An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceutics


Current State of Breast Cancer Diagnosis, Treatment, and Theranostics
Arya bhushan.
1 Ladue Horton Watkins High School, St. Louis, MO 63124, USA; moc.liamg@4002nahsuhba
2 Department of Biomedical and Pharmaceutical Sciences, College of Pharmacy, University of Rhode Island, Kingston, RI 02881, USA; ude.iru@sevlasnoga
Andrea Gonsalves
Jyothi u. menon, associated data.
Not applicable.
Breast cancer is one of the leading causes of cancer-related morbidity and mortality in women worldwide. Early diagnosis and effective treatment of all types of cancers are crucial for a positive prognosis. Patients with small tumor sizes at the time of their diagnosis have a significantly higher survival rate and a significantly reduced probability of the cancer being fatal. Therefore, many novel technologies are being developed for early detection of primary tumors, as well as distant metastases and recurrent disease, for effective breast cancer management. Theranostics has emerged as a new paradigm for the simultaneous diagnosis, imaging, and treatment of cancers. It has the potential to provide timely and improved patient care via personalized therapy. In nanotheranostics, cell-specific targeting moieties, imaging agents, and therapeutic agents can be embedded within a single formulation for effective treatment. In this review, we will highlight the different diagnosis techniques and treatment strategies for breast cancer management and explore recent advances in breast cancer theranostics. Our main focus will be to summarize recent trends and technologies in breast cancer diagnosis and treatment as reported in recent research papers and patents and discuss future perspectives for effective breast cancer therapy.
1. Introduction
Breast cancer has a very long history as it was first reported by the ancient Egyptians more than 3500 years ago in about 1500 B.C [ 1 ]. Today, breast cancer is the second most prevalent type of cancer and is a leading cause of most cancer-related deaths in women in the United States [ 2 ]. Around 281,550 women are projected to be diagnosed with breast cancer in 2021, and 43,600 women are predicted to die due to breast cancer in the US, according to the American Cancer Society. Early diagnosis of the disease is crucial for effective treatment and positive prognosis, as significantly lower probability of dying and higher survival rate is observed in patients with smaller tumors at the time of diagnosis [ 3 ]. Early detection of breast cancer and accurate lesion assessment are, therefore, the primary focus of all imaging modalities. At present the two major pillars to be addressed for effective management of breast cancer disease include: (i) diagnosis of breast cancer in its earliest stages and (ii) providing timely treatment after diagnosis to save lives.
Imaging of the breast is utilized almost exclusively for detection, diagnosis, and clinical management of cancers and for the assessment of the integrity of breast implants ( Figure 1 ) [ 4 ]. As a conventional medical imaging modality, ultrasound has played a key role in breast cancer detection, image-guided biopsy, and lymph-node diagnosis for many years. Mammography, ultrasonography, magnetic resonance imaging (MRI), scintimammography, single photon emission computed tomography (SPECT), and positron emission tomography (PET) are other commonly used imaging modalities [ 5 , 6 , 7 ]. Based on the diagnosis and assessment of the extent of breast cancer, the need for preoperative (neoadjuvant) systemic therapy is determined. Targeted and effective therapies with minimal off-target side effects are needed for breast cancer treatment. As breast cancer is a global problem, major emphasis also needs to be put on diminishing worldwide disparities in terms of access to diagnosis, multimodal treatment, and novel drugs.

Representation of the various imaging techniques that can be used in breast cancer diagnosis.
For this review, we conducted a literature search within the Google Scholar and PubMed databases using the keywords: “Breast Cancer”, “Imaging”, and “Treatment” in the title field, with dates from 2000 to 2021. After reading the abstracts, we manually selected the relevant papers for this review. In this review article, we examine various detection techniques for breast cancer, provide an in-depth analysis on the therapies for different subtypes of breast cancer, and investigate recent trends and the future of breast cancer theranostics.
2. Techniques for Diagnosis or Detection of Breast Cancer
Early diagnosis is a key to successful breast cancer treatment. T1 tumors measuring less than 2 cm in diameter have a 10-year survival of approximately 85%, while T3 tumors—essentially the result of delayed diagnosis—have a 10-year survival of less than 60% [ 8 ]. Imaging techniques commonly used for detection of breast cancer are summarized in Table 1 .
Summary of various imaging modalities for screening of breast cancer.
2.1. Mammography
A mammogram is an x-ray of the breast that can reveal benign or malignant abnormalities. It is obtained by applying a small dose of radiation through the breast post compression between two plates to produce an x-ray image. Mammograms can be utilized for both screening and diagnosis [ 31 ]. Mammogram screening is performed as an attempt to detect any early signs of breast cancer, even before symptoms occur, to decrease mortality by early diagnosis. Diagnostic mammogram assists in detecting breast cancer if a woman experiences symptoms, for instance, a lump that can be felt in her breast [ 32 ]. In 2009, new mammography screening guidelines were issued by the U.S. Preventive Services Task Force (USPSTF) with a recommendation that routine screening mammography for women under age 50 is not needed, whereas its earlier stance was in accordance with American Cancer Society guidelines, which recommended mammography every one to two years for all women age 40 and older [ 31 , 32 , 33 , 34 ]. In addition, since radiologists assess information subjectively, breast density cannot be utilized to infer the information ingrained in a mammogram [ 35 ]. For instance, patients may have appreciably different mammograms, each with vastly different outcomes, but have the same breast density assessment value. In previous studies, mammography results have been used to develop statistics related to glandular tissue volume. However, these automated methods of evaluating breast density are not sufficient to predict breast cancer prevalence [ 36 ]. Recently, gold-based nanoformulations have shown promise in significantly enhancing the contrast in mammographic images [ 37 ]. Mammographic density can improve the accuracy of breast cancer risk models. More accurate risk prediction can also be achieved by a mammography-based deep learning (DL) model [ 36 ].
2.2. Magnetic Resonance Imaging
Breast MRI is a non-invasive and non-ionizing diagnostic imaging tool that employs low-energy radio frequency waves and a magnetic field to obtain detailed images of structures within the breast [ 38 ]. MRI can be used to measure the size of the cancer and look for metastasized tumors in women who have been previously diagnosed with breast cancer. Tumors with size less than or equal to 2 cm have been accurately identified and measured using MRI. However, larger breast tumors are often overestimated due to the abnormal breast tissue encompassing the actual lesion, which can lead to greater mastectomy rates [ 39 , 40 ]. Goldsmith et al. first described the use of nuclear MRI for the breast 40 years ago [ 41 ]. Several uses of MRI for the breast, including screening the high-risk population, have been recommended by the American College of Radiology [ 42 ]. MRI has the ability to detect suspected breast malignancies that often escape clinical, mammographic, and ultrasound detection [ 37 ]. Fe 3 O 4 , gadolinium(III)-, and Mn(II)-based contrast agents are commonly used for preoperative assessment, especially to visualize axillary lymph nodes of the breast [ 43 ]. To reduce the possibility of off-target toxic effects and increase specificity towards breast cancer, these contrast agents may be encapsulated within breast cancer-targeting polymeric carriers [ 44 , 45 ]. Because of the high sensitivity and lower specificity of breast MRI, it is widely used in breast cancer diagnostics, thus, resulting in an increase in incidental findings. It is imperative that these findings be histologically assessed before any surgical intervention [ 46 , 47 ].
2.3. Dynamic Contrast Enhanced MRI (DCE-MRI)
Dynamic contrast-agent-enhanced breast MRI works by analyzing the temporal enhancement pattern of a tissue following the intravenous injection of a paramagnetic contrast agent. This non-invasive imaging technique quantitatively determines the extent of tissue vascularization, interstitial space composition, and differentiation of lesions [ 48 ]. This imaging modality is useful to depict tumor angiogenesis with overall recurrence and overall survival of breast cancer patients [ 49 , 50 , 51 ]. As a result, lymph node metastasis that occurs due to greater angiogenesis in breast cancer can also be predicted using this imaging modality. DCE-MRI, when combined with a computer-aided diagnosis technology, such as texture analysis, can also be used to identify estrogen receptor positive (ER+) breast cancer subtypes [ 52 ]. DCE-MRI technique is non-invasive and three-dimensional, which allows visualization of the extent of disease before morphological alterations and helps to predict the overall response either before the start of therapy or early during treatment [ 53 , 54 ]. Unlike mammography, DCE-MRI is not limited by breast tissue density. However, a central limitation of DCE-MRI is that it is non-specific [ 55 ].
2.4. Magnetic Resonance Elastography
Magnetic resonance elastography (MRE) can be used to obtain details on tissue mechanical properties in vivo [ 56 ]. Following application of an external stress, breast MRE, a non-invasive, non-ionizing, and cross-sectional imaging modality, can quantitate the viscoelastic properties of breast tissues [ 57 ]. Breast cancers often have a higher stiffness due to increase in the number of cells, collagen, and proteoglycans compared to the normal surrounding tissues and benign lesions [ 58 , 59 ]. Although manual palpation is commonly used for routine screening of the breast, it lacks specificity and sensitivity. This is where the limitations of manual palpation can be overcome by MRE scanning of the breast [ 60 , 61 , 62 ]. While the initial results are encouraging, the most significant limitation for MRE in breast cancer is spatial resolution and detection of small focal lesions due to the overlap in the soft malignant tumors and stiff benign lesions elasticity ranges [ 63 ].
2.5. Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) is a form of unenhanced MRI that uses the diffusion of water molecules to generate contrast in MR images to address some of the shortcomings faced by regular breast MRI [ 64 , 65 , 66 ]. The potential benefits of DWI techniques include improved differentiation of benign and malignant breast lesions and assessment and prediction of therapeutic efficacy [ 67 ]. DWI has enabled the identification of breast cancer particularly in dense breasts. However, the sensitivity of DWI tends to be variable compared to contrast-enhanced MRI [ 68 ]. Technical innovations are helping to overcome many of the image quality issues that have limited widespread use of DWI for breast [ 69 ]. While DWI may be an accurate and nonradioactive imaging technique, it has still not achieved its full potential. Detailed investigations and clinical trials are now warranted to prove DWI’s ability to facilitate the diagnostic work-up of the diseases.
2.6. Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy (MRS) can measure a chemical “spectrum” in the region using high magnetic field strengths (typically 11–14 T) on body fluids, cell extracts, and tissue samples, providing additional information about the chemical content in the region [ 70 , 71 ]. The in vivo 1H MRS protocol with the addition of MRI procedure further increases the overall acquisition time by approximately 10 min and has the advantage to improve the diagnostic accuracy of clinical breast MR [ 72 , 73 ]. The MRS specificity has been reported to be approximately 88%; however, the requirement of slightly larger lesions and poor sensitivity to detect total choline (tCho) (a phosphocholine metabolite elevated in breast malignancies and used as a diagnostic biomarker) signal is one of the limitations of this imaging modality [ 16 , 74 , 75 ]. There has been considerable progress on breast MRS in the last decade; however, multiple factors can potentially limit MRS, like optimization of analysis methods and complexity of acquisition procedures, that need to be addressed before including this imaging modality in a clinical setting.
2.7. Positron Emission Tomography (PET) Scanning and PET in Conjunction with Computer-aided Tomography (CT) Scanning (PET-CT)
Positron emission tomography (PET) imaging has been widely adopted as an important clinical modality for oncology. Even though many types of PET radiotracers have been developed to non-invasively interrogate in vivo tumor metabolism, 2-deoxy-2-(18F)fluoro-D-glucose (FDG) is the most widely used US FDA approved PET radiotracer that takes advantage of the enhanced glucose metabolism of cancer cells [ 76 ]. Cancerous cells are highly proliferative and have a higher glucose metabolism rate than normal cells. FDG PET radiotracers enter cells via the glucose transporter and are, thus, taken up in greater amounts by tumor cells than by healthy cells [ 77 ]. FDG uptake inversely correlates with prognosis [ 76 , 77 , 78 ].
PET-CT is a combination of PET (a nuclear medicine technique) and CT that produces highly detailed views of the body. The improved spatial resolution and sensitivity of PET scanners dedicated to breast (positron emission mammography) has allowed its clinical application in the study of primary tumors [ 79 , 80 ]. Numerous studies have shown that hybrid imaging with 18 F-FDG PET/CT provides information about the cellular glucose uptake, which is elevated in malignant lesions [ 81 , 82 , 83 , 84 ]. Jørgensen et al. observed significantly reduced uptake of 18 F-FDG by tumor cells following nanoparticle-assisted photothermal therapy, indicating that it can be effectively used as a marker to assess treatment responses [ 85 ]. Physicians use PET-CT studies to diagnose and stage the cancer, plan treatment, evaluate the effectiveness of treatment, and manage ongoing care.
2.8. Molecular Image-Guided Sentinel Node Biopsy
Sentinel lymph node biopsy (SLNB) is a revolutionary, minimally-invasive method to determine whether metastasis has occurred in early-stage breast cancer patients. Depending on the nodal metastatic status, SLNB is usually conducted to select the optimal therapeutic approach [ 86 ]. SLNB technique is well known for its significantly reduced post-operative complications associated with conventional axillary lymph node dissection [ 87 , 88 ]. This makes effective SLNB management key towards successful breast cancer diagnosis and treatment. Accurate SLNB guidance should limit the amount of invasive procedures needed and determine if multiple-basin drainage is occurring through localizing sentinel lymph nodes, thus, improving the staging accuracy in women with invasive breast cancer [ 89 ].
2.9. Breast Specific Gamma Imaging
Breast specific gamma imaging (BSGI), a molecular breast imaging approach, is a specialized nuclear medicine imaging test that allows detection of sub-centimeter and mammographically occult breast cancer with a sensitivity and specificity comparable to MRI [ 26 ]. In BSGI, a radiotracer such as Technetium Tc99m Sestamibi is injected into the patient’s bloodstream and the breast is visualized using a special camera [ 90 , 91 , 92 ]. Unlike mammography, BSGI is unaffected by breast density [ 93 , 94 ]. The modern BSGI has improved sensitivity for the detection of sub-centimeter lesions compared to scintimammography [ 95 ]. The major drawback of this technique is that, since the whole body gets exposed to the radiation, it is not possible to employ this for frequent breast cancer screening [ 96 ].
2.10. Ultrasound
Although mammography is a gold standard for breast cancer imaging, because of its limitations regarding dense breasts, another supplementing screening tool is required. Ultrasound is a supplemental tool that may be utilized to analyze some breast changes in women with dense breast tissues, as well as suspicious areas not seen on a mammogram [ 97 ]. Advantages of this technique include its wide availability, as well as no patient exposure to radiation. At the same time, however, it is limited by a number of factors. Most notably, it may fail to detect microcalcifications, and it may miss some early signs of cancer. Because of this limitation, this technique is not used to screen for breast cancer and is reserved for special situations. The fusion of ultrasound with other modalities [ 98 , 99 ]. such as ultrasound imaging techniques and ultrasound-guided biopsy, provides important tools for the management of breast cancer patients. Ultrasound elastography is now a routine noninvasive tool used to measure the consistency or hardness of the tissues to differentiate benign and malignant breast lesions [ 100 , 101 ]. Contrast-enhanced ultrasound and other modalities fused with ultrasound are other tools that may be useful in the noninvasive prediction of prognostic factors of breast cancer [ 102 ]. Complementary high resolution ultrasound is excellent for detecting breast lesions when in expert hands [ 103 ]. On the basis of existing literature, it was found that fusion of other modalities with ultrasound may be an effective primary detection tool for breast lesions, particularly in low- and middle-income countries with low-resource settings and where mammography and other expensive techniques are not available [ 104 ].
From the literature discussed above, we can see that, although ultrasound and mammography remain the most commonly used conventional methods of diagnosing breast cancer, other modalities such as DCE-MRI, MRE, PET, PET-CT, SLNB, and BSGI are now being considered for efficient collection of data. For example, most mammography methods can only be used to gather information about one breast, while MRI can be used to collect data from both breasts at the same time. Use of contrast agents can also enhance the quality of the data obtained for breast cancer diagnostics.
3. Current Treatment and Novel Therapies for Different Subtypes of Breast Cancer
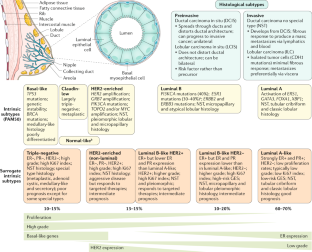
Breast cancer diagnosis by breast examination, mammography, breast ultrasound, MRI, and other imaging modalities can help identify tumors and other abnormalities in the tissue, as described above. These imaging modalities can help find a lump, an area of microcalcification, a suspicious area on ultrasound, or a gadolinium-enhanced area on MRI. Once breast cancer is identified using one of the diagnostic modalities discussed above, immediate and rigorous treatment must be provided to remove the tumor and prevent further spread of the cancer. One of the major challenges for breast cancer treatment is its heterogeneous nature, which affects the response to therapy [ 105 ]. By evaluating the presence of biomarkers such as hormone receptors (HRs), excess levels of human epidermal growth factor receptor 2 (HER2) protein, and/or extra copies of the HER2 gene [ 106 , 107 ], treatments that are most effective against a particular type of breast cancer can be determined and administered ( Figure 2 ). Based on the upregulation of genes, there are five main intrinsic or molecular subtypes of breast cancer:
- Luminal A breast cancer is low grade, HER2– and HR+ (estrogen- and/or progesterone-receptor positive), that has low levels of the protein Ki-67, which are responsible for controlling how fast cancer cells proliferate. Luminal A cancers tend to proliferate slowly with an excellent prognosis compared to other cancers.
- Luminal B breast cancer is a molecular subtype of breast cancer in which the tumors are HR+ (progesterone-receptor and/or estrogen-receptor positive) and show elevated levels of the protein Ki-67 while being either HER2– or HER2+. Luminal B cancer subtype is associated with faster proliferation rate and tends to be more aggressive compared to Luminal A breast cancer, making its prognosis slightly worse [ 108 ].
- Triple-negative/basal-like breast cancer is HR– (estrogen-receptor and progesterone-receptor negative) and HER2–. Women with BRCA1 gene mutations are more prone to develop this form of cancer [ 109 ].
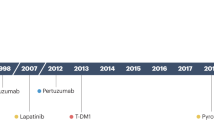
- HER2-enriched breast cancer is a molecular subtype of breast cancer in which tumors are HER2+ and HR– (i.e., negative for estrogen- and progesterone-receptor). This subtype is associated with a tendency to proliferate at a more rapid rate than luminal cancers [ 91 ]. However, patients are successfully treated with drugs targeting the HER2 protein, such as Tykerb (lapatinib), Herceptin (trastuzumab), Perjeta (pertuzumab), and Enhertu (fam-trastuzumab-deruxtecan-nxki) [ 110 ].
- Normal-like breast cancer is identical to luminal A cancer as it is HER2–, HR+ (estrogen- and/or progesterone-receptor positive) with reduced levels of the Ki-67 protein. The prognosis of normal-like breast cancer is, however, slightly worse than the luminal A cancer.

Novel FDA-approved targeted therapies for the treatment of molecular subtypes of breast cancer.
There are several FDA-approved drugs currently used in the treatment of breast cancer ( Table 2 ). The prodrug tamoxifen (brand name: Nolvadex) is a partial agonist that blocks estrogen uptake by the estrogen receptor (ER) [ 111 , 112 ]. Studies have shown that the risk of ER+ breast cancer recurrence can be reduced by half with tamoxifen [ 113 ]. However, similar to most anti-cancer therapies, tamoxifen has known side-effects and has been found to be associated with a number of increased health risks, such as endometrial cancer, blood clots, and stroke [ 114 , 115 ]. Aromatase inhibitors (AIs) block estrogen from being produced in postmenopausal women, suppressing the conversion of androgens to estrogens, thus, resulting in estrogen depletion. Three generations of AIs have been developed. The first-generation (e.g.: aminoglutethimide) and second-generation AIs (e.g., fadrozole and vorozole) are less selective with decreased production of cortisol and aldosterone, in addition to aromatase. They are also poorly tolerated with limited clinical efficacy [ 116 ]. On the other hand, third-generation AIs (e.g.: anastrozole (brand name: Arimidex), letrozole (brand name: Femara), and exemestane (brand name: Aromasin)) are highly selective for the enzyme aromatase and are tolerated fairly well. As a result, they have surpassed tamoxifen as first-line therapy for postmenopausal women with HR+ metastatic breast cancer with excellent response rates and delayed progression. AIs have additionally shown incremental improvement in disease-free survival, lower local and metastatic recurrence rates, and a lower incidence of contralateral breast cancer over tamoxifen [ 117 ].
List of therapeutic drugs used in the treatment of different types of breast cancer, and their status.
Luteinizing hormone-releasing hormone (LH-RH) analogs (goserelin and leuprolide) suppress the production of hormone from the ovaries [ 121 ]. LH-RH agonists act by pituitary desensitization and receptor downregulation, thereby suppressing gonadotrophin release. LH-RH exerts direct anticancer activity on malignant tissue that is independent from the suppression of the ovarian steroid synthesis and secretion [ 145 , 146 ]. Fulvestrant, a selective estrogen receptor degrader (SERD), is another drug that is suitable for breast cancer patients refractory to previous hormonal therapy. This is the first selective ER down regulator that is available clinically. This pure anti-estrogen results in degradation of ER alpha (α), has no agonistic effects, and has also demonstrated activity in tamoxifen-resistant breast cancer models [ 122 ]. Fulvestrant is the first SERD to enter into the clinical arena and a suitable backbone for combination therapy with new targeted agents for endocrine treatment of breast cancer. Preclinical studies have demonstrated that fulvestrant downregulates the expression of ERα in ER+ breast cancer cell lines without decreasing ERα gene (ESR1) transcripts along with inhibition of ER-responsive genes [ 147 ]. Fulvestrant can additionally block the non-genomic actions of estradiol on the G-protein coupled estrogen receptor (GPER), an alternate ER with a structure distinct from the two canonical ERs (ERα and ERβ) that is expressed in 50–60% of breast cancer, and which has been surmised to be related to the development of resistance towards tamoxifen in ERα+ breast cancer patients [ 148 ]. The proliferation of ER+ breast cancer cells is prevented through these processes. Additionally fulvestrant is also effective in those cell lines that are resistant to tamoxifen [ 149 , 150 ]. Patient derived xenograft models of ER+ breast cancer corroborated fulvestrant’s antitumor activity. Thus, we can conclude that it is more efficacious compared to tamoxifen or estrogen withdrawal [ 151 ]. Endocrine drugs work by different mechanisms, and thus, they are usually used as a combinational therapy for better anticancer efficacy. Nevertheless, there are conflicting results reported. It is generally believed that patients with endocrine-therapy-naïve advanced breast cancer and those with highly endocrine-sensitive tumors may benefit the most from combination endocrine therapy [ 152 , 153 , 154 , 155 ]. Several other biomarkers have emerged as potential targets for breast cancer therapy as described below.
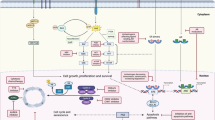
3.1. Cyclin-Dependent Kinases 4/6 (CDK4/6) Pathway
CDK4/6 are pivotal drivers for cell proliferation as they combine with cyclin D proteins, which regulate cell processes during the G1 phase of the cell cycle. Complete understanding of this cell cycle regulation may lead to promising cancer therapies [ 124 ]. Numerous studies are being carried out to explore drugs inhibiting CDK4/6 and assess the efficacy and drug safety for the treatment of breast cancer [ 156 ]. As a result of severe adverse events and less activity, the development of pan-CDK inhibitor flavopiridol [ 157 ] was subsequently discontinued, and then, highly specific inhibitors, namely, ribociclib (LEE011), palbociclib (PD0332991), and abemaciclib (LY2835219), were extensively researched and developed [ 124 , 125 , 126 ]. US FDA has approved palbociclib and ribociclib for the treatment of HR+, HER2–, or metastatic breast cancer. Recent clinical trial data suggest that significantly improved clinical outcome of palbociclib was achieved when combined with letrozole or fulvestrant [ 158 , 159 , 160 , 161 ].
3.2. Phosphoinositide 3-kinase (PI3K) Pathway
The PI3K pathway, also called phosphatidylinositol 3-kinases, is the most commonly activated signaling pathway in human cancer. They are a family of enzymes that are involved in cellular functions linking oncogenes and multiple receptor classes and constitute a critical signal transduction system [ 162 ]. The phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway (PI3K/AKT/mTOR pathway) plays a key role in cancer [ 126 ]. Pan-PI3Ki bind to PI3K isoforms in a selective and ATP competitive manner. The combination of PI3K inhibitors with aromatase inhibitors has been used as second-line treatment for HR+/HER− advanced breast cancer. A potent and highly specific oral pan-class I PI3K inhibitor (pan-PI3Ki), buparlisib is currently under investigation in patients with a variety of solid tumors, including breast cancer [ 127 , 128 ]. According to a new study, toxicities associated with buparlisib make it a poor option for the treatment of patients with HR+, HER2– advanced breast cancer that progressed on or after mTOR inhibitor therapy. The efficacy of the agent, however, suggests that PI3K inhibitors, along with endocrine therapy, remain a reasonable approach in patients with PIK3CA mutations [ 128 ].
Another pan-PI3Ki that displays equipotent inhibition of the p110α and –δ PI3K isoforms and less potent inhibition of p110β and –γ isoforms is pictilisib [ 129 ]. In a phase I dose-escalation clinical trial of 60 patients with advanced solid tumors ( {"type":"clinical-trial","attrs":{"text":"NCT00876109","term_id":"NCT00876109"}} NCT00876109 ), pictilisib was found to be overall safe in patients but with severe side-effects, such as hyperglycemia, rash, and pneumonitis [ 140 ]. Additionally, pilaralisib, also known as XL147, is an orally bioavailable small molecule with potential antineoplastic activity [ 131 ]. XL147 selectively targets and binds reversibly to class 1 PI3Ks thereby inhibiting tumor cell proliferation within tumors that are susceptible. Tumorigenesis is often related to the activation of the PI3K signaling pathway. In a Phase I/II dose-escalation study, pilaralisib (SAR245408), or voxtalisib (SAR245409), a PI3K and mammalian target of rapamycin inhibitor, in combination with letrozole, was evaluated for its efficacy, safety, and pharmacokinetics in HR+, HER2–, non-steroidal AI-refractory, recurrent, or metastatic breast cancer. As compared to voxtalisib, patients who were administered with pilaralisib demonstrated increased glucose levels compared to those who were administered voxtalisib. In conclusion, a limited efficacy and an acceptable safety profile in endocrine–therapy-resistant HR+, HER2– metastatic breast cancer was observed in patients treated with pilaralisib or voxtalisib combined with letrozole, as shown [ 132 ].
3.3. Targeting HER2+ Breast Cancers
HER2+ breast cancer (HER2+ BC) is characterized by drug resistance and a high rate of metastasis. Targeted therapy drugs have been shown to greatly improve the prognosis of HER2+ BC patients, but drug resistance or severe side effects have limited the clinical application of targeted therapy drugs. Various strategies are being researched to overcome drug resistance and to attain a more effective treatment. The HER2 oncogene (HER2, HER2/neu, c-erbB-2) is situated on chromosome-17 [ 163 , 164 ], and the main function of this oncogene is to encode transmembrane receptor tyrosine kinase [ 165 ]. Tyrosine kinase receptors play a key role in mediating various cellular functions, such as cell motility, proliferation, metabolism, and differentiation, that are based on cell-to-cell communication [ 140 ]. These receptors consist of a singular transmembrane helix, extracellular ligand domain and an intracellular region of a tyrosine kinase domain, juxtamembrane region, and a carboxy terminal tail [ 140 ]. Tyrosine kinase inhibitors competitively inhibit tyrosine phosphorylation and block tyrosine kinase enzyme activity, thus, resulting in downregulation of many cellular functions [ 166 ]. Neratinib (NERLYNX, Puma Biotechnology, Inc., CA, USA), an irreversible tyrosine kinase inhibitor (TKI) of HER1/HER2/HER4, has been reported to significantly improve the 2-year invasive disease-free survival after trastuzumab-based adjuvant therapy in HER2+ BC [ 137 ]. Neratinib, in combination with capecitabine, was approved by the US FDA on 25 February 2020 for treating patients with advanced or metastatic HER2+ BC previously treated with two or more anti-HER2 based regimens in the metastatic setting. Another example of TKI is Lapatinib, which competitively inhibits ATP-binding sites intracellularly and reversibly blocks phosphorylation of HER1 and HER2 [ 167 ]. A phase III study of lapatinib, in combination with an anti-neoplastic drug paclitaxel, demonstrated an increase in the survival rate of patients with HER2 metastasis breast cancer [ 168 ]. Another drug moiety, tucatinib, exhibited greater selectivity for HER2 in a phase I study of advanced disease patients along with reduced occurrence of diarrhea, as reported by patients that received other TKIs [ 169 ].
Compared with HER2– tumors, HER2+ BC is an aggressive subtype that demonstrates unique epidemiological, clinical, and prognostic differences with poor response to standard chemotherapy regimens [ 170 ]. About 30% of breast cancer patients have been evaluated for the expression of HER2, which is generally recognized as a marker for invasive disease that is likely to be highly metastatic, drug resistant, and to spread rapidly [ 171 , 172 , 173 ]. There has been remarkable advancements in therapies for managing HER2+ BC in the last 20 years, specifically, targeted treatments that are HER2 expression level dependent [ 174 ]. A humanized monoclonal antibody (mAb), trastuzumab (herceptin), targeted towards the HER2 ectodomain, has demonstrated activity in HER2-overexpressing breast cancer patients. Trastuzumab effectively inhibited basal and induced HER2 cleavage, resulting in the generation of phosphorylated p95 [ 171 ]. Another mAb, pertuzumab, binds to a different epitope of the HER2 dimerization domain than trastuzumab, preventing interactions with other receptors in the HER2 family that lead to cell growth inhibition [ 175 , 176 ]. The direct inhibitory action on the extracellular domain of HER2 has largely contributed to the HER2-directed mAbs antitumor efficacy.
Patritumab, a human anti-HER3 mAb, through inhibiting the formation of HER2/HER3 heterodimers, has shown anticancer activity in preclinical models. It was found to exhibit favorable efficacy and acceptable tolerability in patients with HER2+ advanced breast cancer [ 138 ]. The pharmacokinetic profile for patritumab was determined based on the target trough level, and efficacy was evaluated based on the overall response rate and progression-free survival.
3.4. Treating Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC) accounts for about 10–15% of all breast cancers [ 177 ]. In TNBC, the cancer cells do not possess estrogen or progesterone receptors and also do not produce too much of the protein HER2 [ 178 ]. As compared to other breast cancer subtypes, TNBC is far more invasive and proliferate and spreads at a much faster rate, and patients have limited treatment options and a worse prognosis [ 179 , 180 ]. Standard chemotherapy remains the mainstay treatment for TNBC. However, metastasis and recurrence rates are higher compared to non-TNBC tumors [ 181 ]. Advanced TNBC patients, when treated with carboplatin with or without a taxane drug (e.g., docetaxel), showed better efficacy and toxicity profile compared to docetaxel. Additionally, in germline BRCA1/2-mutated breast cancer patients, carboplatin displayed a response rate twice as high compared to docetaxel. This implies the importance of determining whether breast cancer patients have BRCA1/2 mutation so that the most effective drug for first-line chemotherapy can be chosen [ 182 ]. TNBC has the fewest therapeutic options among all breast cancer subtypes due to the lack of well-defined molecular targets [ 181 ]. Identification of new therapeutic targets and development of effective targeted agents is, hence, urgently needed.
Sacituzumab govitecan is the first antibody–drug conjugate approved by the US FDA in the treatment of relapsed or refractory metastatic TNBC. It was developed by coupling a monoclonal antibody that targets anti-trophoblast cell-surface antigen 2 (Trop-2) with SN-38—an active metabolite of irinotecan, which is a topoisomerase I inhibitor. Approval was based on findings in the phase I/II multicenter IMMU-132-01 trial (ClinicalTrials.gov identifier {"type":"clinical-trial","attrs":{"text":"NCT01631552","term_id":"NCT01631552"}} NCT01631552 ) [ 183 ]. Another drug, enhertu, is an antibody and topoisomerase inhibitor conjugate that targets and attaches to HER2+ cancer cells [ 142 ]. Enhertu is approved for treating adults with unresectable or metastatic HER2-positive breast cancer [ 184 ].
Kadcyla, also known as T-DM1, is an agent approved by the US FDA to treat patients with HER2-positive metastatic breast cancer that have been previously treated with herceptin and taxane chemotherapy (neoadjuvant treatment). T-DM1 is an antibody–drug conjugate targeted therapy in which emtansine is conjugated to Herceptin [ 141 ].
The immunotherapy medicine pembrolizumab (brand name: Keytruda) is a human monoclonal IgG4-ĸ antibody that is highly selective against the programmed cell death 1 receptor (PD-1). The addition of pembrolizumab to first-line chemotherapy significantly extended progression-free survival among patients with metastatic TNBC or TNBC that has resurged and cannot be surgically removed [ 143 ]. Recently, USFDA granted accelerated approval of pembrolizumab in combination with chemotherapy for treating TNBC patients.
Recently, the combination of atezolizumab plus nab-paclitaxel has been approved by FDA as first-line therapy in patients with PD-L1+ TNBC [ 144 ]. We can, therefore, see that several diagnostic/ imaging and therapeutic options are currently available for breast cancer management. There has been increasing interest in recent times to combine diagnostic and therapeutic components within a single system for effective and personalized breast cancer management. Strategies being investigated in this direction are described in the next section.
4. Recent Trends in Breast Cancer Theranostics
Traditionally, cancer management is based on identifying tumor lesions through an appropriate diagnostic imaging modality, followed by treatment with chemotherapy, radiotherapy, or surgery. However, the disadvantages of these treatments include possibility of incomplete surgical resection, off-target toxicities, low local drug concentrations at the disease site, and limited drug penetration into tumors due to abnormal vasculature, which causes elevated interstitial pressure and blood flow stasis [ 185 , 186 ]. Moreover, conventional methods of assessing drug kinetics involves assessing drug concentration in plasma, which is not a reliable method to evaluate chemotherapeutic pharmacokinetics [ 187 , 188 ]. Over the past two decades, personalized medicine has received significant interest as it can be used to tailor treatment according to patient needs and characteristics, thus, minimizing side-effects, resulting in the emergence of theranostics, which is a relatively new research area [ 189 ]. Theranostics is a field of research where a combination of diagnostic agents and therapeutic agents are used to provide patient-centered care for the treatment of cancer and other diseases by providing real-time monitoring of the drug that will assist in altering cancer treatment regimens for better therapeutic efficacy [ 190 ]. Accurate diagnosis is crucial for an early therapeutic intervention, failure of which results in delayed treatment and increased risk of mortality [ 191 ].
Theranostic nanotechnology or nanotheranostics is an area where an integrated nanotherapeutic system can be used to simultaneously diagnose, deliver targeted therapy, and monitor the therapeutic response [ 192 ]. A single nanoparticle formulation, conjugated with targeting ligands, therapeutic agents, and a fluorophore/contrast agent, can be visualized using different imaging modalities as it crosses biological barriers to target receptors upregulated by cancer cells and finally releases the drug in the tumor environment in a controlled manner ( Figure 3 ).

Schematic diagram representing theranostic approaches in breast cancer management.
Nanotheranostics is being widely explored today as a method of effectively managing breast cancer. Nanotheranostic formulations can be tracked using different imaging modalities following administration so that their targeted accumulation and treatment at the site of the cancer can be monitored. Lipid-based carriers, such as liposomes and micelles, are often used due to their versatility and biocompatibility. Gregoriou et al. recently developed theranostic micelles using Pluronic F127 block copolymer and Vitamin E-TPGS that showed promise as a method of targeted delivery of the phytochemical resveratrol to treat breast cancer. Coumarin-6—a fluorophore, can be incorporated to impart imaging capabilities to the system [ 193 ]. Wang et al. targeted EGFR+ TNBCs using a quantum-dot-containing micellar formulation tagged with an anti-EGFR nanobody. The micelles could be imaged using the near-infrared fluorescent quantum dots and could release the anti-cancer drug aminoflavone. Significant tumor regression was observed in orthotopic TNBC mouse models with EGFR+ tumors following administration of the theranostic micelles by i.v. injection [ 194 ]. Parhi et al. functionalized lipid-based NPs with trastuzumab to target HER2+ breast cancer cells. The NPs (~72 nm) contained rapamycin (anti-cancer drug) and quantum dots (imaging). In vitro studies on SKBR 3 breast cancer cells grown as a two-dimensional monolayer and as three-dimensional spheroids confirmed greater cellular uptake and therapeutic efficacy than native drug or unmodified NPs [ 195 ]. Albumin NPs have also been investigated as a delivery vehicle for theranostic applications. A human serum albumin-based NP formulation (~151 nm) encapsulating doxorubicin (DOX, chemotherapeutic drug) and gadolinium III (MRI contrast agent) was developed recently and studied against TNBC xenografts grown on the chorioallantoic membrane of fertilized chick eggs. Persistent NP presence was observed in tumor tissues for at least 15 h, where the NPs significantly reduced the proliferative Ki-67-positive fraction of cells in the xenografts compared to native DOX [ 196 ].
Theranostic formulations developed using different polymers have also been successfully investigated in the treatment of breast cancer and its metastases. For example, Li et al. developed a novel terpolymer using poly(methacrylic acid) and PS 80 covalently grafted onto starch, which was then used to deliver DOX and multiple imaging agents—gadolinium (MRI contrast agent) and near-infrared fluorophore HF750 (fluorescence imaging), for the treatment of brain metastases of breast cancer. The NPs, when delivered by tail vein injection, could selectively accumulate and induce apoptosis in cancer cells while not affecting normal brain cells in a brain metastatic breast cancer SCID mouse model [ 197 ]. Poly lactic-co-glycolic acid (PLGA) is a polymer that has been FDA-approved for many medical applications and is widely used in nanoparticle-based drug delivery strategies. Recently, PLGA NPs were developed and coated with platelet membranes to form nanoplatelets containing DOX, as well as multiple imaging agents—perfluoropentane (PFP for ultrasonic imaging), nanocarbon (for photoacoustic imaging and photothermal therapy), and fluorescence imaging. Upon delivery of the NPs to 4T1 breast-tumor-bearing mice and laser irradiation, the light was converted to heat energy by the NPs, which had a photothermal effect. The heat also led to PFP vaporization for enhanced ultrasonic imaging and release of DOX for therapy [ 198 ]. Dong et al. were able to successfully develop a dual-modal gold-nanoshelled PLGA magnetic hybrid nanoparticle formulation that was encapsulated with perfluorooctyl bromide and superparamagnetic iron oxide nanoparticles and conjugated to anti-HER2 antibodies (HER2-GPH NPs). They were able to monitor the accumulation of these particles using ultrasound and magnetic resonance while the targeted antibody aided the binding of photothermal agents to the HER2-positive breast cancer cells. These particles were able to successfully induce cell death on exposure to near-infrared irradiation [ 199 ].
Metal-based NPs have also been explored in breast cancer nanotheranostics. Ruthenium (Ru) agents also display high anti-cancer activity with limited cytotoxicity towards normal cells and are, therefore, an attractive alternative to platinum-based compounds for anti-cancer therapy [ 200 ]. Ru-based compounds can also be employed as imaging agents by binding to the DNA through non-covalent interactions [ 201 ] and are, thus, useful tools in theranostic applications. Shen et al. reported the development of a liposome-based theranostic formulation containing Ru-polypyridine complex. The liposome carrier enhanced the cellular internalization of Ru in cancer cells. Intravenous (i.v.) administration of these nanocarriers in orthotopic murine model of MDA-MB-231 human breast cancer exhibited high accumulation of the particles within the tumor 2 h post injection, along with a dramatic decrease in the TNBC tumor growth [ 202 ]. We have previously developed theranostic nanoformulations that can co-deliver a ruthenium compound (therapy) along with a radionuclide (imaging) to epidermal growth factor (EGFR)-positive cancer cells [ 103 ]. This formulation is also suitable for the treatment of TNBCs, which tend to overexpress EGFR.
In addition to cancer cells, the tumor microenvironment also consists of several other cell types, including fibroblasts and immune cells, that can play a decisive role in the effective distribution of the NPs within the tumor. Strategies that allow for the penetration of NPs into the tumor microenvironment are, therefore, attractive. Zeng et al. developed novel HER2-DOX-superparamagnetic iron oxide nanoparticles (NP) with a gold shell as a theranostic approach for the diagnosis and targeted identification of HER2+ BC. The accumulation of these particles in the tumors of BT474 breast cancer nude mice was highest after 2h of i.v. injection, which was detected by MRI. Additionally, the gold shell-mediated photothermal effect led to remodeling of the tumor microenvironment by decreasing cancer-associated fibroblasts, which resulted in the improved antitumor efficacy of DOX [ 203 ]. NPs usually tend to accumulate in tumor tissues as a result of enhanced permeability and retention effect exerted by long-circulating nanoparticles. However, the size of the particles plays a critical role in maintaining these properties. Small nanoparticles easily penetrate deep into tumor lesions; however, they are pulled back into the blood stream during circulation, while large particles, on the other hand, are retained easily but tend to have poor penetration ability [ 204 ]. Liu et al. successfully developed a CD44 targeted tumor-specific hyaluronidase-degradable hyaluronic acid, cationic bovine serum albumin-protected gold nanocluster that was loaded with indocyanine green for tumor fluorescence imaging and a chemotherapeutic drug paclitaxel. On subcutaneous injection of the NPs in tumor-bearing Balb/c mice, these particles displayed size-reducible properties as a result of the presence of hyaluronidase leading to highly homogenous intra-tumor distribution of the NPs [ 205 , 206 ].
Nanotheranostic formulations can also be used to provide hyperthermia in cells to promote cell membrane permeabilization causing the destruction of the tumorous mass. Burke et al. used near infrared stimulation of multiwalled carbon nanotubes for photothermal therapy, which led to increased permeability of cell membranes and rapid cell death. This system has the potential to be used for theranostic applications if combined with an anti-cancer agent [ 207 ]. Another promising strategy in theranostics is using an injectable thermoresponsive hydrogel for local therapy of breast cancer. Wu et al. demonstrated that injecting a supramolecular thermoresponsive hydrogel such as poly( N -acryloyl glycinamide- co -acrylamide) hydrogel along with polydopamine (PDA) coated-gold nanoparticles (AuNPs) and loading the carrier with DOX exhibited an excellent photothermal effect, along with sustained release of the anticancer drug [ 208 ].
It is clear from the above research that breast cancer nanotheranostics is a rapidly growing area that holds great promise as a method of combining cutting-edge technologies within a single platform to deliver breast cancer therapies in a targeted, sustained, and effective manner. We can integrate contrast agents for different imaging modalities and an anti-cancer therapeutic agent into a single formulation for targeted theranostic drug delivery, which can minimize patient discomfort while providing personalized medicine.
