical relationship challenges were explained by differing cultural backgrounds, REC requirements and participant values, conflicting requirements between HIC and LMIC RECs and funding procedures. However, to our knowledge, no studies have addressed ethical challenges in global research on health system response to VAW.
The misapplication of statistical methods can directly violate ethical medical research principles. For example, p-hacking to ... affecting the integrity of research Table 1: Statistical issues that can lead to data interpretation errors. In summary, as the biomedical research community increasingly relies on statistical methodologies ...
President Biden on Monday signed an executive order to expand the federal government's research into women's health, including midlife conditions like menopause, arthritis and heart disease ...
Researchers recently reviewed 69 articles focused on the management implications of the Covid-19 pandemic that were published between March 2020 and July 2023 in top journals in management and ...
Today, the Honourable Mark Holland, Minister of Health, announced the 2024 cohort of Applied Public Health Chairs, the program's fourth cohort, with an investment of $13.8 million from the Canadian Institutes of Health Research and the Public Health Agency of Canada. The Chairs will lead high-quality research programs to tackle pressing public health challenges, work with decision makers ...
The knowledge gained from this will be mobilised across local health services with the aim of developing a comprehensive care model to effectively address the challenges identified. This new model will be co-designed by stakeholders and local communities and lead to future research and transformation of local health services.
Maternal mental health disorders such as suicide and opioid overdose are responsible for nearly 1 in 4 maternal deaths in the US, research shows. Prasit photo/Moment RF/Getty Images/File
Taken together, the characteristics identified in the study provide a comprehensive set of priorities that policymakers and urban planners can use as a guide to improve young city dwellers' mental health. Among them: Youth-focused mental health and educational services could support young people's emotional development and self-efficacy.
The Partnership is supporting research in a wide range of health issues affecting women in the military, including cancers, mental and behavioral health, and the unique health care needs of Active ...