
An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Alcohol Research: Current Reviews (ARCR)
ARCR, a peer-reviewed scientific journal published by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, marks its 50th anniversary in 2024. Explore our "News & Notes" webpage for more on this historic accomplishment.

Recent Articles
Liz Simon, Brianna L. Bourgeois, and Patricia E. Molina
Julie A. Kable 1,2 and Kenneth Lyons Jones 3
Grace Chang
News and Notes

25 January 2024
ARCR Celebrates Its 50th Anniversary
2024 marks the 50th anniversary of Alcohol Research: Current Reviews (ARCR), an open-access, peer-reviewed journal published by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) at the National Institutes of Health.
- Open access
- Published: 13 November 2019
Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review
- Susan A. Rombouts 1 ,
- James Conigrave 2 ,
- Eva Louie 1 ,
- Paul Haber 1 , 3 &
- Kirsten C. Morley ORCID: orcid.org/0000-0002-0868-9928 1
Systematic Reviews volume 8 , Article number: 275 ( 2019 ) Cite this article
7242 Accesses
3 Citations
Metrics details
Alcohol use disorder (AUD) is highly prevalent and accounts globally for 1.6% of disability-adjusted life years (DALYs) among females and 6.0% of DALYs among males. Effective treatments for AUDs are available but are not commonly practiced in primary health care. Furthermore, referral to specialized care is often not successful and patients that do seek treatment are likely to have developed more severe dependence. A more cost-efficient health care model is to treat less severe AUD in a primary care setting before the onset of greater dependence severity. Few models of care for the management of AUD in primary health care have been developed and with limited implementation. This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
We will conduct a systematic review to synthesize studies that evaluate the effectiveness of models of care in the treatment of AUD in primary health care. A comprehensive search approach will be conducted using the following databases; MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present).
Reference searches of relevant reviews and articles will be conducted. Similarly, a gray literature search will be done with the help of Google and the gray matter tool which is a checklist of health-related sites organized by topic. Two researchers will independently review all titles and abstracts followed by full-text review for inclusion. The planned method of extracting data from articles and the critical appraisal will also be done in duplicate. For the critical appraisal, the Cochrane risk of bias tool 2.0 will be used.
This systematic review and meta-analysis aims to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings. The evidence will define which models are most promising and will guide further research.
Protocol registration number
PROSPERO CRD42019120293.
Peer Review reports
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [ 1 ]. The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4–2.0) of total DALYs globally among females and 6.0% (5.4–6.7) among males, resulting in alcohol use being the seventh leading risk factor for both premature death and disability-adjusted life years (DALYs) [ 2 ]. Among people aged 15–49 years, alcohol use was the leading risk factor for mortality and disability with 8.9% (95% UI 7.8–9.9) of all attributable DALYs for men and 2.3% (2.0–2.6) for women [ 2 ]. AUD has been linked to many physical and mental health complications, such as coronary heart disease, liver cirrhosis, a variety of cancers, depression, anxiety, and dementia [ 2 , 3 ]. Despite the high morbidity and mortality rate associated with hazardous alcohol use, the global prevalence of alcohol use disorders among persons aged above 15 years in 2016 was stated to be 5.1% (2.5% considered as harmful use and 2.6% as severe AUD), with the highest prevalence in the European and American region (8.8% and 8.2%, respectively) [ 1 ].
Effective and safe treatment for AUD is available through psychosocial and/or pharmacological interventions yet is not often received and is not commonly practiced in primary health care. While a recent European study reported 8.7% prevalence of alcohol dependence in primary health care populations [ 4 ], the vast majority of patients do not receive the professional treatment needed, with only 1 in 5 patients with alcohol dependence receiving any formal treatment [ 4 ]. In Australia, it is estimated that only 3% of individuals with AUD receive approved pharmacotherapy for the disorder [ 5 , 6 ]. Recognition of AUD in general practice uncommonly leads to treatment before severe medical and social disintegration [ 7 ]. Referral to specialized care is often not successful, and those patients that do seek treatment are likely to have more severe dependence with higher levels of alcohol use and concurrent mental and physical comorbidity [ 4 ].
