Your session is about to expire
Clinical research roles: how to become a study coordinator.
A study coordinator is a central player in clinical research. In this article, we’ll go through the role of a clinical study coordinator, including their responsibilities, and required qualifications.

What is a clinical study coordinator?
Also known as a research coordinator or clinical trial coordinator, a study coordinator has in-depth knowledge of the complete study protocol, including the eligibility criteria, drug intervention, baseline measures, and primary outcomes. They work across the three major aspects of clinical research: study management, subject care, and patient advocacy.
What is the role of a study coordinator in clinical trials?
The principal role of a study coordinator is serving as a central point of contact for multiple stakeholders within a clinical study, transferring data, information, and concerns across to relevant trial personnel, as shown below. [ 1 ] They oversee the daily operations of clinical trials, working closely with the principal investigator (PI) and research team to ensure the study is conducted according to Good Clinical Practice (GCP) guidelines, protocol, and regulatory requirements.
Some of the responsibilities of a clinical study coordinator include but are not limited to the following:
- Recruiting and enrolling study participants, including everything from running campaigns through to randomizing patients
- Collecting informed consent and addressing any concerns participants may have
- Coordinating study visits and procedures; scheduling study visits, coordinating with study participants and healthcare providers to ensure they are available for visits, and ensuring that all study procedures are performed according to the protocol and GCP guidelines
- Maintaining accurate study, regulatory, and IRB and documentation, ensuring compliance and good clinical study practice
- Preparing trial documentation for IRB approval and other governing agencies
- Coordinating and documenting training sessions for research staff
- Overseeing inventory and medical equipment, ensuring trial sites have the appropriate supplies
- Writing case reports and reporting results to key stakeholders such as review boards and funding agencies
- Supervising research activities to ensure they comply with federal and local guidelines
- Documenting and reporting any adverse events or non-compliance issues participants may face
- Ensuring all ethical and legal guidelines are followed throughout the clinical trial
Study coordinator skills and qualifications
Academic requirements for study coordination include a Bachelor’s degree in Clinical Research from a CHEA-accredited institution or an undergraduate with a major in clinical research. However, many in this field also have a Bachelor’s degree in science, chemistry, biology, nursing, or other health science-related fields.
In addition to academic requirements, clinical experience is a significant factor in finding success in study coordination. Even though a study coordinator is not directly responsible for administering the trial’s intervention or personally handling patient care, many clinical research coordinator job description listings require expertise in healthcare and clinical settings.
Many who pursue a career in clinical study coordination start as research nurses as they already have a foundation in patient care. Advanced degrees such as a Master’s degree or Ph.D. are not necessary but are a bonus and can help secure a higher pay grade.
Certifications
The Association of Clinical Research Professionals (ACRP) and Society of Clinical Research Associates (SOCRA) offer additional certifications that can boost qualifications, including:
- Certified Clinical Research Coordinator (CCRC)
- Certified Clinical Research Associate (CCRA)
- Certified Clinical Research Professional (CCRP)
A CCRC certification formally recognizes the research professional can facilitate and coordinate clinical research activities according to good clinical practice guidelines under the direction of a principal research investigator. [ 2 ]
A CCRA certification formally recognizes the research professional is experienced in supervising and monitoring progress and conduct on behalf of a study sponsor. [3]
Both certificates have two eligibility pathways, as described below:
- 3,000 hours of verifiable clinical research experience
- A clinical research degree and 1,500 hours of verifiable clinical research experience
The CCRP certification recognizes the research professional with the abilities and experiences to conduct clinical studies per international good clinical practice guidelines. [ 4 ]
It offers three eligibility criteria as listed below:
- Have two years of experience as a full-time clinical research professional
- OR 3,500 hours as part-time experience in the last 5 years
- Have an Associate’s, Undergraduate, or Graduate degree in Clinical Research
- AND one year of full-time clinical research experience OR 1,750 hours part-time
- Have an Associate’s or Bachelor’s degree in health science, science, or pharmacy-related field
- AND an Undergraduate or graduate certification in Clinical Research with a minimum of 144 credit hours from a higher learning institution
A good clinical study coordinator also needs to demonstrate the following abilities:
- Excellent communication, interpersonal and organizational skills
- Leadership, time management, and motivational skills
- Collaborative skills with an ability to work independently
- Strong research skills and high attention to detail
- Analytical and critical thinking
- Problem-solving capabilities
Study coordinator training program
Several academic institutes and organizations offer clinical research training programs that prospective study coordinators can take to improve their skills. These include the following:
- Clinical Research Coordinator (CRC) with the CITI Program [ 5 ]
- Study Coordinator Training at the Research Compliance Center at UNC [ 6 ]
- Certified Clinical Research Coordinator Training with the Clinical Research Society [ 7 ]
Where to find study coordinator jobs
A clinical study coordinator works in CROs, drug development and pharmaceutical companies, and hospitals that participate in clinical trials.
Many of these companies and agencies will list an opening for a clinical research coordinator (job description included) on their websites and social media accounts, such as Facebook and LinkedIn. Other resources include job boards and online job directories.
Study coordinator salary
The mean clinical trial coordinator salary is approximately $50,735 per year, with a base salary ranging from $39,000 to $77,000. [ 8 ]
However, this salary can significantly increase with certifications and experience reaching upwards of $90,000 annually. Additionally, salaries vary greatly from state to state. For example, trial sites in California and Connecticut offer higher salaries due to the high volume of clinical research projects. [ 9 ]
Study coordinator job outlook
While the prospects of study coordination had slowed down considerably in 2020, it is back on the rise. Currently, the outlook for a clinical study coordinator is strong, particularly in regions that are seeing growth in clinical research. The demand for those with experience in multi-center trials and remote technologies is also high as clinical research moves towards a more patient-centric paradigm.
Other Trials to Consider
Manipulation of spatiotemporal coordination during walking
Education program, talk stem familia treatment condition, child life preparation with vr, counseling only intervention, endobronchial ultrasound (ebus), reflective supervision enhancement, supporting transition resilience of newcomer groups (strong), coaching and advancement for peer providers (capp), intervention, popular categories.
OCD Clinical Trials 2024
Parkinson Clinical Trials 2024
Tourette Syndrome Clinical Trials 2024
Paid Clinical Trials in Austin, TX
Paid Clinical Trials in San Diego, CA
Paid Clinical Trials in Long Beach, CA
Paid Clinical Trials in Pennsylvania
Achromatopsia Clinical Trials 2023
Osteopenia Clinical Trials 2023
Retinal Vein Occlusion Clinical Trials 2023
Popular guides.
- Top Courses
- Online Degrees
- Find your New Career
- Join for Free
The Career Path of a Clinical Research Coordinator
Learn about the clinical research coordinator role, sometimes called a clinical trials manager. Discover pathways to work in clinical research, salaries, and typical employers.
![study coordinator clinical research [Featured Image]: Clinical Research Coordinators wearing lab coats and gloves, sitting in a lab, working on a desktop computer, and using a microscope, to analyze data conducting a clinical trial.](https://d3njjcbhbojbot.cloudfront.net/api/utilities/v1/imageproxy/https://images.ctfassets.net/wp1lcwdav1p1/1EknjKuQM15Jx6T9xBMj3f/d6661990a29d32d26b10023ff44340b1/GettyImages-563374209.jpg?w=1500&h=680&q=60&fit=fill&f=faces&fm=jpg&fl=progressive&auto=format%2Ccompress&dpr=1&w=1000)
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private companies, clinical research coordinators can seek various positions, and salary is above average compared to similar professions.
What is a clinical research coordinator?
A clinical research coordinator—sometimes known as a clinical trial manager—supervises clinical research and drug trials, such as interventions involving drugs, devices, or procedures promoting behavior changes. In this position, you would recruit and screen participants, coordinate the day-to-day running of the trials, collect data, and produce reports.
A clinical research coordinator is also responsible for ensuring a trial's safety and keeping the materials used safe during and after the study. You ensure meeting certain regulations and ethical standards and track patient health and progress.
Where does a clinical research coordinator sit in a clinical research team?
A clinical research coordinator reports to a principal investigator, who is responsible for the overall design and management of the study. In contrast, a clinical researcher organizes the day-to-day running of the trials. As a clinical research coordinator, you will also work closely with sponsors and staff within their department responsible for finance, personnel, and compliance. You will typically oversee a clinical research team, including doctors, nurses, other medical staff, and assistants.
What are the primary duties and responsibilities of a clinical research coordinator?
Clinical research coordinators work in various workplaces, from hospitals to private businesses, and your duties will vary according to where you work and what you are trialing. However, typical responsibilities include:
Coordinating and overseeing the running of clinical trials
Recruiting and screening participants for trials
Managing documents and records of participants and trials
Ensuring trials are delivered following regulatory, government, and ethical regulations and requirements
Ensuring the safety of participants, supplies, and materials
Performing cost analysis and preparing budgets
Designing and delivering training to staff and participants
Collecting data and maintaining detailed records
Acting as a point of contact for study participants to ask any questions or express concerns
Promoting the study through meetings, events, and seminars
Overseeing the research team
Career pathway steps
To work in clinical research, you need to hold a degree and have a certain amount of experience. You can pursue certifications and graduate qualifications to further your chances of a position.
Bachelor's degree
A bachelor’s degree is generally step one for anyone who wants to work as a clinical research coordinator. A Bachelor of Science in a relevant subject, such as clinical research administration or a matter under the umbrella of health sciences, public health, or microbiology, is recommended.
Read more: Bachelor of Science (BS) Degree: What It Is and How to Earn One
Work experience
Experience in health care or clinical research is essential for most positions. Some professionals come from a background in nursing or health care. If you have limited experience, getting some through an internship, voluntary assignment, or an entry-level role in a hospital or research position is a good idea. Experience is essential to be able to work towards certain industry-specific certifications.
Master's degree or graduate certificate
All you need to work as a clinical research coordinator is a bachelor’s degree and experience. However, some employers prefer a graduate degree, and having a graduate certificate or a master’s degree will help set you apart from other applicants without this education level.
You have several options to consider, and there is no set course. Still, it is most advantageous to select a subject like clinical research, clinical administration, or clinical research management to combine the elements of clinical research and a level of leadership. Specializations like medical technology are also options, as are more general course subjects like life sciences or health sciences.
When choosing a course, essential factors to consider are whether there is a good mix of theory and practical experience and whether the course covers clinical and managerial subjects to give you a broad range of expertise. If you are already working and gaining experience, consider the flexibility of studying part-time or online to achieve your educational goals.
In addition to a master’s degree, you can also study for a graduate certificate in a relevant subject. These courses tend to be part of the curriculum for a master’s degree, and you can transfer credits, so it’s a great way to gain a certificate while building towards a master’s degree.
Read more: How to Get a Master's Degree
Certification
It isn’t essential but recommended that you get a clinical research certification if you want to advance your career in clinical research. To become certified, you need to be working in the field. Certification verifies your skills and competence, potentially boosting career prospects and salary. The Association of Clinical Research Professionals (ACRP-CP) provides a variety of certifications for clinical research professionals:
ACRP Certified Professional (ACRP-CP): To validate your knowledge and skills as a clinical research professional
Clinical Research Coordinator (CCRC): To validate your knowledge and skills as a clinical research coordinator
Clinical Research Associate (CCRA): To validate your knowledge and skills as a clinical research associate
Principal Investigator (CPI): To validate your knowledge and skills as a principal investigator
ACRP Medical Device Professional Subspecialty (ACRP-MDP): To validate your knowledge of medical device trials
ACRP Project Manager Subspecialty: To validate your knowledge of project management within clinical research
The Society of Clinical Research Associates (SOCRA) also provides its Certified Clinical Research Professional credential to validate your experience, education, and knowledge as a clinical research professional.
How much do clinical research coordinators get paid?
The average clinical research coordinator's salary is $56,504, according to Glassdoor [ 1 ]. This can reach over $89,000 [ 1 ] for more senior positions. Salaries vary according to employer, industry, and type of trial.
Who are typical employers of clinical research coordinators?
A clinical research coordinator can work in various places, including health care organizations, pharmaceutical companies, research hospitals, and government and private companies. You may be employed to research new medicines for market, genetic diseases, illness prevention, and behavioral prevention.
If you’re ready to take your next steps towards a career as a clinical research coordinator, you can start with a short course on Design and Interpretation of Clinical Trials delivered by John Hopkins University on Coursera. This can give you a good grounding in understanding clinical trials and research.
Related articles
Clinical Managers: What They Do + How to Become One
Is Health Care a Good Career Path? Outlook, Jobs, and More
How to Become a Clinical Research Associate
Article sources
Glassdoor. “ Clinical Research Coordinator Salaries , https://www.glassdoor.com/Salaries/us-clinical-research-coordinator-salary-SRCH_IL.0,2_IN1_KO3,32.htm.” Accessed March 9, 2023.
Keep reading
Coursera staff.
Editorial Team
Coursera’s editorial team is comprised of highly experienced professional editors, writers, and fact...
This content has been made available for informational purposes only. Learners are advised to conduct additional research to ensure that courses and other credentials pursued meet their personal, professional, and financial goals.
- Clinical Research Coordinator Roles and Responsibilities
Position Role Sponsored Program Administration Financial Management Effort Reporting Conflicts of Interest Human Research Participant Protection Environmental Health and Safety Human Gene Transfer Export Controls
Position Role
The Clinical Research Coordinator (CRC) is a specialized research professional working with and under the direction of the clinical Principal Investigator (PI). While the Principal Investigator is primarily responsible for the overall design, conduct, and management of the clinical trial, the CRC supports, facilitates and coordinates the daily clinical trial activities and plays a critical role in the conduct of the study. By performing these duties, the CRC works with the PI, department, sponsor, and institution to support and provide guidance on the administration of the compliance, financial, personnel and other related aspects of the clinical study.
The clinical research coordinator reports primarily to the Principal Investigator with associated responsibilities to the department head, division administrator or program administrator.
Sponsored Program Administration
General administrative.
- Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Assists Principal Investigator to assure that all key personnel or persons ‘engaged’ in the research project have met training requirements in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Cooperates with university compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and sponsors.
- Collaborates with PI and institution to respond to any audit findings and implement approved recommendations.
- Cooperates with university and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
Preparation of Scientific Proposal
- Assists the PI in study feasibility assessments as requested.
Proposal Budget
- Collaborates with the PI and department to prepare a categorized budget and justification. Confirms accuracy and completeness of budgeted costs.
Protocol Preparation & Review
- Reviews and comprehends the protocol.
- Attends investigator meetings as required or requested by the PI.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol.
- Prepares other study materials as requested by the PI. These study materials include, but are not limited to, the informed consent document, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Establishes and organizes study files, including but not limited to, regulatory binders, study specific source documentation and other materials.
Award Acceptance (Terms & Conditions)
- Reviews and develops a familiarity with the contract or award terms and conditions. Works with the PI to assure that the study is in compliance with all terms and conditions, including but not limited to education, IRB (HRPO) approval, conflict of interest disclosure, health and safety protections for participants and staff and any financial terms or conditions.
Conduct of Research
- Reviews and develops a familiarity with the protocol, e.g., study proceedings and timelines, inclusion and exclusion criteria, confidentiality, privacy protections.
- Assists PI in communication of study requirements to all individuals involved in the study. Provides appropriate training and tools for study team members. Documents date of training and signatures of study personnel trained on study specific training log.
- Collects documents needed to initiate the study and submit to the sponsor (e.g., FDA Forms 1572, CVs, etc.).
- Works with the PI to develop and implement recruitment strategies in accordance with HRPO (IRB) requirements and approvals.
- Conducts or participates in the informed consent process including interactions with the HRPO (IRB) and discussions with research participants, including answering any questions related to the study. Obtains appropriate signatures and dates on forms in appropriate places. Assures that amended consent forms are appropriately implemented and signed.
- Screens subjects for eligibility using protocol specific inclusion and exclusion criteria, documenting each potential participant’s eligibility or exclusion.
- Registers participants to the appropriate coordinating center (if multi-site study).
- Registers each participant in the billing matrix to ensure billing of study procedures to the appropriate funding source.
- Coordinates participant tests and procedures.
- Collects data as required by the protocol. Assures timely completion of Case Report Forms.
- Maintains study timelines.
- Maintains adequate inventory of study supplies. If handling investigational drugs/devices, follows the sponsor protocol and/or Washington University Policy on Investigational Drug/Device Accountability.
- Completes study documentation and maintains study files in accordance with sponsor requirements and University policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms.
- Retains all study records in accordance with sponsor requirements and university policies and procedures.
- Maintains effective and ongoing communication with sponsor, research participants and PI during the course of the study.
- Assists PI in preparation of any modifications to the scientific protocol in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Works with the PI to manage the day to day activities of the study including problem solving, communication and protocol management.
- Promotes the ethical conduct of research by reporting good faith suspicions of misconduct in research as defined within Washington University’s Research Integrity Policy and other misconduct as described in Washington University’s Code of Conduct.
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with Federal regulations and University and sponsoring agency policies and procedures. Assists in the registration (if required) of the study at ClinicalTrials.gov and maintains current information on the site.
Project Closeout
- Assists the Principal Investigator in submission of accurate and timely closeout documents to applicable federal agencies, university entities, and the sponsoring agency in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Arranges secure storage of study documents that will be maintained according to university policy or for the contracted length of time, whichever is longer.
Financial Management
- Reviews and accepts/corrects the billing matrix as set up by the Center for Applied Research Science (CARS) to facilitate billing of study procedures to the appropriate research fund.
- Coordinates appropriate and timely payments to participants (if applicable) in accordance with university policies and procedures.
Effort Reporting
- Reviews, adjusts and legally certifies personnel activity reports if applicable. Completes effort reporting certification within the timeframe specified by Sponsored Project Accounting.
Conflicts of Interest
- Takes appropriate steps to avoid conflicts of interest, or the appearance of conflicts of interest, between financial or other personal interests and the goals and policies of the university.
- Complies with applicable school, university, and sponsoring agency conflict of interest policies and procedures. Discloses all financial conflicts of interest to the appropriate supervisor.
- Cooperates with university compliance and monitoring efforts related to conflicts of interest and reports instances of noncompliance to the appropriate compliance office.
Human Research Participant Protection
- Assists Principal Investigator in protection of the rights and welfare of all human research participants involved in research in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists Principal Investigator in assuring that all key personnel involved in human research have completed the required education for the protection of human research participants in accordance with federal regulations and university and sponsoring agency policies and procedures. Maintains proof of all such education for all engaged members of the study team. Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with Federal regulations and University and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Cooperates with university compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and/or sponsors.
- Collaborates with PI and institution to respond to any audit findings and implement approved recommendations. Cooperates with university and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol. Prepares other study materials as requested by the PI. These study materials include but are not limited to the informed consent document, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Establishes and organizes study files, including but not limited to, regulatory binders, case report forms, study specific source documentation.
Informed Consent
- Assists in preparation of all documents related to the informed consent process.
- Assists Principal Investigator in preparation and submission of informed consent documents to HRPO for review and approval.
- Collects documents needed to initiate the study for submission to the sponsor (e.g., FDA Forms 1572, CVs, etc.).
- Conducts or participates in the informed consent process including interactions with the HRPO (IRB), discussions with research participants, including answering any questions related to the protocol. Obtains appropriate signatures and dates on forms in appropriate places. Assures that amended consent forms are appropriately implemented and signed.
- Collects data as required by the protocol. Assures timely completion of Case Report Forms.
- Completes study documentation and maintains study files in accordance with sponsor requirements and University policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms, etc.
- Retains all study records in accordance with sponsor requirements and University policies and procedures.
- Assists PI in preparation and submission of any modifications to the scientific protocol in accordance with federal regulations and university and sponsoring agency policies and procedures.
Protected Health Information
- Adheres to and supports all Federal regulations and University policies and procedures instituted to safeguard protected health information (PHI).
- Completes the appropriate level of training regarding the access, use, and disclosure of PHI in accordance with Federal regulations and University and sponsoring agency policies and procedures. Assists PI to assure that all personnel complete appropriate training.
- Cooperates with University compliance and monitoring efforts regarding the access, use, and disclosure of PHI and reports instances of noncompliance to the appropriate compliance office.
Unanticipated Problems
- Assists the Principal Investigator in promptly reporting any unanticipated problems involving risks to research participants or others to the HRPO (Washington University’s IRB).
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists in the registration (if required) of the study at ClinicalTrials.gov and maintains current information on the site.
- Arranges secure storage of study documents that will be maintained according to University policy or for the contracted length of time, whichever is longer.
Environmental Health and Safety
- Assists Principal Investigator in assuring that individuals handling hazardous or regulated materials are well trained in proper safety procedures and have completed required environmental health and safety training in accordance with federal, state, and local regulations and university and sponsoring agency policies and procedures.
- Works with Environmental Health and Safety to ensure that all facilities used are in compliance with all applicable regulations. Maintains copies of any applicable facility audits and equipment inspection/service reports.
Human Gene Transfer
- Assists Principal Investigator in assuring that all key personnel involved in human research have completed the required education for the protection of human research participants in accordance with Federal regulations and University and sponsoring agency policies and procedures. Maintains proof of all such education for all engaged members of the study team. Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with Federal regulations and University and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Cooperates with University compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and/or sponsors.
- Cooperates with University and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol. Prepares other study materials as requested by the PI. These study materials include but are not limited to the informed consent documents, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Engages participants in the informed consent process according to the HRPO approved process.
Award Acceptance (Terms & Conditions)
- Reviews and develops a familiarity with the contract or award terms and conditions. Works with the PI to assure that the study is in compliance with all terms and conditions, including but not limited to education, IRB (HRPO) approval, conflict of interest disclosure, health and safety protections for participants and staff and any financial terms or conditions.
- Maintains adequate inventory of study supplies. If handling investigational drugs/devices, follows the sponsor and/or Washington University Policy on Investigational Drug/Device Accountability.
- Completes study documentation and maintains study files in accordance with sponsor requirements and university policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms, etc.
- Adheres to and supports all federal regulations and university policies and procedures instituted to safeguard protected health information (PHI).
- Completes the appropriate level of training regarding the access, use, and disclosure of PHI in accordance with federal regulations and university and sponsoring agency policies and procedures. Assists PI to assure that all personnel complete appropriate training.
- Cooperates with university compliance and monitoring efforts regarding the access, use, and disclosure of PHI and reports instances of noncompliance to the appropriate compliance office.
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with Federal regulations and University and sponsoring agency policies and procedures.
Export Controls
- Develops awareness of export control regulations and complies as appropriate.
Revised January 2009 | Created 2007
- Vice Chancellor for Research
- Annual Report & Metrics
- Faculty Resources
- Institutional Data
- Chancellor Roles and Responsibilities
- Dean Roles and Responsibilities
- Department Administrator Roles and Responsibilities
- Department Head/Chair Roles and Responsibilities
- Principal Investigator Roles and Responsibilities
- Vice Chancellor for Finance Roles and Responsibilities
- Vice Chancellor for Research Roles and Responsibilities
- Center for Applied Research Services Roles and Responsibilities
- Committee on Research Integrity Roles and Responsibilities
- Conflict of Interest Review Committee Roles and Responsibilities
- Division of Comparative Medicine Roles and Responsibilities
- Environmental Health and Safety Roles and Responsibilities
- Export Control Roles and Responsibilities
- HIPAA Roles and Responsibilities
- Human Research Protection Office Roles and Responsibilities
- Human Research QA/QI Roles and Responsibilities
- Institutional Animal Care and Use Committee Roles and Responsibilities
- Office of General Counsel Roles and Responsibilities
- Office of Sponsored Research Services Roles and Responsibilities
- Office of Technology Management Roles and Responsibilities
- Sponsored Projects Accounting Roles and Responsibilities
- University Compliance Office Roles and Responsibilities
- Ongoing Projects
The Role of a Clinical Research Coordinator