5. Conclusions and Future Outlook
In this review, we have highlighted some of the common methods of breast cancer diagnosis and treatment and the role of the emerging area of breast cancer theranostics in integrating diagnostics and therapy within a single platform to provide patient-specific therapy. Early detection and treatment of breast cancer is crucial in the reduction of breast cancer mortality rate. The methods of diagnosis and treatment of breast cancer has undergone tremendous changes over the past two decades, and the focus is on managing and treating the disease with minimal patient discomfort, increased patient compliance, and reduced off-target side effects. Nanomedicine allows for the targeted delivery and controlled release of the encapsulated drugs at the tumor site, thus, altering the bioavailability and drug pharmacokinetics while simultaneously enhancing permeability and retention in the tumor and minimizing severe side-effects to the healthy cells. Theranostics has emerged as an invaluable tool in personalized medicine as these multifunctional platforms can be used for the simultaneous detection, treatment, and management of cancers. Despite the undeniable potential of nanotheranostic formulations, there are several factors to be taken into consideration while developing and testing these platforms and before taking them into the market. A major challenge is in the manufacturing, scale-up, and reproducibility owing to the complexity of incorporating multiple functionalities into a single platform while maintaining its dimensions in the nanoscale range. Extensive research needs to be conducted to determine the optimal dose that can simultaneously produce a strong signal for imaging while maintaining the desired drug release kinetics for therapy. The platform must also have minimal or negligible toxic interactions with the surrounding biological tissues. Depth of penetration is a significant challenge when using imaging modalities with theranostic formulations; therefore, imaging agents that can be used to obtain high resolution images independent of tissue depth are preferred. The materials used to develop the theranostic system must be optimized to prevent release of the incorporated imaging agent and premature release of the encapsulated therapeutics. While stimuli-responsive “smart” materials may be used for on-demand release of therapeutics in response to changes in the surrounding environment (e.g., temperature, pH, magnetic field), this introduces more complexity to the system and can possibly impede its clinical translation. Different type of breast tumors can upregulate different receptors on their surfaces, and the theranostic system will need to be optimized against each type of breast cancer in order to provide personalized therapy. Nevertheless, it is evident that theranostic nanomedicine holds tremendous potential for breast cancer diagnosis and targeted, personalized treatment. Since theranostics is an emerging research area, we can expect to see new multifunctional formulations enter clinical trials in the near future that can be tracked following administration and provide targeted and effective breast cancer therapy.
Author Contributions
Conceptualization, A.B.; writing—original draft preparation, A.B. and A.G.; writing—reviewing & editing, A.B., A.G. and J.U.M.; funding acquisition, J.U.M. All authors have read and agreed to the published version of the manuscript.
This work received funding support from the Rhode Island Institutional Development Award (IDeA) Network of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Current State of Breast Cancer Diagnosis, Treatment, and Theranostics
Affiliations.
- 1 Ladue Horton Watkins High School, St. Louis, MO 63124, USA.
- 2 Department of Biomedical and Pharmaceutical Sciences, College of Pharmacy, University of Rhode Island, Kingston, RI 02881, USA.
- PMID: 34069059
- PMCID: PMC8156889
- DOI: 10.3390/pharmaceutics13050723
Breast cancer is one of the leading causes of cancer-related morbidity and mortality in women worldwide. Early diagnosis and effective treatment of all types of cancers are crucial for a positive prognosis. Patients with small tumor sizes at the time of their diagnosis have a significantly higher survival rate and a significantly reduced probability of the cancer being fatal. Therefore, many novel technologies are being developed for early detection of primary tumors, as well as distant metastases and recurrent disease, for effective breast cancer management. Theranostics has emerged as a new paradigm for the simultaneous diagnosis, imaging, and treatment of cancers. It has the potential to provide timely and improved patient care via personalized therapy. In nanotheranostics, cell-specific targeting moieties, imaging agents, and therapeutic agents can be embedded within a single formulation for effective treatment. In this review, we will highlight the different diagnosis techniques and treatment strategies for breast cancer management and explore recent advances in breast cancer theranostics. Our main focus will be to summarize recent trends and technologies in breast cancer diagnosis and treatment as reported in recent research papers and patents and discuss future perspectives for effective breast cancer therapy.
Keywords: breast cancer; breast specific gamma imaging; imaging modalities; mammography; theranostics; triple-negative breast cancer.
Publication types
Grants and funding.
- P20 GM103430/GM/NIGMS NIH HHS/United States
- P20GM103430/GM/NIGMS NIH HHS/United States
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 September 2019
- Breast cancer
- Nadia Harbeck 1 ,
- Frédérique Penault-Llorca 2 ,
- Javier Cortes 3 , 4 ,
- Michael Gnant 5 ,
- Nehmat Houssami 6 ,
- Philip Poortmans 7 , 8 ,
- Kathryn Ruddy 9 ,
- Janice Tsang 10 &
- Fatima Cardoso 11
Nature Reviews Disease Primers volume 5 , Article number: 66 ( 2019 ) Cite this article
88k Accesses
1441 Citations
449 Altmetric
Metrics details
- Cancer therapy
- Genetic predisposition to disease
- Radiotherapy
- Tumour biomarkers
Breast cancer is the most frequent malignancy in women worldwide and is curable in ~70–80% of patients with early-stage, non-metastatic disease. Advanced breast cancer with distant organ metastases is considered incurable with currently available therapies. On the molecular level, breast cancer is a heterogeneous disease; molecular features include activation of human epidermal growth factor receptor 2 (HER2, encoded by ERBB2 ), activation of hormone receptors (oestrogen receptor and progesterone receptor) and/or BRCA mutations. Treatment strategies differ according to molecular subtype. Management of breast cancer is multidisciplinary; it includes locoregional (surgery and radiation therapy) and systemic therapy approaches. Systemic therapies include endocrine therapy for hormone receptor-positive disease, chemotherapy, anti-HER2 therapy for HER2-positive disease, bone stabilizing agents, poly(ADP-ribose) polymerase inhibitors for BRCA mutation carriers and, quite recently, immunotherapy. Future therapeutic concepts in breast cancer aim at individualization of therapy as well as at treatment de-escalation and escalation based on tumour biology and early therapy response. Next to further treatment innovations, equal worldwide access to therapeutic advances remains the global challenge in breast cancer care for the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives
Antonio Marra, Sarat Chandarlapaty & Shanu Modi

A careful reassessment of anthracycline use in curable breast cancer
Sara Alsterlind Hurvitz, Nicholas P. McAndrew, … Dennis J. Slamon
Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer
Jun Ma, Jack Junjie Chan, … Yoon-Sim Yap
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406 , 747–752 (2000).
Article CAS PubMed Google Scholar
Cardoso, F. et al. European Breast Cancer Conference manifesto on breast centres/units. Eur. J. Cancer 72 , 244–250 (2017).
Article PubMed Google Scholar
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 , 394–424 (2018).
Bray, F. et al. Cancer Incidence in Five Continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 137 , 2060–2071 (2015).
Mariotto, A. B., Etzioni, R., Hurlbert, M., Penberthy, L. & Mayer, M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol. Biomark. Prev. 26 , 809–815 (2017).
Article Google Scholar
Ren, J.-X., Gong, Y., Ling, H., Hu, X. & Shao, Z.-M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res. Treat. 173 , 225–237 (2019).
Torre, L. A., Siegel, R. L., Ward, E. M. & Jemal, A. Global cancer incidence and mortality rates and trends — an update. Cancer Epidemiol. Biomark. Prev. 25 , 16–27 (2016).
Ginsburg, O. et al. The global burden of women’s cancers: a grand challenge in global health. Lancet 389 , 847–860 (2017).
Allemani, C. et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385 , 977–1010 (2015).
Winters, S., Martin, C., Murphy, D. & Shokar, N. K. Breast cancer epidemiology, prevention, and screening. Prog. Mol. Biol. Transl Sci. 151 , 1–32 (2017).
Hossain, M. S., Ferdous, S. & Karim-Kos, H. E. Breast cancer in South. Asia: a Bangladeshi perspective. Cancer Epidemiol. 38 , 465–470 (2014).
PubMed Google Scholar
Leong, S. P. L. et al. Is breast cancer the same disease in Asian and western countries? World J. Surg. 34 , 2308–2324 (2010).
Article PubMed PubMed Central Google Scholar
Bhoo Pathy, N. et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore–Malaysia hospital-based breast cancer registry. Breast 20 , S75–S80 (2011).
Raina, V. et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in North India. Indian J. Cancer 42 , 40 (2005).
Agarwal, G., Pradeep, P. V., Aggarwal, V., Yip, C.-H. & Cheung, P. S. Y. Spectrum of breast cancer in Asian women. World J. Surg. 31 , 1031–1040 (2007).
Li, C. I., Malone, K. E. & Daling, J. R. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol. Biomark. Prev. 11 , 601–607 (2002).
Google Scholar
Wong, F. Y., Tham, W. Y., Nei, W. L., Lim, C. & Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 38 , 39 (2018).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst . 107 , https://doi.org/10.1093/jnci/djv048 (2015).
DeSantis, C. E. et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women: Breast Cancer Statistics, 2015. CA Cancer J. Clin. 66 , 31–42 (2016).
DeSantis, C. E., Ma, J., Goding Sauer, A., Newman, L. A. & Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast Cancer Statistics, 2017. CA Cancer J. Clin. 67 , 439–448 (2017).
Shiovitz, S. & Korde, L. A. Genetics of breast cancer: a topic in evolution. Ann. Oncol. 26 , 1291–1299 (2015).
CAS PubMed PubMed Central Google Scholar
Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet 358 , 1389–1399 (2001).
Brewer, H. R., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res. Treat. 165 , 193–200 (2017).
Huen, M. S. Y., Sy, S. M. H. & Chen, J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat. Rev. Mol. Cell Biol. 11 , 138–148 (2010).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317 , 2402 (2017).
Balmana, J., Diez, O., Rubio, I. T. & Cardoso, F., On behalf of the ESMO Guidelines Working Group. BRCA in breast cancer: ESMO clinical practice guidelines. Ann. Oncol. 22 , vi31–vi34 (2011).
Paluch-Shimon, S. et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 27 , v103–v110 (2016).
Daly, M. B. et al. Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J. Natl Compr. Cancer Netw. 14 , 153–162 (2016).
Forbes, C., Fayter, D., de Kock, S. & Quek, R. G. W. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, GENETIC COUNSELING and treatment of BRCA -mutated breast cancer. Cancer Manag. Res. 2019 , 2321–2337 (2019).
Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377 , 523–533 (2017).
Litton, J. K. et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379 , 753–763 (2018).
FDA. FDA approves olaparib germline BRCA-mutated metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer (2018).
FDA. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. Fda.gov https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (2018).
Pasche, B. Recent advances in breast cancer genetics. Cancer Treat. Res. 141 , 1–10 (2008).
Cobain, E. F., Milliron, K. J. & Merajver, S. D. Updates on breast cancer genetics: clinical implications of detecting syndromes of inherited increased susceptibility to breast cancer. Semin. Oncol. 43 , 528–535 (2016).
Crawford, B. et al. Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res. Treat. 163 , 383–390 (2017).
Article CAS PubMed PubMed Central Google Scholar
Taylor, A. et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J. Med. Genet. 55 , 372–377 (2018).
Althuis, M. D., Dozier, J. M., Anderson, W. F., Devesa, S. S. & Brinton, L. A. Global trends in breast cancer incidence and mortality 1973–1997. Int. J. Epidemiol. 34 , 405–412 (2005).
Colditz, G. A., Sellers, T. A. & Trapido, E. Epidemiology — identifying the causes and preventability of cancer? Nat. Rev. Cancer 6 , 75–83 (2006).
Britt, K., Ashworth, A. & Smalley, M. Pregnancy and the risk of breast cancer. Endocr. Relat. Cancer 14 , 907–933 (2007).
Siwko, S. K. et al. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells — implications for pregnancy-induced protection against breast cancer. Stem Cells 26 , 3205–3209 (2008).
Hilakivi-Clarke, L., de Assis, S. & Warri, A. Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J. Mammary Gland. Biol. Neoplasia 18 , 25–42 (2013).
Danaei, G., Vander Hoorn, S., Lopez, A. D., Murray, C. J. & Ezzati, M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366 , 1784–1793 (2005).
Chen, W. Y., Rosner, B., Hankinson, S. E., Colditz, G. A. & Willett, W. C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306 , 1884 (2011).
Singletary, K. W. & Gapstur, S. M. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286 , 2143 (2001).
Smith-Warner, S. A. et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279 , 535 (1998).
Bandera, E. V., Maskarinec, G., Romieu, I. & John, E. M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv. Nutr. 6 , 803–819 (2015).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention: breast cancer, inflammation, and obesity. CA Cancer J. Clin. 67 , 378–397 (2017).
Shieh, Y. et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 21 , 48 (2019).
Suzuki, Y., Tsunoda, H., Kimura, T. & Yamauchi, H. BMI change and abdominal circumference are risk factors for breast cancer, even in Asian women. Breast Cancer Res. Treat. 166 , 919–925 (2017).
Del Pup, L., Codacci-Pisanelli, G. & Peccatori, F. Breast cancer risk of hormonal contraception: counselling considering new evidence. Crit. Rev. Oncol. Hematol. 137 , 123–130 (2019).
Busund, M. et al. Progestin-only and combined oral contraceptives and receptor-defined premenopausal breast cancer risk: the Norwegian Women and Cancer Study. Int. J. Cancer 142 , 2293–2302 (2018).
Mørch, L. S. et al. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 377 , 2228–2239 (2017).
Ganz, P. A. et al. Supportive care after curative treatment for breast cancer (survivorship care): resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast 22 , 606–615 (2013).
Burris, J. L., Armeson, K. & Sterba, K. R. A closer look at unmet needs at the end of primary treatment for breast cancer: a longitudinal pilot study. Behav. Med. 41 , 69–76 (2015).
Coughlin, S. S., Yoo, W., Whitehead, M. S. & Smith, S. A. Advancing breast cancer survivorship among African-American women. Breast Cancer Res. Treat. 153 , 253–261 (2015).
Bodai, B. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 19 , 48–79 (2015).
Ho, P. J., Gernaat, S. A. M., Hartman, M. & Verkooijen, H. M. Health-related quality of life in Asian patients with breast cancer: a systematic review. BMJ Open 8 , e020512 (2018).
Miyashita, M. et al. Unmet information needs and quality of life in young breast cancer survivors in japan. Cancer Nurs. 38 , E1–E11 (2015).
Bombonati, A. & Sgroi, D. C. The molecular pathology of breast cancer progression. J. Pathol. 223 , 307–317 (2011).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486 , 353–360 (2012).
Lopez-Garcia, M. A., Geyer, F. C., Lacroix-Triki, M., Marchió, C. & Reis-Filho, J. S. Breast cancer precursors revisited: molecular features and progression pathways: molecular evolution of breast cancer. Histopathology 57 , 171–192 (2010).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534 , 47–54 (2016).
Yates, L. R. & Desmedt, C. Translational genomics: practical applications of the genomic revolution in breast cancer. Clin. Cancer Res. 23 , 2630–2639 (2017).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20 , 71–88 (2019).
Ediriweera, M. K., Tennekoon, K. H. & Samarakoon, S. R. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov. Today 24 , 685–702 (2019).
Munster, P. N. et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer 104 , 1828–1835 (2011).
Zhou, Y., Wang, Y., Zhang, K., Zhu, J. & Ning, Z. Reverse effect of chidamide on endocrine resistance in estrogen receptor-positive breast cancer. J. Shenzhen Univ. Sci. Eng. 35 , 339 (2018).
Jiang, Z. et al. Phase III trial of chidamide, a subtype-selective histone deacetylase (HDAC) inhibitor, in combination with exemestane in patients with hormone receptor-positive advanced breast cancer [abstract]. Ann. Oncol. 29 , 283O_PR (2018).
Williams, C. & Lin, C.-Y. Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience 7 , 370 (2013).
PubMed PubMed Central Google Scholar
Levin, E. R. & Pietras, R. J. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res. Treat. 108 , 351–361 (2008).
Santen, R. J. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J. Clin. Endocrinol. Metab. 96 , 308–319 (2011).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 109 , 2796–2801 (2012).
Solinas, C., Carbognin, L., De Silva, P., Criscitiello, C. & Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast 35 , 142–150 (2017).
Nagarajan, D. & McArdle, S. Immune landscape of breast cancers. Biomedicines 6 , 20 (2018).
Article PubMed Central CAS Google Scholar
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13 , 228–241 (2016).
Dieci, M. V. et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 52 , 16–25 (2018).
Boudreau, A., van’t Veer, L. J. & Bissell, M. J. An ‘elite hacker’: breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adhes. Migr. 6 , 236–248 (2012).
Smyth, M. J., Dunn, G. P. & Schreiber, R. D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90 , 1–50 (2006).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 , 1565–1570 (2011).
Buonomo, O. C. et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLOS ONE 12 , e0184680 (2017).
Article PubMed PubMed Central CAS Google Scholar
Gobbini, E. et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 96 , 17–24 (2018).
Santé Publique France. Breast cancer [French]. Santepubliquefrance.fr https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein (2019).
Zhang, K. et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag. Res. 10 , 2573–2580 (2018).
Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32 , 169–184.e7 (2017).
Gingras, I., Salgado, R. & Ignatiadis, M. Liquid biopsy: will it be the ‘magic tool’ for monitoring response of solid tumors to anticancer therapies? Curr. Opin. Oncol. 27 , 560–567 (2015).
Aurilio, G. et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 50 , 277–289 (2014).
Independent, U. K. Panel on breast cancer screening. the benefits and harms of breast cancer screening: an independent review. Lancet 380 , 1778–1786 (2012).
Nelson, H. D. et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 244–255 (2016).
Lauby-Secretan, B. et al. Breast-cancer screening — viewpoint of the IARC Working Group. N. Engl. J. Med. 372 , 2353–2358 (2015).
Houssami, N. Overdiagnosis of breast cancer in population screening: does it make breast screening worthless? Cancer Biol. Med. 14 , 1–8 (2017).
Suhrke, P. et al. Effect of mammography screening on surgical treatment for breast cancer in Norway: comparative analysis of cancer registry data. BMJ 343 , d4692–d4692 (2011).
Stang, A., Kääb-Sanyal, V., Hense, H.-W., Becker, N. & Kuss, O. Effect of mammography screening on surgical treatment for breast cancer: a nationwide analysis of hospitalization rates in Germany 2005–2009. Eur. J. Epidemiol. 28 , 689–696 (2013).
IARC Handbooks of Cancer Prevention. Breast Cancer Screening (Volume 15). Iarc.fr http://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2016 (2016).
Nelson, H. D. et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164 , 256–267 (2016).
Carter, J. L., Coletti, R. J. & Harris, R. P. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 350 , g7773 (2015).
Saslow, D. et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57 , 75–89 (2007).
Phi, X.-A. et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥ 50 years: evidence from an individual patient data meta-analysis. J. Clin. Oncol. 33 , 349–356 (2015).
Sardanelli, F. et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur. J. Cancer 46 , 1296–1316 (2010).
Melnikow, J. et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. preventive services task force. Ann. Intern. Med. 164 , 268–278 (2016).
Houssami, N. & Lee, C. I. The impact of legislation mandating breast density notification — review of the evidence. Breast 42 , 102–112 (2018).
Marinovich, M. L., Hunter, K. E., Macaskill, P. & Houssami, N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J. Natl Cancer Inst. 110 , 942–949 (2018).
Irwig, L., Macaskill, P. & Houssami, N. Evidence relevant to the investigation of breast symptoms: the triple test. Breast 11 , 215–220 (2002).
Houssami, N., Ciatto, S., Turner, R. M., Cody, H. S. & Macaskill, P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 254 , 243–251 (2011).
Morrow, M., Waters, J. & Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 378 , 1804–1811 (2011).
Srigley, J. R. et al. Standardized synoptic cancer pathology reporting: a population-based approach. J. Surg. Oncol. 99 , 517–524 (2009).
World Heath Organisation. WHO Classification of Tumours of the Breast, Fourth Edition. (World Health Organization, 2012).
Elston, C. W. & Ellis, I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19 , 403–410 (1991).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Nccn.org https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2018).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 28 , 1700–1712 (2017).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24 (Suppl. 6), vi7-vi23 (2013).
Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28 , 2784–2795 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36 , 2105–2122 (2018).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J. Natl Cancer Inst. 103 , 1656–1664 (2011).
Rakha, E. A. et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 118 , 3670–3680 (2012).
Barrio, A. V. & Morrow, M. Appropriate margin for lumpectomy excision of invasive breast cancer. Chin. Clin. Oncol. 5 , 35–35 (2016).
Chung, A. et al. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Ann. Surg. Oncol. 22 , 422–427 (2015).
Schulman, A. M. et al. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) Mastery SM database following the SSO-ASTRO “no ink on tumor” guidelines. Ann. Surg. Oncol. 24 , 52–58 (2017).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract. Radiat. Oncol. 6 , 287–295 (2016).
Morrow, M. et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J. Clin. Oncol. 34 , 4040–4046 (2016).
Moran, M. S. et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 88 , 553–564 (2014).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J. Clin. 67 , 93–99 (2017).
Tao, L. et al. Breast cancer mortality in older and younger breast cancer patients in California. Cancer Epidemiol. Biomark. Prev. 28 , 303–310 (2018).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26 , 259–271 (2015).
Green, A. R. et al. Nottingham Prognostic Index Plus: validation of a clinical decision making tool in breast cancer in an independent series. J. Pathol. Clin. Res. 2 , 32–40 (2016).
Candido dos Reis, F. J. et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 19 , 58 (2017).
Phung, M. T., Tin Tin, S. & Elwood, J. M. Prognostic models for breast cancer: a systematic review. BMC Cancer 19 , 230 (2019).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26 (Suppl. 5), v8-v30 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 , 164–172 (2014).
Cardoso, F. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375 , 717–729 (2016).
Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373 , 2005–2014 (2015).
Sparano, J. A. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 379 , 111–121 (2018).
Harris, L. N. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34 , 1134–1150 (2016).
Krop, I. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 35 , 2838–2847 (2017).
Nitz, U. et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J. Clin. Oncol. 37 , 799–808 (2019).
Sestak, I. Risk stratification in early breast cancer in premenopausal and postmenopausal women: integrating genomic assays with clinicopathological features. Curr. Opin. Oncol. 1 , 29–34 (2018).
McLaughlin, S. A. Surgical management of the breast: breast conservation therapy and mastectomy. Surg. Clin. North Am. 93 , 411–428 (2013).
Margenthaler, J. A. & Ollila, D. W. Breast conservation therapy versus mastectomy: shared decision-making strategies and overcoming decisional conflicts in your patients. Ann. Surg. Oncol. 23 , 3133–3137 (2016).
Buchholz, T. A., Mittendorf, E. A. & Hunt, K. K. Surgical considerations after neoadjuvant chemotherapy: breast conservation therapy. J. Natl Cancer Inst. Monogr. 2015 , 11–14 (2015).
Houssami, N., Macaskill, P., Luke Marinovich, M. & Morrow, M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann. Surg. Oncol. 21 , 717–730 (2014).
Morrow, M., Harris, J. R. & Schnitt, S. J. Surgical margins in lumpectomy for breast cancer — bigger is not better. N. Engl. J. Med. 367 , 79–82 (2012). This commentary and the meta-analysis by Houssami et al. (2014) settled the decade-long discussions about surgical resection margins and are, therefore, landmark contributions.
Tan, M. P., Sitoh, N. Y. & Sim, A. S. The value of intraoperative frozen section analysis for margin status in breast conservation surgery in a nontertiary institution. Int. J. Breast Cancer https://doi.org/10.1155/2014/715404 (2014).
Boughey, J. C. et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery 156 , 190–197 (2014).
Haloua, M. H. et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann. Surg. 257 , 609–620 (2013).
Benelli, L. A new periareolar mammaplasty: the ‘round block’ technique. Aesthetic Plast. Surg. 14 , 93–100 (1990).
Clough, K. B., Kaufman, G. J., Nos, C., Buccimazza, I. & Sarfati, I. M. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann. Surg. Oncol. 17 , 1375–1391 (2010).
Yao, K., Winchester, D. J., Czechura, T. & Huo, D. Contralateral prophylactic mastectomy and survival: report from the national cancer data base, 1998–2002. Breast Cancer Res. Treat. 142 , 465–476 (2013).
Vila, J., Gandini, S. & Gentilini, O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast 24 , 175–181 (2015).
Lucci, A. et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J. Clin. Oncol. 25 , 3657–3663 (2007).
Krag, D. N. et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 11 , 927–933 (2010). This large clinical trial confirms that there is no overall survival difference between sentinel lymph node biopsy and axillary lymph node dissection.
Veronesi, U. et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Engl. J. Med. 349 , 546–553 (2003).
Giuliano, A. E. et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann. Surg. 264 , 413–420 (2016).
Balic, M., Thomssen, C., Würstlein, R., Gnant, M. & Harbeck, N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care 14 , 1–8 (2019).
Kaidar-Person, O., Meattini, I. & Poortmans, P. M. P. Between uncertainties and overtreatment. Int. J. Radiat. Oncol. 104 , 15–16 (2019).
Kuehn, T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14 , 609–618 (2013).
King, T. A. & Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 12 , 335–343 (2015).
Giuliano, A. E. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305 , 569–575 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378 , 1707–1716 (2011). This meta-analysis underlines that the contribution of radiation therapy should always be the standard approach for breast-conserving therapy .
Article CAS Google Scholar
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383 , 2127–2135 (2014). This meta-analysis helps us to better identify those patients who would benefit most from radiation therapy after mastectomy .
Jatoi, I., Benson, J. R. & Kunkler, I. Hypothesis: can the abscopal effect explain the impact of adjuvant radiotherapy on breast cancer mortality? NPJ Breast Cancer 4 , 8 (2018).
Bartelink, H. et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 16 , 47–56 (2015).
Poortmans, P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet 383 , 2104–2106 (2014).
Poortmans, P. M. et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N. Engl. J. Med. 373 , 317–327 (2015).
Whelan, T. J. et al. Regional nodal irradiation in early-stage breast cancer. N. Engl. J. Med. 373 , 307–316 (2015).
Thorsen, L. B. J. et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J. Clin. Oncol. 34 , 314–320 (2016).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 29 , 2153–2153 (2018).
Oliai, C. & Hurvitz, S. A. The debate over post-mastectomy radiotherapy should continue: breast cancer. Nat. Rev. Clin. Oncol. 12 , 567–568 (2015).
Recht, A. et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Ann. Surg. Oncol. 24 , 38–51 (2017).
Dodwell, D. et al. Abstract GS4-02: regional lymph node irradiation in early stage breast cancer: an EBCTCG meta-analysis of 13,000 women in 14 trials. in General Session Abstracts GS4-02-GS4-02 https://doi.org/10.1158/1538-7445.SABCS18-GS4-02 (American Association for Cancer Research, 2019).
Kunkler, I. H., Canney, P., van Tienhoven, G. & Russell, N. S. MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group. Elucidating the role of chest wall irradiation in ‘intermediate-risk’. breast cancer: The MRC/EORTC SUPREMO trial. Clin. Oncol. R. Coll. Radiol. 20 , 31–34 (2008).
CAS PubMed Google Scholar
Poortmans, P., Aznar, M. & Bartelink, H. Quality indicators for breast cancer: revisiting historical evidence in the context of technology changes. Semin. Radiat. Oncol. 22 , 29–39 (2012).
Osman, S. O. S., Hol, S., Poortmans, P. M. & Essers, M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother. Oncol. 112 , 17–22 (2014).
Essers, M., Poortmans, P. M., Verschueren, K., Hol, S. & Cobben, D. C. P. Should breathing adapted radiotherapy also be applied for right-sided breast irradiation? Acta Oncol. 55 , 460–465 (2016).
Poortmans, P. M. P., Arenas, M. & Livi, L. Over-irradiation. Breast 31 , 295–302 (2017).
Blamey, R. W. et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur. J. Cancer 49 , 2294–2302 (2013).
McGuire, S. E. et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 68 , 1004–1009 (2007).
Mamounas, E. P. et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J. Clin. Oncol. 30 , 3960–3966 (2012).
Krug, D. et al. Individualization of post-mastectomy radiotherapy and regional nodal irradiation based on treatment response after neoadjuvant chemotherapy for breast cancer: a systematic review. Strahlenther. Onkol. 194 , 607–618 (2018).
Amoroso, V. et al. International Expert Consensus on Primary Systemic Therapy in the Management of Early Breast Cancer: Highlights of the Fifth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy (2013). J. Natl Cancer Inst. Monogr. 2015 , 90–96 (2015).
Offersen, B. V. et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother. Oncol. 118 , 205–208 (2016).
Haviland, J. S. et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 14 , 1086–1094 (2013).
Whelan, T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362 , 513–520 (2010).
Wang, S.-L. et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 20 , 352–360 (2019).
Brouwers, P. J. A. M. et al. Predictors for poor cosmetic outcome in patients with early stage breast cancer treated with breast conserving therapy: results of the Young Boost trial. Radiother. Oncol. 128 , 434–441 (2018).
Polgár, C. et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the groupe européen de curiethérapie-european society for therapeutic radiology and oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 94 , 264–273 (2010).
Correa, C. et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO Evidence-Based. Consensus Statement. Pract. Radiat. Oncol. 7 , 73–79 (2017).
Miranda, F. A. et al. Accelerated partial breast irradiation: current status with a focus on clinical practice. Breast J. https://doi.org/10.1111/tbj.13164 (2018).
Marta, G. N. et al. Effectiveness of different accelerated partial breast irradiation techniques for the treatment of breast cancer patients: systematic review using indirect comparisons of randomized clinical trials. Rep. Pract. Oncol. Radiother. 24 , 165–174 (2019).
Veronesi, U. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 14 , 1269–1277 (2013).
Vaidya, J. S. et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383 , 603–613 (2014).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378 , 771–784 (2011).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet 379 , 432–444 (2012). This meta-analysis demonstrates the benefits of adjuvant chemotherapy in early breast cancer .
Rastogi, P. et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J. Clin. Oncol. 26 , 778–785 (2008).
Francis, P. A. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379 , 122–137 (2018).
Gnant, M. et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 26 , 313–320 (2015).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386 , 1341–1352 (2015). This meta-analysis demonstrates the benefit of the two individual options for adjuvant endocrine therapy in postmenopausal patients with early breast cancer .
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377 , 1836–1846 (2017).
Gray, R. et al. Increasing the dose density of adjuvant chemotherapy by shortening intervals between courses or by sequential drug administration significantly reduces both disease recurrence and breast cancer mortality: an EBCTCG meta-analysis of 21,000 women in 16 randomised trials [abstract]. SABCS GS1-GS01 (2018).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375 , 1925–1936 (2016).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375 , 1738–1748 (2016).
Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 35 , 3638–3646 (2017).
Mackey, J. R. et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann. Oncol. 27 , 1041–1047 (2016).
Del Mastro, L. et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2×2 factorial, randomised phase 3 trial. Lancet 385 , 1863–1872 (2015).
Article PubMed CAS Google Scholar
Blum, J. L. et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 35 , 2647–2655 (2017).
Gray, R. et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393 , 1440–1452 (2019).
Gianni, L. et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17 , 791–800 (2016).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380 , 617–628 (2018).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377 , 122–131 (2017).
Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1688–1700 (2017).
Tolaney, S. M. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 372 , 134–141 (2015).
Tolaney, S. M. et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J. Clin. Oncol. 35 , 511–511 (2017).
Earl, H. M. et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393 , 2599–2612 (2019).
Pivot, X. et al. Either 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 14 , 741–748 (2013).
Joensuu, H. et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 4 , 1199 (2018).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353 , 1659–1672 (2005).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382 , 1021–1028 (2013).
Hahnen, E. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 3 , 1378–1385 (2017).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33 , 13–21 (2015).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376 , 2147–2159 (2017).
Gnant, M. et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386 , 433–443 (2015).
Gnant, M. et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20 , 339–351 (2019).
Coleman, R. E. et al. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study [abstract]. J. Clin. Oncol. 36 (Suppl.), a501 (2018).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386 , 1353–1361 (2015).
Coleman, R. E. et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J. Bone Oncol. 13 , 123–135 (2018).
Cardoso, F. et al. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann. Oncol. 29 , 1634–1657 (2018).
Golse, N. & Adam, R. Liver metastases from breast cancer: what role for surgery? Indications and results. Clin. Breast Cancer 17 , 256–265 (2017).
Xie, Y. et al. Surgery of the primary tumor improves survival in women with stage IV breast cancer in southwest China: a retrospective analysis. Medicine 96 , e7048 (2017).
Shien, T. & Doihara, H. Resection of the primary tumor in stage IV breast cancer. World J. Clin. Oncol. 5 , 82–85 (2014).
Badwe, R. et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 16 , 1380–1388 (2015).
Soran, A., Ozbas, S., Kelsey, S. F. & Gulluoglu, B. M. Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (Protocol MF07-01): a study of Turkish Federation of the National Societies for Breast Diseases. Breast J. 15 , 399–403 (2009).
Fitzal, F. et al. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE Trial. Ann. Surg . https://doi.org/10.1097/SLA.0000000000002771 (2018).
Barinoff, J. et al. Primary metastatic breast cancer in the era of targeted therapy — prognostic impact and the role of breast tumour surgery. Eur. J. Cancer 83 , 116–124 (2017).
Shien, T. et al. A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (JCOG1017 PRIM-BC). J. Clin. Oncol. 35 , TPS588–TPS588 (2017).
Cameron, D. Removing the primary tumour in metastatic breast cancer. Lancet Oncol. 16 , 1284–1285 (2015).
Dare, A. J. et al. Surgical Services for Cancer Care. in Cancer: Disease Control Priorities , Third Edition (Volume 3) (eds. Gelband, H., Jha, P., Sankaranarayanan, R. & Horton, S.) (The International Bank for Reconstruction and Development/The World Bank, 2015).
Phillips, C., Jeffree, R. & Khasraw, M. Management of breast cancer brain metastases: a practical review. Breast 31 , 90–98 (2017).
Thavarajah, N. et al. Continued success in providing timely palliative radiation therapy at the rapid response radiotherapy program: a review of 2008–2012. Curr. Oncol. 20 , e206–e211 (2013).
Chow, E. et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 15 , 164–171 (2014).
Sologuren, I., Rodríguez-Gallego, C. & Lara, P. C. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 3 , 18-31–31 (2014).
Morgan, S. C. & Parker, C. C. Local treatment of metastatic cancer — killing the seed or disturbing the soil? Nat. Rev. Clin. Oncol. 8 , 504–506 (2011).
Morgan, S., Caudrelier, J.-M. & Clemons, M. Radiotherapy to the primary tumor is associated with improved survival in stage IV breast cancer [abstract]. SABCS P4 , 16–06 (2012).
Bernier, J. Immuno-oncology: allying forces of radio- and immuno-therapy to enhance cancer cell killing. Crit. Rev. Oncol. Hematol. 108 , 97–108 (2016).
Fietz, T. et al. Palliative systemic therapy and overall survival of 1,395 patients with advanced breast cancer — rResults from the prospective German TMK cohort study. Breast. 34 , 122–130 (2017).
Rugo, H. S. et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J. Clin. Oncol. 34 , 3069–3103 (2016).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379 , 1926–1936 (2018).
Miles, D. W. et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 24 , 2773–2780 (2013).
Giordano, S. H. et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 2078–2099 (2014).
Partridge, A. H. et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 3307–3329 (2014).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379 , 2108–2121 (2018).
Marinovich, M. L. et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 21 , 669–677 (2012).
Avril, S. et al. 18 F-FDG PET/CT for monitoring of treatment response in breast cancer. J. Nucl. Med. 57 , 34S–39SS (2016).
Marinovich, M. L. et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl Cancer Inst. 105 , 321–333 (2013).
Marinovich, M. L. et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis. BMC Cancer 15 , 662 (2015).
Humbert, O. et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 20 , 94–104 (2015).
Pennant, M. et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol. Assess. 14 , 1–103 (2010).
Shachar, S. S. Assessing treatment response in metastatic breast cancer. Am. J. Hematol. Oncol . 12 , (2016).
Lee, C. I. et al. Comparative effectiveness of imaging modalities to determine metastatic breast cancer treatment response. Breast 24 , 3–11 (2015).
Pagani, O. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 371 , 107–118 (2014).
Francis, P., Regan, M. & Fleming, G. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372 , 1672–1673 (2015).
Mao, J. J. et al. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J. Clin. Oncol. 33 , 3615–3620 (2015).
Elkins, G. et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J. Clin. Oncol. 26 , 5022–5026 (2008).
Loprinzi, C. L. et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356 , 2059–2063 (2000).
Niravath, P. Aromatase inhibitor-induced arthralgia: a review. Ann. Oncol. 24 , 1443–1449 (2013).
Barton, D. L. et al. Impact of vaginal dehydroepiandosterone (DHEA) on vaginal symptoms in female cancer survivors: Trial N10C1 (Alliance). J. Clin. Oncol. 32 , 9507–9507 (2014).
Razvi, Y. et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 27 , 87–95 (2019).
Gulati, G. et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 37 , 1671–1680 (2016).
Smith, E. M. L. et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309 , 1359–1367 (2013).
Hershman, D. L. et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 32 , 1941–1967 (2014).
Hanai, A. et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: prospective self-controlled trial. J. Natl Cancer Inst. 110 , 141–148 (2018).
Kadakia, K. C., Rozell, S. A., Butala, A. A. & Loprinzi, C. L. Supportive cryotherapy: a review from head to toe. J. Pain Symptom Manage. 47 , 1100–1115 (2014).
Hou, S., Huh, B., Kim, H. K., Kim, K.-H. & Abdi, S. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician 21 , 571–592 (2018).
Ahmed, R. L., Schmitz, K. H., Prizment, A. E. & Folsom, A. R. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res. Treat. 130 , 981–991 (2011).
Gillespie, T. C., Sayegh, H. E., Brunelle, C. L., Daniell, K. M. & Taghian, A. G. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland. Surg. 7 , 379–403 (2018).
Runowicz, C. D. et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J. Clin. Oncol. 34 , 611–635 (2016).
Velikova, G. et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol. 19 , 1516–1529 (2018).
Hofmann, D. et al. WSG ADAPT — adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 14 , 261 (2013).
Robertson, J. F. R., Dowsett, M. & Bliss, J. M. Peri-operative aromatase inhibitor treatment in determining or predicting long-term outcome in early breast cancer — the POETIC Trial (CRUK/07/015) [abstract]. SABCS GS1-03 (2017).
Ellis, M. J. et al. Ki67 Proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 trial (Alliance). J. Clin. Oncol. 35 , 1061–1069 (2017).
Hölzel, D. et al. Improved systemic treatment for early breast cancer improves cure rates, modifies metastatic pattern and shortens post-metastatic survival: 35-year results from the munich cancer registry. J. Cancer Res. Clin. Oncol. 143 , 1701–1712 (2017).
Hölzel, D. et al. Survival of de novo stage IV breast cancer patients over three decades. J. Cancer Res. Clin. Oncol. 143 , 509–519 (2017).
Angus, L. et al. The genomic landscape of 501 metastatic breast cancer patients [abstract]. SABCS GS1-07 (2018).
Desmedt, C. et al. Unraveling lobular breast cancer progression and endocrine resistance mechanisms through genomic and immune characterization of matched primary and metastatic samples [abstract]. SABCS GS1–06 (2018).
Baselga, J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 904–916 (2017).
André, F. et al. Alpelisib for PIK3CA -mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380 , 1929–1940 (2019).
Baselga, J. et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. J. Clin. Oncol. 36 , LBA1006–LBA1006 (2018).
Kim, S.-B. et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18 , 1360–1372 (2017).
Schmid, P. et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 36 (15 Suppl.), 1007 (2018).
Jones, R. H. et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): a randomized, double-blind, placebo-controlled, phase II trial [abstract]. J. Clin. Oncol. 37 (no. 15_suppl), 1005–1005 (2019).
Yardley, D. A. et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 31 , 2128–2135 (2013).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA Topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22 , 5097–5108 (2016).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20 , 816–826 (2019).
Burris III, H. A., Giaccone, G. & Im, S. A. Updated findings of a first-in-human phase 1 study of margetuximab, an Fc-optimized chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors [abstract]. Am. Soc. Clin. Oncol. Meet. 33 (no. 15_suppl), A523 (2015).
Rugo, H. S. et al. SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstr 1000 (2019).
Hyman, D. M., Piha-Paul, S. & Rodon, J. Neratinib in HER2- or HER3-mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 ‘basket’ study [abstract]. Am. Assoc. Cancer Res. Meet . CT001 (2017).
Saura, C. et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial [abstract]. J. Clin. Oncol. 37 (Suppl.), Abstract 1002 (2019).
Gucalp, A. et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 19 , 5505–5512 (2013).
Cortes, J., Crown, J. & Awada, A. Overall survival (OS) from the phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) signaling inhibitor, in AR+ advanced triple-negative breast cancer (aTNBC) [abstract]. Eur. Cancer Congr. 51 (Suppl. 3), 1802 (2015).
Gelmon, K. A. et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12 , 852–861 (2011).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 34 , 2460–2467 (2016).
Schmid, P., Cruz, C. & Braiteh, F. S. Atezolizumab in metastatic triple-negative breast cancer: long-term clinical outcomes and biomarker analyses [abstract]. Am. Assoc. Cancer Res. 77 , A2986 (2017).
André, F. et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): results of the phase 3 SOLAR-1 trial [abstract]. ESMO LBA3 PR (2018).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 , 189–194 (2018).
Hartley, R. L., Stone, J. P. & Temple-Oberle, C. Breast cancer in transgender patients: a systematic review. Part 1: male to female. Eur. J. Surg. Oncol. 44 , 1455–1462 (2018).
Cardoso, F. et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 29 , 405–417 (2017).
PubMed Central Google Scholar
Di Oto, E. et al. X chromosome gain is related to increased androgen receptor expression in male breast cancer. Virchows Arch. 473 , 155–163 (2018).
Severson, T. M. & Zwart, W. A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr. Relat. Cancer 24 , R27–R34 (2017).
Deb, S. et al. PIK3CA mutations are frequently observed in BRCAX but not BRCA2-associated male breast cancer. Breast Cancer Res. 15 , R69 (2013).
Gucalp, A. et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res. Treat. 173 , 37–48 (2019).
Korde, L. A. et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J. Clin. Oncol. 28 , 2114–2122 (2010).
Cardoso, F. et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30 , 1194–1220 (2019).
Bareche, Y. et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 29 , 895–902 (2018).
Lehmann, B. D. & Pietenpol, J. A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 24 , S36–S40 (2015).
Lehmann, B. D. et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLOS ONE 11 , e0157368 (2016).
Burstein, M. D. et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 21 , 1688–1698 (2015).
Siu, A. L. & on behalf of the U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 164 , 279 (2016).
Klarenbach, S. et al. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Can. Med. Assoc. J. 190 , E1441–E1451 (2018).
Oeffinger, K. C. et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314 , 1599 (2015).
European Commission Initiative on Breast Cancer. Recommendations from European Breast Guidelines Europa.eu https://ecibc.jrc.ec.europa.eu/recommendations/list/Professional (2019).