Identifying and treating early stage AUDs in primary care settings can prevent condition worsening. This may reduce the need for more complex and more expensive specialized care. The high prevalence of AUD in primary health care and the chronic relapsing character of AUD make primary care a suitable and important location for implementing evidence-based interventions. Successful implementation of treatment models requires overcoming multiple barriers. Qualitative studies have identified several of those barriers such as limited time, limited organizational capacity, fear of losing patients, and physicians feeling incompetent in treating AUD [ 8 , 9 , 10 ]. Additionally, a recent systematic review revealed that diagnostic sensitivity of primary care physicians in the identification of AUD was 41.7% and that only in 27.3% alcohol problems were recorded correctly in primary care records [ 11 ].
Several models for primary care have been created to increase identification and treatment of patients with AUD. Of those, the model, screening, brief interventions, and referral to specialized treatment for people with severe AUD (SBIRT [ 12 ]) is most well-known. Multiple systematic reviews exist, confirming its effectiveness [ 13 , 14 , 15 ], although implementation in primary care has been inadequate. Moreover, most studies have looked primarily at SBIRT for the treatment of less severe AUD [ 16 ]. In the treatment of severe AUD, efficacy of SBIRT is limited [ 16 ]. Additionally, many patient referred to specialized care often do not attend as they encounter numerous difficulties in health care systems including stigmatization, costs, lack of information about existing treatments, and lack of non-abstinence-treatment goals [ 7 ]. An effective model of care for improved management of AUD that can be efficiently implemented in primary care settings is required.
Review objective
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings. We aim to evaluate the effectiveness of the models of care in increasing engagement and reducing alcohol consumption.
By providing this overview, we aim to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings.
The systematic review is registered in PROSPERO international prospective register of systematic reviews (CRD42019120293) and the current protocol has been written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) recommended for systematic reviews [ 17 ]. A PRISMA-P checklist is included as Additional file 1 .
Eligibility criteria
Criteria for considering studies for this review are classified by the following:
Study design
Both individualized and cluster randomized trials will be included. Masking of patients and/or physicians is not an inclusion criterion as it is often hard to accomplish in these types of studies.
Patients in primary health care who are identified (using screening tools or by primary health care physician) as suffering from AUD (from mild to severe) or hazardous alcohol drinking habits (e.g., comorbidity, concurrent medication use). Eligible patients need to have had formal assessment of AUD with diagnostic tools such as Diagnostic and Statistical Manual of Mental Disorders (DSM-IV/V) or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and/or formal assessment of hazardous alcohol use assessed by the Comorbidity Alcohol Risk Evaluation Tool (CARET) or the Alcohol Use Disorders Identification test (AUDIT) and/or alcohol use exceeding guideline recommendations to reduce health risks (e.g., US dietary guideline (2015–2020) specifies excessive drinking for women as ≥ 4 standard drinks (SD) on any day and/or ≥ 8 SD per week and for men ≥ 5 SD on any day and/or ≥ 15 SD per week).
Studies evaluating models of care for additional diseases (e.g., other dependencies/mental health) other than AUD are included when they have conducted data analysis on the alcohol use disorder patient data separately or when 80% or more of the included patients have AUD.
Intervention
The intervention should consist of a model of care; therefore, it should include multiple components and cover different stages of the care pathway (e.g., identification of patients, training of staff, modifying access to resources, and treatment). An example is the Chronic Care Model (CCM) which is a primary health care model designed for chronic (relapsing) conditions and involves six elements: linkage to community resources, redesign of health care organization, self-management support, delivery system redesign (e.g., use of non-physician personnel), decision support, and the use of clinical information systems [ 18 , 19 ].