Clinical research plays a crucial role in advancing medical knowledge and improving patient care. At the heart of every successful clinical research study is a Clinical Research Coordinator (CRC). As a CRC, you serve as the linchpin between researchers, study participants, and regulatory bodies.
In this comprehensive guide, we will explore the responsibilities, qualifications, challenges, and rewards of being a Clinical Research Coordinator. Whether you are considering a career in clinical research or already working in the field, this article provides valuable insights to help you succeed.
Responsibilities of a Clinical Research Coordinator
As a Clinical Research Coordinator, your responsibilities are diverse and demanding. You serve as the primary point of contact for study participants, ensuring their safety and well-being throughout the research process. You are responsible for recruiting and enrolling eligible participants, obtaining informed consent, and collecting accurate data. Additionally, you must adhere to strict regulatory guidelines and Good Clinical Practice ( GCP ) standards to ensure the integrity and validity of the study results.
Monitoring participants' progress, managing adverse events, and maintaining detailed records are also crucial aspects of your role as a CRC. To effectively carry out these responsibilities, strong organizational and communication skills are essential. You must be able to multitask, prioritize, and work well under pressure. Attention to detail is paramount, as any errors or oversights can compromise the validity of the study. As a CRC, you are also expected to stay updated on the latest research protocols and regulatory requirements to ensure compliance and contribute to the successful completion of the study.
Qualifications and Education Required to Become a Clinical Research Coordinator
While specific qualifications may vary depending on the institution or organization, a minimum educational requirement for most Clinical Research Coordinator positions is a bachelor's degree in a relevant field such as life sciences, nursing, or pharmacy. A solid foundation in biological sciences and research methodologies is crucial to understanding the complexities of clinical research. A master's degree in clinical research or a related field can further enhance your qualifications and open up opportunities for career advancement.
Apart from formal education, relevant work experience is highly valued in the field of clinical research. Prior experience in a research setting, such as working as a research assistant or in a healthcare role, can provide valuable insight into the research process and make you a more competitive candidate. Additionally, possessing knowledge of regulatory guidelines, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH-GCP ) guidelines, is advantageous.
Certification Options for Clinical Research Coordinators
While certification is not always mandatory, obtaining a certification as a Clinical Research Coordinator can enhance your professional credibility and increase your job prospects. Several organizations offer certification programs for CRCs, such as the Association of Clinical Research Professionals ( ACRP ) and the Society of Clinical Research Associates ( SoCRA ). To obtain certification, you typically need to meet certain eligibility criteria, which may include a combination of education, work experience, and passing a certification exam.
These certification programs cover a wide range of topics, including research ethics, study design, data management, and regulatory compliance. By obtaining certification, you demonstrate your commitment to maintaining high standards of practice and staying up-to-date with industry best practices.
Tips for Creating an Effective Clinical Research Coordinator Resume
In a competitive job market, a well-crafted resume can make all the difference in securing a Clinical Research Coordinator position. Here are some tips to help you create an effective resume that highlights your skills and qualifications:
1. Start with a compelling summary: Begin your resume with a concise summary that highlights your relevant experience, qualifications, and career goals. This section should grab the attention of potential employers and encourage them to read further.
2. Emphasize your research experience: Highlight your research experience, including any previous roles as a research assistant or involvement in clinical trials. Describe your responsibilities, methodologies used, and any noteworthy achievements.
3. Showcase your knowledge of regulations and guidelines: Demonstrate your familiarity with regulatory guidelines, such as ICH-GCP, and any additional certifications you have obtained. This shows your commitment to ethical research practices and compliance.
4. Highlight your organizational and communication skills: As a CRC, strong organizational and communication skills are crucial. Provide examples of how you have effectively managed multiple tasks, coordinated with various stakeholders, and maintained accurate documentation.
5. Include relevant technical skills: Depending on the specific requirements of the position, include any relevant technical skills such as proficiency in electronic data capture systems, statistical software, or data analysis tools. These skills can set you apart from other candidates.
Remember to tailor your resume to each specific job application, focusing on the skills and qualifications that are most relevant to the position. Proofread your resume carefully to ensure it is error-free and presents you in the best possible light.
Common Interview Questions for Clinical Research Coordinator Positions
Preparing for a job interview is essential to present yourself confidently and effectively. Here are some common interview questions for Clinical Research Coordinator positions, along with tips on how to answer them:
1. Tell us about your experience in clinical research: Be prepared to discuss your previous roles and responsibilities in clinical research, emphasizing your ability to manage study participants, collect accurate data, and ensure compliance with regulatory guidelines.
2. How do you handle challenges in clinical research?: Demonstrate your problem-solving skills by sharing examples of challenging situations you have encountered and how you successfully resolved them. Emphasize your ability to adapt to unexpected circumstances and maintain a high level of professionalism.
3. How do you ensure participant safety and informed consent?: Highlight your understanding of the importance of participant safety and informed consent in clinical research. Explain your approach to obtaining and documenting informed consent, as well as your strategies for monitoring participant well-being.
4. How do you manage time and prioritize tasks?: Showcase your organizational and time management skills by describing how you handle multiple tasks, prioritize responsibilities, and meet deadlines. Provide examples of how you have effectively managed your workload in previous roles.
5. What are your strategies for maintaining accurate and detailed documentation?: Stress the importance of accurate documentation in clinical research and describe your methods for ensuring meticulous record-keeping. Discuss your attention to detail and your ability to maintain confidentiality.
Remember to practice your responses to these questions beforehand, focusing on providing concise and well-thought-out answers. Also, prepare questions to ask the interviewer to demonstrate your interest in the role and organization.
Challenges and Rewards of Being a Clinical Research Coordinator
Working as a Clinical Research Coordinator comes with its own set of challenges and rewards. It is essential to be aware of both aspects to make an informed decision about pursuing a career in this field.
Challenges:
1. Time management: Balancing multiple tasks and deadlines can be challenging, especially when working on multiple studies simultaneously. Strong organizational skills and the ability to prioritize effectively are crucial.
2. Regulatory compliance: Adhering to strict regulatory guidelines and ensuring compliance with ethical standards can be complex. Staying updated on the latest regulations and guidelines is essential to avoid any non-compliance issues.
3. Participant recruitment: Recruiting and enrolling eligible participants can be challenging, particularly when dealing with specific inclusion and exclusion criteria. A proactive and strategic approach to participant recruitment is necessary.
Rewards:
1. Contribution to medical advancements: As a Clinical Research Coordinator, you play a vital role in advancing medical knowledge and improving patient care. The data and insights you collect contribute to the development of new treatments and therapies.
2. Personal and professional growth: Working in clinical research provides continuous opportunities for learning and professional development. You gain valuable experience in research methodologies, data management, and regulatory compliance.
3. Making a difference: By ensuring participant safety and well-being, you make a meaningful impact on the lives of study participants. Clinical research coordinators are instrumental in bringing new treatments and therapies to patients in need.
The challenges and rewards of being a Clinical Research Coordinator often go hand in hand. The satisfaction of overcoming challenges and contributing to medical advancements can be immensely rewarding and fulfilling.
Continuing Education and Professional Development Opportunities
Continuing education and professional development are crucial for Clinical Research Coordinators to stay updated on the latest research methodologies, regulations, and best practices. Here are some opportunities for ongoing learning and growth:
1. Workshops and conferences: Attend workshops and conferences related to clinical research to expand your knowledge, network with industry professionals, and stay informed about the latest advancements in the field.
2. Online courses and webinars: Take advantage of online courses and webinars offered by reputable organizations and institutions. These courses cover a wide range of topics, from research ethics to data analysis.
3. Association membership: Join professional associations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates ( SoCRA ). These associations offer resources, networking opportunities, and certification programs.
4. Advanced degrees: Consider pursuing an advanced degree, such as a master's or doctoral degree, in clinical research or a related field. This can provide in-depth knowledge and open up opportunities for leadership roles in the field.
Continuing education not only enhances your skills and knowledge but also demonstrates your commitment to professional growth and maintaining high standards of practice.
Resources and Associations
As a Clinical Research Coordinator, it is essential to stay connected with the wider clinical research community and have access to valuable resources. Here are some notable associations and resources for CRCs:
1. Association of Clinical Research Professionals ( ACRP ): ACRP is a global membership association that provides educational resources, networking opportunities, and certification programs for clinical research professionals.
2. Society of Clinical Research Associates ( SoCRA ): SoCRA offers certification programs, training resources, and networking opportunities for clinical research professionals. They also publish a quarterly journal, "The Monitor," which provides valuable insights and updates in the field.
3. ClinicalTrials.gov : ClinicalTrials.gov is a public database maintained by the U.S. National Library of Medicine. It provides information on clinical trials worldwide, allowing CRCs to stay updated on ongoing and upcoming studies.
4. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH ): The ICH website provides access to guidelines and standards for the conduct of clinical research. Familiarize yourself with these guidelines to ensure compliance and ethical conduct.
By utilizing these resources and actively engaging with professional associations, you can stay informed about the latest industry developments, connect with peers, and access valuable tools and support.
The role of a Clinical Research Coordinator is diverse, demanding, and rewarding. As a CRC, you play a crucial role in advancing medical knowledge, ensuring participant safety, and contributing to the development of new treatments and therapies. By understanding the responsibilities, qualifications, and challenges of the role, you can position yourself for success in the field of clinical research.
Continuously seek opportunities for professional growth, stay updated on the latest regulations and best practices, and actively engage with the clinical research community. With dedication, passion, and a commitment to excellence, you can thrive as a Clinical Research Coordinator and make a significant impact in the field of clinical research.
Sign up for post alerts
Icon & you the potential of together..
Careers that improve the lives of patients, our clients and each other. Are you ready to make a difference?
Related jobs
Mexico, Benito Juarez (PRA)
Full Service - Project Management
Mexico City
Benito Juarez
Remote Working
Office Based
Business Area
ICON Full Service & Corporate Support
Job Categories
Project/ Program Management
Description
ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech
Expiry date