Dawood, S. et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann. Oncol. 22 , 515–523 (2011).
Cserni, G., Charafe-Jauffret, E. & van Diest, P. J. Inflammatory breast cancer: the pathologists’ perspective. Eur. J. Surg. Oncol. 44 , 1128–1134 (2018).
Cheang, M. C. U. et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 20 , 474–482 (2015).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012). This research establishes the contemporary method of classifying breast cancer into clinically relevant molecular subtypes.
Hoadley, K. A., Andre, F., Ellis, M. J. & Perou, C. M. Breast cancer intrinsic subtypes (Poster). Nat. Rev. Clin. Oncol . https://www.nature.com/documents/nrclinonc_posters_breastcancer.pdf (2014).
Desmedt, C. et al. Genomic characterization of primary invasive lobular breast cancer. J. Clin. Oncol. 34 , 1872–1881 (2016).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163 , 506–519 (2015).
Vasudev, P. & Onuma, K. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch. Pathol. Lab. Med. 135 , 1606–1610 (2011).
Martelotto, L. G. et al. Genomic landscape of adenoid cystic carcinoma of the breast. J. Pathol. 237 , 179–189 (2015).
Goss, P. E. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med. 375 , 209–219 (2016).
Liang, M. et al. Association between CHEK2*1100delC and breast cancer: a systematic review and meta-analysis. Mol. Diagn. Ther. 22 , 397–407 (2018).
Wang, X. et al. Breast cancer risk and germline genomic profiling of women with neurofibromatosis type 1 who developed breast cancer. Genes. Chromosomes Cancer 57 , 19–27 (2018).
McCart Reed, A. E. et al. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications: prognostic features of metaplastic breast cancer. J. Pathol. 247 , 214–227 (2019).
Wendt, C. & Margolin, S. Identifying breast cancer susceptibility genes — a review of the genetic background in familial breast cancer. Acta Oncol. 58 , 135–146 (2019).
Couch, F. J. et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 3 , 1190 (2017).
Nguyen, J. et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J. Comp. Eff. Res. 4 , 157–166 (2015).
McLachlan, S. A., Devins, G. M. & Goodwin, P. J. Factor analysis of the psychosocial items of the EORTC QLQ-C30 in metastatic breast cancer patients participating in a psychosocial intervention study. Qual. Life Res. 8 , 311–317 (1999).
Bjelic-Radisic, V. et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients (EORTC QLQ-BC23) — EORTC QLQ-BR45. Ann. Oncol. 29 , viii58–viii86 (2018).
Ganz, P. A., Kwan, L., Stanton, A. L., Bower, J. E. & Belin, T. R. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J. Clin. Oncol. 29 , 1101–1109 (2011).
Revicki, D. A. et al. Predicting EuroQol (EQ-5D) scores from the patient-reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Qual. Life Res. 18 , 783–791 (2009).
Hays, R. D., Bjorner, J. B., Revicki, D. A., Spritzer, K. L. & Cella, D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 18 , 873–880 (2009).
Bevans, M., Ross, A. & Cella, D. Patient-reported outcomes measurement information system (PROMIS): efficient, standardized tools to measure self-reported health and quality of life. Nurs. Outlook 62 , 339–345 (2014).
Download references
Acknowledgements
The authors thank N. Radosevic-Robin (Jean Perrin Comprehensive Cancer Centre, France) for her assistance in preparing Fig. 1. N. Houssami receives research support through a National Breast Cancer Foundation (NBCF, Australia) Breast Cancer Research Leadership Fellowship. K.R. acknowledges research funding from the Clinical and Translational Sciences Award (CTSA) grant number KL2 TR002379 from the National Centre for Advancing Translational Sciences, a component of the US National Institutes of Health.
Author information
Authors and affiliations.
LMU Munich, University Hospital, Department of Obstetrics and Gynecology, Breast Center and Comprehensive Cancer Center (CCLMU), Munich, Germany
Nadia Harbeck
Department of Pathology and Biopathology, Jean Perrin Comprehensive Cancer Centre, UMR INSERM 1240, University Clermont Auvergne, Clermont-Ferrand, France
Frédérique Penault-Llorca
IOB Institute of Oncology, Quironsalud Group, Madrid and Barcelona, Spain
Javier Cortes
Vall d´Hebron Institute of Oncology, Barcelona, Spain
Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
Michael Gnant
Sydney School of Public Health, Faculty of Medicine and Health, University of Sydney, Sydney, Australia
Nehmat Houssami
Department of Radiation Oncology, Institut Curie, Paris, France
Philip Poortmans
Université PSL, Paris, France
Department of Oncology, Mayo Clinic, Rochester, MN, USA
Kathryn Ruddy
Hong Kong Breast Oncology Group, The University of Hong Kong, Hong Kong, China
Janice Tsang
Breast Unit, Champalimaud Clinical Center/Champalimaud Foundation, Lisbon, Portugal
Fatima Cardoso
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (all authors); Epidemiology (J.T.); Mechanisms/pathophysiology (F.P.-L.); Diagnosis, screening and prevention (N. Houssami); Management (N. Harbeck, F.C., M.G., P.P., J.C. and N. Houssami); Quality of life (K.R.); Outlook (all authors); Overview of the Primer (N. Harbeck and F.C.).
Corresponding author
Correspondence to Nadia Harbeck .
Ethics declarations
Competing interests.
N. Harbeck reports honoraria for lectures and/or consulting from Agendia, Amgen, Astra Zeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal and Seattle Genetics. F.P.-L. declares personal financial interests in Abbvie, Agendia, Astrazeneca, BMS, Genomic Health, Janssen, Lilly, Merck Lifa, MSD, Myriad, Nanostring, Novartis, Pfizer and Roche; institutional financial interests in Astrazeneca, BMS, Genomic Health, MSD, Myriad, Nanostring and Roche; and congress invitations from Abbvie, Astrazeneca, BMS, MSD and Roche. J.C. has received honoraria from Celgene, Chugai, Eisai, Novartis, Pfizer, Roche and Samsung; has served as a consultant for Astrazeneca, Biothera, Celgene, Daichii Sankyo, Erytech Pharma, Merus, Polyphor, Roche and Seattle Genetics; has received research funding from Ariad, Astrazeneca, Baxalta GMBH, Bayer, Eisai, Guardant Health, Merch Sharp & Dohme, Pfizer, Puma and Roche; and has stocks in MedSIR. M.G. reports honoraria from Amgen, AstraZeneca, Celgene, Eli Lilly, Medison, Nanostring Technologies, Novartis and Roche; advisory fees from Accelsoir; research funding from AstraZeneca, Novartis, Pfizer and Roche; and travel expenses from Amgen, AstraZeneca, Celgene, Eli Lilly, Ipsen, Medison, Novartis and Pfizer. K.R. declares previous ownership of Merck and Pfizer stock (October 2016–February 2018). J.T. reports honoraria and consultancy or advisory roles for AstraZeneca, Astellas, De Novo, Eisai, Foundation Medicine, Nanostring, Novartis, Pfizer and Roche. F.C. declares consultancy roles for Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Genentech, GE Oncology, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Seattle Genetics and Teva. The remaining authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks T. Howell, P. Neven, M. Toi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ABC Global Alliance: https://www.abcglobalalliance.org
Adjuvant! Online: www.adjuvantonline.com
European Organization for Research and Treatment of Cancer: https://qol.eortc.org/modules/
EuroQol 5-Dimensions: https://euroqol.org/
Functional Assessment of Cancer Therapy: http://www.facit.org/FACITOrg
Patient-Reported Outcomes Measurement Information System: http://www.healthmeasures.net/explore-measurement-systems/promis
Short Form Health Survey-36: http://www.rand.org/health/surveys_tools/mos/36-item-short-form.html
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Harbeck, N., Penault-Llorca, F., Cortes, J. et al. Breast cancer. Nat Rev Dis Primers 5 , 66 (2019). https://doi.org/10.1038/s41572-019-0111-2
Download citation
Accepted : 22 July 2019
Published : 23 September 2019
DOI : https://doi.org/10.1038/s41572-019-0111-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Usp22 supports the aggressive behavior of basal-like breast cancer by stimulating cellular respiration.
- Evangelos Prokakis
- Husam Bamahmoud
- Florian Wegwitz
Cell Communication and Signaling (2024)
Targeting cholesterol impairs cell invasion of all breast cancer types
- Mauriane Maja
- Marie Verfaillie
- Donatienne Tyteca
Cancer Cell International (2024)
Identification of a Notch transcriptomic signature for breast cancer
- Eike-Benjamin Braune
- Felix Geist
- Urban Lendahl
Breast Cancer Research (2024)
Synergistic anticancer effect of Pistacia lentiscus essential oils and 5-Fluorouracil co-loaded onto biodegradable nanofibers against melanoma and breast cancer
- Obaydah Abd Alkader Alabrahim
- Hassan Mohamed El-Said Azzazy
Discover Nano (2024)
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
- Sahar Safaei
- Manouchehr Fadaee
- Tohid Kazemi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Advances in Breast Cancer Research
A polyploid giant cancer cell (PGCC) from triple-negative breast cancer.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat breast cancer. They are also looking at how to address disparities and improve quality of life for survivors of the disease.
This page highlights some of what's new in the latest research for breast cancer, including new clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Breast Cancer
Breast cancer is one of a few cancers for which an effective screening test, mammography , is available. MRI ( magnetic resonance imaging ) and ultrasound are also used to detect breast cancer, but not as routine screening tools for people with average risk.
Ongoing studies are looking at ways to enhance current breast cancer screening options. Technological advances in imaging are creating new opportunities for improvements in both screening and early detection.
One technology advance is 3-D mammography , also called breast tomosynthesis . This procedure takes images from different angles around the breast and builds them into a 3-D-like image. Although this technology is increasingly available in the clinic, it isn’t known whether it is better than standard 2-D mammography , for detecting cancer at a less advanced stage.
NCI is funding a large-scale randomized breast screening trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) , to compare the number of advanced cancers detected in women screened for 5 years with 3-D mammography with the number detected in women screened with 2-D mammography.
Two concerns in breast cancer screening, as in all cancer screening, are:
- the potential for diagnosing tumors that would not have become life-threatening ( overdiagnosis )
- the possibility of receiving false-positive test results, and the anxiety that comes with follow-up tests or procedures
As cancer treatment is becoming more individualized, researchers are looking at ways to personalize breast cancer screening. They are studying screening methods that are appropriate for each woman’s level of risk and limit the possibility of overdiagnosis.
For example, the Women Informed to Screen Depending on Measures of Risk (WISDOM) study aims to determine if risk-based screening—that is, screening at intervals that are based on each woman’s risk as determined by her genetic makeup, family history , and other risk factors—is as safe, effective, and accepted as standard annual screening mammography.
WISDOM is also making a focused effort to enroll Black women in the trial. Past studies tended to contain a majority of White women and therefore, there is less data on how screening can benefit Black women. Researchers are taking a number of steps to include as many Black women as possible in the study while also increasing the diversity of all women enrolled.
Breast Cancer Treatment
The mainstays of breast cancer treatment are surgery , radiation , chemotherapy , hormone therapy , and targeted therapy . But scientists continue to study novel treatments and drugs, along with new combinations of existing treatments.
It is now known that breast cancer can be divided into subtypes based on whether they:
- are hormone receptor (HR) positive which means they express estrogen and/or progesterone receptors ( ER , PR )
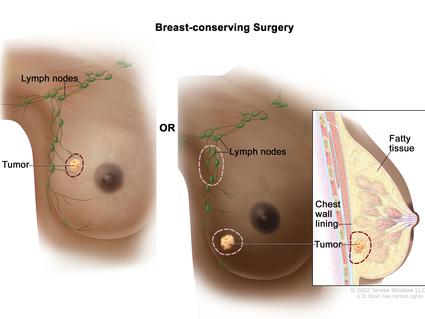
Shortening Radiation Therapy for Some with Early Breast Cancer
A condensed course was as effective and safe as the standard course for women with higher-risk early-stage breast cancer who had a lumpectomy.
As we learn more about the subtypes of breast cancer and their behavior, we can use this information to guide treatment decisions. For example:
- The NCI-sponsored TAILORx clinical trial. The study, which included patients with ER-positive, lymph node-negative breast cancer, found that a test that looks at the expression of certain genes can predict which women can safely avoid chemotherapy.
- The RxPONDER trial found that the same gene expression test can also be used to determine treatment options in women with more advanced breast cancer. The study found that some postmenopausal women with HR positive, HER-2 negative breast cancer that has spread to several lymph nodes and has a low risk of recurrence do not benefit from chemotherapy when added to their hormone therapy.
- The OFSET trial is comparing the addition of chemotherapy to usual treatment ( ovarian function suppression plus hormone therapy) to usual treatment alone in treating premenopausal estrogen receptor (ER)-positive/HER2-negative breast cancer patients who are at high risk of their cancer returning. This will help determine whether or not adding chemotherapy helps prevent the cancer from returning.
Genomic analyses, such as those carried out through The Cancer Genome Atlas (TCGA) , have provided more insights into the molecular diversity of breast cancer and eventually could help identify even more breast cancer subtypes. That knowledge, in turn, may lead to the development of therapies that target the genetic alterations that drive those cancer subtypes.
HR-Positive Breast Cancer Treatment
Hormone therapies have been a mainstay of treatment for HR-positive cancer. However, there is a new focus on adding targeted therapies to hormone therapy for advanced or metastatic HR-positive cancers. These treatments could prolong the time until chemotherapy is needed and ideally, extend survival. Approved drugs include:

Drug Combo Effective for Metastatic Breast Cancer in Younger Women
Ribociclib plus hormone therapy were superior to standard chemotherapy combos in a recent trial.
- Palbociclib (Ibrance) , ribociclib (Kisqali) , and everolimus (Afinitor) have all been approved by the FDA for use with hormone therapy for treatment of advanced or metastatic breast cancer. Ribociclib has been shown to increase the survival of patients with metastatic breast cancer . It has also shown to slow the growth of metastatic cancer in younger women when combined with hormone therapy.
- Elacestrant (Orserdu) is approved for HR-positive and HER2-negative breast cancer that has a mutation in the ESR1 gene, and has spread. It is used in postmenopausal women and in men whose cancer has gotten worse after at least one type of hormone therapy.
- Abemaciclib (Verzenio) can be used with or after hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancer. In October 2021, the Food and Drug Administration ( FDA ) approved abemaciclib in combination with hormone therapy to treat some people who have had surgery for early-stage HR-positive, HER2-negative breast cancer.
- Alpelisib (Piqray) is approved to be used in combination with hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancers that have a mutation in the PIK3CA gene .
- Sacituzumab govitecan-hziy (Trodelvy) is used for HR-positive and HER2-negative breast cancer that has spread or can't be removed with surgery. It is used in those who have received hormone therapy and at least two previous treatments. It has shown to extend the amount of time that the disease doesn't get worse ( progression-free survival ) and also shown to improve overall survival .
HER2-Positive Breast Cancer Treatment
The FDA has approved a number of targeted therapies to treat HER2-positive breast cancer , including:
- Trastuzumab (Herceptin) has been approved to be used to prevent a relapse in patients with early-stage HER2-positive breast cancer.
- Pertuzumab (Perjeta) is used to treat metastatic HER2-positive breast cancer, and also both before surgery ( neoadjuvant ) and after surgery ( adjuvant therapy ).
- Trastuzumab and pertuzumab together can be used in combination with chemotherapy to prevent relapse in people with early-stage HER2-positive breast cancer. Both are also used together in metastatic disease, where they delay progression and improve overall survival.
- Trastuzumab deruxtecan (Enhertu) is approved for patients with advanced or metastatic HER2-positive breast cancer who have previously received a HER2-targeted treatment. A 2021 clinical trial showed that the drug lengthened the time that people with metastatic HER2-positive breast cancer lived without their cancer progressing. The trial also showed that it was better at shrinking tumors than another targeted drug, trastuzumab emtansine (Kadcyla).
- Tucatinib (Tukysa) is approved to be used in combination with trastuzumab and capecitabine (Xeloda) for HER2-positive breast cancer that cannot be removed with surgery or is metastatic. Tucatinib is able to cross the blood–brain barrier, which makes it especially useful for HER2-positive metastatic breast cancer, which tends to spread to the brain.
- Lapatinib (Tykerb) has been approved for treatment of some patients with HER2-positive advanced or metastatic breast cancer, together with capecitabine or letrozole.
- Neratinib Maleate (Nerlynx) can be used in patients with early-stage HER2-positive breast cancer and can also be used together with capecitabine (Xeloda) in some patients with advanced or metastatic disease.
- Ado-trastuzumab emtansine (Kadcyla) is approved to treat patients with metastatic HER2-positive breast cancer who have previously received trastuzumab and a taxane . It's also used in some patients with early-stage HER2-positive breast cancer who have completed therapy before surgery ( neoadjuvant ) and have residual disease at the time of surgery.
HER2-Low Breast Cancer
A newly defined subtype, HER2-low, accounts for more than half of all metastatic breast cancers. HER2-low tumors are defined as those whose cells contain lower levels of the HER2 protein on their surface. Such tumors have traditionally been classified as HER2-negative because they did not respond to drugs that target HER2.
However, in a clinical trial, trastuzumab deruxtecan (Enhertu) improved the survival of patients with HER2-low breast cancer compared with chemotherapy , and the drug is approved for use in such patients.

Immunotherapy Improves Survival in Triple-Negative Breast Cancer
For patients whose tumors had high PD-L1 levels, pembrolizumab with chemo helped them live longer.
Triple-Negative Breast Cancer Treatment
Triple-negative breast cancers (TNBC) are the hardest to treat because they lack both hormone receptors and HER2 overexpression , so they do not respond to therapies directed at these targets. Therefore, chemotherapy is the mainstay for treatment of TNBC. However, new treatments are starting to become available. These include:
- Sacituzumab govitecan-hziy (Trodelvy) is approved to treat patients with TNBC that has spread to other parts of the body . Patients must have received at least two prior therapies before receiving the drug.
- Pembrolizumab (Keytruda) is an immunotherapy drug that is approved to be used in combination with chemotherapy for patients with locally advanced or metastatic TNBC that has the PD-L1 protein. It may also be used before surgery (called neoadjuvant ) for patients with early-stage TNBC, regardless of their PD-L1 status.
- PARP inhibitors, which include olaparib (Lynparza) and talazoparib (Talzenna) , are approved to treat metastatic HER2-negative or triple-negative breast cancers in patients who have inherited a harmful BRCA gene mutation. Olaparib is also approved for use in certain patients with early-stage HER2-negative or triple-negative breast cancer.
- Drugs that block the androgen receptors or prevent androgen production are being tested in a subset of TNBC that express the androgen receptor.
For a complete list of drugs for breast cancer, see Drugs Approved for Breast Cancer .
NCI-Supported Breast Cancer Research Programs
Many NCI-funded researchers working at the NIH campus, as well as across the United States and world, are seeking ways to address breast cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some are more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in breast cancer.
TMIST is a randomized breast screening trial that compares two Food and Drug Administration (FDA)-approved types of digital mammography, standard digital mammography (2-D) with a newer technology called tomosynthesis mammography (3-D).
The Breast Specialized Programs of Research Excellence (Breast SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Breast SPOREs support the development of new therapies and technologies, and studies to better understand tumor resistance, diagnosis, prognosis, screening, prevention, and treatment of breast cancer.
The NCI Cancer Intervention and Surveillance Modeling Network (CISNET) focuses on using modeling to improve our understanding of how prevention, early detection, screening, and treatment affect breast cancer outcomes.
The Confluence Project , from NCI's Division of Cancer Epidemiology and Genetics (DCEG) , is developing a research resource that includes data from thousands of breast cancer patients and controls of different races and ethnicities. This resource will be used to identify genes that are associated with breast cancer risk, prognosis, subtypes, response to treatment, and second breast cancers. (DCEG conducts other breast cancer research as well.)
The Black Women’s Health Study (BWHS) Breast Cancer Risk Calculator allows health professionals to estimate a woman’s risk of developing invasive breast cancer over the next 5 years. With the NCI-funded effort, researchers developed a tool to estimate the risk of breast cancer in US Black women. The team that developed the tool hopes it will help guide more personalized decisions on when Black women—especially younger women—should begin breast cancer screening.
The goal of the Breast Cancer Surveillance Consortium (BCSC) , an NCI-funded program launched in 1994, is to enhance the understanding of breast cancer screening practices in the United States and their impact on the breast cancer's stage at diagnosis, survival rates, and mortality.
There are ongoing programs at NCI that support prevention and early detection research in different cancers, including breast cancer. Examples include:
- The Cancer Biomarkers Research Group , which promotes research in cancer biomarkers and manages the Early Detection Research Network (EDRN) . EDRN is a network of NCI-funded institutions that are collaborating to discover and validate early detection biomarkers. Within the EDRN, the Breast and Gynecologic Cancers Collaborative Group conducts research on breast and ovarian cancers.
- NCI's Division of Cancer Prevention houses the Breast and Gynecologic Cancer Research Group which conducts and fosters the development of research on the prevention and early detection of breast and gynecologic cancers.
Breast Cancer Survivorship Research
NCI’s Office of Cancer Survivorship, part of the Division of Cancer Control and Population Sciences (DCCPS), supports research projects throughout the country that study many issues related to breast cancer survivorship. Examples of studies funded include the impact of cancer and its treatment on physical functioning, emotional well-being, cognitive impairment , sleep disturbances, and cardiovascular health. Other studies focus on financial impacts, the effects on caregivers, models of care for survivors, and issues such as racial disparities and communication.
Breast Cancer Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention , screening , and treatment .
Breast Cancer Research Results
The following are some of our latest news articles on breast cancer research and study updates:
- Can Some People with Breast Cancer Safely Skip Lymph Node Radiation?
- Study Adds to Debate about Mammography in Older Women
- Pausing Long-Term Breast Cancer Therapy to Become Pregnant Appears to Be Safe
- A Safer, Better Treatment Option for Some Younger Women with Breast Cancer
- Shorter Course of Radiation Is Effective, Safe for Some with Early-Stage Breast Cancer
- Pembrolizumab Improves Survival in Advanced Triple-Negative Breast Cancer
View the full list of Breast Cancer Research Results and Study Updates .
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Breast Cancer Treatment : A Review
- 1 Dana-Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts
- JAMA Patient Page Breast Cancer Treatment Adrienne G. Waks, MD; Eric P. Winer, MD JAMA
- JAMA Insights Treatment of Nonmetastatic Breast Cancer Kathryn J. Ruddy, MD, MPH; Patricia A. Ganz, MD JAMA
Importance Breast cancer will be diagnosed in 12% of women in the United States over the course of their lifetimes and more than 250 000 new cases of breast cancer were diagnosed in the United States in 2017. This review focuses on current approaches and evolving strategies for local and systemic therapy of breast cancer.
Observations Breast cancer is categorized into 3 major subtypes based on the presence or absence of molecular markers for estrogen or progesterone receptors and human epidermal growth factor 2 ( ERBB2 ; formerly HER2 ): hormone receptor positive/ ERBB2 negative (70% of patients), ERBB2 positive (15%-20%), and triple-negative (tumors lacking all 3 standard molecular markers; 15%). More than 90% of breast cancers are not metastatic at the time of diagnosis. For people presenting without metastatic disease, therapeutic goals are tumor eradication and preventing recurrence. Triple-negative breast cancer is more likely to recur than the other 2 subtypes, with 85% 5-year breast cancer–specific survival for stage I triple-negative tumors vs 94% to 99% for hormone receptor positive and ERBB2 positive. Systemic therapy for nonmetastatic breast cancer is determined by subtype: patients with hormone receptor–positive tumors receive endocrine therapy, and a minority receive chemotherapy as well; patients with ERBB2 -positive tumors receive ERBB2 -targeted antibody or small-molecule inhibitor therapy combined with chemotherapy; and patients with triple-negative tumors receive chemotherapy alone. Local therapy for all patients with nonmetastatic breast cancer consists of surgical resection, with consideration of postoperative radiation if lumpectomy is performed. Increasingly, some systemic therapy is delivered before surgery. Tailoring postoperative treatment based on preoperative treatment response is under investigation. Metastatic breast cancer is treated according to subtype, with goals of prolonging life and palliating symptoms. Median overall survival for metastatic triple-negative breast cancer is approximately 1 year vs approximately 5 years for the other 2 subtypes.
Conclusions and Relevance Breast cancer consists of 3 major tumor subtypes categorized according to estrogen or progesterone receptor expression and ERBB2 gene amplification. The 3 subtypes have distinct risk profiles and treatment strategies. Optimal therapy for each patient depends on tumor subtype, anatomic cancer stage, and patient preferences.
Read More About
Waks AG , Winer EP. Breast Cancer Treatment : A Review . JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 09 April 2024
Long-term survival after neoadjuvant therapy for triple-negative breast cancer under different treatment regimens: a systematic review and network meta-analysis
- Zhilin Liu 1 na1 ,
- Jinming Li 1 na1 ,
- Fuxing Zhao 1 na1 ,
- Dengfeng Ren 1 ,
- Zitao Li 1 ,
- Yongzhi Chen 1 ,
- Shifen Huang 1 ,
- Zhen Liu 1 ,
- Yi Zhao 1 ,
- Miaozhou Wang 1 ,
- Huihui Li 2 ,
- ZhengBo Xu 3 ,
- Guoshuang Shen 1 &
- Jiuda Zhao 1
BMC Cancer volume 24 , Article number: 440 ( 2024 ) Cite this article
229 Accesses
Metrics details
Triple-negative breast cancer (TNBC) is a life-threatening subtype of breast cancer with limited treatment options. Therefore, this network meta-analysis (NMA) aimed to evaluate and compare the effect of various neoadjuvant chemotherapy (NCT) options on the long-term survival of patients with TNBC.
PubMed, Embase, Medline, Cochrane Library, Web of Science, and major international conference databases were systematically searched for randomized controlled trials (RCTs) on the efficacy of various NCT options in patients with TNBC. Searches were performed from January 2000 to June 2023. Study heterogeneity was assessed using the I 2 statistic. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used to evaluate disease-free survival (DFS) and overall survival (OS). Odds ratios (ORs) and 95% CIs were used to evaluate the pathologic complete response (pCR). The primary outcome was DFS.
We conducted an NMA of 21 RCTs involving 8873 patients with TNBC. Our study defined the combination of anthracyclines and taxanes as the preferred treatment option. On this basis, the addition of any of the following new drugs is considered a new treatment option: bevacizumab (B), platinum (P), poly-ADP-ribose polymerase inhibitors (PARPi), and immune checkpoint inhibitor (ICI). Based on the surface under the cumulative ranking curve (SUCRA) values, the top three SUCRA area values of DFS were taxanes, anthracycline, and cyclophosphamide (TAC; 89.23%); CT (84.53%); and B (81.06%). The top three SUCRA area values of OS were CT (83.70%), TAC (62.02%), and B-containing regimens (60.06%). The top three SUCRA area values of pCR were B + P-containing regimens (82.7%), ICI + P-containing regimens (80.2%), and ICI-containing regimens (61.8%).
Conclusions
This NMA showed that standard chemotherapy is a good choice with respect to long-term survival. Moreover, B associated with P-containing regimens is likely to be the optimal treatment option for neoadjuvant TNBC in terms of pCR.
Peer Review reports
Introduction
The latest global cancer burden data released by the World Health Organization International Agency for Research on Cancer in 2020 indicated that the number of new breast cancer cases reached 2.26 million worldwide, exceeding the total number (2.2 million) of lung cancer cases [ 1 ]. Breast cancer has replaced lung cancer to become the world’s most prevalent cancer [ 2 ]. It poses a great threat to the physical and mental health of patients worldwide. Breast cancer treatment is a very long and complex process, and the cost is also very high, and even some patients give up treatment because they cannot afford the treatment cost, and further worsen the condition. Triple-negative breast cancer (TNBC) is a subtype of breast cancer characterized by the lack of receptor-estrogen and progesterone expression and amplification of human epidermal growth factor receptor 2 [ 3 , 4 ]. Clinically, TNBC is one of the most aggressive subtypes of breast cancer, accounting for approximately 15%–20% of all breast cancers [ 5 ]. Endocrine therapy with hormone receptor and targeted therapy to block human epidermal growth factor receptor 2 (HER-2) have proven ineffective for patients with TNBC [ 6 ]. The clinical course of TNBC is aggressive, with a high probability of visceral and brain metastases, and its prognosis is the worst among the breast cancer subtypes [ 7 , 8 ]. The BRCA 1/2 gene is particularly strongly associated with triple-negative breast cancer. In the Chinese population, the BRCA 1/2 mutation rate is less than 1% in the general population and about 3% in all breast cancer patients, and up to 17.3% in triple-negative breast cancer. From another perspective, approximately 60%-80% of breast cancer patients carrying the BRCA 1 mutation are triple-negative breast cancer, while approximately 25% of breast cancer patients carrying the BRCA 2 mutation have triple-negative breast cancer [ 9 , 10 ].
Anthracyclines, cyclophosphamides, and taxanes are the preferred neoadjuvant chemotherapy (NCT) for TNBC [ 11 , 12 ]. NCT can reduce the micrometastasis, shrink the tumor, reduce the stage, and increase the chance of breast preservation treatment, which improve the radical cure and breast preservation rate and obtain the drug sensitivity information [ 13 , 14 ]. Studies confirm that achieving pathological complete response (pCR) after a neoadjuvant treatment with TNBC has a good predictive value for long-term survival benefits [ 15 ]. Currently, platinum (P) and poly-ADP-ribose polymerase inhibitors (PARPi) play important antitumor roles in NCT for TNBC, and their efficacy is significant in young patients, especially with BRCA gene mutations. As a DNA cross-linking agent, P cross-connects with the DNA after entering the tumor cells, which interferes with DNA replication of the tumor cells, leading to double-strand DNA breaks of the tumor cells, and then killing the tumor cells. Several single-arm or randomized controlled clinical studies including GeparSixto, CALGB40603, BrighTNess, NeoCART have confirmed the efficacy and safety of P-containing chemotherapy regimens for the treatment of TNBC [ 16 , 17 , 18 , 19 ].
Immune checkpoint inhibitor (ICI) therapy is directed against the interaction between the programmed death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) [ 20 , 21 ]. PD-1 is a co-inhibitory molecule expressed by activated T cells when antigen-presenting cells or tumor cells are combined with PD-L1, which further lead to inhibiting the T-cell activation and suppressing the body’s antitumor immune response. Moreover, the view of PD-1/PD-L1 ICI can improve the suppressed antitumor immune response to relieve the body’s immune response inhibition state, further realizing the antitumor effects [ 22 , 23 ]. ICI may enhance the endogenous anticancer immunity after increasing the release of tumor-specific antigens through chemotherapy. Most current studies show that ICI treatment has a better therapeutic effect and lesser toxicity in TNBC [ 24 , 25 ]. Moreover, the vascular endothelial growth factor (VEGF) is an important regulator of tumor angiogenesis and metastasis [ 26 , 27 ]. Bevacizumab (B) is a recombinant human monoclonal antibody against VEGF that plays various roles in the tumor blood vessels by specifically binding to VEGF and blocking its interaction with receptors [ 28 ]. Relevant studies have reported that adding B based on chemotherapeutic drugs can improve the pCR. Antivascular therapy combined with immunotherapy showed an excellent antitumor activity of different cancers [ 29 , 30 ]. Liu et al . showed that antiangiogenic therapy can improve the sensitivity of PD-L1 expression and the infiltration of PD-1/PD-L1 immunotherapy, playing a synergistic sensitization effect and improving the disease-free survival (DFS) and overall survival (OS) of patients with TNBC [ 31 , 32 ].
Although numerous NCT regimens are currently being used for TNBC, the clinical efficacy of different treatment regimens, especially in terms of long-term survival, remains unclear. Therefore, we conducted a Bayesian meta-analysis of randomized controlled trials (RCTs) to evaluate the effectiveness of different treatment regimens (long-term survival and pCR), thereby providing evidence-based medical information on NCT for TNBC in clinical practice.
Search strategy
This network meta-analysis (NMA) was performed according to the preferred reporting items for systematic reviews and meta-analyses statement [ 33 ]. PubMed, EMBASE, Medline, Cochrane Library, Web of Science, main oncology conference of American Society of Clinical Oncology, the European Society of Medical Oncology, and San Antonio Breast Cancer Symposium databases were searched for high-quality RCTs from January 2000 to June 2023. The search was performed using the following keywords without any restrictions: (triple-negative breast cancer OR triple negative breast neoplasm OR er-negative pr-negative her2-negative breast cancer OR TNBC) AND (neoadjuvant therapy OR neoadjuvant treatment OR neoadjuvant chemotherapy OR neoadjuvant chemotherapy treatment) AND (DFS OR disease free survival) AND (OS OR overall survival) AND (pCR OR pathological complete response). The reference lists of relevant studies, reviews, and meta-analyses were manually screened for potentially eligible publications.
Selection criteria
Eligible trials included those that prospectively compared at least two arms of different neoadjuvant chemotherapeutic regimens in patients with TNBC. Inclusion criteria were as follows: patients with pathologically confirmed TNBC; those with clinical stages of II and III (T1c, N1-2 or T2-4, and N0-2); and those who did not receive surgical NCT. The study end-points included event-free survival (EFS) or DFS, OS, and pCR. The exclusion criteria were as follows: studies involving patients with metastatic TNBC; non-RCTs; articles not written in English; and studies with no data regarding EFS or DFS, OS, and pCR. If several publications from the same trial were identified, only the most recent or complete publications were included.
Data extraction
Eight reviewers were divided into four groups to independently screen the articles (ZL and JL, FZ and QX, DR and ZL, and YC and SH), perform data extraction (ZL and JL and LZ and ZY), and assess the risk of bias (ZL and JL and LZ and MW). Disagreements were resolved by discussion, with assistance from a third party (GS or JZ) if necessary. The following information was recorded: study, author–year, journal, country, arms, medicine, clinical stage, trial phase, TNBC definition, sample size, and study outcomes (EFS or DFS, OS, and pCR).
Explanation of treatment regimens and outcome definitions
Currently, the standard treatment options for TNBC are not yet established, and NCT with anthracycline and purple line represents the cornerstone historical standard for TNBC treatment [ 34 ]. Our study defined the combination of anthracyclines and taxanes as the preferred treatment option. On this basis, any addition of other therapeutic drugs is a new treatment option.
Statistical analysis
Hazards ratio (HR) and odds ratio (OR) were used to estimate pooling effect sizes. For pairwise meta-analysis, the Cochrane Q statistic and the I 2 test were used to calculate heterogeneity. Statistical heterogeneity was defined as P of < 0.1 and/or I 2 of > 50%. A pairwise meta-analysis was performed using a random-effects model or a fixed-effect model depending on the presence of statistical heterogeneity. All pairwise meta-analyses were performed using the Review Manager version 5.3. Results are reported as HR, OR, and corresponding 95% confidence intervals (CIs). All P -values were two sided, and differences with P < 0.05 were considered statistically significant. A Bayesian NMA was performed using the Aggregate Data Drug Information System version 1.16.6 ( http://www.drugis.org ). Node splitting analyses were performed to verify the consistency between direct and indirect evidence. If no significant inconsistency was detected, a consistency model was used to analyze the relative effects of the interventions. Otherwise, an inconsistency model was applied. The “gemtc” package of the R (v14.1) software was used for sorting chats and analyze the data. The NMA results are presented as HR and its corresponding 95% CIs. The “network” packages of the Stata (v14.2) software were used for sorting chats and data analysis. The NMA results are presented as OR and corresponding 95% CIs. The rank probability for each treatment was calculated to determine the treatment ranking. When assessing the merit of the drug efficacy, the surface under the cumulative ranking curve (SUCRA) values was used. It has a value of 0 to 1, and higher SUCRA values indicate better efficacy of the agent.
Study selection and characteristics of the included studies
Figure 1 illustrates the study retrieval process. A total of 10,000 results were obtained from the database, and 1500 studies were automatically removed by Zotero. Based on titles and abstracts, 120 suitable full-text studies were screened, and 31 studies were excluded due to the lack of assessment results. Ultimately, 21 studies involving 8873 patients were included in our reticulated meta-analysis [ 16 , 17 , 18 , 19 , 25 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. Table 1 summarizes the characteristics of the included RCTs. A total of 18 phase III trials and 3 phase II trials were identified. This study evaluated nine treatment regimens in the form of network maps: standard chemotherapeutic agents, TAC (taxanes, anthracycline, and cyclophosphamide), TC (taxanes and cyclophosphamide), B, P, B + P, P + PARPi, ICI, and ICI + P (Fig. 2 ).
A flowchart of the study selection process
Network plots for eligible comparisons were included in the network meta-analysis. A Network diagram of the disease-free survival (DFS). B Network diagram of the overall survival (OS). C Network diagram of the pathological complete response (pCR)
Of the 21 studies, 20 reported data on DFS, with 3 studies including standard chemotherapy, 8 studies including P-containing regimen, 1 study including B + P-containing regimen, 4 studies including B-containing regimen, 1 study including P + PARPi-containing regimen, 2 studies including ICI-containing regimen, and 1 study including ICI + P-containing regimen, all of which were NCTs. Results showed that CT compared with P (HR, 0.8; 95% CI, 0.68–0.94), B + ICI (HR, 0.29; 95% CI, 0.12–0.73), and B + P (HR, 0.43; 95% CI, 0.23–0.8) had a significant benefit of DFS. Figure 3 A summarizes the results of DFS analysis.
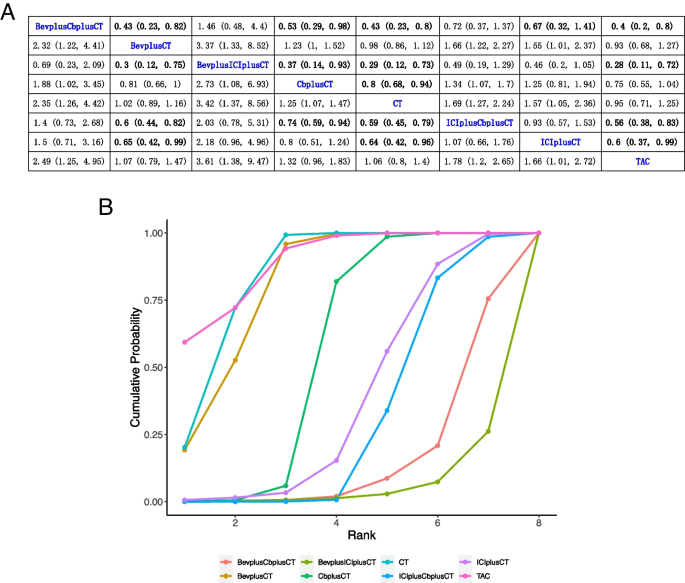
Bayesian network meta-analysis for disease-free survival (DFS). A League comparison table. Data are expressed as hazards ratio (HR) and 95% confidence interval (CI). HR of < 1 supports column definition processing, whereas HR of > 1 supports row definition processing. B Plot of sequencing probabilities for nine DFS schemes. The larger the area of the curve and the X-axis, the higher the recommended treatment
A cumulative ranking of the nine treatment regimens was also analyzed. The results showed that TAC (89.23%), CT (84.53%), B (81.06%), and P (55,30%) ranked first to forth, while ICI (37.86%), ICI + P (30.94%), B + P (15.48%), and B + ICI (5.58%) ranked fifth to eighth (Fig. 3 B).
Of the 21 studies, 17 reported data on OS, with 3 studies including B-containing regimen, 3 studies including standard chemotherapy, 8 studies including P-containing regimen, 2 studies including ICI-containing regimen, and 1 study including PARPi + P-containing regimen, all of which were NCTs. Results showed that PARPi + P-containing regimen compared with B (HR, 0.24; 95% CI, 0.06–0.99), P (HR, 0.24; 95% CI, 0.07–0.89), and standard chemotherapy (HR, 0.21; 95% CI, 0.05–0.8) had a significant benefit of OS. Figure 4 A summarizes the results of OS analysis.
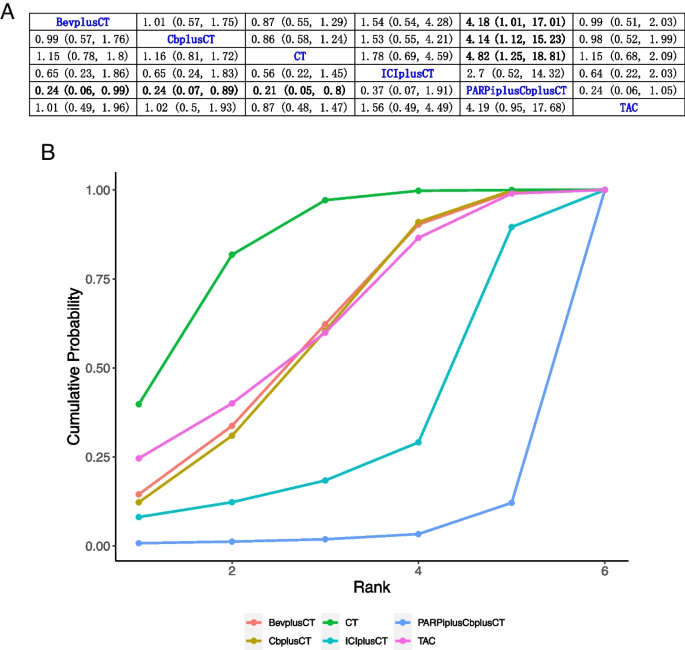
Bayesian network meta-analysis of the overall survival (OS). A League comparison table. Data are expressed as hazards ratio (HR) and 95% confidence interval (CI). HR of < 1 supports the column definition processing, whereas HR of > 1 supports the row definition processing. B Plot of sequencing probabilities for nine OS schemes. The larger the area of the curve and the X-axis, the higher the recommended treatment
A cumulative ranking of the nine treatment regimens was also analyzed. The results showed that CT (83.70%), TAC (62.02%), and B-containing regimens (60.06%) ranked first to third, while P-containing regimens (58.89%), ICI-containing regimens (31.48%), and PARPi + P-containing regimens (3.85%) ranked forth to sixth (Fig. 4 B).
All 21 included trials reported pCR, with 3 studies including standard chemotherapy, 8 studies including P-containing regimen, 1study including B + P-containing regimen, 4 studies including B-containing regimen, 1 study including P + PARPi-containing regimen, 2 studies including ICI-containing regimen, and 2 studies including ICI + P-containing regimen, all of which were NCTs. The incidence of pCR in the PARPi + P-containing regimen (OR, 0.43; 95% CI, − 0.02 to 0.89), P-containing regimen (OR, 0.43; 95% CI, 0.24–0.62), and B-containing regimen (OR, 0.34; 95% CI, 0.06–0.63) was significantly higher than that of standard chemotherapeutic agents. Figure 5 A summarizes the results of pCR analysis.