As numerous articles have already assessed the treatment model SBIRT, this model of care will be excluded from our review unless the particular model adds a specific new aspect. Also, the article has to assess the effectiveness of the model rather than assessing the effectiveness of the particular treatment used. Because identification of patients is vital to including them in the trial, a care model that only evaluates either patient identification or treatment without including both will be excluded from this review.
Model effectiveness may be in comparison with the usual care or a different treatment model.
Included studies need to include at least one of the following outcome measures: alcohol consumption, treatment engagement, uptake of pharmacological agents, and/or quality of life.
Solely quantitative research will be included in this systematic review (e.g., randomized controlled trials (RCTs) and cluster RCTs). We will only include peer-reviewed articles.
Restrictions (language/time period)
Studies published in English after 1 January 1998 will be included in this systematic review.
Studies have to be conducted in primary health care settings as such treatment facilities need to be physically in or attached to the primary care clinic. Examples are co-located clinics, veteran health primary care clinic, hospital-based primary care clinic, and community primary health clinics. Specialized primary health care clinics such as human immunodeficiency virus (HIV) clinics are excluded from this systematic review. All studies were included, irrespective of country of origin.
Search strategy and information sources
A comprehensive search will be conducted. The following databases will be consulted: MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present). Initially, the search terms will be kept broad including alcohol use disorder (+synonyms), primary health care, and treatment to minimize the risk of missing any potentially relevant articles. Depending on the number of references attained by this preliminary search, we will add search terms referring to models such as models of care, integrated models, and stepped-care models, to limit the number of articles. Additionally, we will conduct reference searches of relevant reviews and articles. Similarly, a gray literature search will be done with the help of Google and the Gray Matters tool which is a checklist of health-related sites organized by topic. The tool is produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 20 ].
See Additional file 2 for a draft of our search strategy in MEDLINE.
Data collection
The selection of relevant articles is based on several consecutive steps. All references will be managed using EndNote (EndNote version X9 Clarivate Analytics). Initially, duplicates will be removed from the database after which all the titles will be screened with the purpose of discarding clearly irrelevant articles. The remaining records will be included in an abstract and full-text screen. All steps will be done independently by two researchers. Disagreement will lead to consultation of a third researcher.
Data extraction and synthesis
Two researchers will extract data from included records. At the conclusion of data extraction, these two researchers will meet with the lead author to resolve any discrepancies.
In order to follow a structured approach, an extraction form will be used. Key elements of the extraction form are information about design of the study (randomized, blinded, control), type of participants (alcohol use, screening tool used, socio-economic status, severity of alcohol use, age, sex, number of participants), study setting (primary health care setting, VA centers, co-located), type of intervention/model of care (separate elements of the models), type of health care worker (primary, secondary (co-located)), duration of follow-up, outcome measures used in the study, and funding sources. We do not anticipate having sufficient studies for a meta-analysis. As such, we plan to perform a narrative synthesis. We will synthesize the findings from the included articles by cohort characteristics, differential aspects of the intervention, controls, and type of outcome measures.
Sensitivity analyses will be conducted when issues suitable for sensitivity analysis are identified during the review process (e.g., major differences in quality of the included articles).
Potential meta-analysis
In the event that sufficient numbers of effect sizes can be extracted, a meta-analytic synthesis will be performed. We will extract effect sizes from each study accordingly. Two effect sizes will be extracted (and transformed where appropriate). Categorical outcomes will be given in log odds ratios and continuous measures will be converted into standardized mean differences. Variation in effect sizes attributable to real differences (heterogeneity) will be estimated using the inconsistency index ( I 2 ) [ 21 , 22 ]. We anticipate high degrees of variation among effect sizes, as a result moderation and subgroup-analyses will be employed as appropriate. In particular, moderation analysis will focus on the degree of heterogeneity attributable to differences in cohort population (pre-intervention drinking severity, age, etc.), type of model/intervention, and study quality. We anticipate that each model of care will require a sub-group analysis, in which case a separate meta-analysis will be performed for each type of model. Small study effect will be assessed with funnel plots and Egger’s symmetry tests [ 23 ]. When we cannot obtain enough effect sizes for synthesis or when the included studies are too diverse, we will aim to illustrate patterns in the data by graphical display (e.g., bubble plot) [ 24 ].