Clinical Monitoring
ICON Strategic Solutions
As a Senior Clinical Research Associate you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence. You will be partnering with one
2023-103968

2023-104179
As Clinical Research Associate (CRA), you will be joining the world's largest & most comprehensive clinical research organization, powered by healthcare intelligence. A CRA is a professional who cont
2023-103904

Related stories

Teaser label
Content type
Publish date
Exploring Lucy's Path: From Science to Communications Lucy's career journey is a testament to the power of adaptability and seizing opportunities as they arise. "I've been in Clinical Research si
We hear from Lucy on her varied career journey, who joined as a Clinical Research Associate in 2008.
.png)
Interviews can be nerve-wracking experiences, but with the right preparation, you can enter the room with confidence and increase your chances of success. Preparing for an interview is crucial a
From mastering common interview questions to what to wear, our blog covers all you need to prepare effectively.

Transferrable Skills in Clinical Research In the ever-evolving field of clinical research, having a diverse skill set is essential for career growth and success. Transferrable skills play a crucia
Explore expert insights and actionable advice to elevate your career in the dynamic field of Clinical Research.
Recently viewed jobs
A better career. A better world. A better you.
Browse popular job categories below or search all jobs above
What does a clinical research coordinator do?
Would you make a good clinical research coordinator? Take our career test and find your match with over 800 careers.
What is a Clinical Research Coordinator?
A clinical research coordinator (CRC) plays an important role in the field of clinical research, ensuring the smooth and efficient conduct of clinical trials and studies. These professionals act as a bridge between the research investigators, sponsors, and participants, overseeing various aspects of the research process. They collaborate closely with physicians, nurses, and other healthcare professionals to guarantee the well-being of study participants while collecting valuable data that contributes to scientific advancements and the development of new treatments and therapies.
To excel as a clinical research coordinator, individuals typically need a strong background in life sciences, healthcare, or a related field. Attention to detail, excellent organizational skills, and the ability to communicate effectively are essential.
What does a Clinical Research Coordinator do?