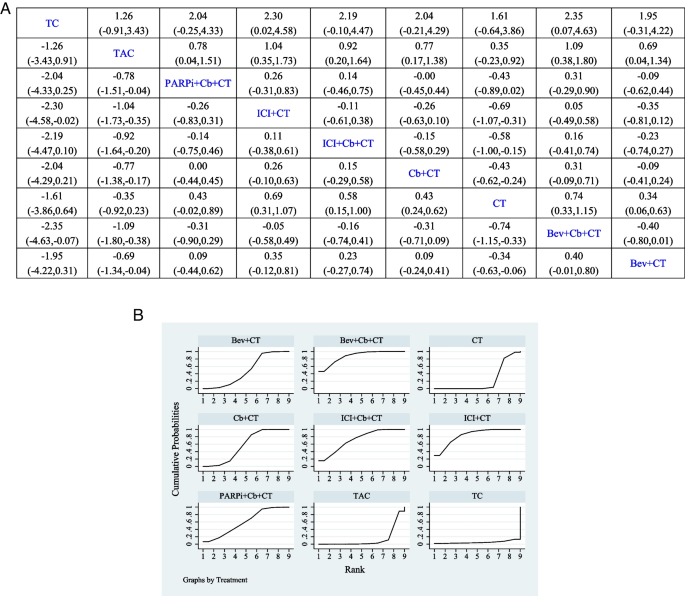
Bayesian network meta-analysis of pathological complete response (pCR). A The league table of comparisons. Data are presented as odds radio (OR) and 95% confidence intervals (CI). An OR of > 1 favors the column-defining treatment, and an OR of < 1 favors the row-defining treatment. B Cumulative sequence diagram of nine pCR schemes. The higher the SUCRA value, the higher the ranking
A cumulative ranking of the nine treatment regimens was also analyzed. The results showed that B + P-containing regimens (82.7%), ICI + P-containing regimens (80.2%), ICI-containing regimens (61.8%), and P-containing regimens (55.0%) ranked first to forth, while PARPi + P-containing regimens (53.5%), B-containing regimens (44.4%), CT (20.5%), TAC (1.8%), and TC (1.5%) ranked fifth to ninth (Fig. 5 B).
Currently, the combination of P, B, PARPi, and ICI based on anthracyclines, cyclophosphamides, and taxanes has paved a new avenue for TNBC treatment [ 50 , 51 , 52 , 53 , 54 ]. However, the long-term survival after neoadjuvant treatment in patients with TNBC under different treatment regimens remains unclear. Therefore, we conducted a Bayesian meta-analysis of RCTs to evaluate the effectiveness of different treatment regimens (long-term survival and pCR) and provide evidence-based medical information on NCT for TNBC in clinical practice. The results showed that, based on SUCRA values, standard chemotherapy is still a better choice for long-term survival consideration compared with NCT for TNBC, and the B + P-containing regimen is most likely the optimal NCT option for TNBC based on pCR results.
In 2022, Li et al [ 53 ]. published an NMA evaluating eight neoadjuvant treatment options for TNBC. The treatment regimen included the combination of P, B, PARPi, and ICI. In this previous study, the observation indicator was pCR; our study added survival indicators to determine the efficacy ranking of several treatment options for TNBC.
This study included 21 RCTs involving 8873 patients with TNBC. Of these, 20 RCTs reported data on DFS; however, only 7 RCTs reported statistical significance for DFS, with 2 studies using standard chemotherapies, 3 studies using P-containing regimens, 1 using ICI-containing regimens, and 1 trial using B + P-containing regimens. Longer survival was also reported in the remaining 13 trials without significant statistical significance. Due to limited DFS data, we treated data regarding EFS, relapse-free survival, and distant DFS reported in these studies as DFS data; however, the significant DFS data remained somewhat unsatisfactory. It may be related to the small number of patients included in the study or the lack of relevant data. When we summarized 20 studies based on SUCRA values, the proportion of studies using standard chemotherapy was relatively high, and the top three treatment options were standard chemotherapy (89.23%), B-containing regimens (81.06%), and P-containing regimens (55.30%).
In our NMA, 17 of 21 trials reported data on OS, but only 5 of them reported statistical significance for OS, which included 1 study using standard chemotherapy, 2 studies using P-containing regimens, 1 study using ICI-containing regimens, and 1 study using B-containing regimens. Longer survival was also reported in the remaining 12 trials, but without significant statistical significance. This may be related to the small number of patients included in the study or the short follow-up time; however, the addition of P, B, and ICI to the standard chemotherapy can partly prolong the OS of patients with TNBC [ 55 , 56 , 57 , 58 , 59 ]. Further large-scale clinical trials are warranted to confirm their efficacy in the future. In terms of OS, when we summarized 17 studies based on SUCRA values, a high proportion of studies were based on standard chemotherapy, and the top three treatment options were standard chemotherapy (83.70%), B-containing regimens (60.06%), and P-containing regimens (58.89%).
All 21 trials reported pCR data, which were shown to be statistically significant. Compared with standard chemotherapeutic agents alone, P-containing regimens, PARPi-containing regimens, or neoadjuvant regimens based on B or ICI showed significant associations with better pCR. Moreover, a recent paired meta-analysis revealed that NCT based on the above regimens significantly improved pCR in patients with TNBC compared with standard chemotherapy [ 53 ], which is consistent with our findings. The results of reticulation analysis based on SUCRA values suggested that B + P-containing regimens are most likely the optimal NCT option for TNBC. The subsequent regimens were ICI + P (80.2%) and ICI (61.8%), and the final recommendation was standard chemotherapy.
This study has some limitations. First, the small number of clinical patients included in these studies or insufficient follow-up time may have caused a bias on the study results. Second, the RCTs included in this study were mainly based on standard chemotherapy, and the proportion of pairs among nine neoadjuvant regimens was small, which may have led to missing indirect contrast data, resulting in inaccurate estimation of the optimal treatment regimen. Third, although we included survival indicators, survival data of different treatment regimens remained insufficient. However, we believe that the use of our carefully pooled data and statistical methods can overcome these limitations of reticulation analysis.
This NMA demonstrated that standard chemotherapy is a good choice with respect to long-term survival, and B-containing regimens are associated with significantly higher pCR rates among patients with neoadjuvant TNBC. Future research should focus on evaluating larger clinical studies to obtain further survival data to help optimize personalized treatment for patients with TNBC.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Triple-negative breast cancer
- Network meta-analysis
Neoadjuvant chemotherapy
Randomized controlled trials
Hazard ratio
Confidence interval
Disease-free survival
Overall survival
Odds ratios
Pathologic complete response
Bevacizumab
Poly-ADP-ribose polymerase inhibitors
Immune checkpoint inhibitor
Surface under the cumulative ranking curve
Programmed death protein 1
Programmed death ligand 1
Vascular endothelial growth factor
Event-free survival
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33.
Article PubMed Google Scholar
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clinicians. 2021;71:209–49.
Article Google Scholar
Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular Classification and Molecular Forecasting of Breast Cancer: Ready for Clinical Application? JCO. 2005;23:7350–60.
Article CAS Google Scholar
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New Strategies for Triple-Negative Breast Cancer—Deciphering the Heterogeneity. Clin Cancer Res. 2014;20:782–90.
Article CAS PubMed PubMed Central Google Scholar
Morris GJ, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84.
Won K, Spruck C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int J Oncol. 2020;57:1245–61.
Dent R, Trudeau M, Pritchard KI, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin Cancer Res. 2007;13:4429–34.
Arvold ND, Taghian AG, Niemierko A, et al. Age, Breast Cancer Subtype Approximation, and Local Recurrence After Breast-Conserving Therapy. JCO. 2011;29:3885–91.
Yin L, Duan J-J, Bian X-W, Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61.
Article PubMed PubMed Central Google Scholar
Derakhshan F, Reis-Filho JS. Pathogenesis of Triple-Negative Breast Cancer. Annu Rev Pathol Mech Dis. 2022;17:181–204.
Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St Gallen International Consensus Guidelines for treatment of early breast cancer. Annals of Oncology. 2021;32:1216–35.
Article CAS PubMed Google Scholar
Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St gallen international expert consensus conference on the primary therapy of early breast cancer. Annals of Oncology. 2017;28:1700–12.
Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362–89.
Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91–113.
Howard FM, Olopade OI. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021;27:8–16.
Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509.
Zhang L, Wu Z, Li J, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer ( NeoCART ): Results from a multicenter, randomized controlled, open-label phase II trial. Intl Journal of Cancer. 2022;150:654–62.
Shepherd JH, Ballman K, Polley MYC, et al. Long term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple negative breast cancer. J Clin Oncol. 2022;40:1323–34.
Hahnen E, Lederer B, Hauke J, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017;3:1378.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810–21.
Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2022;387:217–26.
Gong Y, Ji P, Yang Y-S, et al. Metabolic-Pathway-Based Subtyping of Triple-Negative Breast Cancer Reveals Potential Therapeutic Targets. Cell Metab. 2021;33:51-64.e9.
Zhu Y, Zhu X, Tang C, Guan X, Zhang W. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2021;1876:188593.
Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:1–18.
Nanda R, Liu MC, Yau C, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020;6:676.
LeVasseur N, Sun J, Gondara L, et al. Impact of pathologic complete response on survival after neoadjuvant chemotherapy in early-stage breast cancer: a population-based analysis. J Cancer Res Clin Oncol. 2020;146:529–36.
Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. JCO. 2016;34:2460–7.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384:164–72.
Roodhart J, Langenberg M, Witteveen E, Voest E. The Molecular Basis of Class Side Effects Due to Treatment with Inhibitors of the VEGF/VEGFR Pathway. CCP. 2008;3:132–43.
Syrigos KN, Karapanagiotou E, Boura P, Manegold C, Harrington K. Bevacizumab-Induced Hypertension: Pathogenesis and Management. BioDrugs. 2011;25:159–69.
Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial[J]. J Immunother Cancer. 2020, 8(1).
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6:e1000100.
Iwase M, Ando M, Aogi K, et al. Long-term survival analysis of addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Res Treat. 2020;180:687–94.
Chen X, Ye G, Zhang C, et al. Superior outcome after neoadjuvant chemotherapy with docetaxel, anthracycline, and cyclophosphamide versus docetaxel plus cyclophosphamide: results from the NATT trial in triple negative or HER2 positive breast cancer. Breast Cancer Res Treat. 2013;142:549–58.
Untch M, Von Minckwitz G, Konecny GE, et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin–cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer—outcome on prognosis. Ann Oncol. 2011;22:1999–2006.
Sharma P, Kimler BF, O’Dea A, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res. 2021;27:975–82.
Zhang L, Wu Z, Li J, et al. Neoadjuvant Docetaxel plus Carboplatin Versus Epirubicin plus Cyclophosphamide Followed by Docetaxel in Triple-negative, Early-stage Breast Cancer (NeoCART): Results from a Multicenter, Randomized Controlled. Open-label Phase II Trial In Review. 2021. https://doi.org/10.21203/rs.3.rs-585170/v1 .
Schneeweiss A, Michel LL, Möbus V, et al. Survival analysis of the randomised phase III GeparOcto trial comparing neoadjuvant chemotherapy of intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for patients with high-risk early breast cancer. Eur J Cancer. 2022;160:100–11.
Gluz O, Nitz U, Kolberg-Liedtke C, et al. De-escalated Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer (TNBC): Impact of Molecular Markers and Final Survival Analysis of the WSG-ADAPT-TN Trial. Clin Cancer Res. 2022;28:4995–5003.
Zhang P, Yin Y, Mo H, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget. 2016;7:60647–56.
Yan W, Wu X, Wang S, et al. Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial. Ther Adv Med Oncol. 2022;14:175883592211071.
Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31:569–81.
Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022;386:556–67.
Loibl S, Schneeweiss A, Huober J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33:1149–58.
Bear HD, Tang G, Rastogi P, et al. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16:1037–48.
Von Minckwitz G, Loibl S, Untch M, et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44–GeparQuinto). Ann Oncol. 2014;25:2363–72.
Nahleh ZA, Barlow WE, Hayes DF, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat. 2016;158:485–95.
Earl HM, Hiller L, Dunn JA, et al. Disease-free and overall survival at 3.5 years for neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer: ARTemis Trial. Annals of Oncology. 2017;28:1817–24.
Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–508.
Li Y, Yang D, Chen P, et al. Efficacy and safety of neoadjuvant chemotherapy regimens for triple-negative breast cancer: a network meta-analysis. Aging. 2019;11:6286–311.
Yin J, Zhu C, Wang G, Gu J. Treatment for Triple-Negative Breast Cancer: An Umbrella Review of Meta-Analyses. IJGM. 2022;15:5901–14.
Li J, Shen G, Wang M, et al. Comparative efficacy and safety of first-line neoadjuvant treatments in triple-negative breast cancer: systematic review and network meta-analysis. Clin Exp Med. 2022;23:1489–99.
Alnimer Y, Hindi Z, Katato K. The Effect of Perioperative Bevacizumab on Disease-Free and Overall Survival in Locally Advanced HER-2 Negative Breast Cancer: A Meta-Analysis. Breast Cancer(Auckl). 2018;12:117822341879225.
Ma X, Wang X, Huang J, et al. Bevacizumab Addition in Neoadjuvant Treatment Increases the Pathological Complete Response Rates in Patients with HER-2 Negative Breast Cancer Especially Triple Negative Breast Cancer: A Meta-Analysis. PLoS ONE. 2016;11:e0160148.
Nahleh Z, Botrus G, Dwivedi A, Jennings M, Nagy S, Tfayli A. Bevacizumab in the neoadjuvant treatment of human epidermal growth factor receptor 2-negative breast cancer: A meta-analysis of randomized controlled trials. mol clin onc 2019. Published online Jan 2. https://doi.org/10.3892/mco.2019.1796 .
Li Z-Y, Zhang Z, Cao X-Z, Feng Y, Ren S-S. Platinum-based neoadjuvant chemotherapy for triple-negative breast cancer: a systematic review and meta-analysis. J Int Med Res. 2020;48:030006052096434.
Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–32.
Sternschuss M, Yerushalmi R, Saleh RR, Amir E, Goldvaser H. Efficacy and safety of neoadjuvant immune checkpoint inhibitors in early-stage triple-negative breast cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2021;147:3369–79.
Download references
Acknowledgements
Not applicable.
This work was supported by Provincial-Level Clinical Key Specialty Construction in Qinghai Province.
Author information
Zhilin Liu, Jinming Li and Fuxing Zhao are contributed equally.
Authors and Affiliations
Breast Disease Diagnosis and Treatment Center, Affiliated Hospital of Qinghai University, Affiliated Cancer Hospital of Qinghai University, People’s Republic of China, Qinghai Provincial Clinical Research Center for Cancer, Qinghai Provincial Institute of Cancer Research, Xining, China
Zhilin Liu, Jinming Li, Fuxing Zhao, Dengfeng Ren, Zitao Li, Yongzhi Chen, Shifen Huang, Zhen Liu, Yi Zhao, Miaozhou Wang, Guoshuang Shen & Jiuda Zhao
Department of Breast Medical Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
Qinghai University, Xining, China
You can also search for this author in PubMed Google Scholar
Contributions
Concept and design: Drs ZL, JL and FZ. Acquisition, analysis, or interpretation of data: Drs DR, ZL and YC. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Drs SH, ZL, YZ and MW. Obtained funding: All authors. Administrative, technical, or material support: All authors. Supervision: Drs GS and JZ. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Guoshuang Shen or Jiuda Zhao .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Z., Li, J., Zhao, F. et al. Long-term survival after neoadjuvant therapy for triple-negative breast cancer under different treatment regimens: a systematic review and network meta-analysis. BMC Cancer 24 , 440 (2024). https://doi.org/10.1186/s12885-024-12222-9
Download citation
Received : 18 October 2023
Accepted : 02 April 2024
Published : 09 April 2024
DOI : https://doi.org/10.1186/s12885-024-12222-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Triple negative breast cancer
- Neoadjuvant therapy
- Long-term survival
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Research article
- Open access
- Published: 01 October 2013
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
- Suzanne A Eccles 17 ,
- Eric O Aboagye 1 ,
- Simak Ali 1 ,
- Annie S Anderson 2 ,
- Jo Armes 7 ,
- Fedor Berditchevski 4 ,
- Jeremy P Blaydes 3 ,
- Keith Brennan 5 ,
- Nicola J Brown 6 ,
- Helen E Bryant 6 ,
- Nigel J Bundred 5 ,
- Joy M Burchell 7 ,
- Anna M Campbell 2 ,
- Jason S Carroll 9 ,
- Robert B Clarke 5 ,
- Charlotte E Coles 34 ,
- Gary JR Cook 7 ,
- Angela Cox 6 ,
- Nicola J Curtin 10 ,
- Lodewijk V Dekker 11 ,
- Isabel dos Santos Silva 12 ,
- Stephen W Duffy 13 ,
- Douglas F Easton 9 ,
- Diana M Eccles 3 ,
- Dylan R Edwards 15 ,
- Joanne Edwards 14 ,
- D Gareth Evans 5 ,
- Deborah F Fenlon 3 ,
- James M Flanagan 1 ,
- Claire Foster 3 ,
- William M Gallagher 16 ,
- Montserrat Garcia-Closas 17 ,
- Julia M W Gee 18 ,
- Andy J Gescher 28 ,
- Vicky Goh 7 ,
- Ashley M Groves 8 ,
- Amanda J Harvey 33 ,
- Michelle Harvie 5 ,
- Bryan T Hennessy 20 ,
- Stephen Hiscox 18 ,
- Ingunn Holen 6 ,
- Sacha J Howell 5 ,
- Anthony Howell 5 ,
- Gill Hubbard 21 ,
- Nick Hulbert-Williams 22 ,
- Myra S Hunter 7 ,
- Bharat Jasani 18 ,
- Louise J Jones 13 ,
- Timothy J Key 23 ,
- Cliona C Kirwan 5 ,
- Anthony Kong 23 ,
- Ian H Kunkler 24 ,
- Simon P Langdon 24 ,
- Martin O Leach 17 ,
- David J Mann 1 ,
- John F Marshall 13 ,
- Lesley Ann Martin 17 ,
- Stewart G Martin 11 ,
- Jennifer E Macdougall 25 ,
- David W Miles 7 ,
- William R Miller 24 ,
- Joanna R Morris 4 ,
- Sue M Moss 13 ,
- Paul Mullan 26 ,
- Rachel Natrajan 17 ,
- James PB O’Connor 5 ,
- Rosemary O’Connor 27 ,
- Carlo Palmieri 31 ,
- Paul D P Pharoah 9 ,
- Emad A Rakha 11 ,
- Elizabeth Reed 29 ,
- Simon P Robinson 17 ,
- Erik Sahai 32 ,
- John M Saxton 15 ,
- Peter Schmid 30 ,
- Matthew J Smalley 18 ,
- Valerie Speirs 19 ,
- Robert Stein 8 ,
- John Stingl 9 ,
- Charles H Streuli 5 ,
- Andrew N J Tutt 7 ,
- Galina Velikova 19 ,
- Rosemary A Walker 28 ,
- Christine J Watson 9 ,
- Kaye J Williams 5 ,
- Leonie S Young 20 &
- Alastair M Thompson 2
Breast Cancer Research volume 15 , Article number: R92 ( 2013 ) Cite this article
131k Accesses
278 Citations
69 Altmetric
Metrics details

Introduction
Breast cancer remains a significant scientific, clinical and societal challenge. This gap analysis has reviewed and critically assessed enduring issues and new challenges emerging from recent research, and proposes strategies for translating solutions into practice.
More than 100 internationally recognised specialist breast cancer scientists, clinicians and healthcare professionals collaborated to address nine thematic areas: genetics, epigenetics and epidemiology; molecular pathology and cell biology; hormonal influences and endocrine therapy; imaging, detection and screening; current/novel therapies and biomarkers; drug resistance; metastasis, angiogenesis, circulating tumour cells, cancer ‘stem’ cells; risk and prevention; living with and managing breast cancer and its treatment. The groups developed summary papers through an iterative process which, following further appraisal from experts and patients, were melded into this summary account.
The 10 major gaps identified were: (1) understanding the functions and contextual interactions of genetic and epigenetic changes in normal breast development and during malignant transformation; (2) how to implement sustainable lifestyle changes (diet, exercise and weight) and chemopreventive strategies; (3) the need for tailored screening approaches including clinically actionable tests; (4) enhancing knowledge of molecular drivers behind breast cancer subtypes, progression and metastasis; (5) understanding the molecular mechanisms of tumour heterogeneity, dormancy, de novo or acquired resistance and how to target key nodes in these dynamic processes; (6) developing validated markers for chemosensitivity and radiosensitivity; (7) understanding the optimal duration, sequencing and rational combinations of treatment for improved personalised therapy; (8) validating multimodality imaging biomarkers for minimally invasive diagnosis and monitoring of responses in primary and metastatic disease; (9) developing interventions and support to improve the survivorship experience; (10) a continuing need for clinical material for translational research derived from normal breast, blood, primary, relapsed, metastatic and drug-resistant cancers with expert bioinformatics support to maximise its utility. The proposed infrastructural enablers include enhanced resources to support clinically relevant in vitro and in vivo tumour models; improved access to appropriate, fully annotated clinical samples; extended biomarker discovery, validation and standardisation; and facilitated cross-discipline working.
Conclusions
With resources to conduct further high-quality targeted research focusing on the gaps identified, increased knowledge translating into improved clinical care should be achievable within five years.
Globally, breast cancer is the most frequently diagnosed cancer in women, with an estimated 1.38 million new cases per year. Fifty thousand cases in women and 400 in men are recorded each year in the UK alone. There are 458,000 deaths per year from breast cancer worldwide making it the most common cause of female cancer death in both the developed and developing world [ 1 ].
In the UK, the age-standardised incidence of breast cancer in women has increased by 6% over the last decade, between 1999 to 2001 and 2008 to 2010 [ 2 ]. It is estimated that around 550,000-570,000 people are living with or after a diagnosis of breast cancer in the UK [ 3 ] and, based on current projections, this figure is expected to triple by 2040 due to an ageing population and continued improvements in survival [ 4 ]. Recent research indicates that the annual cost of breast cancer to the UK economy is £1.5bn, with just over a third of that cost (£0.6bn) from healthcare alone [ 5 ]. Yet the annual spend on breast cancer research by partners of the National Cancer Research Institute has reduced in recent years despite the level of cancer research spend being generally maintained [ 6 ].
In 2006, the charity Breast Cancer Campaign facilitated a meeting of leading breast cancer experts in the United Kingdom to explore which gaps in research, if filled, would make the most impact on patient benefit. The subsequent paper [ 7 ] has helped shape the direction of breast cancer research since that time. One overarching need identified was the ‘lack of access to appropriate and annotated clinical material’, which directly led to the formation of the UK’s first multi-centre, breast-specific tissue bank [ 8 ].
This new gap analysis represents an expanded, evidence-based follow-on developed collaboratively by clinicians, scientists and healthcare professionals. The aim is to ensure that the roadmap for breast cancer research remains a relevant, consensual and authoritative resource to signpost future needs. It builds upon the previous gap analysis by briefly reviewing the current status of key areas, critically assessing remaining issues and new challenges emerging from recent research findings and proposes strategies to aid their translation into practice. Whilst a survey of progress during the last five years is not the intention of this article, the preparatory detailed discussions and data analysis could provide the basis for such a retrospective review.
During 2012, Breast Cancer Campaign facilitated a series of workshops, each covering a specialty area of breast cancer (Figure 1 ). These working groups covered genetics, epigenetics and epidemiology; molecular pathology and cell biology; hormonal influences and endocrine therapy; imaging, detection and screening; current and novel therapies and associated biomarkers; drug resistance; invasion, metastasis, angiogenesis, circulating tumour cells, cancer ‘stem’ cells; breast cancer risk and prevention; living with and managing breast cancer and its treatment. Working group leaders and their multidisciplinary teams (comprising a representative cross-section of breast cancer clinicians, scientists, and healthcare professionals) participated in iterative cycles of presentation and discussion, offering a subjective consideration of the recent relevant peer-reviewed literature. Summary reports were prepared by each group, collated, condensed and edited into a draft, which was critically appraised by an external Executive Advisory Board of international experts. This position paper highlights the key gaps in breast cancer research that were identified, together with detailed recommendations for action.
Gap analysis methodology. The flow chart illustrates the concept, processes and procedures devised to generate the gap analysis review.
Genetics, epigenetics and epidemiology
Current status, genetic predisposition.
Our knowledge of the heritability of breast cancer has increased significantly since 2007. Known breast cancer genes (BRCA1, BRCA2, CHEK2, ATM, PALB2, BRIP1, TP53, PTEN, CDH1 and STK11) make up 25 to 30% of the heritability [ 9 ]. Genome-wide association studies (GWAS) and the recent international collaborative analyses have confirmed 77 common polymorphisms individually associated with breast cancer risk, which add a further 14% [ 9 – 11 ]. Evidence from an Illumina collaborative oncological gene-environment study (iCOGS) experiment suggests that further single nucleotide polymorphisms (SNPs) may contribute at least 14% to the heritability, leaving only approximately 50% as ‘missing heritability’ (Figure 2 ).
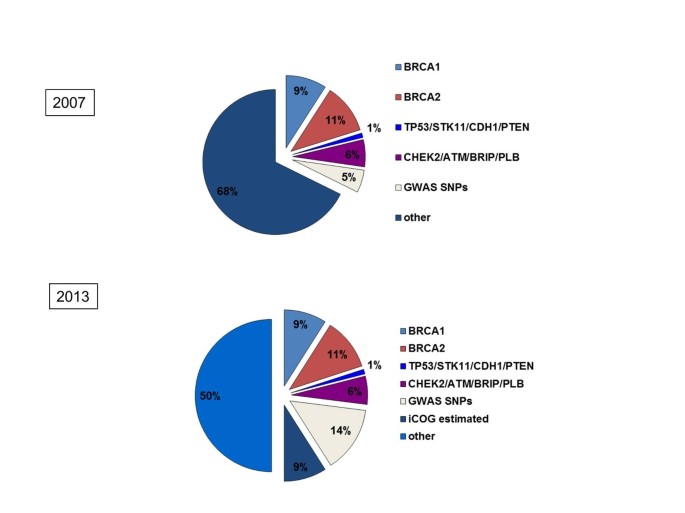
Familial cancer genetics. The proportion of the familial component of breast cancers that can be ascribed to specific genetic defects. The difference between June 2007 and 2013 shows the impact of genome-wide association studies (GWAS) that have now identified 77 common low-risk SNPs. Courtesy of Professor Douglas Easton (University of Cambridge). Reprinted by permission from Macmillan Publishers Ltd: Nature Genetics (45,345-348), copyright 2013.
If we assume the risk estimates for polygenic markers are log additive, the cumulative risk associated with these SNPs has a median of 9% to age 80 (95% confidence intervals 5 to 15%). In the familial setting, we have learnt that common genetic SNPs can modify the risk associated with BRCA2, which may be relevant when considering risk-reducing surgery [ 12 , 13 ].
BRCA1 and BRCA2
There is improved understanding of the function of BRCA1 and BRCA2 in relation to DNA repair and therapeutic responses. For example, BRCA2 functions in RAD51 loading and BRCA1 in countering 53BP1-mediated blocking of homologous recombinational (HR)-DNA repair; hence poly (ADP-ribose) polymerase (PARP) inhibitors have been developed and trialled against BRCA-driven cancers [ 14 ]. Several additional genes associated with breast cancer risk are part of the BRCA network and there is a clear relationship with the Fanconi pathway [ 9 ]. Genes in this network point to reduced HR-DNA repair as the mechanism underlying cancer susceptibility, although the precise functions of associated signalling proteins (for example PTEN, CHK2, ATM and N-terminal BRCA1) that relate to cancer development are unknown. Gene interactions of some higher risk alleles are recognised to be sub-multiplicative, whereas low risk alleles are log-additive [ 15 ]. Some susceptibility SNPs may function at the level of chromatin remodelling/enhancer activity related to nearby gene expression.
Epigenetics
Epigenetic alterations are frequent and cancer-specific methylation in circulating tumour (ct)DNA in serum can be used as an early detection biomarker, or as a prognostic indicator [ 16 , 17 ]. The recent ENCODE study provided a wide-ranging analysis of epigenetic marks on a small fraction of the genome [ 18 ]. The first candidate gene epigenetic risk factor that could usefully be included in breast cancer risk models (once fully validated) has been identified [ 19 ]. Epigenetic factors also provide molecular measures of long-term exposure to potentially oncogenic agents. Epigenetic alterations are reversible; preclinical and recent clinical testing of epigenetic-targeted therapies such as etinostat (a DNA methylation inhibitor) and vorinostat (a histone deacetylase inhibitor) indicate that such drugs may prove effective in combination with other therapies [ 20 , 21 ].
Psychosocial considerations
Predictive genetic testing for breast cancer predisposition genes can increase distress in the short term (which reduces over time) for those identified as gene carriers, whilst non-carriers report lower levels of concern following genetic testing [ 22 ]. A number of interventions have now been developed and tested to support the genetic testing process and have been shown to reduce distress, improve the accuracy of the perceived risk of breast cancer, and increase knowledge about breast cancer and genetics [ 23 ]. Examples introduced since the last gap analysis include education using tailored information technology to prepare women for genetic counselling [ 24 ]; interventions to support women’s decisions about whether or not to have genetic testing [ 25 ] and support for gene carriers thus identified [ 12 ].
What are the key gaps in our knowledge and how might they be filled?
Moderate risk alleles.
Remaining ‘moderate risk’ alleles will be found within the short term by exome sequencing and extended GWAS studies will identify additional lower risk alleles. If up to 28% of the risk from known SNPs could be explained, while the median of the risk distribution changes little, confidence limits would change dramatically, such that the women in the top 5% at risk would have >15% lifetime risk, compared with <3% lifetime risk at the lower end. A prospective analysis will be required to show that genetic risk assessment can predict risk when combined with mammographic screening. We need to determine if or how common SNPs modify the contributions of BRCA1-associated and moderate risk genes (such as CHEK2, ATM) and whether this is influenced by oestrogen levels or risk management using, for example, lifestyle or chemopreventive approaches.
Functional implications of unclassified variants in BRCA1/BRCA2, fine-mapping of risk-associated variants (from GWAS) and understanding the functional impact of the more common SNPs such as TOX3 and the role of FOXA1 remain to be determined. Similarly, deconvoluting the functional interactions between susceptibility genes and known breast cancer-associated proteins require systems biology approaches. Can we achieve a clear clinical use of the knowledge gained by GWAS, SNP and BRCA studies by validation of risk models incorporating SNPs and moderate risk alleles (in particular in the familial setting) to improve risk management? A randomised trial for population screening with mammography stratified on individual genetic risk estimates (combined with other key risk factors) is warranted.
BRCA1 and 2
A scheme to define categories of risk for variants in BRCA (and other) cancer genes is needed to provide specific clinical recommendations. BRCA variants of uncertain significance occur in approximately 5% of all genetic tests for BRCA1/BRCA2 mutations [ 26 ]. A range of in silico and functional assays is available to provide evidence for or against a genetic variant being pathogenic. A calculation combining all lines of evidence can estimate the posterior probability that a particular gene variant is predisposing to disease. The expression of breast cancer genes in normal breast tissue and pathways that may underlie cancer risk (such as DNA damage response) could be used to identify tractable markers and to direct treatment choice. Additional BRCA-deficient human tumour cell lines and animal models of breast cancer are required.
There is a gap in our understanding of cause or consequence between epigenetic traits and gene transcription. Translational studies are needed to investigate epigenetic patterns in clinical material and from clinical trials to identify and validate prognostic markers. The extent to which epigenetic markers can be incorporated into risk models alongside genetic and lifestyle factors is not yet known. Understanding how cancer risk factors impact on the epigenome and whether this provides a mechanism for increased risk associated with those exposures is poorly understood.
Further research is needed to support informed decision making about risk management options and to assess the psychosocial implications of changing behaviour and anxiety about cancer [ 27 ]. Interventions to support discussions with those newly diagnosed with breast cancer are being developed to improve understanding of risk to individuals and their families [ 28 ]. Interventions are also required to support conversations within the family about genetic risk and its implications, given that the onus is often on the patient [ 29 ]. Research involving women at increased genetic risk for breast cancer should assess the psychosocial impact on partners and the implications for their relationships [ 30 ]. Evidence from this research needs to inform services and direct resources to support those at increased risk of breast cancer.
Risk and prevention
Risk estimation.
We know little about the exact cause(s) of the majority of breast cancers. The major challenge for prevention is to identify women at risk as precisely as possible and then to apply measures such as chemoprevention and lifestyle changes. Current models can predict probable numbers of breast cancer cases in specific risk factor strata, but have modest discriminatory accuracy at the individual level [ 31 ]. The publication of more than 70 common genetic susceptibility factors via large-scale collaborative efforts [ 10 , 32 ] and the realisation that mammographic density is a major risk factor is important, but the major gap in our knowledge is how to incorporate these factors into our current risk prediction models [ 33 ].
Automated methods for estimation of mammographic density require further evaluation for its potential use as a biomarker for risk stratification in screening and changes in density as a biomarker of responsiveness to preventive approaches. Studies of chest irradiation for lymphomas and carcinogens in rodent models suggest the importance of exposure to radiation during puberty [ 34 , 35 ].
There is a need to assess the value of several new approaches to discovering biomarkers including adductomics, transcriptomics, metabolomics [ 36 ] and epigenomics and to determine how well-established measurements (for example oestrogen levels) can be incorporated into risk models [ 37 ].
Chemoprevention
An overview of all trials of selective oestrogen receptor modulators (SERMs) as chemopreventive agents indicates that risk is reduced by 38% for up to 10 years from the start of five years’ treatment [ 38 ]. An issue is predicting those women who will benefit from SERM treatment. Lasofoxifene appears to be the most active SERM and its further development is desirable [ 39 ]. In postmenopausal women, the MA P3 trial indicated that exemestane reduced risk by 65% after 35 months median follow-up [ 40 ] requiring confirmation with additional aromatase inhibitor (AI) prevention studies. The value of low-dose tamoxifen and fenretinide also needs to be established [ 41 ]. Since SERMs and AIs reduce only oestrogen receptor positive (ER+ve) disease, there is a need for agents to prevent ER negative (ER-ve) disease, to distinguish between ER- and progesterone receptor (PR)-related disease [ 42 ] and to develop better animal models [ 43 ]. There is a need to confirm that oestrogen-only hormone replacement therapy (HRT) reduces risk whereas combined HRT increases risk in the Women’s Health Initiative (WHI) trials and to establish the mechanism of this dichotomy [ 44 , 45 ].
Lifestyle changes
Most studies related to breast cancer risk and lifestyles are observational. Favourable changes in lifestyle including reduction of calorie excess, increasing exercise, reducing alcohol intake and less environmental exposures to disturbance of circadian rhythm could reduce breast cancer by one third [ 46 – 49 ]. Communicating the potential benefits of lifestyle change, identifying teachable moments and using health services to endorse lifestyle change for prevention will require additional studies to determine why health beliefs translate poorly into action [ 50 ].
Marked adult weight gain in premenopausal women is associated with a doubling of risk of postmenopausal breast cancer compared with no or little weight gain [ 51 ]. Conversely, weight loss of 3kg or more is associated with a 25 to 40% reduction of cancer in older women compared with those who continue to gain weight. [ 52 – 54 ]. It is not clear whether to focus on all overweight women, those with gynoid or abdominal obesity or those with metabolic syndrome. Weight gain after surgery for breast cancer increases risk of relapse [ 55 ]; there is a need for further randomised trials to determine whether reducing weight in the overweight, or preventing weight gain after surgery prevents relapse. Weight management strategies seeking efficacy in the long term may be particularly difficult to sustain.
The effect of individual components of diet is controversial. The risk of ER-ve tumours may be reduced by high vegetable intake [ 56 ] while lowering fat intake may reduce both breast cancer risk and relapse after surgery. However, two of the three randomised trials of lower fat intake are confounded by concomitant weight loss [ 57 , 58 ] and the one study without weight loss showed no effect of reduction of fat intake on breast cancer relapse after surgery [ 59 ].
There is evidence for breast cancer prevention with habitual exercise [ 60 ]. Observational evidence shows that a physically active lifestyle after cancer treatment prevents relapse and reduces the risk of all-cause mortality [ 61 ]. The optimal exercise regime and timing are uncertain and randomised trials are required to assess the preventive benefits. There is a need to understand the mechanism of the apparent beneficial effects of caloric restriction and exercise.
Effective and sustainable lifestyle changes (diet, exercise and weight) need to be agreed and effective routes to initiation and maintenance identified. Further work needs to be undertaken in chemoprevention strategies and adherence to effective agents.
Prospective cohort studies are needed to develop and validate risk models, which may need to incorporate polygenic risks, mammographic density and measures of body composition. Risks may be refined by the discovery and validation of novel biomarkers such as epigenetic markers [ 19 ] and prospective validation of known markers such as serum oestrogen [ 62 , 63 ]. Effectiveness and cost-effectiveness, analyses to evaluate possible personalised screening and prevention programmes [ 64 ] and pilot studies to evaluate delivery options followed by large randomised trials are required. Polygenic and other biomarkers should be used to distinguish between the development of ER +ve, ER+ve/PR +ve and ER–ve cancers.
Many breast cancers arise in women without apparent risk factors; current studies suggest that polygenic risk factors and mammographic density add only a little to the Gail model [ 65 ]. Precision is required using polygenic approaches to decide whether or not to give preventive tamoxifen. Currently, about 10% of breast cancers arise in women with a 10-year risk above 5%. Taking this at-risk group and increasing the frequency of screening would be of some benefit, but more effective risk-adapted screening will depend upon a better definition of risk.
Further improvement and cost-effectiveness of the NHS breast cancer screening programme could include tomography, ultrasound and automated methods for the measurement of volumetric mammographic density (using software programs such as Quantra or Volpara) and automatically using these for risk stratification to adapt screening interval to risk. Experimentally, there are now opportunities for determining whether high breast density alters the response of breast epithelial cells to DNA damage or oncogene activation. This may provide prognostic value if we can define novel biomarkers to distinguish which women with high mammographic density will develop cancer [ 66 , 67 ].
Uptake of tamoxifen and raloxifene is variable and optimal methods need to be developed to explain risk, the benefit/risk ratio of treatment and to identify women who will benefit. The benefit from tamoxifen may be determined by changes in mammographic density [ 68 ] but needs confirmation. Identification of women who could develop ER-ve tumours should become possible (for example by polygenic scores). Work is required to corroborate the efficacy of lasofoxifene; the use of AIs in the preventive setting should be clarified by the International Breast Cancer Intervention Study II (IBIS II) trial, while the use of low-dose tamoxifen and retinoids also await trial results. Further studies are required to develop new preventive agents; those which might be pursued further include rexinoids, omega 3 fatty acids, sulphorophane, antiprogestins and insulin-like growth factor 1 (IGF1) inhibitors [ 409 ].
The widespread introduction of preventive agents depends upon efficient methods for identifying risk and effective counselling. Neither has been widely taken up, particularly in postmenopausal women, but the recently published NICE guidelines may signal a change for the use of tamoxifen in chemoprevention. Identification within screening programmes may be a valid approach [ 64 ]. However, since trials of chemoprevention require long duration and are costly, the development of biomarkers as indicators of effectiveness and their acceptance by regulatory agencies is attractive.
Lifestyle change for breast cancer prevention
A precise definition of interventions for diet and exercise and the relative importance for reduction of ER+ve or ER-ve breast cancer is unclear. The effect of caloric restriction by age and the duration of interventions remain unknown as do the underlying mechanisms of action. Identifying successful methods to translate prevention evidence into public health policy including effective behaviour change programmes and convincing clinicians to change practice in favour of prevention are required. Most evidence for lifestyle change is observational and confirmatory data from prospective randomised controlled trials (RCTs) with long-term follow-up and clinical endpoints may be needed. A breast cancer prevention trial using exercise would require a sample size of 25,000 to 35,000 and an eight to ten-year follow-up to observe a 20 to 25% decrease in risk for a moderate-to-vigorous physical activity programme. Such a large-scale study is not currently possible so the focus has been on a RCT of exercise in breast cancer patients to determine how exercise influences survival. The AMBER cohort study in 1,500 breast cancer patients measures physical activity, fitness and other indicators to determine exactly how physical activity influences survival [ 69 ].
Nevertheless, the beneficial effects demonstrated in randomised trials to prevent diabetes and cardiovascular disease need to be balanced against the enormous size and cost that would be required for such trials in breast cancer. For secondary prevention of disease recurrence after surgery, trials are due to report on caloric restriction and exercise in 2014 and 2018 [ 70 , 71 ].
There are teachable moments within the breast screening programmes for links to prevention through changes in lifestyle [ 50 , 64 ]. Reduction in alcohol consumption using community/class/cultural approaches, analogous to those for smoking, needs to be explored using social marketing approaches within a research context. It is likely that energy restriction and exercise will not be a complete answer to prevention and efforts should be made to design lifestyle prevention trials with and without energy restriction mimetic agents such as mTOR inhibitors, resveratrol, and metformin. mTOR inhibitors such as everolimus (RAD001) are effective in advanced breast cancer [ 72 ] although toxicities will prevent its use as a preventive agent; rapamycin in animal models reduces tumour incidence and increases longevity [ 73 ]. There is a need to translate these important findings into the clinic, perhaps by low dose or intermittent regimens to avoid toxicity [ 74 ]. Metformin is in clinical trial as an adjuvant for breast cancer treatment and demonstration of effectiveness in this situation could lead to assessment for prevention including in prediabetic populations [ 75 ].
Molecular pathology
Breast cancer classification and issues of heterogeneity.
During the last five years several high-profile studies have significantly advanced the molecular subclassification of breast cancer (reviewed in [ 76 ] and [ 77 ]). Intratumoral heterogeneity in both pre-malignant and invasive breast cancer is well documented. It is likely that both genetic and epigenetic instability, combined with microenvironmental and therapy-induced selective pressures lead to clonal evolution, which continues during metastatic progression. However, whether heterogeneity arises from cancer stem cell plasticity and a hierarchy of aberrant differentiation or stochastic events is a moot point (Figure 3 ). Genomic studies have been used to develop both prognostic biomarkers and to identify biomarkers to predict response to therapy. Nevertheless, ‘driver’ genetic changes in breast cancer will need to be filtered from the background, clinically inconsequential changes [ 78 ].