Critical appraisal of studies
All studies will be critically assessed by two researchers independently using the Revised Cochrane risk-of-bias tool (RoB 2) [ 25 ]. This tool facilitates systematic assessment of the quality of the article per outcome according to the five domains: bias due to (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. An additional domain 1b must be used when assessing the randomization process for cluster-randomized studies.
Meta-biases such as outcome reporting bias will be evaluated by determining whether the protocol was published before recruitment of patients. Additionally, trial registries will be checked to determine whether the reported outcome measures and statistical methods are similar to the ones described in the registry. The gray literature search will be of assistance when checking for publication bias; however, completely eliminating the presence of publication bias is impossible.
Similar to article selection, any disagreement between the researchers will lead to discussion and consultation of a third researcher. The strength of the evidence will be graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [ 26 ].
The primary outcome measure of this proposed systematic review is the consumption of alcohol at follow-up. Consumption of alcohol is often quantified in drinking quantity (e.g., number of drinks per week), drinking frequency (e.g., percentage of days abstinent), binge frequency (e.g., number of heavy drinking days), and drinking intensity (e.g., number of drinks per drinking day). Additionally, outcomes such as percentage/proportion included patients that are abstinent or considered heavy/risky drinkers at follow-up. We aim to report all these outcomes. The consumption of alcohol is often self-reported by patients. When studies report outcomes at multiple time points, we will consider the longest follow-up of individual studies as a primary outcome measure.
Depending on the included studies, we will also consider secondary outcome measures such as treatment engagement (e.g., number of visits or pharmacotherapy uptake), economic outcome measures, health care utilization, quality of life assessment (physical/mental), alcohol-related problems/harm, and mental health score for depression or anxiety.
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
Given the complexities of researching models of care in primary care and the paucity of a focus on AUD treatment, there are likely to be only a few studies that sufficiently address the research question. Therefore, we will do a preliminary search without the search terms for model of care. Additionally, the search for online non-academic studies presents a challenge. However, the Gray Matters tool will be of guidance and will limit the possibility of missing useful studies. Further, due to diversity of treatment models, outcome measures, and limitations in research design, it is possible that a meta-analysis for comparative effectiveness may not be appropriate. Moreover, in the absence of large, cluster randomized controlled trials, it will be difficult to distinguish between the effectiveness of the treatment given and that of the model of care and/or implementation procedure. Nonetheless, we will synthesize the literature and provide a critical evaluation of the quality of the evidence.
This review will assist the design and implementation of models of care for the management of AUD in primary care settings. This review will thus improve the management of AUD in primary health care and potentially increase the uptake of evidence-based interventions for AUD.
Availability of data and materials
Not applicable.
Abbreviations
Alcohol use disorder
Alcohol Use Disorders Identification test
Canadian Agency for Drugs and Technologies in Health
The Comorbidity Alcohol Risk Evaluation
Cochrane Central Register of Controlled Trials
Diagnostic and Statistical Manual of Mental Disorders
Human immunodeficiency virus
10 - International Statistical Classification of Diseases and Related Health Problems
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
Screening, brief intervention, referral to specialized treatment
Standard drinks
World Health Organization
WHO. Global status report on alcohol and health: World health organization; 2018.
The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016. a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012.
Article Google Scholar
WHO. Global strategy to reduce the harmful use of alcohol: World health organization; 2010.
Rehm J, Allamani A, Elekes Z, Jakubczyk A, Manthey J, Probst C, et al. Alcohol dependence and treatment utilization in Europe - a representative cross-sectional study in primary care. BMC Fam Pract. 2015;16:90.