Duties and Responsibilities Clinical research coordinators have a wide array of duties and responsibilities aimed at ensuring the successful implementation and completion of clinical trials. Their roles are multifaceted and demanding, requiring a combination of organizational, interpersonal, and scientific skills. Some key duties and responsibilities include:
- Protocol Management: CRCs are responsible for understanding and implementing the research protocol thoroughly. They ensure that all aspects of the study adhere to the protocol, including participant eligibility criteria, investigational product administration, and data collection procedures.
- Participant Recruitment and Informed Consent: CRCs actively recruit eligible participants for the clinical trial. They explain the study details, benefits, and risks to potential participants and obtain their informed consent to participate in the research.
- Data Collection and Management: CRCs collect accurate and comprehensive data from participants during the study, maintaining detailed records. They may use electronic data capture systems to ensure data accuracy and integrity.
- Clinical Procedures: Depending on their qualifications and the nature of the study, CRCs might perform certain clinical procedures such as drawing blood, taking vital signs, or administering investigational drugs under the supervision of a licensed healthcare provider.
- Regulatory Compliance: CRCs ensure that the study complies with all relevant regulations, including guidelines set by the Food and Drug Administration (FDA) and the Institutional Review Board (IRB). They assist in preparing and submitting regulatory documents for study approval and conduct regular audits to maintain compliance.
- Safety Monitoring: CRCs monitor participants for adverse events and ensure appropriate and timely reporting of these events to regulatory authorities and sponsors.
- Collaboration: CRCs work closely with principal investigators, research nurses, pharmacists, and other healthcare professionals involved in the study. They facilitate effective communication among team members to ensure the smooth progress of the research.
- Administrative Tasks: CRCs are often responsible for managing the administrative aspects of the study, including scheduling participant visits, organizing meetings, and maintaining study-related documentation.
- Quality Control: CRCs perform quality control checks to ensure that the collected data is accurate, complete, and in compliance with the study protocol. They also participate in monitoring visits conducted by sponsors or regulatory agencies.
- Education and Support: CRCs provide education and support to study participants, addressing their concerns and questions throughout the trial. They serve as a primary point of contact between participants and the research team.
Types of Clinical Research Coordinators Clinical research coordinators can specialize in various areas within the field of clinical research, each requiring specific skills and expertise. Here are some common types of CRCs based on their specialized roles:
- Clinical Research Nurse Coordinator: These CRCs are registered nurses (RNs) with specialized training in clinical research. They often have a strong background in patient care and are responsible for both the clinical and research aspects of the studies. Clinical Research Nurse Coordinators may administer medications, perform clinical procedures, and monitor participants' health throughout the trials.
- Regulatory Affairs Coordinator: Regulatory CRCs focus on ensuring that the clinical trials comply with all applicable regulations and guidelines. They prepare and submit documents for regulatory approval, liaise with regulatory authorities, and keep the research team informed about any changes in regulations that might affect the study.
- Data Coordinator: Data CRCs are responsible for collecting, managing, and analyzing the data generated during clinical trials. They ensure data accuracy, integrity, and confidentiality. These professionals often work closely with statistical teams and use various software tools for data analysis.
- Recruitment Coordinator: Recruitment CRCs specialize in participant recruitment and retention. They develop strategies to identify eligible participants, engage with potential candidates, explain the study details, and address concerns. Their role is crucial in ensuring that studies meet their enrollment goals.
- Pediatric Clinical Research Coordinator: Specializing in pediatric clinical trials, these CRCs have expertise in working with children and adolescents. They understand the unique ethical and logistical challenges associated with pediatric research and ensure that the trials are conducted safely and ethically in younger populations.
- Oncology Research Coordinator: Oncology CRCs work specifically on cancer-related clinical trials. They collaborate with oncologists and other specialists to coordinate complex cancer studies, which often involve novel therapies and treatments.
- Quality Assurance Coordinator: Quality Assurance CRCs focus on ensuring that the clinical trial processes and data collection adhere to quality standards and protocols. They conduct internal audits, develop quality control procedures, and assist in preparing for external audits from regulatory authorities or sponsors.
- Site Management Coordinator: Site Management CRCs oversee the operations of clinical trial sites. They coordinate activities among various research sites, ensuring consistency in protocol implementation, data collection, and reporting. Site Management CRCs also facilitate communication between different sites and the central research team.
Are you suited to be a clinical research coordinator?
Clinical research coordinators have distinct personalities . They tend to be investigative individuals, which means they’re intellectual, introspective, and inquisitive. They are curious, methodical, rational, analytical, and logical. Some of them are also enterprising, meaning they’re adventurous, ambitious, assertive, extroverted, energetic, enthusiastic, confident, and optimistic.
Does this sound like you? Take our free career test to find out if clinical research coordinator is one of your top career matches.
What is the workplace of a Clinical Research Coordinator like?
Clinical research coordinators work in a variety of settings, primarily in environments related to healthcare, research, and pharmaceutical industries. Here are the typical workplaces for CRCs:
Academic Medical Centers: Many CRCs are employed at universities and academic medical centers, where they collaborate with researchers, physicians, and other healthcare professionals to conduct clinical trials. These settings often involve a combination of patient care and research activities.
Hospitals: Hospitals, especially those with research-focused departments, employ CRCs to coordinate and manage clinical trials. In hospital settings, CRCs work closely with medical staff and patients, often in specialized departments like oncology, cardiology, or neurology.
Clinical Research Organizations (CROs): CROs are specialized companies hired by pharmaceutical, biotechnology, and medical device companies to conduct clinical trials on their behalf. CRCs in CROs manage multiple clinical trials across different therapeutic areas and work with various sponsors and research sites.
Pharmaceutical and Biotechnology Companies: CRCs can work directly for pharmaceutical and biotechnology companies, overseeing in-house clinical trials to test new drugs, treatments, or medical devices. In this setting, CRCs collaborate closely with scientists, regulatory affairs professionals, and project managers.
Government and Nonprofit Organizations: Some CRCs are employed by government agencies (such as the National Institutes of Health) and nonprofit organizations (such as research foundations or advocacy groups) that fund and conduct clinical research. These professionals are involved in a wide range of studies aimed at advancing scientific knowledge and public health.
Private Research Clinics: Private research clinics specialize in conducting clinical trials. CRCs in these settings work on a variety of studies and often have a more streamlined and focused work environment compared to larger medical institutions.
Home-based Work: With advancements in technology, some CRCs may have the flexibility to work remotely for certain tasks, such as data analysis, regulatory document preparation, or administrative tasks. However, a significant portion of their work still involves on-site activities, especially those related to direct participant interaction and clinical procedures.
Clinical Research Coordinators are also known as: CRC

Clinical Research Coordinator
Clinical Research Coordinator Certification
Clinical research coordinator jobs are in high demand. Learn how to become a clinical research coordinator with our clinical research coordinator training program. Get advanced ccrc certification to be successful in this growing field.

Clinical Research Coordinator Training FAQs
Introduction to crc.
CME Handout
Duties and Responsibilities of Clinical Research Coordinators
Employment Advancement for Clinical Research Coordinators
Process Map of A Sponsored Clinical Trial Study
Orientation Manual for Clinical Research Coordinator
Protocols and Guidelines
SOPs and MOPs
SOP Template
MOP Outline
MOP Example
Clinical Research Coordinator Toolkit
Routine Site Visit Report
Adverse Event Tracking Log
Chart Audit Tool
Regulatory File Review Tool
Monitoring Log
An Introduction to Clinical Research
An Overview of ICH GCP
Code of Federal Regulations
CFR 21 Part 11
Sponsor/CRO Responsibilities
ICH GCP E6 Sections 2-4 Principles, IRB, & Investigator Roles
ICH GCP Section 4.8 Informed Consent
Reporting Responsibilities of the Investigators
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated
Ethics of Research Involving Pregnant Women and Fetuses
Ethics of Research Involving Prisoners
ICH GCP E6 and E2A - Adverse Events
Safety of Human Subjects in Clinical Research
ICH GCP 5.5 Trial Management – Data Handling and Record Retention
a) Common Terminology Used In Clinical Research
b) Commonly Used Abbreviations and Terms in Clinical Research
ICH GCP Quiz
Advanced Clinical Trials Foundations
Designs of Clinical Trials
Phases of Clinical Trials
Stakeholders in Clinical Research and Their Relationships
Contract Research Organization- CRO
Randomized Controlled Trials
Types of Monitoring Visits
Site and Investigator Selection
Site Initiation Visit (SIV)
Site Qualification Visit
Routine Monitoring Visit
Site Close Out Visit
Source Documents and Informed Consent Forms
Quality Monitoring Quiz Modules 1-15
Inclusion Exclusion Criteria in Clinical Research
Interactive Voice Response System - IVRS
The Trial Protocol
Protocol Deviations and Violations
Institutional Review Board
Quality Control in Clinical Research
Blinding in Clinical Trials
Communication between Blinded and Unblinded Staff
Investigational Product Storage and Dispensing
Investigational Product Accountability in Clinical Trials
Quality Monitoring Quiz
Adverse Drug Reactions
Basics of Adverse Event Monitoring
Adverse Event Reporting
Safety Reporting Requirements for Sponsor Investigators of An IND
IND and NDA Process
Guidelines for Designing and Completing Case Report Forms
Do’s and Don’ts of a Case Report Form Design
Clinical Trial Management System-CTMS

Compliance and Regulations
Regulatory Documents in Clinical Research
Regulatory Affairs
Essential Regulatory Documents Guidance and Binder Tabs (Part 1)
Essential Regulatory Documents Guidance and Binder Tabs (Part 2)
Electronic Regulatory Submission and Review
Financial Disclosure- Duties and Strategies for Clinical Studies
Financial Disclosures and Conflicts of Interest in Clinical Research
FDA Form 1572 - Part 1
FDA Form 1572 - Part 2
Delegation of Authority Log – DOAL
Investigators Brochures
Protocol Continuing
IND Application
Trial Master File and DIA Model
Trial Master File Reference Guide
Regulatory Training Quiz (20 Questions)
Audit and Inspections
Audits and Inspections in Clinical Trials
FDA Warning Letter
Site FDA Audit Inspection Checklist
How to Survive Through an FDA Inspection
Do and Don’ts during an FDA Inspection
Audits and Inspection Quiz
Subject Recruitment and Retention
Compliance Requirements in Clinical Trials
Subject Recruitment and Retention (Part 1)
Subject Recruitment and Retention (Part 2)
Increasing Subject Compliance in Clinical Trials
Ethical Consideration Associated with Investigator Payment and Patient Recruitment
Advertisement Aid in Subject Recruitment and Retention
Misconduct and Fraud
Scientific Misconduct in Research and How to Prevent It
Misconduct in Research – Detecting Falsification
Statistics and Data Management of Clinical Trials
Data Management In Clinical Research
Good Clinical Data Management Protocol
Financial Management of Clinical Trials
Financial Management Fundamentals
Developing A Trial Budget
Budget Worksheet
Final Examination
Competency Exam (52 Questions)

About this course
- 100 lessons
- 6.5 hours of video content
CRC Certification Graduate Case Study
From No Research Experience to Columbia University CRC
Impact on Confidence and Employability: It significantly enhanced Lisa-Pierre's confidence in applying for Clinical Research Coordinator (CRC) positions, despite lacking direct US work experience.
Career Advancement: The course enabled Lisa-Pierre to kickstart her career as a CRC at Columbia University, and additional job offers subsequently.
Recommendations for Others: She advises taking the course to gain needed research knowledge for one's resume and to facilitate a career transition into the field, especially for those without research degrees.
F a vorite Aspects of the Course: She appreciated the self-paced online format, which allowed her flexibility to learn alongside her other commitments. The ability to revisit lessons and access support was particularly beneficial.
Satisfaction with Course Content: She was impressed with the course's comprehensive overview of the CRC role and clinical research processes, finding it informative on topics she was previously unfamiliar with and a solid foundation comparable to on-the-job training. (March 2024 Graduate Case Study).
CCRPS Reviews
Excellent course, karim heras.
I am an IMG who is just waiting for the residency cycle to open up and needed to continue to keep up with my clinical experience. This course is very complet...
I am an IMG who is just waiting for the residency cycle to open up and needed to continue to keep up with my clinical experience. This course is very complete, it have all the information needed to completely understand what goes on in clinical trials. It covers all the basic information and further explores and explains more of the important aspects duties and responsibilities of clinical research. The staff has been very courteous and patient with all my questions and needs.
CCRPS Program
Deborah conradi.
What an amazing program for clinical research certification! I totally endorse this product and am very impressed with the quality and educational materials!...
What an amazing program for clinical research certification! I totally endorse this product and am very impressed with the quality and educational materials! thank you
Introduction chapter
Serena kurumety.
I like the thoroughness and detail information of CRC role. I appreciate the examples of information that should be included in resume as well as in Linke...
I like the thoroughness and detail information of CRC role. I appreciate the examples of information that should be included in resume as well as in LinkedIn ; for me this is the weakness. " I'm not good at marketing my self "
FRANCISCO MCNALLY
Excellent course with quality of presentation and information, precise and clear.
easy to learn
Ederlin pimentel, good descriptions do explains quite well.
Clinical research coordinators help to manage and monitor clinical trials. These trials must follow protocols established by the FDA or other relevant regulatory bodies. In order to be a certified clinical research coordinator, one must be certified by an organization, such as CCRPS.
Clinical research coordinators are responsible for developing the protocol, recruiting patients, collecting data, and making sure subjects are safe during a clinical trial. They must make sure that all regulations such as HIPAA and GCP are followed during the study.
Clinical research coordinators are also responsible for managing budgets and preparing reports. They make sure information is correct and that they follow protocol guidelines. They may be responsible for training staff on proper procedures and how to use various software programs.
Become familiar with the field of clinical research if you want to become a Certified Clinical Research Coordinator (CRC). This means reading about clinical trials and the role of a CRC. It is also helpful to get formal education in this field, for example by pursuing a degree or clinical research coordinator certification, CRC Certification, health sciences or a related field. You can also take courses specifically focused on clinical research coordinator training.
- Get relevant experience by volunteering in sponsors’ offices, participating in investigator meetings, or attending conferences related to the industry.
- When you have fulfilled all educational requirements, your next step is to obtain a clinical research coordinator certificate.
- CCRPS offers the only advanced clinical research coordinator certification program for CRCs at all levels.
Clinical research coordinator certification
By obtaining a clinical research coordinator certification, you will be able to maximize your salary potential. Clinical research coordinators are typically in high demand and the average salary for those with certification is $51,594 per year.
- To get job as a clinical research coordinator, it will help if you are a certified clinical research coordinator.
- Clinical research coordinator certifications provide instruction on topics related to this field, including GCP compliance, protocol design, data collection methods, and more.
- Certification helps become eligible for promotions or higher positions within their organization and to keep up with the latest changes in medical protocols.
Clinical research coordinator salary
Average clinical research coordinator salary is between $46,000 and $70,000 per year. The highest paying states for clinical research coordinators include California ($78,500), New York ($77,800), Massachusetts ($76,300) and Connecticut ($75,600). CRC salaries vary based on research organization and whether it is public or private.
- Those with higher education credentials may earn even more; for example, a Master's degree could result in a salary of up to $90,000 annually.
- More experience are likely to make more money than clinical research coordinator I salary.
- A Clinical Research Coordinator I typically earns an hourly wage of $19.00-$27.50. CCRC certification can increase your position.
CRC Certification FAQs
- Mayo Clinic Careers
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Radiology Imaging
Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Diversity, Equity & Inclusion
- Employees with Disabilities