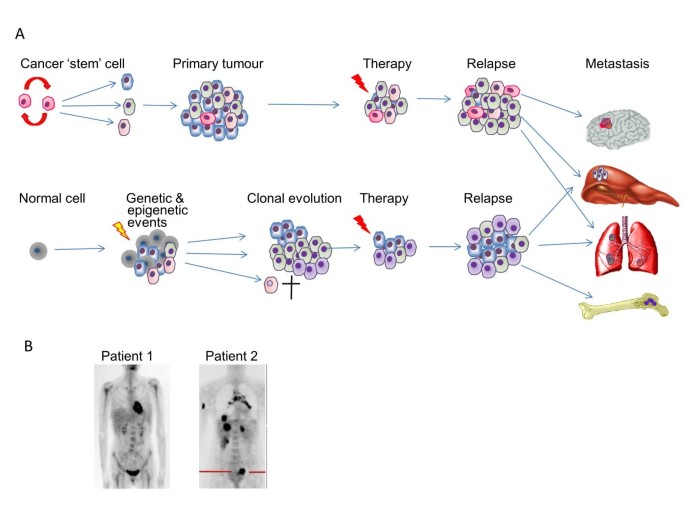
Tumour heterogeneity. (A) Recent molecular and genetic profiling has demonstrated significant intratumoural heterogeneity that can arise through genomic instability (leading to mutations), epigenetic events and/or microenvironmental influences. The stem cell hypothesis proposes that tumour-initiating cells are pluripotent and can thus give rise to progeny of multiple phenotypes; alternatively heterogeneity could be due to stochastic events. Temporal heterogeneity can be exacerbated by therapy (theoretically due to clonal evolution as some clones are eliminated whilst others expand). The significant molecular/genetic differences between cells in different areas within individual cancers, between primary and metastatic tumours (and potentially between cancer cells that successfully colonise different organs) have implications for the reliability of primary tumour biopsies for diagnosis, seeking biomarkers for treatment planning and responses to therapy. In addition, there is substantial inter-tumour heterogeneity. (B) shows images of two patients who presented with breast cancers of identical histological type and biochemical parameters. Four years later, one patient is clear of disease, while the other has evidence of multiple distant metastases, illustrative of between-patient heterogeneity in terms of response to therapy (clinical images kindly provided by Professor William Gallagher, with thanks to Dr Rut Klinger and Dr Donal Brennan (UCD Conway Institute).
Exploring the diversity and inter-tumour heterogeneity of breast cancer has led to the development of a novel classification that integrates genomic and transcriptomic information to classify 10 subtypes with distinct clinical outcomes [ 79 ]. Triple-negative breast cancer (TNBC) in particular is now recognised to demonstrate heterogeneity at the molecular, pathological and clinical levels. [ 80 ]. Such analyses, together with advanced next-generation sequencing have significant implications for improved understanding of basic tumour biology and will potentially enable the identification of new molecular targets for personalised treatment plans [ 81 , 82 ] Additionally, identification of non-coding RNAs is showing potential in diagnosis, prognosis and therapy [ 83 ].
Microenvironmental influences and tumour - host interactions
Breast development is critically reliant upon cell polarity [ 84 ], choreographed cell death pathways and interactions between epithelial cells and stroma; all processes which when deregulated are implicated in oncogenesis and tumour progression [ 85 – 87 ]. The tumour microenvironment, comprising a community of both malignant and non-malignant cells, significantly influences breast cancer cell behaviour [ 88 , 89 ]. Recently, progress has been made in understanding the bidirectional interplay between tumours and surrounding stromal cells/extracellular matrix (ECM), which can potentiate resistance to targeted therapies including endocrine therapy [ 90 , 91 ]. Consequently, components of the tumour microenvironment may represent targets for therapeutic intervention alongside the tumour to improve response to treatment [ 92 ].
Hypoxia reflects dynamic microenvironmental conditions in solid tumours, limits responses to radiotherapy [ 93 ] and some chemotherapeutic and anti-endocrine agents [ 94 , 95 ], drives genomic instability and is generally associated with progression to invasive/metastatic disease [ 96 , 97 ]. Tumour-stromal interactions change under hypoxic conditions to promote tumour progression via the activity of enzymes such as LOX [ 98 ], angiogenic factors and infiltrating macrophages [ 99 , 100 ]. A stem-like breast cancer cell subpopulation with an epithelial-mesenchymal transition (EMT) phenotype is expanded during repetitive hypoxia/reoxygenation cycles [ 101 ]. Hypoxia also contributes to cancer stem cell plasticity and niche formation [ 102 ] potentially explaining the relationship between hypoxia and chemotherapy resistance [ 103 ]. Finally, at the physiological level, host metabolic, inflammatory and immunological factors can impact on cancer development and progression, and these processes are further modified by the physical environments in which we live (Figure 4 ).

Microenvironmental influences on breast cancer. Breast cancer biology, progression and response to therapy is influenced at many levels from epigenetic effects on gene expression (for example methylation) through soluble and cell-mediated stromal interactions, intratumoural inflammatory and angiogenic components, hypoxia, host endocrinological and immunological status through to exposure to multiple agents in the environment in which we live.
What are the key gaps in our knowledge and how might these be filled?
Normal breast development and the origins of cancer.
It is not known how many breast epithelial cell subpopulations function as stem cells (capable of self-renewal) or progenitor cells (which proliferate expansively) [ 104 – 106 ]. Clearer understanding of cell lineages, changes in transcription factor expression during breast development and definition of the nature of stem and progenitor cells is fundamental to delineating relationships between normal and malignant cells.
Current cancer stem cell (CSC) assays have limitations: dormant cells cannot be detected and cell subpopulations that give rise to clones in vivo may not be active in ‘mammosphere’ cultures. There is no clear consensus on markers that define functional breast CSC in mouse and human. Indeed, they may not represent a fixed subpopulation, but instead exist in specific niches in flexible equilibrium with non-CSCs, with the balance depending on interactions between them as well as external selective pressures [ 107 – 109 ]. Understanding this plasticity [ 110 ] and its therapeutic implications are key areas for future investigation.
Breast cancer subtypes: genomics and bioinformatics
Several large-scale, cross-sectional, integrated molecular studies have established comprehensive molecular portraits of invasive primary breast cancers [ 111 – 114 ]. The International Cancer Genome Consortium (ICGC), The Cancer Genome Atlas (TCGA) and individual studies have released sequence data; however, gaining access to and interrogating this information requires expert bioinformatic collaborations. Relating these advances in genomic knowledge to improving clinical care has yet to be achieved. Knowledge of genetic, epigenetic and host factors underpinning distinct subtypes of breast cancer (plus their associated aberrant signalling pathways) and predictive biomarkers will be essential in targeting new therapeutic agents to the right patients.
For ductal carcinoma in situ (DCIS), an increased understanding is required of molecular markers of prognosis, thus providing key information to avoid overtreatment. We need to know which DCIS lesions will recur if adequate surgery is performed with wide, clear margins. Biological markers of DCIS should aim at defining which lesions are likely to progress, in order to avoid radiotherapy or even surgery if the risk of invasive cancer is sufficiently remote [ 115 ]. Markers for response to radiotherapy or endocrine therapy and the need for these therapies (particularly in low-risk patients) remain unclear.
Tumour microenvironment and stromal influences
Paget’s venerable ‘seed and soil’ analogy - recognising that tumour-initiating cells require a permissive host environment to thrive - is beginning to be deciphered at the molecular level. [ 42 ]. The composition and biophysical characteristics of the breast matrisome [ 116 ] and how it controls different stages of gland development and in early breast cancer requires definition. It is important to identify the transcription factors that define luminal and myoepithelial cells and to understand whether additional microenvironmental factors such as the ECM and fibroblast growth factor (FGF), Notch or Wnt signalling can switch their fate. Specialised niches defined by specific cell-cell/cell-matrix interactions in the microenvironment together with soluble, ECM-bound and microvesicle-associated host factors regulate CSC activation [ 117 ]. Further research on such CSC niches, their role in dormancy and the complex relationships between CSCs and metastasis is essential [ 118 – 120 ].
Stromal changes predict early progression of disease [ 121 ] and in-depth knowledge of how these conditions can be manipulated for therapeutic benefit is required [ 122 ]. Advances in the field of mechanotransduction are shedding light on the mechanisms by which altered matrix density or ‘stiffness’ can influence cell behaviour, and enzymes such as lysyl oxidases (LOX) are potential targets for therapy [ 123 ].
There is a need for better biomarkers of hypoxia including gene expression profiles [ 124 ] serum proteins, circulating tumour cells (CTCs) or functional imaging that could be used non-invasively in patients to enable more rigorous testing of its prognostic/predictive value. Although hypoxia-targeted therapies have proven disappointing to date, new approaches are emerging. In common with other targeted therapies for systemic disease, methods for measuring efficacy will need to be redesigned [ 124 – 126 ].
Tumours have an increased dependence on aerobic glycolysis. We need to understand how hypoxia affects the tumour metabolome and thus may determine therapeutic responses [ 96 ]. The dependence of metabolically adapted breast cancer cells on altered biochemical pathways presents new therapeutic targets linked to aerobic glycolysis, acidosis and the hypoxic response [ 127 , 128 ]. Since these pathways also interact with classical survival and proliferation signalling pathways via PKB/mTOR, there are opportunities to develop new combinatorial therapeutic strategies.
Breast cancer development and progression
Mammary stem cells.
There is increased understanding of stem cell hierarchies and their potential roles in breast development [ 129 – 131 ], but debate continues on the relationship between normal stem and progenitor cells, their dysregulation in cancer and the nature of putative CSCs [ 132 – 135 ]. Most data suggest that breast CSCs are a defined population with basal-like or mesenchymal-like features [ 136 – 138 ]. There is emerging data from cell line models that the CSC state is dynamic and can be induced by the tumour microenvironment [ 110 ], and this requires further investigation in human cancers. It is not known whether there are differences in CSC phenotype between breast cancer subtypes such as luminal vs. TNBC [ 139 , 140 ]. An emerging consensus is that CSCs initiate metastases and tumour regrowth after therapy, but do not necessarily generate the majority cell population in primary tumours.
Circulating tumour cells
Blood-borne tumour cells are routinely identified in breast cancer patients but their scoring can depend upon the method used [ 141 ]. Their relationship to disseminated tumour cells (DTCs) in tissues is unclear, although a recent publication showed that the presence of CD44+CD24 -/lo cells (putative CSCs) in the bone marrow is an independent adverse prognostic indicator in patients with early stage breast cancer [ 142 ]. A population of CTCs from patients with primary luminal cancer (expressing EPCAM, CD44, CD47 and MET) generated multi-site metastases when injected into mice. Hence it is likely that a subset of CTCs have metastatic potential [ 143 ], which may equate to CSCs. CTCs may occur in heterogeneous emboli of multiple cell types; perhaps those containing stem-like cells and/or ‘feeder’ cells are more likely to survive and grow at distant sites.
This key hallmark of breast cancer occurs when cancer cells access lymphatic and vascular systems, enabling dissemination via lymph nodes and then via the venous and arterial vascular system to distant organs. Once the disease has spread, it becomes life-threatening and patients require systemic treatment. Metastatic relapse typically occurs many months to decades after surgery, thus we need a greater understanding of the processes that occur following tumour cell dissemination, including the phenomenon of dormancy. Recent mathematical modelling using relapse data has provided interesting insights and proposals for hypothesis testing [ 144 ]. CTCs and DTCs that generate metastases are, by definition, tumour-initiating cells; hence their study needs to relate to CSC research [ 145 , 146 ]. Since the last gap analysis, there has been a paradigm shift in this area with the discovery of ‘pre-metastatic niches’ (analogous to stem cell niches) in organs destined to develop metastases [ 147 , 148 ].
In addition, seminal research using animal models has identified tumour and host genes associated with metastatic capacity (quite distinct from tumorigenic potential), and also organotropism [ 149 – 151 ]. The relevance of these experimental observations to human breast cancer and the translation of these findings into clinical studies require confirmation but may provide additional predictive value [ 152 ].
Reversible EMT, regulated by many factors including transforming growth factor beta (TGFβ) signalling, Slug and Snail transcription factors and hypoxia may be linked to invasion, dissemination and drug resistance [ 153 – 156 ]. The role of EMT in human cancer metastasis is still controversial and the underlying molecular mechanisms are not fully understood [ 157 ]. However, mesenchymal/stromal gene signatures have been identified which relate to TNBC subtypes, bone metastasis and resistance to neoadjuvant therapies [ 158 ].
Circulating tumour cells and nucleic acids
It is unclear whether CTCs originate from primary tumours, micro-metastases or multiple primary and secondary sites. Indeed, CTCs from distant metastases can potentially reseed the primary tumour [ 159 , 160 ]. More research is needed to define the origins of these cells. Importantly, analysis of CTCs needs to be carried out as far as possible in the clinical context, where their biology can be correlated with patient outcomes. CTCs and ctDNA are particularly useful where accessible breast cancer material is not available, or to obtain serial samples during therapy, providing a window on response and relapse.
To enable further progress, systems and protocols for isolating and characterising CTCs need to be rigorously defined and standardised, with an analysis of whether all systems identify/isolate the same cells (or indeed all CTCs, since EMT may preclude identification using epithelial markers [ 141 , 161 – 163 ]). We need to know the proportion of live, quiescent and apoptotic CTCs, their characteristics and malignant potential and to understand their relationship to the primary tumour and whether different subsets of CTCs have different predictive value.
The use of ctDNA is increasing as a potentially useful further source of information on breast cancer biology and response to therapy [ 164 – 166 ]. miRNAs identified in the systemic circulation (free or exosome-associated) [ 167 ] may also serve as diagnostic or prognostic biomarkers and/or as therapeutic targets. Indeed, it has been suggested that exosomes themselves, with their emerging roles in bidirectional signalling, immune suppression, subversion of targeted therapy and potentiation of metastasis [ 168 ] could be removed (for example by plasmapheresis) for therapeutic benefit [ 169 ].
Metastatic disease
Metastasis is the major cause of treatment failure, but it is far from clear why some patients with apparently similar disease succumb and not others [ 170 ]. We need to identify key signalling pathways linked to organotropism [ 171 ] and to develop new therapies for micro-and macro-metastatic disease [ 172 ]. Given the multiple breast cancer subtypes (and associated oncogenic drivers), it will be important to try to align genotypes/epigenotypes to metastatic patterns, in order to predict likely sites of relapse. Treatment decisions are generally based on the profile of the primary cancer, but information about the evolution of the disease from CTC, DTC or (where possible) metastases at different sites is essential, since both gains and losses of potential therapeutic targets have been observed in these distinct tumour cell populations.
We need to understand how the host microenvironment at secondary sites influences tumour cell survival and to define similarities and differences between ‘permissive’ microenvironments in organs favoured by breast cancer cells such brain, bone or liver. We have learned a good deal since the last gap analysis about the ‘vicious cycle’ of bone metastasis, whereby tumour cell interactions within this unique microenvironment mutually promote metastatic outgrowth and bone remodelling via hormonal, immunological and inflammatory mediators. These findings need to be translated into new therapies targeting both tumour and host components [ 173 ] with the paradigm extended to other specialised sites such as brain [ 174 ].
Current therapies
Clinical therapies.
Current clinical therapies for breast cancer are offered on an individual patient basis via a multidisciplinary team and comprise surgery, radiotherapy and drug therapies targeting oncogenic processes. Selection of therapy is based on Level 1 evidence from large RCTs or meta-analyses of such RCTs [ 175 – 177 ]. Increasingly, correlative translational studies are integrated prospectively into clinical trials, aiming to define the optimal target population and provide insight into mechanisms of resistance. The individualisation of treatment, optimal duration of treatments, prediction of metastasis or drug resistance remain challenging and reflect incomplete understanding of the underlying biology of breast cancer. However, up-to-date guidelines are useful to determine the best therapy for individual patients [ 178 ].
Immunohistochemical (IHC) analyses for selecting therapeutic options generally lack reproducibility and standardization resulting in poor concordance between laboratories. The Quality Assurance programme for ER, PR and human epidermal growth factor receptor 2 (HER2) in the UK has to some extent addressed this, but for other biomarkers, including Ki67, there clearly remain problems. We need to develop standardised protocols for better quantification of biomarkers [ 179 ], especially optimised methods of sample collection/storage to ensure that unstable or transient biomarkers (such as phosphoproteins or histone marks) are retained. This is especially important for predictive markers such as HER2, together with those which report on the efficacy of HER2-directed therapies and other emerging targets.
Health inequalities remain in relation to treatment. Older people diagnosed with cancer are more likely to experience undertreatment, potentially having poorer clinical outcomes than younger women for example [ 180 , 181 ]. Indeed, there is a lack of data to inform decision making about treatment for the elderly patient with breast cancer in part attributable to their under-representation in trials, but clinical teams may make inadvertent ageist decisions [ 182 , 183 ]. In addition, breast cancer and its treatment can have a considerable impact on women and their families [ 184 ]. Psychological distress is common, although not inevitable, and is associated with poorer quality of life [ 185 , 186 ]. Regular distress screening is recommended as a core component of good quality cancer care [ 187 , 188 ] in order to provide appropriate support.
Surgery remains the primary treatment for most women, with breast conservation (plus whole breast radiotherapy) providing similar outcomes to mastectomy. Following mastectomy, breast reconstruction should be considered, although uptake is incomplete. Axillary surgery has moved from clearance via node sampling techniques to sentinel node biopsy as the preferred means for assessment of axillary metastasis in early breast cancer. Neoadjuvant therapy, initially implemented to down-stage inoperable cancers, is increasingly used to assess drug efficacy in individuals and to reduce the extent of surgery required in good responders [ 189 ].
Radiotherapy
Radiotherapy is both clinically effective and cost-effective in the adjuvant and palliative settings. The Oxford overview of adjuvant radiotherapy trials [ 177 ] showed a halving of risk of first recurrence in all risk groups and favourable effects of local control on long-term survival. There is long-term confirmation of the value of boost irradiation to the site of excision after breast-conserving surgery in all subgroups, including women >60 years [ 190 ]. The long-term safety and efficacy of hypo-fractionated radiotherapy after breast-conserving surgery and mastectomy for operable breast cancer has recently been confirmed: (10-year results of Canadian [ 191 ] and Standardisation of Breast Radiotherapy (START) trials also suggesting generalisability to all subgroups of patients [ 192 , 193 ].
Trials of partial breast irradiation evaluating intraoperative radiotherapy in comparison to external beam radiotherapy [ 194 , 195 ] or brachytherapy [ 196 ] have short follow-up, but guidelines on partial breast irradiation [ 197 , 198 ] have encouraged off-study use of partial breast irradiation in advance of clinical trial results. Omission of postoperative radiotherapy after breast-conserving surgery in older, lower-risk women suggests the differential in local recurrence rates may be acceptable with a cumulative in breast recurrence of 2.5% in breast conservation surgery alone vs. 0.7% for surgery and postoperative radiotherapy (median follow-up 53 months age 55 to 75 years [ 199 ]) and at 10 years local recurrence, nine for conservation alone vs. 2% for surgery and radiotherapy in the =/>70 years, ER+ve group [ 200 ].
Decision making
Clinical decision-making tools to support individualised treatment can influence patients’ treatment choices and experiences [ 201 ] and communication training for oncology professionals is now widely available throughout the UK to improve the delivery of information and support to patients [ 202 ]. A recent national survey of over 40,000 patients with a broad range of cancers identified the fact that younger patients and ethnic minorities in particular reported substantially less positive experiences of involvement in decision making [ 203 ].
Overtreatment
A significant number of patients are overtreated to achieve the improved survival overall in early breast cancer, since we cannot define individual risks of disease recurrence or sensitivity to treatment. For survivors, the long-term side effects of treatment may be significant; individualised treatment so that patients only receive the treatment they require to achieve cure remains elusive. This is relevant to surgery, radiotherapy, chemotherapy and endocrine therapy.
With the widespread adoption of sentinel node biopsy (SNB)-limiting surgery to the axilla has substantially reduced arm morbidity [ 204 ]. A detailed understanding of underlying tumour biology is required to support decisions around surgical management, (for example axillary node clearance or not after positive sentinel nodes). No further axillary surgery even for one to two positive nodes [ 205 ] and the equivalence of axillary clearance to axillary radiotherapy for local disease recurrence (despite the differing morbidities) in the presence of a low disease burden [ 206 ] demonstrate further progress in this surgical setting. However, the optimal design of radiation treatment fields for SNB-positive patients is not known.
For postoperative radiotherapy after breast-conserving therapy, we do not have reliable ways of identifying low risk, particularly in elderly patients for whom radiotherapy might be omitted. While even low-risk patients have an approximately 50% reduction in first recurrence [ 177 ], the absolute gain for low-risk breast cancer patients (older age, small, ER+ve cancers) after breast-conserving surgery is very modest. We need reliable molecular markers of identifying such low-risk groups or individuals.
Further work is required to clarify whether the response to neoadjuvant chemotherapy can be used to guide the selection of patients for regional nodal irradiation [ 207 ] or whether patients who are clinically node positive before neoadjuvant chemotherapy and are converted to node negative after neoadjuvant chemotherapy on SNB require axillary nodal irradiation.
Individualisation of treatment
Understanding the optimal treatment strategies for an individual patient remains elusive. A number of genomic (for example Mammaprint, Oncotype Dx, PAM50) and immunohistochemical (for example IHC 4) tests have been developed to predict prognosis and latterly, response to chemotherapy; however, prospective trial evidence is still awaited [ 208 ]. Recently, serum metabolite profiling using a combination of nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography-mass spectrometry (LC-MS) correctly identified 80% of breast cancer patients whose tumours failed to respond adequately to chemotherapy, showing promise for more personalized treatment protocols [ 209 ].
Increased understanding of the dynamic changes that occur over time is critical and will require repeated assessment of tumour profiles. Genomic tests predict response to endocrine or chemotherapy and those at highest risk of relapse [ 210 – 212 ], but prospective trials are required to determine whether axillary clearance or chemotherapy can be avoided in node-positive patients. Similarly, biological markers of radiosensitivity (tumour and normal tissue) require better characterisation and implementation into clinical strategies to allow personalisation of treatment and avoidance of late radiation-induced toxicity [ 213 ].
CNS metastatic disease
As a result of improved outcome for patients with metastatic breast cancer (MBC), central nervous system (CNS) metastatic disease is an increasing therapeutic challenge [ 214 ]. Optimal treatment strategies have yet to be defined including sequencing or combination of stereotactic and whole brain radiotherapy, systemic treatments, intrathecal treatment approaches for leptomeningeal disease and prophylactic interventions.
Bone metastatic disease
Bisphosphonates reduce the risk of developing breast cancer in osteoporotic and osteopenic women by approximately 30% and the risk of recurrence in early breast cancer when used at the time of diagnosis [ 215 , 216 ].The interaction between the internal endocrine environment and the effect of bisphosphonates is complex and poorly understood. While negative results overall were reported in the large UK AZURE trial [ 217 ] women more than five years postmenopausal benefitted, consistent with data from the NSABP-34 trial [ 218 ]. In premenopausal women, bisphosphonates can abrogate the bone loss associated with use of an AI. In addition, recurrence and death rates were reduced when used in combination with either tamoxifen or an AI after treatment with the LHRH agonist goserelin (ABCSG12: [ 219 ]. Taken together, these studies suggest that a bisphosphonate may have its greatest effect in a low-oestrogen environment.
The impact of bone-targeted therapy on extra-skeletal metastases and locoregional relapse also highlights the need to better understand experimental observations concerning reseeding of tumours from dormant cells within the bone microenvironment [ 220 ]. Additionally, the role of RANK-RANKL signalling in mammary stem cell biology allows for the possibility that targeting this pathway with agents such as denosumab may offer a prevention strategy for bone metastasis [ 221 , 222 ].
Oligometastatic disease
The role of localised treatment of oligometastatic disease for example in the form of selective stereotactic body radiotherapy, radiofrequency ablation or surgery is currently unclear. The impact of irradiating the primary tumour, biological communications between treated primary site and distant metastases and whether radiation therapy can convert the primary tumour into an in situ vaccine [ 223 ] are relatively unexplored. Prospective randomised trials are required, which should ideally incorporate comprehensive molecular studies to define subtypes most likely to respond; a related question is how to treat primary breast cancer in patients presenting with metastatic disease.
The molecular basis of chemo-radiosensitivity, biomarkers (including specific gene signatures, proteomic markers) of tumour and/or normal tissue sensitivity is required to allow selection of patients who may benefit from adjuvant radiotherapy and avoid toxicity to those who will not. Explanations for the mechanism(s) of favourable impacts of locoregional control from radiotherapy (RT) on survival are needed [ 224 ] and may include in vivo real time biosensors of tumour biology to capture transient changes in the tumour microenvironment that drive metastasis.
Hypofractionated adjuvant radiotherapy
Even shorter-dose fractionation schedules (that is one week of whole breast radiotherapy) might achieve equivalent locoregional control with comparable toxicity [ 225 , 226 ]. Partial breast irradiation appears promising, but the long-term safety and efficacy is still uncertain [ 197 , 198 ]. In addition, it appears likely that there is a subgroup of low-risk, older patients from whom postoperative radiotherapy can be safely omitted [ 227 , 228 ]. The role of postmastectomy radiotherapy in intermediate risk breast cancer [ 229 ], axillary irradiation in sentinel node positive macro- or micro-metastases [ 230 ] or boost dose in DCIS following breast-conserving surgery [ 231 ] are all currently unclear. Further definition of the role of stereotactic body radiotherapy, accounting for tumour motion [ 232 ], in combination with neoadjuvant systemic therapy, to liver or bone metastases for oligometastatic disease are required. Similarly, the optimal dose fractionation for locally advanced disease needs to be established [ 233 ].
Molecularly targeted therapies
Anti-endocrine agents.
Multiple lines of clinical and translational evidence have increased our knowledge of the risk of recurrence, particularly for ER+ve disease [ 212 , 234 – 236 ]. The optimal duration of treatment remains incompletely defined but several RCTs have provided important new data: eight to ten years of adjuvant treatment for ER+ve breast cancers is more effective than five years of letrozole or tamoxifen [ 237 – 239 ].
Endocrine therapy resistance
Comprehensive guidelines to define endocrine resistance have now been agreed [ 240 ]. Clinical studies of various agents alone and in combination with signalling inhibitors have been completed since the last gap analysis. [ 241 – 243 ]. The biology of ERs, including the importance of phosphorylation [ 244 ], ER co-regulators [ 245 ], cross-talk with kinases [ 246 ] and altered ER-binding events [ 247 ] nevertheless requires further elucidation. MicroRNAs regulate ER activity and endocrine responses, [ 248 ], while epigenetic events promote ER loss or tumour suppressor silencing [ 249 ]. Cancer stem cells may also be implicated in endocrine resistance [ 250 ].
The multiple cell-signalling changes driving resistance and associated disease progression, nevertheless reveal potential cancer cell vulnerabilities [ 251 ] for example mTOR [ 72 ], EGFR/HER2 [ 252 ] and Src kinase [ 253 ]. New methodologies such as large-scale siRNA screens have also provided novel therapeutic targets such as CDK10 and fibroblast growth factor receptor 1(FGFR1) [ 254 , 255 ].
Oncogenic signalling inhibitors
Several molecularly targeted therapies have been licensed since the last gap analysis including lapatinib and pertuzumab in HER2+ cancers [ 31 ] and the mTOR inhibitor everolimus in ER+ve disease [ 72 , 256 ], which can overcome endocrine resistance [ 257 ]. Agents targeting signal transduction pathways (notably HER2) have had a significant impact in the treatment of certain breast cancer subtypes [ 258 ]. However, there is still limited understanding of the oncogenic pathways that control the progression of premalignant breast diseases or rare, but often aggressive, breast cancers (for example metaplastic breast cancer) [ 259 ]. Molecules may have distinct functions in different cellular contexts, therefore rigorous target validation is critical [ 260 , 261 ]; if a signalling protein has a scaffold function, disruption of protein-protein interactions may be required for efficacy. This requires a detailed biophysical analysis of protein structures and their key interactions.
For HER-2 positive disease, dual HER-receptor blockade is more effective than monotherapy and may help prevent or overcome resistance [ 262 , 263 ]. Two years of adjuvant trastuzumab offers no benefit over one year [ 264 ] but the utility of shorter trastuzumab therapy is, as yet, unconfirmed [ 265 ]. In metastatic breast cancer, serum metabolomic analyses may help to select patients with HER2+ cancers with greater sensitivity to paclitaxel plus lapatinib [ 266 ]. Multiple clinical trials are evaluating PI3K pathway inhibitors; other new agents under development include HSP90 inhibitors (for example NVP-AUY922 and ganetespib); panHER, irreversible inhibitors including neratinib and afatinib; monoclonal antibodies directed against human epidermal growth factor receptor 3 (HER3) and Src inhibitors such as saracatinib.
Resistance to signalling inhibitors
Resistance to targeted signal transduction agents is common, arising via multiple mechanisms including utilisation of compensatory feedback loops or alternative signalling pathways. Systems biology applications have begun to describe these dynamic changes [ 267 , 268 ], and are critical to identify key target points for effective therapeutic intervention.
Robust guidelines (akin to REMARK) are not yet employed in studies assessing the efficacy of novel therapeutics. Such rigour is essential to ensure that both appropriate models and quantitative outputs are fully utilised. The best drug combinatorial approaches could then be developed based on mechanistic insight into opportunities afforded by synthetic lethality [ 269 , 270 ]. More sophisticated experimental models of DNA-damage response (DDR) defects and those that accurately reflect mechanisms of therapy resistance will enable the design of targeted therapies to overcome these clinically relevant issues.
Drug responses
We lack a comprehensive understanding of the exact mechanisms (both on- and off-target) by which drugs exert anti-cancer effects in vivo ; this is exacerbated by our incomplete appreciation of networks, cross-talk and redundancy in cell signalling. Given that multiple inhibitors of specific pathways are now available (for example PI3K/PKB/mTOR), harmonised approaches to prioritisation of specific inhibitors/inhibitor classes and of research objectives in clinical trials are required.
Clinical determinants of intrinsic and acquired resistance
There is incomplete understanding of the role of diverse gene expression, epigenetic, protein and non-coding RNA changes in the heterogeneous manifestations of clinical resistance, [ 271 ]. There is a lack of equivalence between clinical, pathological, proliferative and molecular resistance that needs to be addressed and single genes or a canonical pathway are unlikely to be responsible. Furthermore, multiple mechanisms have also been implicated in acquired resistance, but their relationship to intrinsic resistance remains to be defined. Figure 5 illustrates the heterogeneity in patterns of gene expression in clinical endocrine resistance, suggesting that at least three major molecular mechanisms could be involved [ 272 ].
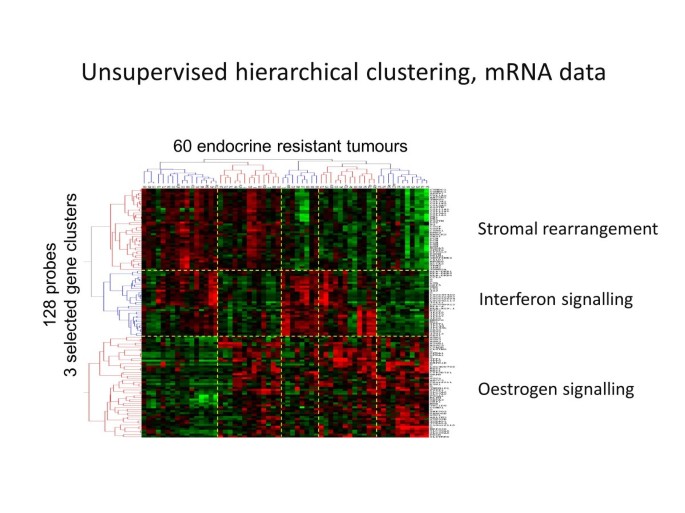
Molecular heterogeneity of endocrine resistance. Unsupervised hierarchical clustering of mRNA from 60 endocrine-resistant breast cancers shows heterogeneity in gene expression suggesting a multiplicity of underlying mechanisms including changes in oestrogen and interferon signalling and stromal genes. Courtesy of Professor William Miller and Dr Alexey Larionov, based on a poster presentation at the thirty-second annual CTRC-AACR San Antonio Breast Cancer Symposium, Dec 10–13, 2009 [ 272 ].
There is a need to understand the clinical impact of additional hormone receptors besides ERα, especially the progesterone receptor (PR): whilst PR is prognostic, the TEAM study has not demonstrated a predictive value [ 273 ]. Similar considerations apply to ERβ [ 274 , 275 ] and the androgen receptor (AR) [ 276 ], since trials of anti-androgens are currently underway in metastatic breast cancer [ 277 ].
It is not clear whether there are differences in ER+ve premenopausal vs. postmenopausal endocrine resistance [ 278 ]. As with other targeted therapies, the microenvironment, therapy-induced signalling reprogramming and stem cells are likely to play key roles. Proteomic profiling and protein functionality are particularly poorly characterised in the clinical resistance setting and such measurements remain challenging but essential.
It is important to define the contribution of CSCs to relapse on endocrine therapy, determine their sensitivity to existing agents or identify the unique signalling pathways that sustain their clonogenic potential. Diagnostic or prognostic tests based on ‘whole’ tumour samples may fail to address these potentially significant minority subpopulations of cells.
The few prospective studies to date have demonstrated that changes in management for one in six patients could be advised based on changes in breast cancer biomarkers on relapse, particularly ER, PR and HER2 [ 279 – 281 ]. Consequently, important clinical questions such as whether changes in the frequency of drug administration or alternating drug therapy could avoid or contribute to this process need to be addressed. Considering host factors such as adherence to medication [ 282 ], drug metabolism [ 283 ] and immune mechanisms [ 284 ], alongside molecular characteristics of tumours and the host microenvironment is essential.
Combinations and sequencing of targeted agents with conventional agents
Despite high-level evidence for isolated treatment situations (for example adjuvant treatment with AIs) [ 210 , 285 , 286 ], these have not been integrated into sequential treatment strategies, for example for adjuvant or first- or second-line palliative treatment. As treatment standards change (with AIs as standard adjuvant therapy), the sequence of tamoxifen as adjuvant therapy with AIs for first-line metastatic ER+ve disease may require adaptation. Such trials apply standard treatments that manufacturers may have little interest in supporting; new ways of supporting these trials will need to be explored.
Models are needed for the longitudinal study of hypoxic ‘microniches’ to inform timing of delivery of sequential targeted therapies or chemotherapy with radiation; to test real-time robotically controlled RT delivery to motion-affected hypoxic regions of primary breast tumours; and RT in combination with novel agents targeting pH regulatory mechanisms. Similarly, novel early-phase clinical trials of preoperative RT + targeted therapy or neoadjuvant hormonal therapy with baseline on-treatment biopsies for markers and gene signatures of radiosensitivity (the window of opportunity design) could complement the development of trials of stereotactic body RT to primary + neoadjuvant systemic therapy for limited-volume metastases in liver and bone.
Practical considerations include the risk/benefit of combining signalling inhibitors with anti-hormones, sequencing of tamoxifen and AIs [ 287 ] and targeting additional steroidogenic enzymes [ 288 ]. Recent randomised clinical studies have demonstrated substantial benefits for combinations of targeted agents such as endocrine therapy and mTOR inhibitors in ER+ve MBC [ 72 ] or horizontal dual HER-receptor blockade [ 289 – 292 ]. This results in several new challenges. Many patients benefit from single agent endocrine therapy or HER2-blockade and could avoid, at least initially, the toxicity of combination therapy if these cancers could be identified. There is a clear need to identify patients who respond adequately to targeted therapy (for example anti-HER-2 agents +/− endocrine agents) and do not need chemotherapy. Rational combinations need to be explored in the appropriate setting, taking into consideration compensatory induction of alternative signal transduction pathways bypassing targeted treatments. Treatment benefits in MBC or the neoadjuvant setting need converting into a potential survival benefit in early breast cancer.
New therapeutic approaches
Although phenotypically similar to BRCA1 mutant breast cancers, TNBC are heterogeneous and lack of expression of ER, PR and HER2 is not a good predictor of homologous recombination repair (HRR) status [ 293 ] Prognostic and predictive biomarkers of response for TNBC are obvious gaps which need to be addressed [ 294 ], complemented by an expanded and representative panel of fully characterised tumour cell lines and models [ 295 ]. More emphasis should be directed at developing markers of drug resistance and markers of resistance to current basal-like breast cancer/TNBC therapies [ 296 ]. Better biomarker-led characterisation could assist in patient stratification and hopefully improved treatment responses. Similarly, additional targets are required for other molecular subtypes that fail to respond to existing therapies.
Lymphangiogenesis and angiogenesis
Current understanding the role of lymphangiogenesis in metastasis (and thus its potential as a therapeutic target akin to neoangiogenesis) is limited [ 297 ]. In contrast, given the morbidity associated with lymphoedema following extensive lymph node dissection, identifying a means of inducing local regeneration of lymphatic vessels postoperatively could be envisaged. The contribution of the lymphatic system to immune responses to tumours is also underexplored [ 298 ]. Better in vitro and in vivo models are required to understand the cellular and molecular complexities of pathological angiogenesis and lymphangiogenesis, tumour cell intravasation, extravasation, organ colonisation and strategies for effective therapeutic interventions [ 299 ].
Anti-angiogenic therapies have been extensively trialled but have not yet lived up to their promise, with bevacizumab no longer approved for breast cancer by the FDA [ 300 – 302 ]. Tumour vasculature is heterogeneous [ 303 ] and multiple, temporally dynamic mechanisms contribute to the lack of durable responses [ 304 ]. The main focus has been vascular endothelial growth factor (VEGF)-driven angiogenesis but there is considerable redundancy in angiogenic signalling pathways [ 305 ]. Also, there are no validated biomarkers of response to anti-angiogenic therapies and it is likely that the vasculature of anatomically dispersed metastases will demonstrate further functional heterogeneity.
Exploiting the immune system
Although generally considered to be immunosuppressive, some chemotherapeutic agents (and indeed monoclonal antibodies) may involve an immune element; thus the combination of immunotherapy and chemotherapy becomes a real possibility [ 306 , 307 ]. In node-positive, ER-/HER2- disease, lymphocytic infiltration was associated with good prognosis in the BIG 02–98 adjuvant phase III trial [ 284 ]. There needs to be a systematic quantification of immune infiltration of breast cancer subtypes and how this relates to tumour progression, response to therapy or changes during treatment.
Cancer immunotherapy is gaining ground, whether antibody-based or cell-based, with an increasing emphasis on targeting the tumour microenvironment (for example macrophages or cancer-associated fibroblast (CAFs)) with DNA vaccines [ 308 ]. In addition, several immunogenic antigens (such as cancer testis antigens) have been detected in poor-prognosis breast cancers, which may serve as targets for therapy or chemoprevention [ 309 , 310 ]. New strategies for enhancing natural immunity or eliminating suppressor functions are required. There is a need for better animal models for evaluating immunotherapeutic strategies and in deciphering possible contributions to lack of responsiveness.
Living with and managing breast cancer and its treatment
Survivorship.
Cancer and its treatment have a considerable and long-term impact on everyday life [ 311 – 313 ]. Consequences may be physical (for example pain, fatigue, lymphoedema, hot flushes, night sweats and sexual problems), or psychological (cognitive function, anxiety, depression, fear of recurrence) and directly affect relationships, social activities and work. The relationship between the cancer patient and his/her partner will have a bearing on the level of distress: if communication is good, psychological distress will be lower [ 314 ]. Women may feel abandoned once treatment is completed with low confidence as a result [ 312 , 315 ]. The current system does not meet their needs [ 184 ] and the National Cancer Survivorship Initiative has been established to investigate new models of aftercare.
A recent framework publication highlights the importance of providing support to enable people to self-manage their aftercare [ 315 ]. Patients benefit from improved sense of control and ability to effect change together with an increased likelihood of seeking health information [ 316 , 317 ].
Living with advanced breast cancer
Quality of life in women with metastatic breast cancer is poor [ 318 ] with many experiencing uncontrolled symptoms [ 319 ]. Pain is a significant problem throughout the illness, not just with the end of life [ 318 ]. Depression, anxiety and traumatic stress also require intervention [ 320 , 321 ]. Those with metastatic breast cancer receiving social support report more satisfaction and a sense of fulfilment. Fewer avoidance-coping strategies are associated with better social functioning and a larger social network. Social stress has been found to increase pain and mood disturbance and has been associated with isolation. In addition, self-image and a decrease in sexual functioning challenge self-esteem and relationships at a time when support is most needed [ 322 ].
The impact of medical management on quality of life and decision making regarding palliative chemotherapy [ 323 , 324 ] and a lack of rehabilitation services [ 325 , 326 ] has been recognised. The convergence of palliative treatments and the end of life may impact on symptom control and care provision as well as place of death [ 327 , 328 ].
Supportive interventions
The main physical symptoms associated with breast cancer treatment are fatigue, pain, hot flushes, night sweats, cognitive and sexual problems and lymphoedema. Some interventions have demonstrated benefit with specific side effects [ 329 – 331 ]. Meta-analysis demonstrates that psychological interventions can reduce distress and anxiety [ 332 ], provide some physiological benefit, but with weak evidence regarding survival benefit [ 333 ]. Overall the evidence focuses on short-term benefit while the longer-term implications are unknown.
Group interventions are less effective in reducing anxiety and depression than individualised interventions such as cognitive behaviour therapy (CBT); [ 334 ], but do result in social and emotional improvements [ 335 ] and greater patient satisfaction [ 336 ]. Psycho-educational interventions show improvements in physical and psychosocial wellbeing [ 337 ] and reduced anxiety [ 338 ].
CBT reduces fatigue [ 339 ], insomnia [ 340 ] improves physical activity and quality of life [ 341 ]. CBT appears to be effective at all stages of breast cancer: group CBT can significantly reduce the impact of menopausal symptoms in breast cancer patients [ 342 , 343 ] with effects maintained over six months. Care packages to help improve coping skills, including group counselling sessions and/or telephone-based prompts has shown supportive care in the extended and permanent phases of survival to be effective [ 344 ]. Mindfulness-based stress reduction and cognitive therapy can improve mood, endocrine-related quality of life, and wellbeing at least in the short term [ 345 ].
Much evidence demonstrates the benefits of physical activity for breast cancer patients [ 346 ]. RCTs show that physical activity interventions during treatment show small to moderate beneficial effects on cardiovascular fitness, muscular strength and can reduce deconditioning. Post treatment, physical activity interventions result in a reduction in body fat and increase in fat-free mass, a moderate to large effect on cardiovascular and muscular strength, small to moderate effect on quality of life, fatigue, anxiety and depression and some evidence of reduced lymphoedema and osteoporosis [ 347 , 348 ].
The translation of physical activity research into clinical practice is a challenge. Currently, exercise-based cancer rehabilitation is not routinely incorporated into breast cancer care. However, from the National Cancer Survivorship Initiative, Macmillan Cancer Support is evaluating around 12 physical activity programmes and evaluating physical, psychological and cost benefits. One exercise intervention during therapy reassessed participants after five years and showed that those from the exercise group were still incorporating approximately 2.5 hours more physical activity a week and were more positive than control patients [ 349 ]. Furthermore, other charities are starting up similar programmes, such as Breast Cancer Care’s ‘Best Foot Forward’. There are very few intervention studies involving women with advanced metastatic cancer; these predominantly focus on supportive-expressive therapy and have been found to reduce distress [ 350 ] but the benefits are not maintained in the long term [ 334 ].
Inadequate translation of research findings into practice
While the problems are well recognised, there is inadequate clinical translation: for example, recognising the benefits of physical activity requires incorporating and testing intervention(s) in clinical practice. There is also a lack of representation and sensitivity to the needs of diverse groups. Similarly, the impact of breast cancer goes beyond the patient; more attention should be paid to their families, partners and children.