Life-changing careers
Search life-changing careers.
Search by Role or Keyword
Enter Location
- United States Applicants
- United Kingdom Applicants
- United Arab Emirate Applicants
- Current Employees
Explore a life-changing career in a position that provides career development and growth, that is multifaceted, rewarding, and where the learning never stops.
As a Clinical Research Coordinator, you will have the opportunity to work with a variety of people, from patients to care teams, enabling you to be a part of the legacy that puts the needs of the patients first.
In addition to driving innovation, the Research shield at Mayo Clinic is committed to creating a diverse environment that is open to unconventional ideas recognizing that diverse research teams consisting of unique contributions make better decisions and are more equipped to adapt to change and solve complex problems – producing better outcomes.
Join us on our path to finding cures where you’ll contribute to the research and development of new treatments, devices, and cutting-edge innovations – making a direct impact on patients’ lives.
Clinical Research Coordinator Jobs
- Associate Clinical Research Coordinator - Cardiovascular Diseases 329950 Rochester, Minnesota
- Senior Clinical Research Coordinator (Cancer) 329399 Jacksonville, Florida
- Clinical Research Coordinator-Early Cancer Therapeutic 329004 Phoenix, Arizona
- Associate Clinical Research Coordinator - NW Wisconsin 328244 Eau Claire, Wisconsin
- Clinical Research Coordinator 324574 Rochester, Minnesota
- Associate Clinical Research Coordinator 324573 Rochester, Minnesota
- Senior Clinical Research Coordinator - Cardiovascular Research 323822 Rochester, Minnesota
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.
Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Advanced Practice Providers Business Education Engineering Executive Facilities Support Global Security Housekeeping Information Technology Internship Laboratory Nursing Office Support Patient Care - Other Pharmacy Phlebotomy Physician Post Doctoral Radiology Imaging Research Scientist Surgical Services Therapy
Location Select Location Albert Lea, Minnesota Austin, Minnesota Barron, Wisconsin Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Decorah, Iowa Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Faribault, Minnesota Holmen, Wisconsin Jacksonville, Florida Kasson, Minnesota La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Prairie du Chien, Wisconsin Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Cloud, Minnesota Saint James, Minnesota Scottsdale, Arizona Sparta, Wisconsin Waseca, Minnesota
Area of Interest Select One Nursing Research Laboratory Medicine & Pathology Radiology Surgery Pharmacy Facilities Psychiatry & Psychology Physical Medicine & Rehabilitation General Services Surgical Technician Cardiovascular Medicine Finance Licensed Practical Nurse (LPN) Respiratory Therapy Emergency Medicine Neurology Environmental Services Mayo Collaborative Services Social Work Ambulance Services Radiation Oncology Anesthesiology & Perioperative Medicine Family Medicine Information Technology Orthopedics Gastroenterology & Hepatology Global Security Medical Oncology Surgical Assistant Urology Administration Cardiovascular Surgery Critical Care Healthcare Technology Management International Transplant Pediatrics Hospital Internal Medicine Mayo Clinic Laboratories Obstetrics & Gynecology Office Support Desk Operations Hematology Ophthalmology Community Internal Medicine Education Oncology Otolaryngology (ENT) Patient Scheduling Biochemistry & Molecular Biology Dermatology Pulmonary/Sleep Medicine Senior Care Engineering Hospice & Palliative Care Digital General Internal Medicine Artificial Intelligence & Informatics Clinical Genomics Clinical Nutrition Endocrinology Infectious Diseases Linen & Central Services Molecular Medicine Neurologic Surgery Physiology & Biomedical Engineering Sports Medicine Cancer Center Development/Philanthropy Epidemiology Immunology Neurosciences Primary Care Business Development Cancer Biology Communications Comparative Medicine Mayo Clinic Platform Nephrology & Hypertension Quality Regenerative Biotherapeutics Rheumatology Addiction Services Center for Individualized Medicine Clinical Trials & Biostatistics Dental Specialities Executive Office Health Care Delivery Research Marketing Media Support Services Molecular Pharmacology & Experimental Therapeutics Spiritual Care Travel Computational Biology Geriatric Medicine & Gerontology Health Information Management Services Human Resources Information Security Legal Occupational/Preventative Medicine Pain Medicine Risk Management Spine Center Volunteer Services
Confirm Email
By submitting your information, you consent to receive email communication from Mayo Clinic.
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
- CE Certificates
- Billing Inquiries
- Purchase Inquiries
Clinical Research Coordinator (CRC)
CRC courses focus on key topics essential to the conduct of clinical research. They are specifically tailored to the needs of clinical research coordinators.
About these Courses
CRC courses provide foundational and advanced role-based training for clinical research professionals. The courses cover information that expands beyond but is directly connected to the Human Subjects Research (HSR) and Good Clinical Practice (GCP) courses.
The Foundations course delivers basic CRC training that organizations may use for onboarding new CRCs. Included are the operational and regulatory essentials that CRCs need. It also provides a basis for learners who will later move on to the advanced course.
In the Advanced course, learners gain a deeper understanding of the CRC’s role by exploring key operational, leadership, regulatory, and technical elements associated with daily work.
These courses were written and peer-reviewed by experts.
Language Availability: English
Suggested Audiences: Clinical Research Coordinators (CRCs), Clinical Research Professionals, Investigators
Basic Courses
Clinical research coordinator (crc) foundations.
This course provides basic CRC training.

Advanced Courses
Clinical research coordinator (crc) advanced.
This course provides clinical research professionals with advanced training tailored to the CRC’s critical role in the conduct of clinical trials.

Combined Courses
Comprehensive crc.
Provides clinical research professionals with basic and advanced training tailored to the CRC’s critical role in the conduct of clinical trials.

Who should take the CRC courses?
CRCs, investigators, and other clinical research professionals.
The foundations course can be used as foundational role-based training for learners needing basic CRC training or organizations needing onboarding training for new CRCs. It may also be useful to those pursuing a career in clinical research.
The advanced course is designed for CRCs who have taken the foundations course or those with two or more years of experience as a CRC. It complements the foundational course, and may be used for professional development and/or as a refresher course.
The combined course is an option that allows independent learners to purchase the foundations and advanced courses together.
How long does it take to complete a CRC course?
The foundations course consists of 11 modules and 4 additional modules of interest, and the advanced course consists of 7 modules.
Each module contains detailed content, images, supplemental materials (such as case studies), and a quiz. Modules vary in length, and learners may require different amounts of time to complete them based on their familiarity and knowledge of the topic. However, they can complete them at their own pace. Modules are each designed to take about 30 to 45 minutes to complete, which means an entire CRC course could take about three to six hours to complete.
Are the CRC courses eligible for CME credits?
Both the CRC Foundations and CRC Advanced courses are eligible for CME credits. For more information, see the CRC Foundations and CRC Advanced course pages.
For more information on how to ensure CME credit availability for learners at your organization, contact [email protected] or 888.529.5929.
How frequently should learners take CRC training?
There is no uniform standard for how frequently CRC training should occur. For a retraining (refresher) cycle, organizations should designate the frequency for their learner groups.
What are the advantages of CITI Program’s CRC training?
The CRC courses provide role-specific, peer-reviewed training written by CRC experts. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for CRC training.
Privacy Overview

Clinical research coordinator
A Clinical Research Coordinator (CRC) manages and conducts the day-to-day activities of a clinical trial . The Principal Investigator (PI) determines the CRC’s specific responsibilities and works closely with the CRC. In general, the CRC ensures the clinical study maintains accordance with the protocol, applicable regulations, and Good Clinical Practice (GCP) and Institutional Review Board (IRB) requirements. Beyond administrative duties, responsibilities of a CRC may include acting as a liaison for the clinical site, ensuring staff are properly trained per the protocol, recruiting and/or registering participants, maintaining study guidelines, and collecting and/or reviewing the data or review before it is entered into a study database .
A CRC usually has a bachelor’s degree in a scientific, health-related, or business administration program. When a CRC is a registered nurse, they may be called Clinical Research Nurse Coordinator (CRNC).
Sourced From National Institute on Aging (NIA) Glossary of Clinical Research Terms Washington University St. Louis: Clinical Research Coordinator Roles and Responsibilities Learn More NCATS Toolkit: Educating Your Disease Community
- Education Home
- Medical Education Technology Support
- Graduate Medical Education
- Medical Scientist Training Program
- Public Health Sciences Program
- Continuing Medical Education
- Clinical Performance Education Center
- Center for Excellence in Education
- Research Home
- Biochemistry & Molecular Genetics
- Biomedical Engineering
- Cell Biology
- Microbiology, Immunology, & Cancer Biology (MIC)
- Molecular Physiology & Biological Physics
- Neuroscience
- Pharmacology
- Public Health Sciences
- Office for Research
- Clinical Research
- Clinical Trials Office
- Funding Opportunities
- Grants & Contracts
- Research Faculty Directory
- Cancer Center
- Cardiovascular Research Center
- Carter Immunology Center
- Center for Behavioral Health & Technology
- Center for Brain Immunology & Glia
- Center for Diabetes Technology
- Center for Immunity, Inflammation & Regenerative Medicine
- Center for Public Health Genomics
- Center for Membrane & Cell Physiology
- Center for Research in Reproduction
- Myles H. Thaler Center for AIDS & Human Retrovirus Research
- Child Health Research Center (Pediatrics)
- Division of Perceptual Studies
- Research News: The Making of Medicine
- Core Facilities
- Virginia Research Resources Consortium
- Center for Advanced Vision Science
- Charles O. Strickler Transplant Center
- Keck Center for Cellular Imaging
- Institute of Law, Psychiatry & Public Policy
- Translational Health Research Institute of Virginia
- Clinical Home
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopaedic Surgery
- Otolaryngology
- Physical Medicine & Rehabilitation
- Plastic Surgery, Maxillofacial, & Oral Health
- Psychiatry & Neurobehavioral Sciences
- Radiation Oncology
- Radiology & Medical Imaging
- UVA Health: Patient Care
- Diversity Home
- Diversity Overview
- Student Resources
- GME Trainee Resources
- Faculty Resources
- Community Resources
- Study Coordinators
In addition to what is offered in this section, please be sure to see Education for all researchers . This section includes IRB education, required CITI training, and other online training opportunities, such as the IRB Learning Shots.
The School Of Medicine Clinical Trials Office (SOM CTO) is a safe haven for the lost and lonely Clinical Research Coordinator (CRC). We provide mentoring for new CRCs, either new to the job or just new to UVA. We also manage a CRC global mailing list to alert coordinators to new issues, tips, continuing education opportunities, etc. We are here at all times as a resource: a place to start to find the answer to your question or guidance in managing your trial(s).
Cancer Center Education
The Office for Clinical Research (OCR) is committed to supporting Cancer Center Clinical study staff in Education and Compliance. We are dedicated to elevating the knowledge base and performance of the Cancer Center research study staff. The OCR assists Investigators and study coordinators at any stage in their trials.
CRC Continuing Education Series
Working with the Center for Organizational Development, the CTO offers training and education focusing on the various aspects of clinical trials. This education is designed to keep CRCs and PIs abreast of current trends in the conduct of clinical trials.
- Continuing education series
- External audio conferences
- Online orientation manual
The SOM CTO offers training and education focusing on the various aspects of clinical trials. This education is designed to keep CRCs and other research team members abreast of current internal research processes and trends in the conduct and ethics of clinical trials.The continuing education consists of webinars and the CRC Continuing Education Series.Each year coordinators are polled for their education requests and a continuing education series is created.
The SOM CTO is a “provider” for contact hours through the Virginia Nurses’ Association, and the continuing education series helps coordinators maintain the education hours they need to maintain their CRC or CRA certification.
CRC Email Distribution List
The CRC email distribution list is an easy way to communicate with each other. Therefore, the CTO uses the email list to share tips, concerns, questions and answers, etc..
To join the email list, contact the Clinical Trials Office .
Professional Research Certifications
There are 2 national organizations that provide education and professional certification of Clinical Research Coordinators: ACRP and SoCRA.
ACRP (Association of Clinical Research Professionals) is the older organization. From their website:
“Headquartered in Alexandria, Virginia, ACRP was founded in 1976 to address the distinct educational and networking needs of research nurses and others who supported the work of clinical investigations. With its own professional society came the recognition of a new distinctive profession — that of the clinical researcher. More than 35 years later, ACRP is a global association comprised of the largest community dedicated to clinical research and development.”
SoCRA (Society of Clinical Research Associates) was founded in 1991. From their website:
SoCRA, as a “professional membership organization, was developed to provide educational programs, certification, and a forum for research professionals to exchange information. SoCRA was originally created to benefit researchers at the site, yet our membership has grown to include monitors, data managers, quality assurance, and regulatory representatives from industry, academia, research centers, NIH and regulatory agencies.”
- Clinical Research Education
- Clinical Trials Unit
- Diversity in Clinical Research
- Investigators & Trainees
- All Researchers
- Research Resources
- Study Management
- Participate in a Trial
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
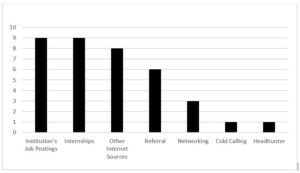
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
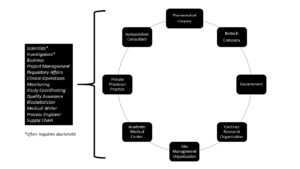
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Embracing the Future: Opportunities and Challenges of AI Integration in Healthcare