CBT is becoming integrated into clinical practice with training for clinical nurse specialists but there is still a need to consider how CBT and other interventions can be better integrated to widen access. Novel interventions must be developed and validated using methods based upon sound theoretical principles, with demonstrable effectiveness (both clinical and financial) that can be deployed as widely as possible to maximise benefit. A clear understanding of the components of interventions that promote uptake, adherence and long-term benefit is required. Funding for research into living with and managing the consequences of breast cancer and its treatment is very limited, adversely impacting the building of research capacity and expertise.
Establishing a multidisciplinary research consortium to develop a theoretical framework to inform research addressing the needs of those living with and managing the broad ranging consequences of breast cancer and its treatment would inform choice of outcome measures, innovative approaches to intervention design and testing. Alternative trial designs to RCTs need to be considered that incorporate patient preferences. It would also be of great benefit to the field to draw up guidance on implementing successful evidence into clinical practice.
Longitudinal studies are required to assess the recovery of health and wellbeing and the long-term adjustment of women and men who have a diagnosis of breast cancer. This will allow investigation of how unmet psychosocial needs and psychological morbidity during diagnosis and treatment relate to quality of life, sexuality, physical wellbeing and the effects of other illnesses later in life. The long-term impacts of breast cancer and therapy on everyday life need further investigation [ 351 ]. There are implications for cardiac functioning, osteoporosis, neuropathy, cognitive dysfunction, lymphoedema and shoulder mobility on the ability to maintain independence [ 352 ].
There is insufficient epidemiological data on the problems of women who have recurrence and metastatic disease. Research into integrated oncology and palliative care models are needed to determine which approaches improve quality of life, psychological wellbeing, palliation of symptoms, treatment decisions and end of life care. The needs of the families of women with advanced metastatic cancer and how to support them and their carers most effectively are unclear. Decision making at the end of life and the development of tools to assist women and healthcare professionals to choose appropriate treatment and place of death is needed.
Specialist breast care nurses have also been found to enhance the supportive care of women with metastatic breast cancer. [ 353 ]. However, there is a need to identify the active components of interventions and an individual’s preference for different types of interventions to determine what works best for him or her.
Development of mindfulness and third-wave approaches (for example Acceptance and Commitment Therapy) may be effective. More RCTs of theory-based interventions for treatment-related symptoms and innovative trial designs are needed (with longer follow-up, analysis of moderators and mediators and identified components) to support women to manage their everyday lives. Interventions to address specific psychological needs such as low self-confidence and fear of recurrence also need to be tested. Interventions are required to support women to increase their physical activity, reduce the risk of recurrence and examine the impact on late effects. The frequency, intensity, type and timing of physical activity for maximum benefit needs to be established. Effective means are required to support women to manage impaired sexuality/sexual function, altered body image, lymphoedema, weight gain [ 354 ], fear of recurrence, hormone therapy-related symptoms [ 341 , 343 , 355 , 356 ], cognitive problems [ 357 ][ 358 ] and post-surgical problems [ 359 , 360 ]. Alternative delivery of intervention needs to be explored, such as self-management, telephone or online support and non-specialist delivery: for example comparison of home-based versus hospital-based interventions on physical activity levels, patient satisfaction and motivation.
Strategic approaches to enable progress
Experimental models of breast cancer, improved tissue culture models.
There is now a greater appreciation of the importance of employing appropriate human cancer cells. [ 361 ]. Commonly used breast cancer cell lines are derived from metastases or pleural effusions and fail to adequately represent the diversity and complexity of breast cancer [ 362 ]. It has proven difficult to establish human tumour cell cultures representative of the major subtypes and to maintain their genomic and phenotypic integrity. In addition, inter-patient variability and inadvertent selection of the most malignant subtypes, skews availability of representative material.
Better representation of breast cancer subtypes is required. Material from normal mammary tissue, premalignant breast conditions, different ER+ve (and rare) subtypes of breast cancers and ideally metastases from all major sites are needed to cover the full spectrum of breast cancer development and progression. Primary or minimally passaged cell cultures will avoid issues of misidentification, contamination or long-term culture artefacts. Ideally, a central repository of well-annotated human primary breast cancer cells, associated host cells and cell lines should be available to researchers linked to a searchable, open-access database. Maintaining breast tumour tissue in culture with its essential characteristics intact will enable prognostic screening and testing of potential therapeutic agents.
Reliable cell-type-specific markers are required and it is also important to be able to recognise cancer stem cell subpopulations (or transient phenotypes). Identification of promoters for distinct cell subpopulations will enhance the number and scope of available in vitro models. [ 363 ] and enable conditional genetic modifications for mechanistic and target validation studies [ 364 ]. Ideally, co-cultures (of both normal and precancerous breast cells) with host cell populations such as fibroblasts, myoepithelial cells, macrophages, adipocytes or vascular endothelial cells are needed for studies of cellular interactions within the appropriate ECM microenvironment.
Three-dimensional culture models can recapitulate the tissue architecture of the breast and its characteristic invasion patterns [ 89 , 365 ] especially if host stromal components are incorporated [ 366 ]. Three-dimensional heterotypic model systems are also enabling dissection of the effect of cell-cell interactions and stromal elements in drug resistance. Three-dimensional cultures require additional refinement, higher throughput, quantitative assays [ 367 ] and a move towards more physiologically relevant conditions, for example by the use of bioreactors, enabling long-term cultures under flow conditions; especially appropriate for invasion assays [ 368 , 369 ].
Animal tumour models
In the last five years there has been an expansion in the use of orthotopic (anatomically correct) breast cancer xenografts [ 370 ] and significant advances in developing patient-derived xenografts (PDX) [ 371 ]. These models better reflect the human cancers from which they were derived and ER+ve tumours respond appropriately to oestrogen ablation [ 372 ]. Increased use of genetically engineered mouse (GEM) models driven by relevant abnormalities such as BRCA mutations, HER2 overexpression and so on have enabled the study of naturally occurring tumours in immunocompetent hosts and evaluation of new targeted therapies such as PARP inhibitors and the emergence of resistance [ 373 ]. Pros and cons of different models are shown in Figure 6 .
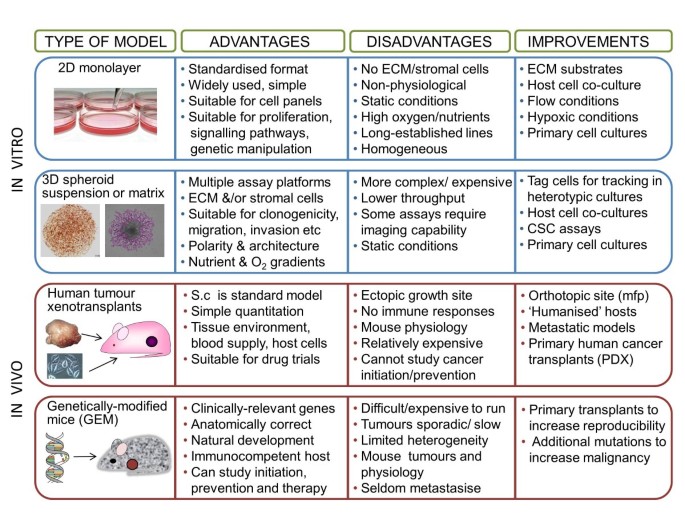
Comparative properties of experimental tumour models. In vitro assays of tumour growth and response to therapy can be conducted in two dimensions or three dimensions - the latter more closely approximating the biology of solid tumours than a simple monolayer. Cultures can be enhanced by the addition of matrix proteins and/or host cells and can be adapted to measure not only tumour cell proliferation, but also additional cancer hallmarks such as invasion. Standard in vivo assays depend upon the transplantation of established human tumour cell lines into athymic (immune-incompetent) hosts. These models are relatively simple and easy to use, but are increasingly complemented by genetically engineered mice harbouring targeted genetic mutations which render them susceptible to developing mammary cancers. The figure summarises key advantages and disadvantages of each model and means by which their clinical relevance and utility might be enhanced. Based on a figure provided courtesy of Claire Nash in Dr Valerie Speirs’ group (University of Leeds).
Expansion of PDX models will be required to cover all the main breast cancer phenotypes [ 374 ] and to address the contribution of ethnic diversity [ 375 ]. Advanced GEM models with multiple genetic abnormalities, able to generate both hormone sensitive and insensitive tumours and in which metastasis occurs at clinically relevant sites will also be a desirable refinement [ 376 , 377 ]. However, all such animal models will require validation of any findings in the clinical setting [ 296 , 378 , 379 ]. Models are also required to investigate mechanisms of the induction of (and escape from) long-term tumour dormancy [ 380 ], a unique feature of breast cancer.
Invasive behaviour does not occur uniformly or synchronously within a tumour [ 381 ] and this heterogeneity is not easily reproduced in vitro . Improved tumour models and methods are required to understand the localised and possibly transient factors involved in temporal and spatial heterogeneity that promote invasion and metastasis.
Models for testing novel targeted agents against disseminated disease
Novel agents designed for systemic administration are rarely tested against established invasive/metastatic disease in preclinical animal models [ 382 , 383 ]. There is an urgent need to develop better models for the discovery and development of therapies targeting metastases that are effective against all sites of disease [ 384 ].
In around 20% of women, complete resection of primary tumours does not prevent distant metastases because dissemination has already occurred. In these cases, agents targeting cell motility or invasion may have limited value. It is therefore critical that preclinical models used for testing such therapies incorporate established micrometastases [ 385 ]. Similarly, there is a preponderance of lung metastasis models in routine use. Other important sites of breast cancer metastasis (for example bone, brain and, liver) are relatively poorly represented, and this needs remedying in preclinical drug evaluation [ 386 – 388 ]. Human tissue (such as bone) transplanted into mice can provide a more relevant microenvironment [ 389 ].
Preclinical or clinical trials focused on tumour shrinkage are not appropriate for testing the efficacy of anti-invasive or anti-metastatic agents that may reduce metastasis without significantly impacting primary tumour growth [ 390 ]. Such approaches would likely fail current response evaluation criteria in solid tumors (RECIST) criteria and show little activity in the neoadjuvant setting or in late stage patients with advanced metastatic disease. The potential to utilise veterinary models for testing novel therapies or RT-systemic therapy combinations and cross-disciplinary collaboration with other scientific disciplines to develop real-time in vivo biosensors of tumour biology offer novel opportunities for significant progress.
Modelling drug resistance
While challenging, establishing cell lines, tissue slice models and PDX from relapsed and resistant cancers should be the ultimate goal in order to provide a window on the mechanisms that occur in patients where therapies fail. This would also allow ex vivo targeting studies, employing signalling analyses and imaging systems to track resistance mechanisms and progression.
Preclinical endocrine resistant models have largely been derived from ER+ve MCF7 cells in vitro , either by transfection of potential signalling molecules such as HER2 or from continuous exposure to anti-endocrine agents. Extensive panels of relapsed human tumour cell lines are required to reflect the heterogeneity of clinical resistant disease. This will allow assessment of the impact of genetic background, duration, sequence and type of endocrine agent (including AI) and rational evaluation of agents to reverse resistance [ 391 ]. It is critical to validate mechanisms identified in vitro with clinical resistance.
Longitudinal clinical samples and associated biological studies
Biobanking has substantially improved and is seen as a significant outcome of the last gap analysis [ 7 ] but the systematic analysis of clinical material collected from serial tumour biopsies/ fine-needle aspiration (FNA) (or ideally less invasive means such as ‘liquid biopsy’) before, during and following resistance development is lacking. Procurement of matched materials remains challenging but is critical to establishing clinically relevant signalling mechanisms that culminate in acquired resistance, allowing tracking of the dynamics and prevalence of molecular events during response through to any subsequent relapse. Care must be taken to provide adequate sampling of inherently heterogeneous tumours in their primary, recurrent and disseminated settings, which may also provide material for study of site-specific metastasis. [ 392 ] and samples must be full annotated, ideally with ‘omics’ profiling and immunohistochemistry. The biopsy of metastatic lesions is challenging and will require systematic introduction of a ‘warm autopsy’ programme [ 393 ]. A more realistic alternative is to further exploit the preoperative neoadjuvant setting, despite the potential issues of heterogeneity and sampling [ 394 ]. Collection of such samples is a particularly valuable resource to address mechanisms of intrinsic resistance and to track early therapy-associated signalling changes (Figure 7 ).
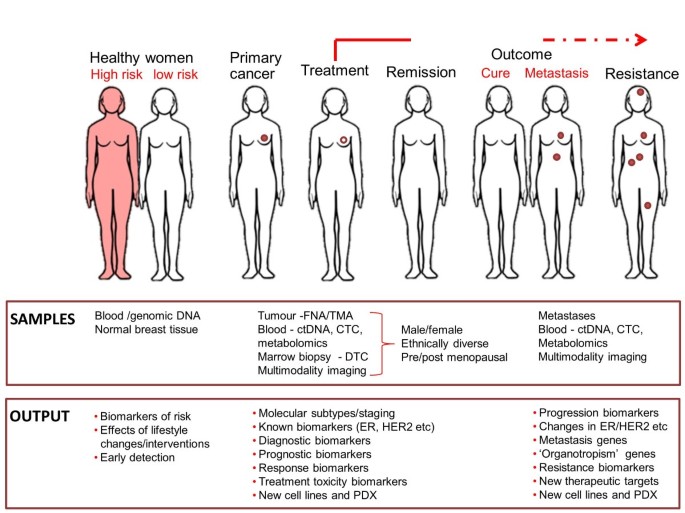
Longitudinal sampling and enhanced biobanks. The longitudinal collection of blood and samples from normal breasts, primary cancers and relapsed/metastatic/treatment-resistant disease is essential in order to address the origins, heterogeneity and evolution of breast cancers. Samples are required from as broad a patient population as possible to understand ethnic, age-related and gender differences in incidence, molecular subtypes, prognosis and response to treatment. Sequential samples (ideally patient-matched) from primary tumours and metastases will enable detailed studies of tumour evolution/progression and provide material for generating new cell lines and patient-derived xenografts for translational research. Multimodality imaging and metabolomic analyses will add further dimensions of valuable information. Based on a figure provided courtesy of Professor William Gallagher, with thanks to Dr Rut Klinger (UCD Conway Institute).
Increased use of clinical relapse material will determine the relevance of preclinical findings and identify potential candidates for detailed mechanistic evaluation in appropriate tumour model systems. Ultimately the goal is to determine if patients can be better stratified to allow rational, personalised choices for further therapy. This aspiration requires better integration between clinicians and scientists, trial providers and pharmaceutical companies and would benefit from data sharing. Tissue-based analyses from clinical trials need to be expanded to incorporate all of the next generation sequencing studies for research. These initiatives need to be co-ordinated with cancer registry/ British Association of Surgical Oncology (BASO) breast cancer data.
Blood samples for early diagnosis, monitoring treatment response, early indicators of disease relapse (and revealing increased heterogeneity) are imperative as our ability to generate new biomarkers through emerging technologies increases. These include detection of CTCs, miRNAs, ctDNA, exosomes, and so on. Serum HER2 measurement may be another promising biomarker with prognostic and predictive value [ 395 – 398 ].
Biomarkers of response or relapse
With the exception of ER and HER2, the availability of biomarkers to accurately identify which patients will receive benefit from targeted treatment, and indicators of patients at high risk of progression or relapse remains limited. Further advances in molecularly targeted and anti-endocrine therapy require clinically applicable predictive biomarkers to enable appropriate patient recruitment and to track responses to treatment [ 399 , 400 ]. These analyses should be applied both to primary tumours and recurrent/metastatic lesions to accommodate the profound heterogeneity within individual cancers, which increases further during disease progression. Understanding which molecular markers are ‘drivers’ of breast cancer and their functional roles at different stages of disease will be key to designing more effective targeted agents.
Validation of predictive markers for drug response could be better facilitated by the routine inclusion of such approaches into clinical trials rather than retrospective analyses of archived material. Any new biomarkers should have well-defined cut-off points, be thoroughly validated and robust. We require biomarkers to identify patients who will not respond to trastuzumab (primary resistance) in addition to the development of secondary acquired resistance. Discriminatory biomarkers are required for combination therapies such as lapatinib and trastuzumab in HER2-positive breast cancers. We lack preclinical data that can predict which combination of anti-HER2 therapies is optimal. There is also a need for biomarkers that can identify patients who may be more suitably treated with a tyrosine kinase inhibitor (TKI) rather than trastuzumab or combination anti-HER2 therapy. New irreversible TKIs currently in clinical trials, (for example afatinib and neratinib) have shown increased potency in preclinical studies - could these now become the mainstay for HER2-positive tumours?
Knowledge of the therapeutic benefits of mTOR inhibitors and of newer PI3K pathway inhibitors in breast cancer subtypes is rudimentary and we have no biomarkers that can be used to optimise their therapeutic index. In addition, knowledge of how important genomic (for example PIK3CA mutations) and proteomic (for example PTEN loss) biomarkers impact the efficacy of specific PI3K pathway inhibitors in the clinical setting is limited. Further preclinical research on the functional proteomic effects of genomic abnormalities in the PI3K pathway in breast cancer is essential.
ER+ve tumour heterogeneity remains a challenge: luminal A vs. luminal B subgroups impact on prognosis; however, the mechanisms of endocrine failure remain largely unknown. In ER+ve disease there is a lack of accepted biomarkers/signatures to distinguish endocrine-sensitive patients from those with intrinsic insensitivity or who will develop early or late resistance.
There is a need to develop non-invasive means of detecting risk of subsequent relapse. In addition to serial tumour samples, serum samples are warranted as these may ultimately provide less invasive indicators of acquisition of resistance. It remains unclear if single or multiple biomarkers or transcriptional profiles are optimal, or even if basic endocrinological markers may prove valuable in the context of predicting resistance.
While imaging (at least with some modalities) is routinely applied to the early detection and follow-up of breast cancers, there is a need to increase the use of functional screening techniques to better understand tumour heterogeneity, identify features associated with response or resistance to treatment and more rapidly translate promising new preclinical methodologies to clinical evaluation. It is important to evaluate emerging imaging biomarkers of primary and metastatic breast cancer and there is a requirement for new, more specific and clinically translatable radiotracers for positron emission tomography/single-photon emission computed tomography (PET/SPECT) [ 401 , 402 ]. We also need to identify and assess the utility of imaging biomarkers associated with other hallmarks of cancer beyond proliferation for example invasion, altered metabolism, hypoxia. Attention needs to be given as to how to validate novel imaging biomarkers in adequately powered multi-centre clinical trials. The funding available from most grant-awarding bodies is insufficient to cover this, suggesting the need to consider larger collaborative trials funded by more than one agency.
Imaging may also be able to report on intratumoural heterogeneity and identify the most significant region (for example more aggressive/invasive areas via diffusion-weighted magnetic resonance imaging (MRI)), to more accurately direct biopsies or radiotherapy. EMT could be addressed by the increased use of cluster, histogram and/or texture analyses, but it will be necessary to define the correct metrics to assess and quantify such phenotypes [ 403 ]. It would be desirable to extend these techniques to define different tumour subtypes such as DCIS, luminal or TNBC non-invasively (which may identify mixed lesions missed by homogenised or limited sample analyses) and assess heterogeneity between metastases. Ideally, imaging studies (both preclinical and clinical) should be co-registered with linked genomic and proteomic information in order to fully interpret the biological relevance of the images obtained [ 404 – 406 ]. However, tissue collection is often not co-ordinated with imaging studies and the added benefit not always appreciated.
A key achievable goal is to non-invasively evaluate predictive biomarkers of therapeutic responses. Increased adoption of more clinically relevant orthotopic xenograft and transgenic murine models of primary and metastatic breast cancer will demand robust preclinical imaging approaches. The use of such models in imaging-embedded trials of novel agents will improve the accuracy of preclinical data, accelerating the development of promising drugs, or enabling early closure of suboptimal programmes. Such refined preclinical trial designs will also prove highly informative in establishing combination and/or sequential treatment regimes.
Clinical trial design and patient involvement
Clinical trial design should be adapted to use preoperative and neoadjuvant models to allow novel therapies to be tested in patients [ 394 , 407 ], identify de novo resistant cancers and investigate how such resistance can be counteracted. These approaches are particularly relevant for therapeutic strategies that target cancer stem cells, residual (dormant) cancer cells or influence the tumour microenvironment. Future trial design will also have to incorporate dynamic strategies, such as using the response to short-term treatment to guide the use of additional preoperative treatment. Given the increasing focus on small target populations (for example molecular subtypes of breast cancer), clinical trial strategies for effective patient stratification or selection based on molecular characteristics are required to allow routine integration into large-scale clinical trials. In addition, the relatively long period between surgery and relapse in breast cancer patients impacts negatively on the economic feasibility of such clinical trials. New thinking will be required to modify clinical trial design, and to consider biomarkers that relate to invasive and metastatic phenotypes, for example as in trials with denosumab where the development of skeletal-related events (SRE) was an accepted and measurable endpoint [ 221 ].
Patient reported outcomes
There is a need to incorporate standardised patient-reported outcome measures (PROMs) both within clinical trials and in everyday clinical practice. Currently, many trial reports are reliant on the common terminology criteria for adverse events (CTCAE) gradings about side effects, which show alarming discrepancies with data actually collected from patients [ 408 ].
Further research is needed to support the use of decision aids around surgery and treatment and to define any benefits. There is also a need for prospective research to identify consequences of treatment and the impact of co-morbidities on the lives of women with breast cancer so that future patients can consider these as part of their decision making. The experiences of minority ethnic groups, younger (<45 years) and older (>70 years) women in relation to their treatment choices and management need further research. Addressing non-adherence to endocrine therapy and understanding the biological mechanisms of significant side effects such as menopausal symptoms are poorly understood. The value of incorporating lifestyle recommendations as part of routine care and its impact on recovery and quality of life should be further explored.
Multidisciplinary collaborations and resources
Increased resources are required to support core (for example biochemical/IHC) as well as new ‘omics technologies; to develop improved in vitro / in vivo / ex vivo model development, serial clinical sample collection, advanced bioinformatic/systems biology analysis, clinical biomarker validation and ‘bench to bedside’ drug development. Stronger multidisciplinary collaborations between laboratory scientists, clinicians, bioinformaticians and engineers (and in turn with funding bodies and industry) must be encouraged. Much better integration of computer science, database engineering, data analytics and visualisation, hardware and software engineering within biological research will be essential to effectively read and translate increasingly complex data. Convincing drug companies of the benefits of a co-ordinated approach (tissue collection before, during and after treatments) in clinical trials of new drugs is problematic, and access of material for research purposes is limited. Companies must be convinced of the benefits of accurate biomarkers to allow for the better stratification of patients. Even though this will limit their target population, this should be offset by higher response rates and faster regulatory approval.
Continued support is required for basic biological research and understanding of cell signalling processes with emphasis on interactions, cross-talk and microenvironmental regulation. It is important that approaches in this area are linked to systematic investigations and precise analyses of cell responses to a wide range (and combination) of inhibitors, tested in clinically relevant breast cancer model systems. A key element is open discussion and learning from negative results to avoid unnecessary duplication of research. Sharing of information, best practice, optimised model systems, technologies and resources is essential, perhaps through developing web-based analysis portals. Such approaches are needed to integrate and interpret diverse sources of data to understand the plasticity of signalling emerging during treatment though to resistance (Figure 8 ).
Integrated vision of multidisciplinary research. Enhanced integration and utilisation of the vast amount of clinical and experimental observations relating to breast cancer is urgently required. Clinical observations generate hypotheses relating to the origins of cancer, its underlying molecular pathology and potential vulnerabilities that could be exploited for therapeutic benefit. Such insights provide opportunities for testing and validation in in vitro, in vivo and in silico models. Drug discovery aims to provide inhibitors of major oncogenic ‘drivers’ for use singly or in combination with conventional therapies; such personalised medicine requires the co-development of predictive and pharmacodynamic biomarkers of response. Results from preclinical therapy studies and clinical trials should be fed back into searchable databases to reveal reasons for treatment failure and allow new strategies to be tested and deployed. Based on a figure provided courtesy of Professor William Gallagher, with thanks to Professor Walter Kolch (UCD Conway Institute).
A co-operative network of advanced radiotherapy facilities, analogous to the Experimental Cancer Medicine Centres is needed to ensure adequate patient numbers for clinical trials. Engaging patients and healthcare teams is critical to enable complex biological studies (especially longitudinal biomarker studies). Lack of academic clinicians (particularly in radiation oncology), radiobiology and physics staff nationally and rising service pressures on NHS staff are all detrimental to delivery of clinical translational research.
While substantial advances have been made in breast cancer research and treatment in the last five years, there remain significant gaps in translating this newly acquired knowledge into clinical improvements.
Understanding the specific functions and contextual interactions of genetic and epigenetic advances and applying this knowledge to clinical practice, including tailored screening, will require deeper understanding of molecular mechanisms and prospective clinical validation. Even with clinically actionable tests, decision making, support for patients and their families and overcoming the barriers to lifestyle change (diet, exercise and weight) alongside chemopreventive strategies are required to optimise health outcomes.
Genomic profiling of sequential clinical samples (primary, relapsed and secondary cancers, CTC, ctDNA, before, during and following therapy) is required to identify specific biomarkers of inter-/intra-tumour spatial and temporal heterogeneity, metastatic potential, sensitivity to radiotherapy and different forms of chemotherapy, de novo or acquired resistance. This will significantly improve patient stratification for existing therapies and identify key nodes in these dynamic processes as potential new therapeutic targets. Validated markers of these processes (including minimally invasive multimodality imaging and metabolomics methodologies) will benefit from synergies between laboratory and clinical interactions. Improved understanding of the interactions, duration, sequencing and optimal combinations of therapy should allow better stratification of patients and reduce overtreatment (or undertreatment) enhancing prevention or survival while reducing morbidity.
Further genetic, epigenetic and molecular profiling of breast cancers and their associated stroma would be significantly enhanced by expanded panels of cell lines representing all major breast cancer subtypes and three-dimensional tumour-host heterotypic co-culture systems. This would enable increased understanding of the molecular drivers behind specific cancer subtypes and their role (together with microenvironmental modifiers) in treatment resistance and metastasis. Deciphering tumour-stromal interactions incorporating metabolic and immunological host mechanisms and intracellular/extracellular signalling pathways would have therapeutic implications for prevention and therapy. Advanced high-content analytical methods will enable consideration of additional key cancer ‘hallmarks’ beyond proliferation (for example cell motility and invasion) and enable screening for inhibitors under more physiologically relevant conditions. Better preclinical animal models (for example genetically engineered mice expressing relevant human oncogenes, which develop widespread metastases; patient-derived xenografts) are required. Such models would enable testing of hypotheses derived from clinical observations and rigorous target validation and evaluation of novel therapies in the metastatic setting (and where desirable in immunocompetent hosts).
Underpinning these advances, optimised multimodality imaging for diagnosis and therapeutic monitoring should enable better evaluation of primary and metastatic disease. Clinically annotated tissues for translational research must be linked to bioinformatics as key contributors to interdisciplinary research, essential for rapid future advances. Increasing numbers of women and men are surviving breast cancer. Alongside advances in understanding the disease and using that knowledge for prevention, earlier detection and successful treatment of breast cancer, interventions to improve the survivorship experience require innovative approaches to address the consequences of diagnosis and treatment.
Top 10 gaps:
Understanding the specific functions and contextual interactions of genetic and epigenetic changes in the normal breast and the development of cancer
Effective and sustainable lifestyle changes (diet, exercise and weight) alongside chemopreventive strategies
Tailored screening approaches including clinically actionable tests
Molecular drivers behind breast cancer subtypes, treatment resistance and metastasis
Mechanisms of tumour heterogeneity, tumour dormancy, de novo or acquired resistance; how to target the key nodes in these dynamic processes
Validated markers of chemosensitivity and radiosensitivity
Interactions, duration, sequencing and optimal combinations of therapy for improved individualisation of treatment
Optimised multimodality imaging for diagnosis and therapeutic monitoring should enable better evaluation of primary and metastatic disease
Interventions and support to improve the survivorship experience including physical symptoms such as hot flushes and lymphoedema
Clinically annotated tissues for translational research including tumour, non-tumour and blood based materials from primary cancers, relapsed and metastatic disease
Proposed strategic solutions:
For significant progress to be made in treating and supporting those impacted by breast cancer (and ultimately preventing and overcoming this disease) basic and translational research scientists in academia and industry, funding bodies, government and patients need to work together to achieve the following key strategic solutions
To reverse the decline in resources targeted towards breast cancer research, funding must be increased and strategically directed to enhance our current knowledge, develop the talent pool, and apply evidence-based findings to improve clinical care
A fully cohesive and collaborative infrastructure must be developed to support breast cancer research; this requires improved access to appropriate, well-annotated clinical material including longitudinal sample collection with expert bioinformatics support and data sharing.
Building on sound investment and infrastructure, all stakeholders (researchers, funders, government, industry and patients) must work together on the clinical development and translation of research knowledge to patient benefit. For example, enhanced, clinically relevant, in vitro and in vivo models are required for evaluation of new therapies together with validated biomarkers, which should then be embedded in clinical practice.
Research funders, government and industry should provide innovative programmes to encourage collaborative cross-disciplinary working practices, including the training of more physician-scientists and integration of physical sciences, technology and engineering.
Improving clinical trial methodologies, including patient involvement, recognising that a changing global environment is required to ensure that all clinical developments can be tested and ultimately implemented for patient benefit.
Abbreviations
Aromatase inhibitor
Androgen receptor
Ataxia telangiectasia mutated
British Association of Surgical Oncology
Cancer-associated fibroblast
Cognitive behavioural therapy
Cyclin-dependent kinase 10
CHK2 checkpoint homolog
Checkpoint kinase 2
Central nervous system
Cancer stem cell
Circulating tumour cell (in blood)
Common terminology criteria for adverse events
Circulating tumour DNA
Ductal carcinoma in situ
DNA damage response
Deoxyribonucleic acid
Disseminated tumour cell (usually in marrow nodes or tissue)
Extracellular matrix
Epithelial-mesenchymal transition
Oestrogen receptor
Fibroblast growth factor
Fibroblast growth factor receptor 1
Fine-needle aspiration
Forkhead box protein A1
Genetically engineered mouse
Genome-wide association studies
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 3
Homologous recombination repair
Hormone replacement therapy
Heat shock protein 90
Ipsilateral breast tumour recurrence
International Cancer Genome Consortium
Illumina collaborative oncological gene-environment study
Insulin-like growth factor 1
Immunohistochemical
Induced pluripotent stem cells
Chromatography-mass spectrometry
Metastatic breast cancer
Magnetic resonance imaging
Nuclear magnetic resonance
Representing the whole HER family
Poly (ADP-ribose) polymerase
Patient-derived xenografts
Positron emission tomography/single-photon emission computed tomography
Phosphatidylinositide-3 kinase
Gene encoding PI3 kinase alpha
Protein kinase B
Progesterone receptor
Patient-reported outcome measures
Randomised controlled trial
Response evaluation criteria in solid tumors
Ribonucleic acid
Selective oestrogen receptor modulators
Short inhibitory RNAs
Sentinel node biopsy
Single nucleotide polymorphism
Skeletal-related events
Standardisation of Breast Radiotherapy (START) trial A
Standardisation of Breast Radiotherapy (START) trial B
The Cancer Genome Atlas
Transforming growth factor beta
Tyrosine kinase inhibitor
Tissue microarray
Triple-negative breast cancer
Vascular endothelial growth factor
Women’s Health Initiative.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM: Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010, 127: 2893-2917.
Article CAS PubMed Google Scholar
Breast cancer incidence statistics. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/incidence/#trends ,
Maddams JBD, Gavin A, Steward J, Elliott J, Utley M, Møller H: Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer. 2009, 101: 541-547.
Article CAS PubMed PubMed Central Google Scholar
Maddams J, Utley M, Moller H: Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer. 2012, 107: 1195-1202.
Leal J: The economic burden of cancer across the European Union. Proceedings of the National Cancer Research Institute Conference: 4–7. 2012, Liverpool, November
Google Scholar
Data package. http://www.ncri.org.uk/includes/Publications/general/Data_package_12.xls ,
Thompson A, Brennan K, Cox A, Gee J, Harcourt D, Harris A, Harvie M, Holen I, Howell A, Nicholson R, Steel M, Streuli C: Evaluation of the current knowledge limitations in breast cancer research: a gap analysis. Breast Cancer Res : BCR. 2008, 10: R26-
Article PubMed PubMed Central Google Scholar
Tissue Bank. http://breastcancertissuebank.org/about-tissue-bank.php ,
Melchor L, Benitez J: The complex genetic landscape of familial breast cancer. Hum Genet. 2013
Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, Wang Q, Dicks E, Lee A, Turnbull C, Rahman N, Fletcher O, Peto J, Gibson L, Dos Santos Silva I, Nevanlinna H, Muranen TA, Aittomäki K, Blomqvist C, Czene K, Irwanto A, Liu J, Waisfisz Q, Meijers-Heijboer H, Adank M, Breast and Ovarian Cancer Susceptibility Collaboration, et al: Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013, 45: 353-361. 361e351-352
Sakoda LC, Jorgenson E, Witte JS: Turning of COGS moves forward findings for hormonally mediated cancers. Nat Genet. 2013, 45: 345-348.
Antoniou AC, Beesley J, McGuffog L, Sinilnikova OM, Healey S, Neuhausen SL, Ding YC, Rebbeck TR, Weitzel JN, Lynch HT, Isaacs C, Ganz PA, Tomlinson G, Olopade OI, Couch FJ, Wang X, Lindor NM, Pankratz VS, Radice P, Manoukian S, Peissel B, Zaffaroni D, Barile M, Viel A, Allavena A, Dall'Olio V, Peterlongo P, Szabo CI, Zikan M, Claes K, et al: Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: implications for risk prediction. Cancer Res. 2010, 70: 9742-9754.
Ingham S, Warwick J, Byers H, Lalloo F, Newman W, Evans D: Is multiple SNP testing in BRCA2 and BRCA1 female carriers ready for use in clinical practice? Results from a large Genetic Centre in the UK. Clin Genet. 2013, 84: 37-42.
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A: Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010, 376: 245-251.
Turnbull C, Seal S, Renwick A, Warren-Perry M, Hughes D, Elliott A, Pernet D, Peock S, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, et al: Gene-gene interactions in breast cancer susceptibility. Hum Mol Genet. 2012, 21: 958-962.
Muller HM, Widschwendter A, Fiegl H, Ivarsson L, Goebel G, Perkmann E, Marth C, Widschwendter M: DNA methylation in serum of breast cancer patients: an independent prognostic marker. Cancer Res. 2003, 63: 7641-7645.
PubMed Google Scholar
Yazici H, Terry MB, Cho YH, Senie RT, Liao Y, Andrulis I, Santella RM: Aberrant methylation of RASSF1A in plasma DNA before breast cancer diagnosis in the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2009, 18: 2723-2725.
Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M, ENCODE Project Consortium: An integrated encyclopedia of DNA elements in the human genome. Nature. 2012, 489: 57-74.
Article CAS Google Scholar
Brennan K, Garcia-Closas M, Orr N, Fletcher O, Jones M, Ashworth A, Swerdlow A, Thorne H, Investigators KC, Riboli E, Vineis P, Dorronsoro M, Clavel-Chapelon F, Panico S, Onland-Moret NC, Trichopoulos D, Kaaks R, Khaw KT, Brown R, Flanagan JM: Intragenic ATM methylation in peripheral blood DNA as a biomarker of breast cancer risk. Cancer Res. 2012, 72: 2304-2313.
Azad N, Zahnow CA, Rudin CM, Baylin SB: The future of epigenetic therapy in solid tumours–lessons from the past. Nat Rev Clin Oncol. 2013, 10: 256-266.
Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, Harris J, Yen RW, Ahuja N, Brock MV, Stearns V, Feller-Kopman D, Yarmus LB, Lin YC, Welm AL, Issa JP, Minn I, Matsui W, Jang YY, Sharkis SJ, Baylin SB, Zahnow CA: Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012, 21: 430-446.
Foster C, Watson M, Eeles R, Eccles D, Ashley S, Davidson R, Mackay J, Morrison PJ, Hopwood P, Evans DG, Psychosocial Study Collaborators: Predictive genetic testing for BRCA1/2 in a UK clinical cohort: three-year follow-up. Br J Cancer. 2007, 96: 718-724.
Hilgart JS, Coles B, Iredale R: Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Syst Rev. 2012, 2: CD003721
Albada A, Werrett J, Van Dulmen S, Bensing JM, Chapman C, Ausems MG, Metcalfe A: Breast cancer genetic counselling referrals: how comparable are the findings between the UK and the Netherlands?. J Comm Gen. 2011, 2: 233-247.
Article Google Scholar
Wakefield CE, Meiser B, Homewood J, Peate M, Taylor A, Lobb E, Kirk J, Young MA, Williams R, Dudding T, Tucker K, AGenDA Collaborative Group: A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Res Treat. 2008, 107: 289-301.
Article PubMed Google Scholar
Lindor NM, Goldgar DE, Tavtigian SV, Plon SE, Couch FJ: BRCA1/2 Sequence variants of uncertain significance: a primer for providers to assist in discussions and in medical management. Oncol. 2013, 18: 518-524.
Hallowell N, Baylock B, Heiniger L, Butow PN, Patel D, Meiser B, Saunders C, Price MA, kConFab Psychosocial Group on behalf of the kConFab I: Looking different, feeling different: women’s reactions to risk-reducing breast and ovarian surgery. Fam Cancer. 2012, 11: 215-224.
Watts KJ, Meiser B, Mitchell G, Kirk J, Saunders C, Peate M, Duffy J, Kelly PJ, Gleeson M, Barlow-Stewart K, Rahman B, Friedlander M, Tucker K, TFGT Collaborative Group: How should we discuss genetic testing with women newly diagnosed with breast cancer? Design and implementation of a randomized controlled trial of two models of delivering education about treatment-focused genetic testing to younger women newly diagnosed with breast cancer. BMC Cancer. 2012, 12: 320-
Chivers Seymour K, Addington-Hall J, Lucassen AM, Foster CL: What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns. 2010, 19: 330-342.
Mireskandari S, Sherman KA, Meiser B, Taylor AJ, Gleeson M, Andrews L, Tucker KM: Psychological adjustment among partners of women at high risk of developing breast/ovarian cancer. Genet Med. 2007, 9: 311-320.
Amir E, Freedman OC, Seruga B, Evans DG: Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010, 102: 680-691.
Dite GS, Mahmoodi M, Bickerstaffe A, Hammet F, Macinnis RJ, Tsimiklis H, Dowty JG, Apicella C, Phillips KA, Giles GG, Southey MC, Hopper JL: Using SNP genotypes to improve the discrimination of a simple breast cancer risk prediction model. Breast Cancer Res Treat. 2013, 139: 887-896.
Eriksson L, Hall P, Czene K, Dos Santos SI, McCormack V, Bergh J, Bjohle J, Ploner A: Mammographic density and molecular subtypes of breast cancer. Br J Cancer. 2012, 107: 18-23.
Swerdlow AJ, Cooke R, Bates A, Cunningham D, Falk SJ, Gilson D, Hancock BW, Harris SJ, Horwich A, Hoskin PJ, Linch DC, Lister TA, Lucraft HH, Radford JA, Stevens AM, Syndikus I, Williams MV: Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a National Cohort Study. J Clin Oncol. 2012, 30: 2745-2752.
Aupperlee MD, Leipprandt JR, Bennett JM, Schwartz RC, Haslam SZ: Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res BCR. 2013, 15: R44-
Denkert C, Bucher E, Hilvo M, Salek R, Oresic M, Griffin J, Brockmoller S, Klauschen F, Loibl S, Barupal DK, Budczies J, Iljin K, Nekljudova V, Fiehn O: Metabolomics of human breast cancer: new approaches for tumor typing and biomarker discovery. Genome Med. 2012, 4: 37-
CAS PubMed PubMed Central Google Scholar
Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, Easton D, Forbes JF, Key T, Hankinson SE, Howell A, Ingle J, Breast Cancer Prevention Collaborative Group: Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007, 14: 169-187.
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix AZ, Mershon J, Mitlak BH, Powles T, Veronesi U, Vogel V, Wickerham DL, SERM Chemoprevention of Breast Cancer Overview Group: Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013, 381: 1827-1834.
LaCroix AZ, Powles T, Osborne CK, Wolter K, Thompson JR, Thompson DD, Allred DC, Armstrong R, Cummings SR, Eastell R, Ensrud KE, Goss P, Lee A, Neven P, Reid DM, Curto M, Vukicevic S, PEARL Investigators: Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010, 102: 1706-1715.
Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H, NCIC CTG MAP.3 Study Investigators: Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011, 364: 2381-2391.
Decensi A, Gandini S, Serrano D, Cazzaniga M, Pizzamiglio M, Maffini F, Pelosi G, Daldoss C, Omodei U, Johansson H, Macis D, Lazzeroni M, Penotti M, Sironi L, Moroni S, Bianco V, Rondanina G, Gjerde J, Guerrieri-Gonzaga A, Bonanni B: Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007, 25: 4201-4209.
Rosner B, Glynn RJ, Tamimi RM, Chen WY, Colditz GA, Willett WC, Hankinson SE: Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013, 178: 296-308.
Uray IP, Brown PH: Chemoprevention of hormone receptor-negative breast cancer: new approaches needed. Recent Results Cancer Res. 2011, 188: 147-162.
Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, Manson JE, Stefanick ML, Ockene J, Sarto GE, Johnson KC, Wactawski-Wende J, Ravdin PM, Schenken R, Hendrix SL, Rajkovic A, Rohan TE, Yasmeen S, Prentice RL, WHI Investigators: Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010, 304: 1684-1692.
Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, Martin L, Ockene J, Rohan T, Schenken R, Wactawski-Wende J: Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012, 13: 476-486.
Wiseman M: The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008, 67: 253-256.
Parkin DM, Boyd L, Walker LC: 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011, 105: S77-S81.
Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, Lessin L, O’Sullivan MJ, Wactawski-Wende J, Yasmeen S, Prentice R: Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women’s health initiative observational study. J Natl Cancer Inst. 2010, 102: 1422-1431.
Hansen J, Stevens RG: Case–control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012, 48: 1722-1729.
Anderson AS, Mackison D, Boath C, Steele R: Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexplored opportunity for endorsing healthy behaviors. Cancer Prev Res. 2013, 6: 165-172.
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC: Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997, 278: 1407-1411.
Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA: Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005, 14: 656-661.
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE: Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006, 296: 193-201.
Teras LR, Goodman M, Patel AV, Diver WR, Flanders WD, Feigelson HS: Weight loss and postmenopausal breast cancer in a prospective cohort of overweight and obese US women. CCC. 2011, 22: 573-579.
Niraula S, Ocana A, Ennis M, Goodwin PJ: Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012, 134: 769-781.
Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles GG, Goodman G, Hakansson N, Hankinson SE, Helzlsouer K, Horn-Ross PL, Inoue M, Krogh V, Lof M, McCullough ML, Miller AB, Neuhouser ML, Palmer JR, Park Y, Robien K, Rohan TE, Scarmo S, Schairer C, Schouten LJ, Shikany JM, Sieri S, et al: Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013, 105: 219-236.
Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, et al: Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006, 295: 629-642.
Chlebowski RT, Rose D, Buzzard IM, Blackburn GL, Insull W, Grosvenor M, Elashoff R, Wynder EL: Adjuvant dietary fat intake reduction in postmenopausal breast cancer patient management. The Women’s Intervention Nutrition Study (WINS). Breast Cancer Res Treat. 1992, 20: 73-84.
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, Faerber S, Gold EB, Hajek RA, Hollenbach K, Jones LA, Karanja N, Madlensky L, Marshall J, Newman VA, Ritenbaugh C, Thomson CA, Wasserman L, Stefanick ML: Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007, 298: 289-298.
Friedenreich CM: Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011, 188: 125-139.
Fontein DB, de Glas NA, Duijm M, Bastiaannet E, Portielje JE, Van de Velde CJ, Liefers GJ: Age and the effect of physical activity on breast cancer survival: A systematic review. Cancer Treat Rev. 2013, 39: 958-965.
Key TJ: Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids. 2011, 76: 812-815.
Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, Huang AJ, Vitolins M, Hubbell FA, Manson JE, Cochrane BB, Lane DS, Lee JS: Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011, 103: 562-570.
Evans DG, Warwick J, Astley SM, Stavrinos P, Sahin S, Ingham S, McBurney H, Eckersley B, Harvie M, Wilson M, Beetles U, Warren R, Hufton A, Sergeant JC, Newman WG, Buchan I, Cuzick J, Howell A: Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention. Cancer Prev Res. 2012, 5: 943-951.
Darabi H, Czene K, Zhao W, Liu J, Hall P, Humphreys K: Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. BCR. 2012, 14: R25-
Brower V: Homing in on mechanisms linking breast density to breast cancer risk. J Natl Cancer Inst. 2010, 102: 843-845.
Martin LJ, Boyd NF: Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. BCR. 2008, 10: 201-
Article PubMed PubMed Central CAS Google Scholar
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM: Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J Natl Cancer Inst. 2011, 103: 744-752.
Courneya KS, Karvinen KH, McNeely ML, Campbell KL, Brar S, Woolcott CG, McTiernan A, Ballard-Barbash R, Friedenreich CM: Predictors of adherence to supervised and unsupervised exercise in the Alberta Physical Activity and Breast Cancer Prevention Trial. J Phys Act Health. 2012, 9: 857-866.
Rack B, Andergassen U, Neugebauer J, Salmen J, Hepp P, Sommer H, Lichtenegger W, Friese K, Beckmann MW, Hauner D, Hauner H, Janni W: The German SUCCESS C Study - the first European lifestyle study on breast cancer. Breast Care (Basel). 2010, 5: 395-400.
Villarini A, Pasanisi P, Traina A, Mano MP, Bonanni B, Panico S, Scipioni C, Galasso R, Paduos A, Simeoni M, Bellotti E, Barbero M, Macellari G, Venturelli E, Raimondi M, Bruno E, Gargano G, Fornaciari G, Morelli D, Seregni E, Krogh V, Berrino F: Lifestyle and breast cancer recurrences: the DIANA-5 trial. Tumori. 2012, 98: 1-18.
CAS PubMed Google Scholar
Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012, 366: 520-529.
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV: Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011, 10: 4230-4236.
Longo VD, Fontana L: Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging. 2011, 3: 1039-1040.
Goodwin PJ, Thompson AM, Stambolic V: Diabetes, metformin, and breast cancer: lilac time?. J Clin Oncol. 2012, 30: 2812-2814.
Reis-Filho JS, Pusztai L: Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011, 378: 1812-1823.
Baird RD, Caldas C: Genetic heterogeneity in breast cancer: the road to personalized medicine?. BMC Med. 2013, 11: 151-
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, et al: Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013, 499: 214-218.
Dawson SJ, Rueda OM, Aparicio S, Caldas C: A new genome-driven integrated classification of breast cancer and its implications. EMBO J. 2013, 32: 617-628.
Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA, Ellis P, Di Leo A, Baselga J, Sotiriou C, Piccart-Gebhart M: Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012, 30: 1879-1887.
Russnes HG, Navin N, Hicks J, Borresen-Dale AL: Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest. 2011, 121: 3810-3818.
Samuel N, Hudson TJ: Translating genomics to the clinic: implications of cancer heterogeneity. Clin Chem. 2013, 59: 127-137.
Jansson MD, Lund AH: MicroRNA and cancer. Mol Oncol. 2012, 6: 590-610.
Akhtar N, Streuli CH: An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013, 15: 17-27.
Bazzoun D, Lelievre S, Talhouk R: Polarity proteins as regulators of cell junction complexes: Implications for breast cancer. Pharmacol Ther. 2013, 138: 418-427.
Lelievre SA: Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective. J Mammary Gland Biol Neoplasia. 2010, 15: 49-63.
Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK: Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol. 2013, 15: 189-200.
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, O'Brien T, Kerin MJ: Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010, 124: 317-326.
Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ: HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010, 122: 35-43.
Pontiggia O, Sampayo R, Raffo D, Motter A, Xu R, Bissell MJ, Joffe EB, Simian M: The tumor microenvironment modulates tamoxifen resistance in breast cancer: a role for soluble stromal factors and fibronectin through beta1 integrin. Breast Cancer Res Treat. 2012, 133: 459-471.
Martinez-Outschoorn UE, Goldberg A, Lin Z, Ko YH, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP: Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther. 2011, 12: 924-938.
Hanahan D, Coussens LM: Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012, 21: 309-322.
He WS, Dai XF, Jin M, Liu CW, Rent JH: Hypoxia-induced autophagy confers resistance of breast cancer cells to ionizing radiation. Oncol Res. 2012, 20: 251-258.
Article PubMed CAS Google Scholar
Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, Candiloro I, Pezzella F, Gatter KC, Millar EK, O'Toole SA, McNeil CM, Crea P, Segara D, Sutherland RL, Harris AL, Fox SB: The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009, 100: 405-411.
Milas L, Hittelman WN: Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol. 2009, 19: 96-105.
Mimeault M, Batra SK: Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013, 17: 30-54.
Rundqvist H, Johnson RS: Hypoxia and metastasis in breast cancer. Curr Top Microbiol Immunol. 2010, 345: 121-139.
Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA: Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008, 103: 1369-1378.
Obeid E, Nanda R, Fu YX, Olopade OI: The role of tumor-associated macrophages in breast cancer progression (Review). Int J Oncol. 2013, 43: 5-12.
Lewis CE, Hughes R: Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. BCR. 2007, 9: 209-
Louie E, Nik S, Chen JS, Schmidt M, Song B, Pacson C, Chen XF, Park S, Ju J, Chen EI: Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. BCR. 2010, 12: R94-
Dittmer J, Rody A: Cancer stem cells in breast cancer. Histol Histopathol. 2013, 28: 827-838.
Mao Q, Zhang Y, Fu X, Xue J, Guo W, Meng M, Zhou Z, Mo X, Lu Y: A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. J Cancer Res Clin Oncol. 2013, 139: 211-222.
Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C: Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011, 479: 189-193.
van Amerongen R, Bowman AN, Nusse R: Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012, 11: 387-400.
de Visser KE, Ciampricotti M, Michalak EM, Tan DW, Speksnijder EN, Hau CS, Clevers H, Barker N, Jonkers J: Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J Pathol. 2012, 228: 300-309.
Smalley M, Piggott L, Clarkson R: Breast cancer stem cells: Obstacles to therapy. Cancer Lett. 2012, 338: 57-62.
Iliopoulos D, Hirsch HA, Wang G, Struhl K: Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011, 108: 1397-1402.
Sarrio D, Franklin CK, Mackay A, Reis-Filho JS, Isacke CM: Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells. 2012, 30: 292-303.
Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D’Alessio AC, Young RA, Weinberg RA: Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013, 154: 61-74.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001, 98: 10869-10874.
Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Piña V, Duke F, Francis J, Jung J, Maffuz-Aziz A, Onofrio RC, Parkin M, Pho NH, Quintanar-Jurado V, Ramos AH, Rebollar-Vega R, Rodriguez-Cuevas S, Romero-Cordoba SL, Schumacher SE, Stransky N: Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012, 486: 405-409.
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, et al: The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012, 486: 395-399.
Cancer Genome Atlas N: Comprehensive molecular portraits of human breast tumours. Nature. 2012, 490: 61-70.
Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW, Davidson NE, Ingle JN, Perez EA, Wood WC, Sparano JA, Badve S: A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013, 105: 701-710.
Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO: The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. MCP. 2012, 11: M111.014647-
Glukhova MA, Streuli CH: How integrins control breast biology. Curr Opin Cell Biol. 2013, 25: 633-641.
Ito Y, Iwase T, Hatake K: Eradication of breast cancer cells in patients with distant metastasis: the finishing touches?. Breast Cancer. 2012, 19: 206-211.
Sampieri K, Fodde R: Cancer stem cells and metastasis. Semin Cancer Biol. 2012, 22: 187-193.
Takebe N, Warren RQ, Ivy SP: Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. BCR. 2011, 13: 211-
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M: Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008, 14: 518-527.
Kalluri R, Zeisberg M: Fibroblasts in cancer. Nat Rev Cancer. 2006, 6: 392-401.
Barker HE, Cox TR, Erler JT: The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012, 12: 540-552.
Favaro E, Lord S, Harris AL, Buffa FM: Gene expression and hypoxia in breast cancer. Genome Med. 2011, 3: 55-
Milani M, Harris AL: Targeting tumour hypoxia in breast cancer. Eur J Cancer. 2008, 44: 2766-2773.
Lundgren K, Holm C, Landberg G: Hypoxia and breast cancer: prognostic and therapeutic implications. CMLS. 2007, 64: 3233-3247.
Ward C, Langdon SP, Mullen P, Harris AL, Harrison DJ, Supuran CT, Kunkler IH: New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev. 2013, 39: 171-179.
Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ: Targeting the metabolic microenvironment of tumors. Adv Pharmacol. 2012, 65: 63-107.
Dos Santos CO, Rebbeck C, Rozhkova E, Valentine A, Samuels A, Kadiri LR, Osten P, Harris EY, Uren PJ, Smith AD, Hannon GJ: Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc Natl Acad Sci U S A. 2013, 110: 7123-7130.
Makarem M, Spike BT, Dravis C, Kannan N, Wahl GM, Eaves CJ: Stem cells and the developing mammary gland. J Mammary Gland Biol Neoplasia. 2013, 18: 209-219.
Visvader JE: Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009, 23: 2563-2577.
Ablett MP, Singh JK, Clarke RB: Stem cells in breast tumours: are they ready for the clinic?. Eur J Cancer. 2012, 48: 2104-2116.
Badve S, Nakshatri H: Breast-cancer stem cells-beyond semantics. Lancet Oncol. 2012, 13: e43-e48.
Kaimala S, Bisana S, Kumar S: Mammary gland stem cells: more puzzles than explanations. J Biosci. 2012, 37: 349-358.
La Porta CA: Thoughts about cancer stem cells in solid tumors. World J Stem Cells. 2012, 4: 17-20.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA: The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008, 133: 704-715.
Polyak K, Weinberg RA: Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009, 9: 265-273.
Scheel C, Weinberg RA: Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells?. Int J Cancer. 2011, 129: 2310-2314.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF: Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003, 100: 3983-3988.
Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB: Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010, 70: 709-718.
Muller V, Riethdorf S, Rack B, Janni W, Fasching P, Solomayer E, Aktas B, Kasimir-Bauer S, Pantel K, Fehm T, DETECT study group: Prognostic impact of circulating tumor cells assessed with the Cell Search AssayTM and AdnaTest BreastTM in metastatic breast cancer patients: the DETECT study. BCR. 2012, 14: R118-
Giordano A, Gao H, Cohen EN, Anfossi S, Khoury J, Hess K, Krishnamurthy S, Tin S, Cristofanilli M, Hortobagyi GN, Woodward WA, Lucci A, Reuben JM: Clinical of cancer stem cells in bone marrow of early breast cancer patients. Ann Oncol. 2013, [Epud ahead of print]
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, Holland-Letz T, Höfner T, Sprick M, Scharpff M, Marmé F, Sinn HP, Pantel K, Weichert W, Trumpp A: Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013, 31: 539-544.
Willis L, Graham TA, Alarcon T, Alison MR, Tomlinson IP, Page KM: What can be learnt about disease progression in breast cancer dormancy from relapse data?. PloS one. 2013, 8: e62320-
Balic M, Lin H, Williams A, Datar RH, Cote RJ: Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert Rev Mol Diagn. 2012, 12: 303-312.
Barriere G, Riouallon A, Renaudie J, Tartary M, Rigaud M: Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer. 2012, 12: 114-
Sceneay J, Smyth MJ, Moller A: The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013, [Epud ahead of print]
Peinado H, Lavotshkin S, Lyden D: The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011, 21: 139-146.
Nguyen DX, Bos PD, Massague J: Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009, 9: 274-284.
Hu G, Kang Y, Wang XF: From breast to the brain: unraveling the puzzle of metastasis organotropism. J Mole Cell Biol. 2009, 1: 3-5.
Hsieh SM, Look MP, Sieuwerts AM, Foekens JA, Hunter KW: Distinct inherited metastasis susceptibility exists for different breast cancer subtypes: a prognosis study. BCR. 2009, 11: R75-
Scheel C, Weinberg RA: Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012, 22: 396-403.
Dave B, Mittal V, Tan NM, Chang JC: Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. BCR. 2012, 14: 202-
Drasin DJ, Robin TP, Ford HL: Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. BCR. 2011, 13: 226-
Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN, Tin S, De Laurentiis M, Parker CA, Alvarez RH, Valero V, Ueno NT, De Placido S, Mani SA, Esteva FJ, Cristofanilli M, Reuben JM: Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012, 11: 2526-2534.
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T: Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. BCR. 2012, 14: R15-
Chui MH: Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer. 2013, 132: 1487-1495.
Marchini C, Montani M, Konstantinidou G, Orru R, Mannucci S, Ramadori G, Gabrielli F, Baruzzi A, Berton G, Merigo F, Fin S, Iezzi M, Bisaro B, Sbarbati A, Zerani M, Galiè M, Amici A: Mesenchymal/stromal gene expression signature relates to basal-like breast cancers, identifies bone metastasis and predicts resistance to therapies. PloS one. 2010, 5: e14131-
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J: Tumor self-seeding by circulating cancer cells. Cell. 2009, 139: 1315-1326.
Comen E, Norton L: Self-seeding in cancer. Recent Res Cancer Res. 2012, 195: 13-23.
Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T, von Ahsen O: Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012, 12: 178-
Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S: Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. BCR. 2011, 13: R59-
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S: Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013, 339: 580-584.
De Mattos-Arruda L, Cortes J, Santarpia L, Vivancos A, Tabernero J, Reis-Filho JS, Seoane J: Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 2013, 10: 377-389.
Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, Marass F, Humphray S, Hadfield J, Bentley D, Chin TM, Brenton JD, Caldas C, Rosenfeld N: Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013, 497: 108-112.
Zhang F, Chen JY: Breast cancer subtyping from plasma proteins. BMC Med Genomics. 2013, 6: S6-
Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L: Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011, 57: 18-32.
Hendrix A, Hume AN: Exosome signaling in mammary gland development and cancer. Int J Dev Biol. 2011, 55: 879-887.
Marleau AM, Chen CS, Joyce JA, Tullis RH: Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012, 10: 134-
Eccles SA, Paon L: Breast cancer metastasis: when, where, how?. Lancet. 2005, 365: 1006-1007.
Eccles: Growth regulatory pathways contributing to organ selectivity of metastasis. Cancer Metastasis: Biologic Basis and Therapeutics. 2011, Cambridge: Cambridge University Press, 204-214.
Chapter Google Scholar
Mina LA, Sledge GW: Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol. 2011, 8: 325-332.
Wilson C, Holen I, Coleman RE: Seed, soil and secreted hormones: potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev. 2012, 38: 877-889.
Fidler IJ: The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011, 21: 107-112.
Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K, Early Breast Cancer Trialists’ Collaborative G: Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012, 379: 432-444.
Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R, Early Breast Cancer Trialists’ Collaborative G: Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011, 378: 1707-1716.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R, Early Breast Cancer Trialists’ Collaborative G: Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011, 378: 771-784.
Senkus EKS, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F: ESMO Guidelines Working Group. Ann Oncol. 2013, doi: 10.1093/annonc/mdt284
Khoury T: Delay to formalin fixation alters morphology and immunohistochemistry for breast carcinoma. Appl Immunohistochem Mol Morphol. 2012, 20: 531-542.
Dale DC: Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol. 2003, 1: 11-17.
Seah MD, Chan PM: Rethinking undertreatment in elderly breast cancer patients. Asian J Surg. 2009, 32: 71-75.
Harder H, Ballinger R, Langridge C, Ring A, Fallowfield LJ: Adjuvant chemotherapy in elderly women with breast cancer: patients’ perspectives on information giving and decision making. Psychooncology. 2013, doi: 10.1002/pon.3338
Ring A, Harder H, Langridge C, Ballinger RS, Fallowfield LJ: Adjuvant chemotherapy in elderly women with breast cancer (AChEW): an observational study identifying MDT perceptions and barriers to decision making. Ann Oncol. 2013, 24: 1211-1219.
Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, Palmer N, Ream E, Young A, Richardson A: Patients’ supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol. 2009, 27: 6172-6179.
Maguire P: Psychological aspects. ABC of Breast Diseases. 2002, London: BMJ Books, 150-153. 2nd Edition. Edited by Dixon M.
Hulbert-Williams N, Neal R, Morrison V, Hood K, Wilkinson C: Anxiety, depression and quality of life after cancer diagnosis: what psychosocial variables best predict how patients adjust?. Psychooncology. 2011, doi: 10.1002/pon.1980
Jacobsen PB: Screening for psychological distress in cancer patients: challenges and opportunities. J Clin Oncol. 2007, 25: 4526-4527.
The International. Psycho-Oncology Society. http://www.ipos-society.org/about/news/standards_news.aspx ,
Thompson AM, Moulder-Thompson SL: Neoadjuvant treatment of breast cancer. Ann Oncol. 2012, 23: x231-x236.
Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Warlam-Rodenhuis CC, Pierart M, Collette L: Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007, 25: 3259-3265.
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C: Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010, 362: 513-520.
Group ST, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin PJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR: The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008, 9: 331-341.
Group ST, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin PJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR: The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008, 371: 1098-1107.
Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, Keshtgar M, Dewar J, Kraus-Tiefenbacher U, Sütterlin M, Esserman L, Holtveg HM, Roncadin M, Pigorsch S, Metaxas M, Falzon M, Matthews A, Corica T, Williams NR, Baum M: Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010, 376: 91-102.
Rampinelli C, Bellomi M, Ivaldi GB, Intra M, Raimondi S, Meroni S, Orecchia R, Veronesi U: Assessment of pulmonary fibrosis after radiotherapy (RT) in breast conserving surgery: comparison between conventional external beam RT (EBRT) and intraoperative RT with electrons (ELIOT). Technol Cancer Res Treat. 2011, 10: 323-329.
Hannoun-Levi JM, Resch A, Gal J, Kauer-Dorner D, Strnad V, Niehoff P, Loessl K, Kovacs G, Van Limbergen E, Polgar C, On behalf of the GEC-ESTRO Breast Cancer Working Group: Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: Multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother Oncol. 2013, doi: 1016/j.radonc.2013.03.026
Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, Julian TB, Marks LB, Todor DA, Vicini FA, Whelan TJ, White J, Wo JY, Harris JR: Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009, 74: 987-1001.
Polgar C, Van Limbergen E, Potter R, Kovacs G, Polo A, Lyczek J, Hildebrandt G, Niehoff P, Guinot JL, Guedea F, Johansson B, Ott OJ, Major T, Strnad V, GEC-ESTRO Breast Cancer Working Group: Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol. 2010, 94: 264-273.
Tinterri C, Gatzemeier W, Zanini V, Regolo L, Pedrazzoli C, Rondini E, Amanti C, Gentile G, Taffurelli M, Fenaroli P, Tondini C, Saccetto G, Sismondi P, Murgo R, Orlandi M, Cianchetti E, Andreoli C: Conservative surgery with and without radiotherapy in elderly patients with early-stage breast cancer: a prospective randomised multicentre trial. Breast. 2009, 18: 373-377.
Hughes KS, Schnaper LA, Cirrincione C, Berry DA, McCormick B, Muss HB, Shank B, Hudis C, Winer EP, Smith BL: ASCO Annual Meeting 2010. 2010, Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer, Journal of Clinical Oncology,
Lipkus IM, Peters E, Kimmick G, Liotcheva V, Marcom P: Breast cancer patients’ treatment expectations after exposure to the decision aid program adjuvant online: the influence of numeracy. Med Decis Making. 2010, 30: 464-473.
Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R: Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet. 2002, 359: 650-656.
El Turabi A, Abel GA, Roland M, Lyratzopoulos G: Variation in reported experience of involvement in cancer treatment decision making: evidence from the National Cancer Patient Experience Survey. Br J Cancer. 2013, 109: 780-787.
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE: Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006, 95: 279-293.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M: Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011, 305: 569-575.
Rutgers EJ, Donker M, Straver ME, Meijnen P, Van De Velde CJ, Mansel RE, Westenberg H, Orzales L, Bouma WH, van der Mijle H, Nieuwenhuijzen P, Sanne C, Veltkamp LS, Messina CGM, Duez NJ, Hurkmans C, Bogaerts J, van Tienhoven G: ASCO Annual Meeting. 2013, Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: final analysis of the EORTC AMAROS trial (10981/22023), Journal of Clinical Oncology,
Smith BD: Using chemotherapy response to personalize choices regarding locoregional therapy: a new era in breast cancer treatment?. J Clin Oncol. 2012, 30: 3913-3915.
Azim HA, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M, Neven P, Symmans WF, Thompson A, Andre F, Loi S, Swanton C: Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013, 24: 647-654.
Wei S, Liu L, Zhang J, Bowers J, Gowda GA, Seeger H, Fehm T, Neubauer HJ, Vogel U, Clare SE, Raferty D: Metabolomics approach for predicting response to neoadjuvant chemotherapy for breast cancer. Mol Oncol. 2013, 7: 297-307.
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S: Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010, 28: 1829-1834.
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North America: Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11: 55-65.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004, 351: 2817-2826.
Coates PJ, Appleyard MV, Murray K, Ackland C, Gardner J, Brown DC, Adamson DJ, Jordan LB, Purdie CA, Munro AJ, Wright EG, Dewar JA, Thompson AM: Differential contextual responses of normal human breast epithelium to ionizing radiation in a mouse xenograft model. Can Res. 2010, 70: 9808-9815.
Arslan UY, Oksuzoglu B, Aksoy S, Harputluoglu H, Turker I, Ozisik Y, Dizdar O, Altundag K, Alkis N, Zengin N: Breast cancer subtypes and outcomes of central nervous system metastases. Breast. 2011, 20: 562-567.
Rennert G, Pinchev M, Rennert HS: Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol. 2010, 28: 3577-3581.
Chlebowski RT, Col N: Bisphosphonates and breast cancer prevention. Anticancer Agents Med Chem. 2012, 12: 144-150.
Coleman RE: Adjuvant bone-targeted therapy to prevent metastasis: lessons from the AZURE study. Curr Opin Support Palliat Care. 2012, 6: 322-329.
Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, Weir LM, Brufsky AM, Dakhil S, Lad T, Baez-Diaz L, Gralow JR, Robidoux A, Perez EA, Zheng P, Geyer CE, Swain S, Costantino JP, Mamounas EP, Wolmark N: Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012, 13: 734-742.
Gnant M, Dubsky P, Hadji P: Bisphosphonates: prevention of bone metastases in breast cancer. Recent Results Cancer Res. 2012, 192: 65-91.
Comen E, Norton L, Massague J: Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011, 8: 369-377.
Azim H, Azim HA: Targeting RANKL in breast cancer: bone metastasis and beyond. Expert Rev Anticancer Ther. 2013, 13: 195-201.
Drooger JC, van der Padt A, Sleijfer S, Jager A: Denosumab in breast cancer treatment. Eur J Pharmacol. 2013, doi: 10.1016/j.ejphar.2013.03.034
Formenti SC, Demaria S: Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012, 84: 879-880.
Liauw SL, Connell PP, Weichselbaum RR: New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013, 5: 173sr172-
Coles CE, Brunt AM, Wheatley D, Mukesh MB, Yarnold JR: Breast radiotherapy: less is more?. Clin Oncol (R Coll Radiol). 2013, 25: 127-134.
Yarnold J, Bentzen SM, Coles C, Haviland J: Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys. 2011, 79: 1-9.
Mannino M, Yarnold JR: Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld?. Radiother Oncol. 2009, 90: 14-22.
Blamey RW, Bates T, Chetty U, Duffy SW, Ellis IO, George D, Mallon E, Mitchell MJ, Monypenny I, Morgan DA, Macmillan RD, Patnick J, Pinder SE: Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer. 2013, 49: 2294-2302.
Kunkler I: Adjuvant chest wall radiotherapy for breast cancer: black, white and shades of grey. Eur J Surg Oncol. 2010, 36: 331-334.
Critchley AC, Thompson AM, Chan HY, Reed MW: Current controversies in breast cancer surgery. Clin Oncol (R Coll Radiol). 2013, 25: 101-108.
Riou O, Lemanski C, Guillaumon V, Lauche O, Fenoglietto P, Dubois JB, Azria D: Role of the radiotherapy boost on local control in ductal carcinoma in situ. Int J Surg Oncol. 2012, 2012: 748196-
PubMed PubMed Central Google Scholar
Kirkbride P, Hoskin PJ: Implementation of stereotactic ablative radiotherapy (stereotactic body radiotherapy). Clin Oncol (R Coll Radiol). 2012, 24: 627-628.
Somaiah N, Yarnold J, Lagerqvist A, Rothkamm K, Helleday T: Homologous recombination mediates cellular resistance and fraction size sensitivity to radiation therapy. Radiother Oncol. 2013, 1008: 155-1561.
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca E, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF, International Ki-67 in Breast Cancer Working Group: Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011, 103: 1656-1664.
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE JR, Wickerham DL, Wolmark N: Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006, 24: 3726-3734.
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velds T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R: A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002, 347: 1999-2009.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Peres ES, Abrams JS, Cameron DA, Palmer MJ, Pater JL, et al: Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005, 97: 1262-1271.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Fores JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Muller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, et al: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013, 381: 805-816.
Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Woldgang J, ABCSG and the GABG: Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005, 366: 455-462.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ: Panel m: Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009, 20: 1319-1329.
Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, Rimm DL, Magill P, Sellers M: Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011, 17: 1147-1159.
Carlson RW, O’Neill A, Vidaurre T, Gomez HL, Badve SS, Sledge GW: A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2012, 133: 1049-1056.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldkirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Dormont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, NeoALTTO Study Team: Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012, 379: 633-640.
Hamilton-Burke W, Coleman L, Cummings M, Green CA, Holliday DL, Horgan K, Maraqa L, Peter MB, Pollock S, Shaaban AM, Smith L, Speirs V: Phosphorylation of estrogen receptor beta at serine 105 is associated with good prognosis in breast cancer. Am J Pathol. 2010, 177: 1079-1086.
O’Hara J, Vareslija D, McBryan J, Bane F, Tibbitts P, Byrne C, Conroy RM, Hao Y, Gaora PO, Hill AD, McIlroy M, Young LS: AIB1:ERalpha transcriptional activity is selectively enhanced in aromatase inhibitor-resistant breast cancer cells. Clin Cancer Res. 2012, 18: 3305-3315.
Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W: Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids. 2009, 74: 586-594.
Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, Ali S, Chin SF, Palmieri C, Caldas C, Carroll JS: Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012, 481: 389-393.
Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H, Nakamura T, Nuovo G, Liu Y, Nephew KP, Croce CM: MicroRNA cluster 221–222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 2010, 102: 706-721.
Dunn BK, Jegalian K, Greenwald P: Biomarkers for early detection and as surrogate endpoints in cancer prevention trials: issues and opportunities. Recent Results Cancer Res. 2011, 188: 21-47.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP: Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010, 140: 62-73.
Giamas G, Filipovic A, Jacob J, Messier W, Zhang H, Yang D, Zhang W, Shifa BA, Photiou A, Tralau-Stewart C, Castellano L, Green AR, Coombes RC, Ellis IO, Ali S, Lenz HJ, Stebbing J: Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nat Med. 2011, 17: 715-719.
Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M: Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009, 27: 5538-5546.
Elsberger B, Paravasthu DM, Tovey SM, Edwards J: Shorter disease-specific survival of ER-positive breast cancer patients with high cytoplasmic Src kinase expression after tamoxifen treatment. J Cancer Res Clin Oncol. 2012, 138: 327-332.
Iorns E, Turner NC, Elliott R, Syed N, Garrone O, Gasco M, Tutt AN, Crook T, Lord CJ, Ashworth A: Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008, 13: 91-104.
Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A, Gillett C, Grigoriadis A, Tutt A, Reis-Filho JS: FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010, 70: 2085-2094.
Higgins MJ, Baselga J: Targeted therapies for breast cancer. J Clin Invest. 2011, 121: 3797-3803.
Gnant M: Overcoming endocrine resistance in breast cancer: importance of mTOR inhibition. Expert Rev Anticancer Ther. 2012, 12: 1579-1589.
Zardavas D, Baselga J, Piccart M: Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013, 10: 191-210.
Moulder S, Moroney J, Helgason T, Wheler J, Booser D, Albarracin C, Morrow PK, Koenig K, Kurzrock R: Responses to liposomal Doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol. 2011, 29: e572-e575.
Hoelder S, Clarke PA, Workman P: Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol. 2012, 6: 155-176.
Kauselmann G, Dopazo A, Link W: Identification of disease-relevant genes for molecularly-targeted drug discovery. Curr Cancer Drug Targets. 2012, 12: 1-13.
Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14: 461-471.
Criscitiello C, Azim HA, Agbor-Tarh D, de Azambuja E, Piccart M, Baselga J, Eidtmann H, Di Cosimo S, Bradbury I, Rubio IT: Factors associated with surgical management following neoadjuvant therapy in patients with primary HER2-positive breast cancer: results from the NeoALTTO phase III trial. Ann Oncol. 2013, 24: 1980-1985.
Goldhirsch A, Piccart-Gebhart MJ, Procter M, Azambuja E de, Weber HA, Untch M, Smith I, Gianni L, Jackisch C, Cameron D, Bell R, Dowsett M, Gelber RD, Leyland-Jones B, Baselga J: The HERA Study Team HERA TRIAL: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up. Cancer Research. 72 (24): December 15, 2012 Supplement 3;
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, Jacquin JP, Jouannaud C, Rios M, Abadie-Lacourtoisie S, Tubiana-Mathieu N, Cany L, Catala S, Khayat D, Pauporte I, Kramar A, PHARE trial investigators: 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013, 14: 741-748.
Tenori L, Oakman C, Claudino WM, Bernini P, Cappadona S, Nepi S, Biganzoli L, Arbushites MC, Luchinat C, Bertini I, Di Leo A: Exploration of serum metabolomic profiles and outcomes in women with metastatic breast cancer: a pilot study. Mol Oncol. 2012, 6: 437-444.
Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, Johnson NL, Granger DA, Jordan NV, Darr DB, Usary J, Kuan PF, Smalley DM, Major B, He X, Hoadley KA, Zhou B, Sharpless NE, Perou C, Kim WY, Gomez SM, Chen X, Jin J, Frye SV, Earp HS, Graves LM, Johnson GL: Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012, 149: 307-321.
Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassiley LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, et al: Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A. 2012, 109: 2724-2729.
Kelly CM, Buzdar AU: Using multiple targeted therapies in oncology: considerations for use, and progress to date in breast cancer. Drugs. 2013, 73: 505-515.
Sultana R, Abdel-Fatah T, Abbotts R, Hawkes C, Albarakati N, Seedhouse C, Ball G, Chan S, Rakha EA, Ellis IO, Madhusudan S: Targeting XRCC1 deficiency in breast cancer for personalized therapy. Cancer Res. 2013, 73: 1621-1634.
Miller WR, Larionov A, Anderson TJ, Evans DB, Dixon JM: Sequential changes in gene expression profiles in breast cancers during treatment with the aromatase inhibitor, letrozole. Pharmacogenomics J. 2012, 12: 10-21.
Larionov AFD, Caldwell H, Sims A, Fawkes A, Murphy L, Renshaw L, Dixon J: Gene expression profiles of endocrine resistant breast tumours. Cancer Res. 2009, 69: 809-810.
Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, Grant M, Hasenburg A, Hille ET, Kay C, Kieback DG, Putter H, Markopoulos C, Kranenbarg E, Mallon EA, Dirix L, Seynaeve C, Rea D: Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011, 29: 1531-1538.
Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G: Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008, 26: 3727-3734.
Yan Y, Li X, Blanchard A, Bramwell VH, Pritchard KI, Tu D, Shepherd L, Myal Y, Penner C, Watson PH, Leygue E, Murphy LC: Expression of both estrogen receptor-beta 1 (ER-beta1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-alpha)-negative early breast cancer (EBC). Ann Oncol. 2013, 24: 1986-1993.
De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Ando S, Fuqua SA: Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010, 121: 1-11.
Garay JP, Park BH: Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012, 2: 434-445.
Fan P, Yue W, Wang JP, Aiyar S, Li Y, Kim TH, Santen RJ: Mechanisms of resistance to structurally diverse antiestrogens differ under premenopausal and postmenopausal conditions: evidence from in vitro breast cancer cell models. Endocrinology. 2009, 150: 2036-2045.
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA: Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010, 12: R92-
Amir E, Clemons M, Purdie CA, Miller N, Quinlan P, Geddie W, Coleman RE, Freedman OC, Jordan LB, Thompson AM: Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012, 38: 708-714.
Moussa O, Purdie C, Vinnicombe S, Thompson AM: Biomarker discordance: prospective and retrospective evidence that biopsy of recurrent disease is of clinical utility. Cancer Biomark. 2012, 12: 231-239.
Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C: Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013, 108: 1515-1524.
Thompson AM, Johnson A, Quinlan P, Hillman G, Fontecha M, Bray SE, Purdie CA, Jordan LB, Ferraldeschi R, Latif A, Hadfield KD, Clarke RB, Ashcroft L, Evans DG, Howell A, Nikoloff M, Lawrence J, Newman WG: Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011, 125: 279-287.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C: Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013, 31: 860-867.
Group BIGC, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, Mauriac L, Forbes J, Price KN, Regan MM, Gelber RD, Coates AS: Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009, 361: 766-776.
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Homberg SB, Dodwell D, Mickiewicz E, Anderson J, Lonning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, et al: Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007, 369: 559-570.
Palmieri C, Shah D, Krell J, Gojis O, Hogben K, Riddle P, Ahmad R, Tat T, Fox K, Porter A, Mahmoud S, Kirschke S, Shousha S, Gudi M, Coombes RC, Leonard R, Cleator S: Management and outcome of HER2-positive early breast cancer treated with or without trastuzumab in the adjuvant trastuzumab era. Clin Breast Cancer. 2011, 11: 93-102.
Fontein DB, Seynaeve C, Hadji P, Hille ET, van de Water W, Putter H, Kranenbarg EM, Hasenburg A, Paridaens RJ, Vannetzel JM, Markopoulos C, Hoxumi Y, Bartlett JM, Jones SE, Rea DW, Nortier JW, van de Velde CJ: Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysis. J Clin Oncol. 2013, 31: 2257-2264.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J: Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012, 30: 2585-2592.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimi V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13: 25-32.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domant J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, NeoALTTO Study Team: Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012, 379: 633-640.
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, CLEOPATRA Study Group: Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012, 366: 109-119.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, Macpherson E, Carmichael J, Oza A: Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12: 852-861.
Cleator S, Heller W, Coombes RC: Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007, 8: 235-244.
Molyneux G, Smalley MJ: The cell of origin of BRCA1 mutation-associated breast cancer: a cautionary tale of gene expression profiling. J Mammary Gland Biol Neoplasia. 2011, 16: 51-55.
Michalak EM, Jonkers J: Studying therapy response and resistance in mouse models for BRCA1-deficient breast cancer. J Mammary Gland Biol Neoplasia. 2011, 16: 41-50.
Ran S, Volk L, Hall K, Flister MJ: Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010, 17: 229-251.
Ferris RL, Lotze MT, Leong SP, Hoon DS, Morton DL: Lymphatics, lymph nodes and the immune system: barriers and gateways for cancer spread. Clin Exp Metastasis. 2012, 29: 729-736.
Gomes FG, Nedel F, Alves AM, Nor JE, Tarquinio SB: Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013, 92: 101-107.
Lenzer J: FDA committee votes to withdraw bevacizumab for breast cancer. BMJ. 2011, 343: d4244-
D’Agostino RB: Changing end points in breast-cancer drug approval–the Avastin story. N Engl J Med. 2011, 365: e2-
Shojaei F: Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012, 320: 130-137.
Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF: Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008, 11: 109-119.
Kerbel RS: Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012, 17: 229-239.
Sitohy B, Nagy JA, Dvorak HF: Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012, 72: 1909-1914.
Chew V, Toh HC, Abastado JP: Immune microenvironment in tumor progression: characteristics and challenges for therapy. J Oncol. 2012, 2012: 608406-
Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, Loi S: Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013, 19: 28-33.
Reisfeld RA: The tumor microenvironment: a target for combination therapy of breast cancer. Crit Rev Oncog. 2013, 18: 115-133.
Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O, Neville A: Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PloS one. 2011, 6: e17876-
Adams S, Greeder L, Reich E, Shao Y, Fosina D, Hanson N, Tassello J, Singh B, Spagnoli GC, Demaria S, Jungbluth AA: Expression of cancer testis antigens in human BRCA-associated breast cancers: potential targets for immunoprevention?. Cancer Immunol Immunother. 2011, 60: 999-1007.
Corner J, Wright D, Hopkinson J, Gunaratnam Y, McDonald JW, Foster C: The research priorities of patients attending UK cancer treatment centres: findings from a modified nominal group study. Br J Cancer. 2007, 96: 875-881.
Hewitt M, Rowland JH, Yancik R: Cancer survivors in the United States: age, health, and disability. J Geront A, Biol Sci Med Sci. 2003, 58: 82-91.
Foster C, Wright D, Hill H, Hopkinson J, Roffe L: Psychosocial implications of living 5 years or more following a cancer diagnosis: a systematic review of the research evidence. Eur J Cancer Care (Engl). 2009, 18: 223-247.
Hubbard G, Menzies S, Flynn P, Adams S, Haseen F, Thomas I, Scanlon K, Reed L, Forbat L: Relational mechanisms and psychological outcomes in couples affected by breast cancer: a systematic review of the literature. BMJ, Supportive and Palliative Care. 2013, 3: 1-7.
Foster C, Fenlon D: Recovery and self-management support following primary cancer treatment. Br J Cancer. 2011, 105: S21-S28.
Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW: Taking CHARGE: A self-management program for women following breast cancer treatment. Psychooncology. 2005, 14: 704-717.
Bloom JR, Stewart SL, D’Onofrio CN, Luce J, Banks PJ: Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change?. J Cancer Surviv. 2008, 2: 190-204.
Reed E, Simmonds P, Haviland J, Corner J: Quality of life and experience of care in women with metastatic breast cancer: a cross-sectional survey. J Pain Symptom Manage. 2012, 43: 747-758.
Aranda S, Schofield P, Weih L, Yates P, Milne D, Faulkner R, Voudouris N: Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. Eur J Cancer Care (Engl). 2005, 14: 211-222.
Hopwood P, Howell A, Maguire P: Psychiatric morbidity in patients with advanced cancer of the breast: prevalence measured by two self-rating questionnaires. Br J Cancer. 1991, 64: 349-352.
Pinder KL, Ramirez AJ, Black ME, Richards MA, Gregory WM, Rubens RD: Psychiatric disorder in patients with advanced breast cancer: prevalence and associated factors. Eur J Cancer. 1993, 29A: 524-527.
Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC: Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry. 2004, 38: 320-326.
Grunfeld EA, Maher EJ, Browne S, Ward P, Young T, Vivat B, Walker G, Wilson C, Potts HW, Westcombe AM, Richards MA, Ramirez AJ: Advanced breast cancer patients’ perceptions of decision making for palliative chemotherapy. J Clin Oncol. 2006, 24: 1090-1098.
Karamouzis MV, Ioannidis G, Rigatos G: Quality of life in metastatic breast cancer patients under chemotherapy or supportive care: a single-institution comparative study. Eur J Cancer Care. 2007, 16: 433-438.
Cheville AL, Troxel AB, Basford JR, Kornblith AB: Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008, 26: 2621-2629.
Headley JA, Ownby KK, John LD: The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs forum. 2004, 31: 977-983.
Asola R, Huhtala H, Holli K: Intensity of diagnostic and treatment activities during the end of life of patients with advanced breast cancer. Breast Cancer Res Treat. 2006, 100: 77-82.
Gagnon B, Mayo NE, Hanley J, MacDonald N: Pattern of care at the end of life: does age make a difference in what happens to women with breast cancer?. J Clin Oncol. 2004, 22: 3458-3465.
Richardson A, Addington-Hall J, Amir Z, Foster C, Stark D, Armes J, Brearley SG, Hodges L, Hook J, Jarrett N, Stamataki Z, Scott I, Walker J, Ziegler L, Sharpe MS: Knowledge, ignorance and priorities for research in key areas of cancer survivorship: findings from a scoping review. Br J Cancer. 2011, 105: S82-S94.
Stanton AL, Luecken LJ, MacKinnon DP, Thompson EH: Mechanisms in psychosocial interventions for adults living with cancer: opportunity for integration of theory, research, and practice. J Consult Clin Psychol. 2013, 81: 318-335.
Fenlon DR, Corner JL, Haviland JS: A randomized controlled trial of relaxation training to reduce hot flashes in women with primary breast cancer. J Pain Symptom Manage. 2008, 35: 397-405.
Osborn RL, Demoncada AC, Feuerstein M: Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med. 2006, 36: 13-34.
Spiegel D, Bloom JR, Kraemer HC, Gottheil E: Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989, 2: 888-891.
Edwards AG, Hulbert-Williams N, Neal RD: Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev. 2008, 3: CD004253
Emilsson S, Svensk AC, Tavelin B, Lindh J: Support group participation during the post-operative radiotherapy period increases levels of coping resources among women with breast cancer. Eur J Cancer Care (Engl). 2012, 21: 591-598.
Hoey LM, Ieropoli SC, White VM, Jefford M: Systematic review of peer-support programs for people with cancer. Patient Educ Couns. 2008, 70: 315-337.
Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR: Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011, 29: 1101-1109.
Capozzo MA, Martinis E, Pellis G, Giraldi T: An early structured psychoeducational intervention in patients with breast cancer: results from a feasibility study. Cancer Nurs. 2010, 33: 228-234.
Gielissen MF, Verhagen CA, Bleijenberg G: Cognitive behaviour therapy for fatigued cancer survivors: long-term follow-up. Br J Cancer. 2007, 97: 612-618.
Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD: Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012, 21: 695-705.
Armes J, Chalder T, Addington-Hall J, Richardson A, Hotopf M: A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer-related fatigue. Cancer. 2007, 110: 1385-1395.