Utilizing Cultural Humility as a Tool to Support Diversity in Clinical Research

Looking at Clinical Trial Technology Through a Site Lens

Clinical Research Coordinator 2
🔍 school of medicine, stanford, california, united states.
The Stanford Center for Clinical Research (SCCR) is a growing academic research organization within the Stanford Department of Medicine. Our mission is to conduct and promote high-impact, innovative clinical research to improve human health.
At SCCR, we strive to find team members who are passionate about their work, flexible, fun, and want to deliver results. We place a high priority on equipping our staff to perform their job efficiently, helping them acquire new skills and grow within the organization. We encourage our team to have a healthy balance between work commitments and life outside of work and provide support to achieve this balance. If you are looking to make a large impact through global-reaching clinical research, we encourage you to apply!
The CRC2 will work closely with the Investigator and Clinical Research Manager (CRM) in a dynamic environment, and be responsible for supporting multiple staff members, including coordinators. The CRC2 will work on multiple studies to develop recruitment and retention strategies, oversee study operation and conduct, oversee regulatory compliance and financial logistics, and ensure overall progress on projects. The position will report project progress to the team and trouble-shoot barriers. Outstanding communication and organizational skills, the ability to proactively manage challenges, a strong background in clinical research, and attention to detail are qualities desired in a successful candidate.
This is an on-site role.
Duties include:
- Oversee subject recruitment and study enrollment goals. Determine effective strategies for promoting/recruiting research participants and retaining participants in long-term clinical trials.
- Oversee data management for research projects. Develop and manage systems to organize, collect, report, and monitor data collection. Extract, analyze, and interpret data.
- Develop project schedules, targets, measurements, and accountabilities, as assigned. Lead team meetings and prepare/approve minutes.
- Formally supervise, train, and/or mentor new staff or students, as assigned, potentially including hiring, preparing or assisting with the preparation of performance evaluations, and performing related duties, in addition to instruction on project work.
- Audit operations, including laboratory procedures, to ensure compliance with applicable regulations; provide leadership in identifying and implementing corrective actions/processes. Monitor Institutional Review Board submissions, and respond to requests and questions.
- Collaborate with principal investigators and study sponsors, monitor and report serious adverse events, and resolve study queries.
- Provide leadership in determining, recommending, and implementing improvements to policies/processes; define best practices.
- Develop study budget with staff and principal investigator, identifying standard of care versus study procedures. Track patient and study specific milestones, and invoice sponsors according to study contract.
- Ensure regulatory compliance. Regularly inspect study document to ensure ongoing regulatory compliance.
- Work with principal investigator to ensure Investigational New Drug applications are submitted to the FDA when applicable. Ensure Institutional Review Board renewals are completed.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
DESIRED QUALIFICATIONS:
- 2-4 years clinical research coordinator experience on industry sponsored and investigator initiated clinical trials.
- Clinical research certification from the Society of Clinical Research Associates, Association of Clinical Research Professionals, or Stanford CROP is preferred.
- Bilingual (Spanish Preferred).
- Flexible schedule and ability to periodically work outside of regular business hours.
EDUCATION & EXPERIENCE (REQUIRED):
- Bachelor’s degree in a related field and two years of experience in clinical research, or an equivalent combination of education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency in Microsoft Office and database applications.
- Experience with research protocols and regulatory or governing bodies, which include HIPAA and FDA regulations, Institutional Review Board requirements, and Good Clinical Practices.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred. May require a valid California Driver’s License.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk-based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
WORKING STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University’s Administrative Guide, http://adminguide.stanford.edu/ .
The expected pay range for this position is $72,000 to $92,000 per annum. Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow. We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture. We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you. Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun. Stroll through historic sculptures, trails, and museums.
- Enviable resources. Enjoy free commuter programs, ridesharing incentives, discounts and more.
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 4923
- Employee Status: Regular
- Requisition ID: 101667
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The Invisible Hand in Clinical Research: The Study Coordinator's Critical Role in Human Subjects Protection
Over the past decade, the number of clinical trials registered with the Food and Drug Administration (FDA) has increased dramatically. 1 The business of clinical research has become more diverse, involving academic institutions, clinician-researchers in community settings, pharmaceutical companies, and contract research organizations. This growth has been accompanied by increasing concerns about the ethical conduct of research. 2 Much of this concern has been directed to procedural issues including institutional review board (IRB) review, data monitoring, and informed consent forms. However, the protection of human subjects cannot be achieved by relying solely on procedural safeguards. There are more nuanced issues related to recruitment and retention of subjects, and to the process of informed consent, 3 that are generated during the interaction between study staff and subjects. It is only through an examination of these relationships 4 that one can more fully define and understand the challenges of protecting subjects in research.
Study coordinators are at the center of the clinical research enterprise. In collaboration with principal investigators, they assist with protocol development, write consent forms, recruit subjects, explain the study to and obtain consent from subjects, coordinate with relevant hospital/clinic units, collect and maintain clinical data, and serve as the main contact person for subjects during a trial. One recent survey identified 128 different activities performed by study coordinators. 5 Studies have shown that adding a coordinator to a research team significantly improves subject recruitment numbers, 6 enhances subject retention, 7 and increases general study efficiency. 8
Much of study coordinators' added efficiency is a result of their central position in clinical research activities. They see themselves as interpreters or liaisons, especially in their relationships with principal investigators and subjects, representing the study to subjects and subjects to fellow researchers, clinical staff, and to relevant institutional and external (e.g., federal government, financial sponsor) actors. Each relationship may require a different role, but more commonly, the study coordinator position requires that one relationship encompass several roles. Of particular interest is how clinical study coordinators reconcile the roles of caregiver and researcher. Some reports focus on study coordinators' successful combination of these two roles, 9 while others emphasize their inherent incompatibility. 10
Despite literature that chronicles their skills and efficiencies, surprisingly little systematic information is available regarding the impact of the study coordinator on the protection of human subjects. In fact, when study coordinators have been in the limelight, it has often been as objects of rebuke. For example, approximately 19 percent of investigators receiving FDA warning letters in 2000, and more than 37 percent in 2001, were cited for failing to supervise their trials. 11 In an anecdotal analysis of twenty of these incidents, almost half of the investigators blamed study coordinators for their citations. 12
Study Coordinators and the Ethical Conduct of Research
Given this ambiguous profile, a key question to answer is the extent to which study coordinators shape the ethical conduct of clinical research and how their multiple roles affect the protection of subjects. To understand these issues, we conducted interviews with study coordinators, asking them to respond to scenarios that presented a series of potential dilemmas. We recruited participants from three types of work settings — an academic medical center, a federal research institution, and private organizations. Our underlying hypothesis was that a variety of orientations toward patient care and clinical research might engender different responses.
Focus group interview study
The study was approved by the IRBs at the University of North Carolina School of Medicine (UNC) and the National Human Genome Research Institute. Informed consent was obtained from all focus group participants. Pilot interviews with thirteen study coordinators at UNC identified key issues. Because these issues proved potentially complex and contingent, we selected focus group discussions, designed to foster conversation about specific topics among people with similar backgrounds, as our method of data collection. The ability to assess participants' reactions to statements by peers, as well as the type of language and phrasing used, give focus groups their special purchase on social interaction. 13 To accommodate potential sensitivity about our questions, an issue raised in pilot interviews, we selected a vignette-based approach, a projective technique that allows respondents to discuss issues without direct personal references. 14 The design of these focus groups was more structured than is usually the case. 15
The first set of vignettes elicited information about study coordinator skills and roles. The groups were presented with two different job advertisements with three potential candidates for each, and were asked to decide who would be the best for the job. Participants also commented on contrasting statements about the compatibility of nursing with clinical research (see Box 1 ).
Compatibility of Nursing and Research: Contrasting: Quotes
The second set of vignettes posed questions about two hypothetical study coordinators recruiting subjects for a hypertension outpatient study and an oncology trial for patients whose disease had failed to respond to conventional therapies. The moderator led the group through a series of questions about the following topics: (1) appraisal of subject motives; (2) recruitment methods; (3) responses to subjects' hope for direct medical benefit in a trial: (4) responses to subjects' desire to withdraw from a study; (5) pressure from investigators and study sponsors to increase enrollment numbers; and (6) difficulties involving other staff in recruitment and subject follow-up (see Table 1 ).
In 2000, we conducted seven 90-minute focus groups: three at the UNC, a public academic medical center with a mix of patient care and clinical research; two at the Warren G. Magnuson Clinical Center (NIH), a government organization dedicated to research; and two private focus groups with participants from the Research Triangle Park area of North Carolina and the Washington, D.C. area. These private sector participants worked as freelance consultants or as employees of research foundations, private research institutes, and contract research organizations; one private sector participant was employed full time by the research arm of a private clinic. There was a total of forty-five participants; 68 percent had nursing backgrounds. Other backgrounds included social work, genetic counseling, general baccalaureate, and public health, with education levels also varying from high school to doctoral degrees (see Table 2 ).
Informed consent was obtained from all participants. Discussions were audio-taped and transcribed. Analysis of the transcripts led to a codebook of twelve index codes (see Table 3 ), each with dozens of subcodes produced through an iterative process that involved several readings of the text and revisions of the codes. Each of the investigators was involved in code development and validation, with three of us (Davis, Hull, and Henderson) coding and reconciling the seven transcripts in teams of two, and bringing disagreements back to the larger group. Transcripts were coded using a qualitative analysis program, NUD.IST version 5. 16
Analysis of the focus groups suggests that study coordinators have a central position, with complex relationships, role expectations, and the potential for conflict among the roles.
Unless otherwise noted, all quoted material in this article is attributable to study coordinators who participated in our study.
The Study Coordinator Position in Clinical Research
Multiple skills and relationships.
The study coordinator position requires a wide variety of skills, including nineteen general skill types, and twenty-five subcategories (see Table 4 ). Most frequently mentioned were: hands-on clinical skills; the ability to advance research goals using a variety of research-related skills; psychosocial skills; communication skills; and complex organizational skills including managing the protocol, functioning as a team member, and coordinating with outside units. Intrinsic to several of these skill sets is the study coordinator's ability to identify necessary relationships with others, inside and outside the research team, and to forge and sustain them. A number of different relationships were identified; the most frequently cited relationships were with supervisors, including principal investigators, and patient-subjects (see Figure 1 ).