Mann E, Smith M, Hellier J, Hunter MS: A randomised controlled trial of a cognitive behavioural intervention for women who have menopausal symptoms following breast cancer treatment (MENOS 1): trial protocol. BMC Cancer. 2011, 11: 44-
Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, Gerritsma MA, Menke-Pluymers MB, Plaisier PW, Rijna H, Lopes Cardozo AM, Timmers G, van der Meij S, van der Veen H, Bijker N, de Widt-Levert LN, Geenen MM, Heuff G, van Dulken EJ, Aaronson NK BE: Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012, 30: 4124-4133.
Thompson J, Cocker H, Coleman RE, Colwell B, Freeman JV, Holmes K, Reed MW, Anthony C, Greenfield D: Breast cancer aftercare; preparing patients for discharge from routine hospital follow-up (PREP). Proceedings of the British Psychosocial Oncology Society Conference: 3–4 December 2009. 2009, Cardiff, Wales: Psycho-Oncology, 19(Suppl. 3):S1–S20 (2010)
Shennan C, Payne S, Fenlon D: What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology. 2011, 20: 681-697.
Campbell KL, Neil SE, Winters-Stone KM: Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2011, 46: 909-916.
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH: An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010, 4: 87-100.
Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, Cerin E, Chan WY, Leung IP, Lam SH, Taylor AJ, Cheng KK: Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012, 344: e70-
Mutrie N, Campbell A, Barry S, Hefferon K, McConnachie A, Ritchie D, Tovey S: Five-year follow-up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects?. J Cancer Surviv. 2012, 6: 420-430.
Classen C, Butler LD, Koopman C, Miller E, DiMiceli S, Giese-Davis J, Fobair P, Carlson RW, Kraemer HC, Spiegel D: Supportive-expressive group therapy and distress in patients with metastatic breast cancer: a randomized clinical intervention trial. Arch Gen Psychiatry. 2001, 58: 494-501.
Watson EK, Rose PW, Neal RD, Hulbert-Williams N, Donnelly P, Hubbard G, Elliott J, Campbell C, Weller D, Wilkinson C: Personalised cancer follow-up: risk stratification, needs assessment or both?. Br J Cancer. 2012, 106: 1-5.
Fenlon D, Frankland J, Foster CL, Brooks C, Coleman P, Payne S, Seymour J, Simmonds P, Stephens R, Walsh B, Addington-Hall JM: Living into old age with the consequences of breast cancer. Eur J Oncol Nurs. 2013, 17: 311-316.
Watts K, Meiser B, Conlon H, Rovelli S, Tiller K, Zorbas H, Lewis C, Neil G, Friedlander M: A specialist breast care nurse role for women with metastatic breast cancer: enhancing supportive care. Oncol Nurs Forum. 2011, 38: 627-631.
Absolom K, Eiser C, Michel G, Walters SJ, Hancock BW, Coleman RE, Snowden JA, Greenfield DM: Follow-up care for cancer survivors: views of the younger adult. Br J Cancer. 2009, 101: 561-567.
Fenlon DR, Corner JL, Haviland J: Menopausal hot flushes after breast cancer. Eur J Cancer Care (Engl). 2009, 18: 140-148.
Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, Hunter MS: Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012, 13: 309-318.
Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA: Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004, 26: 955-969.
Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA: Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003, 60: 951-959.
Oliveri JM, Day JM, Alfano CM, Herndon JE, Katz ML, Bittoni MA, Donohue K, Paskett ED: Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv. 2008, 2: 233-242.
Fourie WJ, Robb KA: Physiotherapy management of axillary web syndrome following breast cancer treatment: discussing the use of soft tissue techniques. Physiotherapy. 2009, 95: 314-320.
Holliday DL, Speirs V: Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13: 215-
Lacroix M, Leclercq G: Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004, 83: 249-289.
Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R: ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012, 180: 599-607.
Yuan H, Myers S, Wang J, Zhou D, Woo JA, Kallakury B, Ju A, Bazylewicz M, Carter YM, Albanese C, Grant N, Shad A, Dritschilo A, Liu X, Schlegel R: Use of reprogrammed cells to identify therapy for respiratory papillomatosis. N Engl J Med. 2012, 367: 1220-1227.
Lee GY, Kenny PA, Lee EH, Bissell MJ: Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007, 4: 359-365.
Calvo F, Sahai E: Cell communication networks in cancer invasion. Curr Opin Cell Biol. 2011, 23: 621-629.
Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA: Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10: 29-
Krishnan V, Shuman LA, Sosnoski DM, Dhurjati R, Vogler EA, Mastro AM: Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three-dimensional cultures. J Cell Physiol. 2011, 226: 2150-2158.
Quail DF, Maciel TJ, Rogers K, Postovit LM: A unique 3D in vitro cellular invasion assay. J Biomol Screen. 2012, 17: 1088-1095.
Ho KS, Poon PC, Owen SC, Shoichet MS: Blood vessel hyperpermeability and pathophysiology in human tumour xenograft models of breast cancer: a comparison of ectopic and orthotopic tumours. BMC Cancer. 2012, 12: 579-
DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, Welm AL, Welm BE, et al: Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Current protocols in pharmacology. Edited by: Enna SJ, John Wiley & Sons . 2013, Chapter 14:Unit14 23
Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, Manuel CA, Edgerton SM, Harrell JC, Elias A, Sartorius CA: Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012, 135: 415-432.
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Conner MJ, Martin NM, Borst P, Jonkers J: High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008, 105: 17079-17084.
Mollard S, Mousseau Y, Baaj Y, Richard L, Cook-Moreau J, Monteil J, Funalot B, Sturtz FG: How can grafted breast cancer models be optimized?. Cancer Biol Ther. 2011, 12: 855-864.
Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, Landis MD, Wiechmann L, Schiff R, Giuliano M, Wong H, Fuqua SW, Contreras A, Gutierrez C, Huang J, Mao S, Pavlick AC, Froehlich AM, Wu MF, Tsimelzon A, Hilsenbeck SG, Chen ES, Zuloaga P, Shaw CA, Rimawi MF, Perou CM, Mills GB, Chang JC, Lewis MT: A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013, 73: 4885-4897.
Borowsky AD: Choosing a mouse model: experimental biology in context–the utility and limitations of mouse models of breast cancer. Cold Spring Harb Perspect Biol. 2011, 3: a009670-
Andrechek ER, Nevins JR: Mouse models of cancers: opportunities to address heterogeneity of human cancer and evaluate therapeutic strategies. J Mol Med. 2010, 88: 1095-1100.
Caligiuri I, Rizzolio F, Boffo S, Giordano A, Toffoli G: Critical choices for modeling breast cancer in transgenic mouse models. J Cell Physiol. 2012, 227: 2988-2991.
Kirma NB, Tekmal RR: Transgenic mouse models of hormonal mammary carcinogenesis: advantages and limitations. J Steroid Biochem Mol Biol. 2012, 131: 76-82.
Uhr JW, Pantel K: Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011, 108: 12396-12400.
Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E: Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009, 11: 1287-1296.
Eccles SA, Welch DR: Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007, 369: 1742-1757.
Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS: Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011, 11: 135-141.
Eckhardt BL, Francis PA, Parker BS, Anderson RL: Strategies for the discovery and development of therapies for metastatic breast cancer. Nature Rev Drug Dis. 2012, 11: 479-497.
Guerin E, Man S, Xu P, Kerbel RS: A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 2013, 73: 2743-2748.
Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, Park JO, Ellenbogen RG, Disis ML, Zhang M: Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano. 2012, 6: 2591-2601.
Fang Y, Chen Y, Yu L, Zheng C, Qi Y, Li Z, Yang Z, Zhang Y, Shi T, Luo J, Liu M: Inhibition of breast cancer metastases by a novel inhibitor of TGFbeta receptor 1. J Natl Cancer Inst. 2013, 105: 47-58.
Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, Johnson M, Flores N, Qian Y, Vega-Valle E, Tasker KS, Rudraraju V, Mittapalli RK, Gaasch JA, Bohn KA, Thorsheim HR, Liewehr DJ, Davis S, Reilly JF, Walker R, Bronder JL, Feigenbaum L, Steinberg S, Camphausen K, Meltzer PS, Richon VM, Smith QR, Steeq PS: Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009, 15: 6148-6157.
Xia TS, Wang J, Yin H, Ding Q, Zhang YF, Yang HW, Liu XA, Dong M, Du Q, Ling LJ, Zha XM, Fu W, Wang S: Human tissue-specific microenvironment: an essential requirement for mouse models of breast cancer. Oncol Rep. 2010, 24: 203-211.
Steeg PS: Perspective: the right trials. Nature. 2012, 485: S58-S59.
Wong AL, Lee SC: Mechanisms of resistance to trastuzumab and novel therapeutic strategies in HER2-positive breast cancer. Int J Breast Cancer. 2012, 2012: 415170-
Polyak K: Heterogeneity in breast cancer. J Clin Invest. 2011, 121: 3786-3788.
Lindell KO, Erlen JA, Kaminski N: Lessons from our patients: development of a warm autopsy program. PLoS medicine. 2006, 3: e234-
Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder-Thompson S, Dewar JA, Thompson AM: Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011, 128: 783-794.
Leary AF, Hanna WM, van de Vijver MJ, Penault-Llorca F, Ruschoff J, Osamura RY, Bilous M, Dowsett M: Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009, 27: 1694-1705.
Witzel I, Loibl S, von Minckwitz G, Mundhenke C, Huober J, Hanusch C, Henschen S, Hauschild M, Lantzsch T, Tesch H, Latos K, Just M, Hilfrich J, Barinoff J, Eulenburg CZ, Roller M, Untch M, Muller V: Monitoring serum HER2 levels during neoadjuvant trastuzumab treatment within the GeparQuattro trial. Breast Cancer Res Treat. 2010, 123: 437-445.
Thureau S, Clatot F, Laberge-Le-Couteulx S, Baron M, Basuyau JP, Blot E: Elevated HER2 extracellular domain level in primary breast cancer with HER2 overexpression predicts early failure of adjuvant trastuzumab. Anticancer Res. 2012, 32: 1429-1433.
Molina R, Escudero JM, Munoz M, Auge JM, Filella X: Circulating levels of HER-2/neu oncoprotein in breast cancer. Clin Chem Lab Med. 2012, 50: 5-21.
Dietel M, Johrens K, Laffert M, Hummel M, Blaker H, Muller BM, Lehmann A, Denkert C, Heppner FL, Koch A, Sers C, Anagnostopoulos I: Predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance. Cancer Gene Ther. 2013, 20: 211-221.
Modur V, Hailman E, Barrett JC: Evidence-based laboratory medicine in oncology drug development: from biomarkers to diagnostics. Clin Chem. 2013, 59: 102-109.
Knowles SM, Wu AM: Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012, 30: 3884-3892.
Capala J, Bouchelouche K: Molecular imaging of HER2-positive breast cancer: a step toward an individualized ‘image and treat’ strategy. Curr Opin Oncol. 2010, 22: 559-566.
Asselin MC, O’Connor JP, Boellaard R, Thacker NA, Jackson A: Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012, 48: 447-455.
Waterton JC, Pylkkanen L: Qualification of imaging biomarkers for oncology drug development. Eur J Cancer. 2012, 48: 409-415.
Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD: Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007, 25: 675-680.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, Zegers CML, Gillies R, Boellard R, Dekker A, Aerts HJ: Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012, 48: 441-446.
Macaskill EJ, Bartlett JM, Sabine VS, Faratian D, Renshaw L, White S, Campbell FM, Young O, Williams L, Thomas JS, Barber MD, Dixon JM: The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res Treat. 2011, 128: 725-734.
Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, Appawu M, Iasonos A, Atkinson T, Goldfarb S, Culkin A, Kris MG, Schrag D: Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009, 101: 1624-1632.
Dietary Fish and Omega 3 Fatty Acids for Breast Cancer Prevention. [ http://clinicaltrials.gov/show/NCT01282580 ]
Download references
Acknowledgements
We would like to acknowledge the helpful contributions to the final manuscript from the Executive Advisory Board: Kevin Brindle, Robert E Coleman, Charles Coombes, Jack Cuzick, Mitchell Dowsett, Lesley Fallowfield, Christine Friedenreich, William J Gullick, Barry Gusterson, Craig Jordan, Sunil Lakhani, Bettina Meiser, Emma Pennery, Rebecca Riggins and Stephen Johnston. We would also like to acknowledge the contributions of the patient advocate representatives Mairead McKenzie and Marion Lewis from Breast Cancer Care’s Service User Research Panel.
SAE acknowledges support from the NIHR RM/ICR Biomedical Research Centre, ICR and Cancer Research UK.
AMT acknowledges support from Breast Cancer Campaign, Breakthrough Breast Cancer and CR-UK.
Breast Cancer Campaign staff Lisa Wilde, Phyllis Quinn and Stuart Griffiths assisted in the design and implementation of the gap analysis initiative and acted as facilitators throughout the process. Geraldine Byrne was responsible for co-ordinating and delivering the logistics and acted as a facilitator at the nine gap analysis workshops that were held at the Breast Cancer Campaign offices.
We thank Dr Alexis Willet who provided editorial assistance on behalf of Punch Consulting.
Author information
Authors and affiliations.
Imperial College London, Exhibition Rd, London, SW7 2AZ, UK
Eric O Aboagye, Simak Ali, James M Flanagan & David J Mann
University of Dundee, Perth Road, Dundee, DD1 4HN, UK
Annie S Anderson, Anna M Campbell & Alastair M Thompson
University of Southampton, University Road, Southampton, SO17 1BJ, UK
Jeremy P Blaydes, Diana M Eccles, Deborah F Fenlon & Claire Foster
University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
Fedor Berditchevski & Joanna R Morris
University of Manchester, Oxford Road, Manchester, M13 9PL, UK
Keith Brennan, Nigel J Bundred, Robert B Clarke, D Gareth Evans, Michelle Harvie, Sacha J Howell, Anthony Howell, Cliona C Kirwan, James PB O’Connor, Charles H Streuli & Kaye J Williams
University of Sheffield, Western Bank, Sheffield, S10 2TN, UK
Nicola J Brown, Helen E Bryant, Angela Cox & Ingunn Holen
Kings College London, Strand, London, WC2R 2LS, UK
Jo Armes, Joy M Burchell, Gary JR Cook, Vicky Goh, Myra S Hunter, David W Miles & Andrew N J Tutt
University College London, Gower Street, London, WC1E 6BT, UK
Ashley M Groves & Robert Stein
Cancer Research UK, Cambridge Research Institute/University of Cambridge, Trinity Lane, Cambridge, CB2 1TN, UK
Jason S Carroll, Douglas F Easton, Paul D P Pharoah, John Stingl & Christine J Watson
Newcastle University, Claremont Road, Newcastle upon Tyne, NE1 7RU, UK
Nicola J Curtin
University of Nottingham, University Park, Nottingham, NG7 2RD, UK
Lodewijk V Dekker, Stewart G Martin & Emad A Rakha
London School of Hygiene and Tropical Medicine, Keppel Street, London, WC1E 2HT, UK
Isabel dos Santos Silva
Queen Mary University of London, Mile End Road, London, E1 4NS, UK
Stephen W Duffy, Louise J Jones, John F Marshall & Sue M Moss
University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK
Joanne Edwards
University of East Anglia, Earlham Road, Norwich, NR4 7TJ, UK
Dylan R Edwards & John M Saxton
University College Dublin, Belfield, Dublin 4, Ireland
William M Gallagher
The Institute of Cancer Research, 15 Cotswold Road, London, SM2 5MG, UK
Suzanne A Eccles, Montserrat Garcia-Closas, Martin O Leach, Lesley Ann Martin, Rachel Natrajan & Simon P Robinson
University of Cardiff, Park Place, Cardiff, CF10 3AT, UK
Julia M W Gee, Stephen Hiscox, Bharat Jasani & Matthew J Smalley
University of Leeds, Woodhouse Lane, Leeds, LS2 9JT, UK
Valerie Speirs & Galina Velikova
Royal College of Surgeons Ireland, 123, St Stephen’s Green, Dublin 2, Ireland
Bryan T Hennessy & Leonie S Young
University of Stirling, Stirling, FK9 4LA, UK
Gill Hubbard
University of Chester, Parkgate Road, Chester, CH1 4BJ, UK
Nick Hulbert-Williams
University of Oxford, Wellington Square, Oxford, OX1 2JD, UK
Timothy J Key & Anthony Kong
University of Edinburgh, South Bridge, Edinburgh, EH8 9YL, UK
Ian H Kunkler, Simon P Langdon & William R Miller
National Cancer Research Institute, 407 St John Street, London, EC1V 4AD, UK
Jennifer E Macdougall
Queen’s University Belfast, University Road, Belfast, BT7 1NN, UK
Paul Mullan
University College Cork, College Road, Cork, Ireland
Rosemary O’Connor
University of Leicester, University Road, Leicester, LE1 4RH, UK
Andy J Gescher & Rosemary A Walker
Princess Alice Hospice, West End Lane, Esher, KT10 8NA, UK
Elizabeth Reed
Brighton and Sussex Medical School, University of Sussex, Brighton, East Sussex, BN1 9PX, UK
Peter Schmid
The University of Liverpool, Brownlow Hill, Liverpool, L69 7ZX, UK
Carlo Palmieri
London Research Institute, 44 Lincoln’s Inn Fields, London, WC2A 3LY, UK
Brunel University, Kingston Lane, Uxbridge, UB8 3PH, UK
Amanda J Harvey
Cambridge University Hospitals NHS Foundation Trust, Hills Road, Cambridge, CB2 0QQ, UK
Charlotte E Coles
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Suzanne A Eccles or Alastair M Thompson .
Additional information
Competing interests.
Dr Galina Velikova: Chair of a working group of the National Cancer Survivorship Initiative led by Macmillan Cancer Support.
Drs Helen Bryant and Dr Nicola Curtin: hold patents for PARP inhibitors.
Professor William Gallagher: co-Founder and part-time Chief Scientific Officer of OncoMark, a molecular diagnostics company.
Dr Martin Leach: director of Specialty Scanners plc, developing MRI-based diagnosis and treatment systems.
Dr Sacha Howell: Advisory Board honoraria from AstraZeneca, Roche, Novartis, Genomic Health and Celgene.
Dr Robert Stein: shareholder in GlaxoSmithKline and chief investigator of the OPTIMA study; travel funds received from Celgene, Roche, BristolMeyersSquibb, SanofiAventis and Novartis; Advisory Board fees from Novartis, Amgen, GSK, Roche and AstraZeneca.
Dr Nigel Bundred has received paid honoraria from Genomic Health.
The remaining authors declare that they have no competing interests.
Authors’ contributions
*denotes recipient of Breast Cancer Campaign funding in the last five years. ≠ denotes current Breast Cancer Campaign Scientific Advisory Board membership. # denotes current Breast Cancer Campaign Board of Trustees membership. Chairs: SAE # and AMT # conceived the overall strategy, designed the workshop formats and authored the manuscript on the basis of the final reports submitted by the nine working groups. Group Leaders: RBC, IDSS, DGE* ≠ , CF ≠ ,WMG ≠ , AH ≠ , IH* ≠ , LJJ*, SPL, SPR ≠ , PS* ≠ , and VS* led their respective groups with the help of the Deputy Group Leaders, co-ordinated responses from a pre-circulated questionnaire, and wrote and submitted final reports. Deputy Group Leaders: EOA, NJB a , JMF* ≠ , JMWG*, AJH*, MH, AK, JRM*, PM* ≠ , ES, MJS* ≠ , ER, and RN* supported the activities of the Group Leaders in contributing to collating workshop presentations and discussions and producing the final reports from each group. Working group members: SA*, ASA , JA*, FB*, JPB*, KB* ≠ , NJB b , HEB ≠ , JMB, AMC*, JSC*, CEC*, GJRC*, AC, NJC, LVD* ≠ , SWD, DFE, DME, DRE*, JE, DFF*, MGC, AJG, VG, AMG, BTH, SH, SJH ≠ , GH, NHW, MSH, BJ, TJK, CCK, IHK*, MOL, DJM, JFM* ≠ , LAM, SGM ≠ , JEM, DWM, WRM, JRM, SMM*, JPBOC, ROC*, CP, PDPP*, EAR ≠ , JMS*, RS ≠ , JS, CHS, ANJT, GV, RAW*, CJW, KJW ≠ and LSY all participated in/contributed to the gap analysis workshops, discussions and in generating the respective reports. NJB a Nigel J Bundred. NJB b Nicola J Brown. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, authors’ original file for figure 5, authors’ original file for figure 6, authors’ original file for figure 7, authors’ original file for figure 8, authors’ original file for figure 9, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Eccles, S.A., Aboagye, E.O., Ali, S. et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res 15 , R92 (2013). https://doi.org/10.1186/bcr3493
Download citation
Received : 08 August 2013
Accepted : 12 September 2013
Published : 01 October 2013
DOI : https://doi.org/10.1186/bcr3493
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast Cancer
- Circulating Tumor Cells (CTCs)
- Current Cancer Stem Cell (CSC)
- Mammographic Density
- Triple-negative Breast Cancer (TNBC)
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
Exploring research progress in studying serum exosomal miRNA-21 as a molecular diagnostic marker for breast cancer
- REVIEW ARTICLE
- Published: 11 April 2024
Cite this article
- Hang Li ORCID: orcid.org/0009-0000-9518-732X 1 na1 &
- Xiao-jing Tie 2 na1
Explore all metrics
Breast cancer is one of the most prevalent malignancies affecting women globally and poses a significant public health challenge. Early clinical detection plays a pivotal role in providing optimal treatment opportunities and favorable prognoses, crucial for reducing breast cancer mortality and enhancing patients’ quality of life. Therefore, the timely identification and diagnosis of breast cancer are imperative. Conventional tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3), serve as reliable methods for actively monitoring disease progression and have become a routine auxiliary diagnostic approach in clinical settings. However, these biomarkers exhibit limitations in sensitivity and specificity, particularly in the early screening and diagnosis of tumors, often yielding results inconsistent with clinical manifestations. In recent years, research has increasingly focused on exosomes released by tumor cells as potential new biomarkers for early stage breast cancer screening. Exosomes carry various components, including tumor-derived proteins, nucleic acids, and lipids. This paper delves into the specific utilization of serum exosomal microRNA-21 (miR-21) as a biomarker for early detection and diagnosis of breast cancer, evaluating its efficacy within this framework.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Carcinoembryonic antigen
Carbohydrate antigen 15-3
Microribonucleic acid-21
Messenger RNAs
Non-coding RNAs
Long non-coding RNAs
B-cell lymphoma 2
Breast cancer susceptibility gene 1
Breast cancer susceptibility gene 2
Tumor suppressor protein p53
Diagnostic odds ratio
Negative likelihood of detection results ratio
Positive likelihood ratio
Confidence interval
Phosphatase and tensin homolog deleted on chromosome 10
The phosphoinositide 3-kinase
Phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin
Michigan Cancer Foundation-7
Programmed cell death protein 4
Epithelial growth factor
Transforming growth factor β
Tumor suppressor gene tropomyosin 1
Mammary serine protease inhibitor
Liquid chromatography/mass spectrometry
Receptor protein against Pseudomonas MACULICOLA1
Human epidermal growth receptor 2
Breast cancer stem cells
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Rabbani F, Yazdiniapour Z, Ghanadian M, Zolfaghari B, Maleki M, Shafiee F. Cytotoxicity and apoptosis assay of novel cyclomyrsinol diterpenes against breast cancer cell lines. World J Tradit Chin Med. 2022;8:273–7.
Article CAS Google Scholar
Tang Z, Xu L, Sun Z, Chen W, Li R. Differential diagnosis of benign and malignant breast lesions using mammography and 3.0T magnetic resonance imaging. Chin Med Equip. 2022;19(10):48–52.
Huang W-H, Chen I-W, Yang C-C, Wang T-Y. The sonography is a valuable tool for diagnosis of microcalcification in screening mammography. Ultrasound Med Biol. 2017;43:S28.
Article Google Scholar
Wang H, Song Q, Zheng G, Cao g. Study on the diagnosis of early breast cancer with molybdenum target and ultrasound Doppler combined with serum tumor markers. China Med Equip. 2024;21(1):82–7.
Google Scholar
Li X, Xu Y, Zhang L. Serum CA153 as biomarker for cancer and noncancer diseases. Prog Mol Biol Transl Sci. 2019;162:265–76.
Article CAS PubMed Google Scholar
Jia L, Li G, Ma N, Zhang A, Zhou Y, Ren L, et al. Soluble POSTN is a novel biomarker complementing CA153 and CEA for breast cancer diagnosis and metastasis prediction. BMC Cancer. 2022;22(1):760.
Article CAS PubMed PubMed Central Google Scholar
Lin Z. Clinical significance of CA15-3, CA125 and CEA joint detection in the diagnosis of breast cancer. Exp Lab Med. 2009;27(3):261–2.
Tanman Ü, Yangın S, Cansaran-Duman D. Determination of dysregulated miRNA expression levels by qRT-PCR after the application of usnic acid to breast cancer. J Anticancer Agents Med Chem. 2020;20(5):548–58.
Ortega MA, Fraile-Martínez O, Guijarro LG, Casanova C, Coca S, Álvarez-Mon M, et al. The regulatory role of mitochondrial microRNAs (MitomiRs) in breast cancer: translational implications present and future. Cancers (Basel). 2020;12(9):2443.
Ren FL, Li P, Wang CM, Chen QH. Application of exosomes in cancer diagnosis and treatment and progress in isolation technology. J Chin Oncol. 2020;26(6):475–80.
Boriachek K, Islam MN, Möller A, Salomon C, Nguyen NT, Hossain MSA, et al. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14(6).
Lauretti E, Dabrowski K, Praticò D. The neurobiology of non-coding RNAs and Alzheimer’s disease pathogenesis: pathways, mechanisms and translational opportunities. Ageing Res Rev. 2021;71:101425.
Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4):dmm047662.
Kim MW, Park S, Lee H, Gwak H, Hyun KA, Kim JY, et al. Multi-miRNA panel of tumor-derived extracellular vesicles as promising diagnostic biomarkers of early-stage breast cancer. Cancer Sci. 2021;112(12):5078–87.
Abdel-Hamid NR, Mohammed EA, Abbas AH, Badr FM. MicroRNA-21 expression in primary breast cancer tissue among Egyptian female patients and its correlation with chromosome 17 aneusomy. Mol Diagn Ther. 2015;19(6):365–73.
Shaaban NZ, Ibrahim NK, Saada HN, El-Rashidy FH, Shaaban HM, Elbakary NM, et al. The implication of microRNAs as non-invasive biomarkers in 179 Egyptian breast cancer female patients. Oncol Res. 2023;30(6):269–76.
Article PubMed PubMed Central Google Scholar
Wang M, Wang Y, Tian X, Wang Q, Huang H, Lu X, et al. Diagnostic and predictive value of liquid biopsy-derived exosome miR-21 for breast cancer: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2023;23(4):315–24.
Yu X, Liang J, Xu J, Li X, Xing S, Li H, et al. Identification and validation of circulating microRNA signatures for breast cancer early detection based on large scale tissue-derived data. J Breast Cancer. 2018;21(4):363–70.
Liang Y, Hao M, Guo R, Li X, Li Y, Yu C, et al. Biomarkers for early screening and diagnosis of breast cancer: a review. Sheng Wu Gong Cheng Xue Bao. 2023;39(4):1425–44.
CAS PubMed Google Scholar
García Z, Kumar A, Marqués M, Cortés I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J. 2006;25(4):655–61.
Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91.
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283(2):1026–33.
Han M, Wang F, Gu Y, Pei X, Guo G, Yu C, et al. MicroRNA- 21 induces breast cancer cell invasion and migration by suppressing smad7 via EGF and TGF-β pathways. Oncol Rep. 2016;35(1):73–80.
Wu MF, Yang J, Xiang T, Shi YY, Liu LJ. miR-21 targets Fas ligand-mediated apoptosis in breast cancer cell line MCF-7. J Huazhong Univ Sci Technol Med Sci. 2014;34(2):190–4.
Wang W, Li J, Zhu W, Gao C, Jiang R, Li W, et al. MicroRNA-21 and the clinical outcomes of various carcinomas: a systematic review and meta-analysis. BMC Cancer. 2014;14:819. https://doi.org/10.1186/1471-2407-14-819 .
Zhu W, Xu B. MicroRNA-21 identified as predictor of cancer outcome: a meta-analysis. PLoS One. 2014;9(8):103373.
Desouky EM, Khaliefa AK, Hozayen WG, Shaaban SM, Hasona NA. Signature of miR-21 and MEG-2 and their correlation with TGF-β signaling in breast cancer. Hum Exp Toxicol. 2023;42:9603271231159800.
El-Toukhy SE, El-Daly SM, Kamel MM, Nabih HK. The diagnostic significance of circulating miRNAs and metabolite profiling in early prediction of breast cancer in Egyptian women. J Cancer Res Clin Oncol. 2023;149(8):5437–51.
Download references
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
No external funding received to conduct this study.
Author information
Hang Li and Xiao-jing Tie have contributed equally to this work.
Authors and Affiliations
Department of Laboratory Medicine, Kaifeng Central Hospital, 85 HeDao Street, Kaifeng, 475000, China
Department of Oncology, Kaifeng Central Hospital, Kaifeng, 475000, China
Xiao-jing Tie
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design of the research: HL, XT. Acquisition of data: HL, XT. Analysis and interpretation of the data: HL. Statistical analysis: HL. Obtaining financing: None. Writing of the manuscript: HL. Critical revision of the manuscript for intellectual content: HL. All authors read and approved the final draft.
Corresponding author
Correspondence to Hang Li .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Li, H., Tie, Xj. Exploring research progress in studying serum exosomal miRNA-21 as a molecular diagnostic marker for breast cancer. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03454-z
Download citation
Received : 20 February 2024
Accepted : 06 March 2024
Published : 11 April 2024
DOI : https://doi.org/10.1007/s12094-024-03454-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Molecular diagnosis
- Find a journal
- Publish with us
- Track your research

Clinical Trials for Metastatic Breast Cancer: What You Need to Know
Learn the pros and cons of joining a clinical trial, and how to find a trial that may be right for you.
Here's what you need to know about joining a clinical trial in metastatic breast cancer.
Know Your Breast Cancer Type
To better understand what trials you might be able to join, it’s important to know the type of metastatic breast cancer you have, explains Rita Nanda, MD , the director of the Breast Oncology Program at University of Chicago Medicine.
If you don’t have any of these targets, that means you have triple-negative breast cancer , and you will be eligible for trials designed for this type of breast cancer.
When breast cancer spreads or recurs, it can be the same type as the first time you had it, or it can change to a different subtype, Dr. Nanda says.
Types of Clinical Trials
Clinical trials aim to answer specific questions about a new therapy or existing treatments that require further study.
- Phase 1 These trials are performed in a small group of people to determine the appropriate dose of the treatment or drug and to examine side effects.
- Phase 2 These trials involve hundreds of people and evaluate the effectiveness of the new treatment. They also further assess its safety and side effects.
- Phase 3 These are often conducted in multiple places across the country, involving thousands of participants. These trials further evaluate the safety, effectiveness, and side effects of the new treatment, and compare the new treatment to the existing standard of care. A request will ultimately be submitted to the FDA for approval to make the drug available to the public.
- Phase 4 These trials take place after a treatment has been approved by the FDA and is being sold to the public. The purpose is to monitor the long-term safety of the new drug in a much larger, more diverse group of people in the real world.
Is Joining a Clinical Trial Right for You? Pros and Cons
“Clinical trials are the way that we improve [cancer] treatment,” says Nanda. But participating in a trial has pros and cons from the patient perspective, she says.
The benefits of joining a clinical trial may include:
- The chance of getting a new, effective drug before it’s commercially available
- Better medical attention than you might usually receive
- Helping other patients by contributing to cancer research
- Sometimes getting paid to participate
The challenges of participating in a trial might include the following:
- Having to travel long distances if the trial isn’t taking place close to home
- Spending more time for extra tests and procedures, such as more blood draws and biopsies
- No guarantee that you will receive the investigational drug or therapy being tested, as treatment is given according to the study plan
- Finding out you are not eligible to participate because of strict rules for entry into a clinical trial
Dr. Lustberg says she discusses clinical trials with her patients often. “[People with metastatic breast cancer] are always on some type of therapy and need serial treatment changes. Any time there is a need to alter something … [we] evaluate all options — standard-of-care therapy and clinical trials.”
Standard-of-care therapy is the “gold standard” that has been proven in clinical trials to be the best treatment currently available for breast cancer, and has been approved by the FDA.
Lustberg notes, however, that not all doctors discuss clinical trials with their patients and, in particular, there is “a lot of unconscious bias on the part” of some physicians when it comes to non-white patients. But, “if given the chance, [many of] these women would want to participate in clinical trials,” she stresses.
If your doctor has never mentioned clinical trials, you can be the one to bring it up and ask about potentially joining a trial.
Do Clinical Trials Increase Survival?
Still, the authors of this study noted that other researchers have found different results, so “the promotion of clinical trials for cancer due to survival benefit must be done with caution,” they say.
Lustberg agrees that not all studies on this topic show the same results, but she insists that the survival data in metastatic breast cancer are more favorable than for some other cancers.
Ultimately, whether to join a trial is a personal decision you make with your doctor.
How to Find Clinical Trials in Metastatic Breast Cancer
New breast cancer treatment breakthroughs can only happen with the aid of clinical trials. Here’s where you can find out about clinical trials you may be eligible for.
- Your doctor: Ask your oncologist if you may be eligible for a clinical trial, and how to find one that’s a good fit for you.
- ClinicalTrials.gov : This online resource is maintained by the National Library of Medicine, where clinical studies are registered and updated. Visit the site and search “actively recruiting” and “metastatic breast cancer,” or a more specific term if you know your breast cancer subtype, and add suitable locations for you. If it’s hard to understand the wording on the site, print out the information or send the link to your doctor so you can discuss it at your next appointment.
- Patient advocacy groups and nonprofit organizations: There are a number of patient support groups for metastatic breast cancer that give information on clinical trials, including BreastCancerTrials.org (which offers a Metastatic Trial Search function), the Metastatic Breast Cancer Alliance , and the Triple Negative Breast Cancer Foundation . You can also visit the American Cancer Society for reliable information on breast cancer clinical trials.
- Online forums: Join online forums and support groups through social media platforms. Search #metastaticbreastcancer, #MBC, #bcsm (for breast cancer social media), or the subtype you have. This should help connect you to other patients and doctors who are posting about this disease.
As of March 2024, more than 300 clinical trials in metastatic breast cancer actively enrolling in the United States were identified on ClinicalTrials.gov.
Beware of False Claims: Always Discuss New Treatments With Your Doctor First
“I’ve had many patients [come to ask me about] a clinical trial because they are part of a Facebook group. Patients find out [about clinical trials] from other patients and from online support groups. We try to help them as much as we can. If they are interested in a specific clinical trial that we don’t have [at our institution], we can try to find them places to go,” Nanda says.
But some patients don’t want to connect with others, Nanda says, and they prefer to handle things on their own or have one-on-one counseling. “Everybody is different, so we have a dedicated social worker to help.”
Lustberg also encourages her patients to look online and tap into other resources to research their cancer. But there can be downsides to searching the web, she says.
“There are often unsubstantiated claims about this supplement or that supplement that might take the place of standard-of-care treatment,” Lustberg says.
And she warns that there are myths about clinical trials in metastatic breast cancer. “I want to make sure that we [doctors] have effectively dispelled those,” she says. One such myth is that patients might get a placebo [dummy pill] in a study, but Lustberg assures patients that this is not the case in metastatic breast cancer.
“Standard of care is always given [if you’re not given the drug being tested]. It would not be ethical otherwise,” Lustberg says. “I don’t want my patients to be afraid to look online by themselves. I just want to help them distinguish what is good.”
Questions to Ask Your Doctor
- What is this clinical trial testing?
- Why is this clinical trial a good fit for me?
- Has this treatment been tested before? If yes, what were the results?
- Do you have other patients enrolled in this trial?
- How long will the trial take?
- What tests and procedures will I have to get? How often will they be done? Where will they be done?
- Will you be providing my care while I am in this trial or will it be someone else?
- What are the potential benefits?
- What are the potential risks? Will the side effects be treatable?
- How will this clinical trial affect my daily life? Will I be able to go to work or school?
- What tests or follow-up visits are required when the trial is over?
- Will insurance cover the tests, procedures, and treatments I need to get during the trial?
- What will happen to my cancer if I don’t join this trial? What will my treatment be? What are the benefits and risks of that treatment?
- Are there other trials I should consider joining?
Editorial Sources and Fact-Checking
Everyday Health follows strict sourcing guidelines to ensure the accuracy of its content, outlined in our editorial policy . We use only trustworthy sources, including peer-reviewed studies, board-certified medical experts, patients with lived experience, and information from top institutions.
- Lee V. Metastatic Breast Cancer. BreastCancer.org. March 9, 2024.
- What Several New Statistics Tell Us About Our Progress Against Breast Cancer. Breast Cancer Research Foundation. April 9, 2021.
- Chow CJ et al. Does Enrollment in Cancer Trials Improve Survival? Journal of the American College of Surgeons . April 2013.
- Breast Cancer Types: What Your Type Means. Mayo Clinic. December 6, 2022.
- What Are Clinical Trials and Studies? National Institute on Aging. March 22, 2023.
Related Topics
- BARD1 Gene Mutation
- Lymphoma Survival Rate
- STK11 Gene Mutation
- TP53 Mutation

COMMENTS
1. Introduction. Breast cancer has a very long history as it was first reported by the ancient Egyptians more than 3500 years ago in about 1500 B.C [].Today, breast cancer is the second most prevalent type of cancer and is a leading cause of most cancer-related deaths in women in the United States [].Around 281,550 women are projected to be diagnosed with breast cancer in 2021, and 43,600 ...
Breast Cancer Research and Treatment is a comprehensive forum dedicated to all aspects of breast cancer research. The journal's focus spans across various disciplines including surgery, radiotherapy, medical oncology, endocrinology, epidemiology, immunology and cell biology. Provides an international platform for the discussion and resolution ...
Introduction. Therapy with curative intent for breast cancer achieves optimal outcomes when it is administered to completion within a defined timeframe; its success depends on appropriate referrals for timely and personalized multimodality treatment after the receipt of a definitive diagnosis. 1 The provision of cancer treatment requires an organized, multidisciplinary approach in which ...
Breast Cancer Research is the highest ranking breast cancer-specific title in the top quartile of oncology journals worldwide. ... assisted by transcriptomics analysis to reveal metabolic characteristics and potential biomarkers associated with treatment response of neoadjuvant therapy with TCbHP regimen in HER2 + breast cancer ... 1.764 - SNIP ...
Our main focus will be to summarize recent trends and technologies in breast cancer diagnosis and treatment as reported in recent research papers and patents and discuss future perspectives for effective breast cancer therapy. Keywords: breast cancer; breast specific gamma imaging; imaging modalities; mammography; theranostics; triple-negative ...
The lifetime risk for breast cancer in men is 1 in 833 compared with 1 in 10 for a woman. Of affected men, 20% have a first-degree family history of cancer; 4-14% of cases in males are ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
This page highlights what's new and recent research in the detection and treatment of breast cancer. New methods to detect breast cancer, such as 3-D mammography, are described. Treatments tailored to breast cancer subtypes are discussed, including targeted therapies.
Worldwide, breast cancer is the most common cancer in women, other than nonmelanoma skin cancer. 1 More than 250 000 new cases of breast cancer were diagnosed in the United States in 2017, and breast cancer will be diagnosed in 12% of all women in the United States over their lifetimes. 2 This review summarizes evidence-based approaches to the systemic and local treatment of the 3 major breast ...
Doxorubicin-based chemotherapy is one of the most frequently used systemic triple-negative breast cancer (TNBC) treatments. The upregulation of EGFR signaling was suggested to be involved in doxorubicin-acquired resistance. Doxorubicin-induced EMT and CSC properties of resistant cells were transferred from doxorubicin-resistant cells to ...
Breast cancer is still the most common cancer worldwide. But the way breast cancer is viewed has changed drastically since its molecular hallmarks were extensively characterised, now including immunohistochemical markers (eg, ER, PR, HER2 [ERBB2], and proliferation marker protein Ki-67 [MKI67]), genomic markers (eg, BRCA1, BRCA2, and PIK3CA), and immunomarkers (eg, tumour-infiltrating ...
Breast cancer is one of the leading causes of cancer-related morbidity and mortality in women worldwide. Early diagnosis and effective treatment of all types of cancers are crucial for a positive prognosis. Patients with small tumor sizes at the time of their diagnosis have a significantly higher survival rate and a significantly reduced probability of the cancer being fatal. Therefore, many ...
Time trends in real-world treatment patterns and survival in patients diagnosed with de novo HER2+ metastatic breast cancer: an analysis of the SONABRE registry. Sandra M. E. Geurts. Khava I. E. Ibragimova. Vivianne C. G. Tjan-Heijnen. Epidemiology Open access 21 February 2024.
new case diagnosed every 18 seconds; additionally, 626,679 women with breast cancer died. The global. incidence of breast cancer has been rising wi th annual. increases of 3.1%, beginning with ...
The latest global cancer burden data released by the World Health Organization International Agency for Research on Cancer in 2020 indicated that the number of new breast cancer cases reached 2.26 million worldwide, exceeding the total number (2.2 million) of lung cancer cases [].Breast cancer has replaced lung cancer to become the world's most prevalent cancer [].
In general, Breast Cancer Research and Treatment will not accept for publication papers in which all of the data shown in the paper were obtained using a single cell line. Indeed, for most studies, experiments involving the use of multiple cell lines (more than 2) is highly recommended.
Breast cancer remains a significant scientific, clinical and societal challenge. This gap analysis has reviewed and critically assessed enduring issues and new challenges emerging from recent research, and proposes strategies for translating solutions into practice. More than 100 internationally recognised specialist breast cancer scientists, clinicians and healthcare professionals ...
Citation 1 Serum HER2 has changed the treatment paradigm for half of patients with advanced breast cancer, and HER2 is currently defined as an expression immunohistochemistry without amplification via in-situ hybridization and therefore remains a clinical challenge in the treatment of breast cancer.
Strategies for effective breast cancer treatment. Sara McLaughlin, M.D., Surgical Oncologist, Department of Surgery: When we think about the treatment of breast cancer, we think about it from a comprehensive strategy of the breast, of what could be going on anywhere else. And then how do we create a treatment plan that addresses all of those ...
A potential mechanism by which copper complexes may exert antitumor effects by causing copper death in breast cancer cells is proposed. Breast cancer, a malignancy originating from the epithelium or ductal epithelium of the breast, is not only highly prevalent in women but is also the leading cause of cancer-related deaths in women worldwide. Research has indicated that breast cancer incidence ...
Breast cancer is one of the most prevalent malignancies affecting women globally and poses a significant public health challenge. Early clinical detection plays a pivotal role in providing optimal treatment opportunities and favorable prognoses, crucial for reducing breast cancer mortality and enhancing patients' quality of life. Therefore, the timely identification and diagnosis of breast ...
Key Takeaways. Clinical trials are the major way to improve existing therapies in metastatic breast cancer. In a clinical trial you may receive great medical care with a new, effective drug that ...
Tanoa Ava Show, 13 APR 2024 - Radio Samoa. Like. Comment