Patient care background
For some, nursing and research are “a wonderful match … because you really get to utilize your nursing background.” In our job application vignettes, the nursing candidates were most often selected because their hands-on skills, clinical expertise, and psychosocial skills were identified as excellent preparation for the job. If not trained as nurses, coordinators need to be patient-oriented. As one participant noted, “If you're going to be dealing with patients at all, you can't totally disassociate yourselves with [sic] what a nurse needs [to know], because at some point you're going to leave the page and talk with a human.” In contrast, a nursing background can be seen as detrimental when it interferes with the research agenda. A private sector group noted that many nurse study coordinators have problems with protocols that deviate from the standard of care. As one participant in the group said, “I have great nurses working for me and [sometimes] I could pull my hair out because I can't get them in the research mode.”
The Three Advocacies
As one of the participans noted, “One criterion for a study coordinator is… the ability to balance all of the issues from the clinical trial side and the medical patient care side, and translate both to the principal investigator, who mostly is medically-oriented, and to the patient involved in the study.” Focus group participants consistently described their position in terms of complex and potentially conflicting obligations to various parties. They identified three critical roles: (1) patient advocacy; (2) subject advocacy; and (3) study advocacy (see Table 5 ). One role is often identified in contrast with another. Participants discussed the moral dimensions of these conflicts. Their discussions focused on relationships; only occasionally did they explicitly describe conflicts as ethical issues.
Patient advocacy
All focus group participants identified patient advocacy as their primary responsibility. Contrasting this with the investigator role, one participant noted: “You're still the patient advocate. You know, you have to think about what your priority is. Enrollment, I think, is the [principal investigator's] priority, but that's not necessarily your priority.” Study coordinators often think in terms of a patient's interests and needs. This commitment to the patient's welfare translates into an advocacy for the patient that follows the subject into the study, 17 and remains a central role for the study coordinator during the study and perhaps thereafter. The salience of the patient advocate role may affect which patients the study coordinator talks to about the study, and whether the study coordinator encourages or discourages interest in participating. Another participant noted that the study coordinator has to be very careful because the patients “look to you as an advocate for them …. They [easily conclude that] you're recommending [what they should do].” Patient advocacy was reflected in the participants' explicit use of the term “patient advocate” as a metaphor, and in comments about “mothering” or “taking care of” patients.
Subject advocacy
Study coordinators are vital to the investigator's ability to enroll and retain subjects. The role as subject advocate emerged in descriptions of the recruitment process, though study coordinators typically continue to use patient rather than subject terminology. Subject advocacy promotes an informed decision to participate in research: it entails the subject gaining an understanding of the study's purpose, of the nature of voluntary consent and the corresponding ability to withdraw from the study, and that declining to participate will not affect their current health care in that setting. One participant summed up a common description of the role, in terms of risk and subject safety: “I think that the subject, if the coordinator is doing the job as it should be done, sees the coordinator as an advocate, understands… that there are risks, but that the coordinator is there to be tuned in to anything that might, you know, indicate that something is going on that might be harmful to the subject.” Although investigators may certainly advocate for subjects, coordinators contrasted their role with the investigator's role, including access to information that investigators do not have: “You're the one listening and dealing with the patient…. They're not there.” For subject advocacy, the metaphor used by study coordinators was “lawyer,” to protect the rights of this individual and to remind investigators of the subject's right to withdraw from the study. Subject advocacy does not survive the end of participation in the study, but the relationships acquired may be satisfying personally, or may be instrumental in future recruitments.
Study advocacy
Study coordinators are hired to advocate for the study. Participants discussed study advocacy in terms of advancing the research goals, and gathering valid clean data via good recruitment and retention of subjects. They also emphasized responsibilities of the enrolled participants: “It's important for subjects to also understand that [commitment].” Beyond that, study coordinators expressed a need to believe in the value of a study. Without that, the job is more difficult, even undesirable. Advancing the study may also advance the investigator and study coordinator's professional careers. Therefore, there are personal stakes, as well as scientific outcomes. The metaphor used here was “policeman of the protocol,” and also “teacher,” because often the study coordinator trained others, including other staff members, in the conduct of the study. Study advocacy does not survive the conduct of the study, but good performance can be professionally advantageous.
Balancing the Roles
Because the three advocacies have different objectives, they must be balanced. Although there are instances when the advocacies are synergistic, the more common result of balancing is one of potential conflict. One advocacy may hinder the advancement of another. For example, when a coordinator carefully maintains an objective stance as a subject advocate, this may conflict with the role of patient advocate, advising patients who are dependent on the study coordinator for guidance. Likewise, patient advocacy can be burdensome in the course of study advocacy, as described by one participant: “It is very, very important to be very objective, so patients understand that if they do enroll in a study, we're not saying that this is going to fix you or cure you. This is research and we're learning from it, you know. We'll do everything to make sure that you're safe … and [we will stop] if we need to, but I think that's where [I get into] conflicts because patients are just so hopeful…. You want patients to be positive, but you need to be realistic too.”
Balancing can be difficult. As noted by one participant: “It takes a while to develop a balance so that you can be the patient advocate but you can also be the researcher, and when you have to be tough, and when you have to say to the doctor that they've had enough…. A lot of it is experience.” And this balancing is necessary throughout the course of a study. As early as protocol development, where the study coordinator assists the investigator in creating a study design reasonable for its subjects, and during the study's implementation, via recruitment, screening, and enrollment, the study coordinator balances concerns for the patient, the subject, and the study. After enrollment, the balancing of advocacies continues as the study coordinator addresses data collection and retention issues such as compliance, managing side-effects, and withdrawals.
We examined how study coordinators balanced their multiple roles in two ways—from a general question about the care giving and researcher roles, and a specific question in the cancer trial vignette about how one should respond to a hopeful patient subject. When the participants considered the contrasting quotes from the literature (see Box 1 ), they provoked a discussion about the general compatibility of nursing and clinical research. Participants argued that the study coordinator position must include both the caring component of nursing and the detached analytical gathering of knowledge that is fundamental to research. “[You have to be clear] that you're doing research and there's a point to it and its not clinical care, but it includes [clinical care]. It includes … being empathetic and education and all the things nurses do in a clinical setting. However, there's a specific point and you have to be somewhat… rigid to get that objective.”
Response to the hopeful patient subject vignette illustrated more particularized relationships and skills. The need for “balancing hope and realism,” was consistently identified as the “tough part of the job.” These study coordinators struggled with their perception that they must be realistic with patients, an aspect of all three advocacies, but without destroying the therapeutic value of patients' hope, a mainstay of patient advocacy. They worried about not misleading patients who could “easily get the wrong impression,” since “just being referred gives them hope.” “When patients are willing to try anything,” study coordinators say they have to protect patients from themselves, and this “makes it kind of hard on the coordinator.” Responding to the hopeful subject, as patient advocates, they want to encourage patients' hope but not take advantage of it. As study advocates, they recognize the value of hope in encouraging subject participation; and as subject advocates, they move away from both of those positions to one of neutrality, providing information but without unduly influencing decision-making.
The picture would be incomplete without recognizing the parallel tension between hope and realism in members of the research team as well. As patient advocates, study coordinators themselves hope that the patient will benefit from participation in a specific study, or at least will benefit from better care while “on study.” Coordinators' “belief in a study,” as study advocates, evinces their hope that the study will work, or that altruism is beneficial for patients. Researchers' hopefulness in individual benefit and in the successful outcome of the study is tempered by subject advocacy with its “hope neutral” realistic focus on key features of research participation: that patients choose to participate for their own reasons, that benefit is not promised, and that subject safety measures are in place.
Influence of workplace setting on balancing roles
We found surprisingly little variation in description of the study coordinator role across the three different work settings. There were, however, some differences in degree of emphasis on one advocacy versus another. For instance, when describing the challenge of balancing hope versus realism for hopeful subjects, the UNC study coordinators emphasized patient advocacy. The NIH coordinators focused on both subject and study advocacy, such as patient safety and dose-tolerance; while the private sector sites emphasized good study results. Private sector participants further advised keeping a certain distance from subjects' hopefulness, to listen and “then go on your way.” Perhaps not surprisingly, study advocacy appeared to be more fully articulated in the NIH and private sectors, settings that are research-oriented and have a more clearly defined study coordinator role, hierarchy, and support system.
The Invisible Hand in Clinical Research
Focus group participants across all settings described themselves as advocating for patients, patients-turned-subjects, and research. While not representative of all study coordinators or research organizations in general, the finding of similar roles across carefully selected case comparisons such as these —when differences are expected by design—is compelling. 18
Previous literature about study coordinators has acknowledged the tension between the dual role of researcher and caregiver. 19 Our results suggest that, in fact, study coordinators have several roles, and more importantly, that these roles must be balanced. Balancing the three roles heightens the potential for conflict as the study coordinator promotes the interests of each part of the triad: the patient, the patient-turned-subject, and the study. Clearly, there are times when one advocacy must advance and the others retreat, and deciding which one to focus upon and which ones to subrogate is the major ethical challenge of the study coordinator position.
The constant movement between different roles, coupled with the potential for influential long-term relationships, also increases the complexity of the position and reinforces its centrality. Coordinator is often noted to be the best descriptor of the job, following a string of labels denoting tasks that range across different relationships and skills, from study conception to follow-up. 20 While recent literature has also focused on the efficiencies of the position, 21 study coordinators still tend to be invisible players in much of the general clinical research and ethics literature.
Why have study coordinators been neglected in the literature on the protection of subjects in research studies? One answer is that the job has been considered an assistant's position, with little authority or autonomy. A profession is often defined by a group's ability to control the application of a body of knowledge and establish training programs that award jurisdiction over that knowledge to recipients. 22 In contrast, the study coordinator is an occupation in transition, lacking agreed upon training requirements or job criteria. Neither the creation of the research nurse subspecialty, 23 or the establishment of a clinical research coordinator certification program by the Association of Clinical Research Professionals (ACRP), has resulted in an independent professional identity for study coordinators. New National Institutes of Health guidelines do require all “key personnel” involved in research to certify receipt of ethics training. 24 With the rapid proliferation of excellent clinical research training courses, the focus is on the investigator; there is no certainty that study coordinators will be included as key personnel and therefore required to receive training in ethical issues related to human subjects protection.
In fact, the challenges we describe that are raised by study coordinators' different advocacies resonate with much that has been written about the dual role faced by physician-investigators. 25 While our purpose was not to examine the similarities of these two roles, the fact that the potential role conflicts seem similar only serves to reinforce the importance of the study coordinator role for the protection of human subjects.
Moreover, because of their central position and their commitment to balancing the three advocacies, study coordinators are uniquely placed to further the goals of human subject protection. The study coordinator is the person with whom subjects interact the most, and the one most able to identify their needs and employ necessary procedural safeguards. Often, challenges that raise ethical issues are most apparent to study coordinators, yet their particular perspective may go unnoticed or be misunderstood. Such misunderstanding may be cast as blame for ethical lapses in the study, an age-old issue for study coordinators starting with Nurse Rivers, a “scientific assistant” in the Tuskegee syphilis study, 26 and persisting today in commentary about FDA warning letters, as described in the introduction. 27
The findings that study coordinators face challenging issues related to human subjects protection demonstrate why it is critical that study coordinators be included in research ethics training programs. Indeed, the focus group discussions revealed participants' keen interest in research ethics education, and in a forum to discuss their ethical issues. These results also suggest that evaluating the adequacy or inadequacy of the protection of human subjects cannot rely solely upon procedural safeguards such as IRB review, data monitoring, or informed consent forms. It must include explicit recognition of the study coordinator's role. Further research to test our findings regarding the role of study coordinators in recruitment, consent, and retention is also indicated. Through greater recognition of the invisible hand in clinical research, we can better address the complex issues of human subjects protection.
Acknowledgments
Kathleen Dietrich played a critical role in the formative stages of this project. Nancy M. E King provided helpful comments on this manuscript. Michele Easter, Holly Gooding, Jon Mize, and Benjamin Morley provided technical assistance. This work was supported by a grant from the National Human Genome Research Institute (NHGRI) (R01HG02087) and the University of North Carolina School of Medicine Faculty Grants Program. The opinions expressed in this article are those of the authors and do not reflect the opinions or policies of NHGRI, National Institutes of Health, or the Department of Health and Human Services. The funding sources had no role in the design or conduct of the study, or in the analysis of data or production of this manuscript.
Study Coordinator
The study coordinator is a specialized research professional working with and under the direction of the clinical Principal Investigator (PI). While the Principal Investigator is primarily responsible for the overall design, conduct, and management of the clinical trial, the study coordinator supports, facilitates and coordinates the daily clinical trial activities and plays a critical role in the conduct of the study. By performing these duties, the study coordinator works with the PI, department, sponsor, and institution to support and provide guidance on the administration of the compliance, financial, personnel and other related aspects of the clinical study.
Created: 02.26.2021
Updated: 03.31.2024
Clinical Research Coordinator
Responsibilities*.
Characteristic Duties and Responsibilities:
Expert level knowledge, skills, and abilities within all 8 competency domains is expected:
- Scientific Concepts and Research Design
- Ethical Participant Safety Considerations
- Investigational Products Development and Regulation
- Clinical Study Operations (GCPs)
- Study and Site Management
- Data Management and Informatics
- Leadership and Professionalism
- Communication and Teamwork
The Clinical Research Coordinator supports clinical research within the Division of Pediatric Nephrology and is expected to understand and support the conduct of research within all federal, state and local regulatory requirements. Candidate will perform independent work under the guidance of the program director and supervisor, while also collaborating as necessary to support overall program success. This coordinator is expected to participate in patient recruitment, consenting, and follow up activities in addition to underlying study coordination tasks.
Routine duties include:
- Support clinical research in Pediatric Nephrology to ensure clinical research related procedures are completed in accordance with good clinical practice guidelines.
- Collaborate within research team of physicians, biostatisticians, program/project managers, study coordinators, data managers, and administrative support.
- Screen, recruit, obtain consent, and retain participants according to research protocols.
- Coordinate pediatric/caregiver and adult patient follow up visits with research and clinical teams.
- Act as the primary point of contact for research participants, working with labs to handle biospecimens and pharmacies to place orders and pick up study drugs for research participants.
- Participate in communication of study results to participants when indicated.
- Serve as liaison between the clinical and research teams.
- Prepare and maintain Institutional Review Board (IRB) applications, consent documents, and recruitment materials for standard and/or ceding applications.
- Perform data collection, data entry, and query management.
- Collect, process, label and store biospecimens including blood, urine, and kidney biopsy tissue.
- Assist research administrative work such as data use agreements, material transfer agreements, and proposal submissions.
- Work with administrative staff to ensure appropriate billing for study-related care.
- Assist the research team in generation of presentations, abstracts, and manuscripts.
- 40% work from home (locally).
Required Qualifications*
- Bachelor's degree in Health Science or an equivalent combination of related education and experience is necessary.
- Certification is required through Association of Clinical Research Professionals (ACRP) as a Certified Clinical Research Coordinator (CCRC) or Society of Clinical Research Association (SOCRA) as a Certified Clinical Research Professionals (CCRP) and must be achieved by 01/01/24. After 01/01/24, certification is required within six months of date of hire.
- Minimum 3 years of directly related experience in clinical research and clinical trials is necessary
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.
- Undergraduate Students
- Masters Students
- PhD/Doctoral Students
- Postdoctoral Scholars
- Faculty & Staff
- Families & Supporters
- Prospective Students
- Explore Your Interests / Self-Assessment
- Build your Network / LinkedIn
- Search for a Job / Internship
- Create a Resume / Cover Letter
- Prepare for an Interview
- Negotiate an Offer
- Prepare for Graduate School
- Find Funding Opportunities
- Prepare for the Academic Job Market (PhD Students Only)
- Search for a Job or Internship
- Advertising, Marketing, and Public Relations
- Arts & Entertainment
- Consulting & Financial Services
- Engineering & Technology
- Government, Law & Policy
- Hospitality
- Management & Human Resources
- Non-Profit, Social Justice & Education
- Retail & Consumer Services
- BIPOC Students & Scholars
- Disabled Students & Scholars
- First-Generation Students & Scholars
- Former Foster Youth
- Formerly Incarcerated Students & Scholars
- International Students & Scholars
- LGBTQ+ Students & Scholars
- Students & Scholars with Dependents
- Transfer Students
- Undocumented Students & Scholars
- Women-Identifying Students & Scholars
Olive View-UCLA Education and Research Institute
Clinical Research Coordinator
- Share This: Share Clinical Research Coordinator on Facebook Share Clinical Research Coordinator on LinkedIn Share Clinical Research Coordinator on X
Help our team conduct federally-funded (Centers for Disease Control/NIH) and infectious disease research studies and Industry-sponsored pharmaceutical clinical trials among patients presenting to the Department of Emergency Medicine, Olive View-UCLA Medical Center and other U.S. Emergency Departments.
The clinical research coordinator will take an active role in assisting in study initiation, including administrative start-up support, regulatory document collection, and other study start-up activities. This position will involve in assisting the other research coordinators and principal investigators in patient screening, recruitment and enrollment, participant scheduling, coordination of research measures and clinical care, accurate and safe completion of protocol required procedures and the prompt and accurate collection and reporting of data in accordance with applicable laws, institutional policies, and study specific protocol, processing and shipment of specimens, ordering and maintaining supplies etc. Current projects involve studies of COVID-19, diabetic foot infections, skin and soft tissue infections, and UTI.
Qualifications:
- Ability to speak Spanish fluently is required (our patient population is mostly Spanish speaking).
- Proficient with Microsoft Office (Word, Excel, Access).
- Ability to work independently and efficiently is required.
- Study location is at Olive View-UCLA Medical Center in Sylmar, CA, so must have some mode of transportation.
- Flexible hours work hours (after training, able to work some weekday evenings and possibly some weekends)
- Prior research experience is preferred, but not required.
Hours per week: full time (30+ hours/week) position
Salary: $20-$27/hour
- Undergraduates
- Ph.Ds & Postdocs
- Prospective Students & Guests
- What is a Community?
- Student Athletes
- First Generation and/or Low Income Students
- International Students
- LGBTQ Students
- Students of Color
- Students with Disabilities
- Student Veterans
- Exploring Careers
- Advertising, Marketing & PR
- Finance, Insurance & Real Estate
- General Management & Leadership Development Programs
- Law & Legal Services
- Startups, Entrepreneurship & Freelance Work
- Environment, Sustainability & Energy
- Media & Communications
- Policy & Think Tanks
- Engineering
- Healthcare, Biotech & Global Public Health
- Life & Physical Sciences
- Programming & Data Science
- Graduate School
- Health Professions
- Business School
- Meet with OCS
- Student Organizations Workshop Request
- OCS Podcast Series
- Office of Fellowships
- Navigating AI in the Job Search Process
- Cover Letters & Correspondence
- Job Market Insights
- Professional Conduct & Etiquette
- Professional Online Identity
- Interview Preparation
- Resource Database
- Yale Career Link
- Jobs, Internships & Other Experiences
- Gap Year & Short-Term Opportunities
- Planning an International Internship
- Funding Your Experience
- Career Fairs/Networking Events
- On-Campus Recruiting
- Job Offers & Salary Negotiation
- Informational Interviewing
- Peer Networking Lists
- Building Your LinkedIn Profile
- YC First Destinations
- YC Four-Year Out
- GSAS Program Statistics
- Statistics & Reports
- Contact OCS
- OCS Mission & Policies
- Additional Yale Career Offices
Associate Clinical Research Coordinator Position at UNC
- Share This: Share Associate Clinical Research Coordinator Position at UNC on Facebook Share Associate Clinical Research Coordinator Position at UNC on LinkedIn Share Associate Clinical Research Coordinator Position at UNC on X
THIS POSTING IS FOR 2 POSITIONS. ALL CANDIDATES WILL BE CONSIDERED FOR BOTH VACANCIES. This position will serve as an Associate Clinical Research Coordinator within Division of Endocrinology at the UNC School of Medicine and is ideal for an individual interested in pursuing a career in medicine and would like to gain experience working in clinical research. The Associate Clinical Research Coordinator is responsible for the execution of clinical research protocols, including leading efforts in gathering, compiling, and reporting data for a variety of research studies. This position will require excellent people skills as the applicant will be working directly with research participants in clinical settings including inpatient and outpatient clinics as well as MRI settings. Activities would include assisting in MRI based research studies, coordinating research subject visits, subject recruitment, research sample collection, data entry, data analysis, and questionnaire administration.
For full position description and application instructions, visit the website: https://unc.peopleadmin.com/postings/276892
The Office of Career Strategy posts job listings for the convenience of students. The University does not endorse or recommend employers and a posting does not constitute an endorsement or recommendation. The University explicitly makes no representations or guarantees about job listings or the accuracy of the information provided by the employer. The University is not responsible for safety, wages, working conditions, or any other aspect of off-campus employment without limitation. It is the responsibility of students to perform due diligence in researching employers when applying for or accepting private, off-campus employment and to thoroughly research the facts and reputation of each organization to which they are applying. Students should be prudent and use common sense and caution when applying for or accepting any position. All concerns and issues related to job and/or internship opportunities, including those posted within the Yale Career Link, should be addressed promptly via email to the Office of Career Strategy.

Office of Career Strategy
Visiting yale.

IMAGES
VIDEO
COMMENTS
Also known as a research coordinator or clinical trial coordinator, a study coordinator has in-depth knowledge of the complete study protocol, including the eligibility criteria, drug intervention, baseline measures, and primary outcomes. They work across the three major aspects of clinical research: study management, subject care, and patient ...
A clinical research coordinator reports to a principal investigator, who is responsible for the overall design and management of the study. In contrast, a clinical researcher organizes the day-to-day running of the trials. As a clinical research coordinator, you will also work closely with sponsors and staff within their department responsible ...
By performing these duties, the CRC works with the PI, department, sponsor, and institution to support and provide guidance on the administration of the compliance, financial, personnel and other related aspects of the clinical study. The clinical research coordinator reports primarily to the Principal Investigator with associated ...
Clinical research plays a crucial role in advancing medical knowledge and improving patient care. At the heart of every successful clinical research study is a Clinical Research Coordinator (CRC). As a CRC, you serve as the linchpin between researchers, study participants, and regulatory bodies. In this comprehensive guide, we will explore the ...
Clinical Research Coordinator Associate (Fixed-term 2 years) New. Stanford University 4.2. Stanford, CA 94305. $27.88 - $36.54 an hour. Weekends as needed + 1. Qualified candidates will coordinate moderately complex aspects of one or more clinical studies working under close direction of the principal investigator and….
4. Consider a master's degree. If you pursue a master's degree in clinical research or a related field, you might qualify for supervisory or management roles. Many universities offer master's programs online to provide greater flexibility to clinical research coordinators working full-time. 5. Pursue certification.
Most research facilities require clinical research coordinators to hold a bachelor's degree in a related medical study such as public health administration, biomedical technologies or microbiology. Courses focused on medical research, ethical practices in medicine and the scientific method help prepare graduates for work in a medical research ...
A clinical research coordinator (CRC) plays an important role in the field of clinical research, ensuring the smooth and efficient conduct of clinical trials and studies. These professionals act as a bridge between the research investigators, sponsors, and participants, overseeing various aspects of the research process. They collaborate closely with physicians, nurses, and other healthcare ...
If the involvement of CSN in clinical trials is a crucial factor, studies have shown that adding a coordinator to a research team significantly improves subject recruitment numbers, subject retention and general study efficiency , increasing general quality in all study phases. A more active involvement of CRCs in the activities of clinical ...
As a Clinical Research Coordinator, you must have knowledge of GCPs in order to ensure the quality and safety of the data collected during the study. • Regulatory compliance: Clinical Research Coordinators must stay up-to-date on any changes in regulations governing clinical trials.
To identify factors associated with job satisfaction and retention, we surveyed a large cohort of clinical research coordinators (CRCs). In recent years, the clinical research coordinator has changed from a semi-permanent role to one that has a high turnover rate. ... research nurse coordinators, and study coordinators. 1 The main tasks that ...
Senior Clinical Research Coordinator (Cancer) 329399 Jacksonville, Florida. Clinical Research Coordinator-Early Cancer Therapeutic 329004 Phoenix, Arizona. Associate Clinical Research Coordinator - NW Wisconsin 328244 Eau Claire, Wisconsin. Clinical Research Coordinator 324574 Rochester, Minnesota.
This article provides a detailed guide to help you enhance your capabilities and thrive as a proficient site study coordinator. 1. Master Study Protocols: Thoroughly understand study protocols ...
ACRP Certification is an ideal solution for managers looking to enhance team knowledge, grow leaders, and signify to others that their study teams are among the best of the best. Clinical research professional with 3,000 hours of verifiable work experience are eligible to sit for the CCRC ® Exam. Complete eligibility criteria is defined in the ...
In the Advanced course, learners gain a deeper understanding of the CRC's role by exploring key operational, leadership, regulatory, and technical elements associated with daily work. These courses were written and peer-reviewed by experts. Language Availability: English. Suggested Audiences: Clinical Research Coordinators (CRCs), Clinical ...
Clinical Research Coordinators (CRCs) are responsible for the organization, coordination, and overall integrity of a research project with humans. Principal and co-investigators provide the overall direction in a clinical study, but CRCs have significant roles in clinical study activities, including Clinical Trial Study Start-Up.
Over time, the role of clinical research coordinators requires increasing autonomy, accountability and responsibility. 16 Clinical research coordinators are also the main point of contact for subjects during a trial, and act as liaisons between study subjects and principal investigators. 15 Thus, they are heavily involved in the day to day ...
A Clinical Research Coordinator (CRC) manages and conducts the day-to-day activities of a clinical trial.The Principal Investigator (PI) determines the CRC's specific responsibilities and works closely with the CRC. In general, the CRC ensures the clinical study maintains accordance with the protocol, applicable regulations, and Good Clinical Practice (GCP) and Institutional Review Board ...
Clinical Coordinator- RN. Alaska Heart Institute 3.3. Anchorage, AK 99508. ( University Area area) $40 - $50 an hour. Easily apply. Assist in clinical policies, reviews and revisions under the guidance of the Clinical Operations Manager. A nonexempt position that functions as a member of the…. Employer.
The Office for Clinical Research (OCR) is committed to supporting Cancer Center Clinical study staff in Education and Compliance. We are dedicated to elevating the knowledge base and performance of the Cancer Center research study staff. The OCR assists Investigators and study coordinators at any stage in their trials.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. ... Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of ...
The CRC2 will work closely with the Investigator and Clinical Research Manager (CRM) in a dynamic environment, and be responsible for supporting multiple staff members, including coordinators. The CRC2 will work on multiple studies to develop recruitment and retention strategies, oversee study operation and conduct, oversee regulatory ...
Over the past decade, the number of clinical trials registered with the Food and Drug Administration (FDA) has increased dramatically. 1 The business of clinical research has become more diverse, involving academic institutions, clinician-researchers in community settings, pharmaceutical companies, and contract research organizations. This growth has been accompanied by increasing concerns ...
The study coordinator is a specialized research professional working with and under the direction of the clinical Principal Investigator (PI). While the Principal Investigator is primarily responsible for the overall design, conduct, and management of the clinical trial, the study coordinator supports, facilitates and coordinates the daily clinical trial activities and plays a critical role in ...
The CRC1 supports the daily clinical trial activities and plays a critical role in the conduct of the study. The Clinical Research Coordinator 1 (CRC1) will be trained and supervised by a Clinical Psychologist and Principal Investigator to deliver lifestyle coaching to individuals enrolled in research trials. Remote coaching will be the main ...
This coordinator is expected to participate in patient recruitment, consenting, and follow up activities in addition to underlying study coordination tasks. Routine duties include: Support clinical research in Pediatric Nephrology to ensure clinical research related procedures are completed in accordance with good clinical practice guidelines.
The clinical research coordinator will take an active role in assisting in study initiation, including administrative start-up support, regulatory document collection, and other study start-up activities. This position will involve in assisting the other research coordinators and principal investigators in patient screening, recruitment and ...
The Associate Clinical Research Coordinator is responsible for the execution of clinical research protocols, including leading efforts in gathering, compiling, and reporting data for a variety of research studies. This position will require excellent people skills as the applicant will be working directly with research participants in clinical ...