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. National trends in alcohol pharmacotherapy: findings from an Australian claims database. Drug Alcohol Depend. 2016;166:254–7.
Article CAS Google Scholar
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. Socioeconomic and geographic disparities in access to pharmacotherapy for alcohol dependence. J Subst Abus Treat. 2017;74:23–5.
Rehm J, Anderson P, Manthey J, Shield KD, Struzzo P, Wojnar M, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51(4):422–7.
Le KB, Johnson JA, Seale JP, Woodall H, Clark DC, Parish DC, et al. Primary care residents lack comfort and experience with alcohol screening and brief intervention: a multi-site survey. J Gen Intern Med. 2015;30(6):790–6.
McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, et al. Can substance use disorders be managed using the chronic care model? review and recommendations from a NIDA consensus group. Public Health Rev. 2014;35(2).
Storholm ED, Ober AJ, Hunter SB, Becker KM, Iyiewuare PO, Pham C, et al. Barriers to integrating the continuum of care for opioid and alcohol use disorders in primary care: a qualitative longitudinal study. J Subst Abus Treat. 2017;83:45–54.
Mitchell AJ, Meader N, Bird V, Rizzo M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: meta-analysis. Br J Psychiatry. 2012;201:93–100.
Babor TF, Ritson EB, Hodgson RJ. Alcohol-related problems in the primary health care setting: a review of early intervention strategies. Br J Addict. 1986;81(1):23–46.
Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):Cd004148.
O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78.
Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–95.
Saitz R. ‘SBIRT’ is the answer? Probably not. Addiction. 2015;110(9):1416–7.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–9.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. Jama. 2002;288(15):1909–14.
CADTH. Grey Matters: a practical tool for searching health-related grey literature Internet. 2018 (cited 2019 Feb 22).
Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta-analysis. 2002;21(11):1539–58.
Google Scholar
Higgins JPT, Thompson SG, Deeks JJ. Altman DG. Measuring inconsistency in meta-analyses. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Higgins JPT, López-López JA, Becker BJ, Davies SR, Dawson S, Grimshaw JM, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858–e.
Higgins, J.P.T., Sterne, J.A.C., Savović, J., Page, M.J., Hróbjartsson, A., Boutron, I., Reeves, B., Eldridge, S. (2016). A revised tool for assessing risk of bias in randomized trials. In: Chandler, J., McKenzie, J., Boutron, I., Welch, V. (editors). Cochrane methods. Cochrane database of systematic reviews, 10 (Suppl 1). https://doi.org/10.1002/14651858.CD201601 .
Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html ).
Download references
Acknowledgements
There is no dedicated funding.
Author information
Authors and affiliations.
Discipline of Addiction Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Susan A. Rombouts, Eva Louie, Paul Haber & Kirsten C. Morley
NHMRC Centre of Research Excellence in Indigenous Health and Alcohol, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
James Conigrave
Drug Health Services, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
KM and PH conceived the presented idea of a systematic review and meta-analysis and helped with the scope of the literature. KM is the senior researcher providing overall guidance and the guarantor of this review. SR developed the background, search strategy, and data extraction form. SR and EL will both be working on the data extraction and risk of bias assessment. SR and JC will conduct the data analysis and synthesize the results. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kirsten C. Morley .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA-P 2015 Checklist.
Additional file 2.
Draft search strategy MEDLINE. Search strategy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rombouts, S.A., Conigrave, J., Louie, E. et al. Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review. Syst Rev 8 , 275 (2019). https://doi.org/10.1186/s13643-019-1157-7
Download citation
Received : 25 March 2019
Accepted : 13 September 2019
Published : 13 November 2019
DOI : https://doi.org/10.1186/s13643-019-1157-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Model of care
- Primary health care
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
A burden of proof study on alcohol consumption and ischemic heart disease
- Sinclair Carr ORCID: orcid.org/0000-0003-0421-3145 1 ,
- Dana Bryazka 1 ,
- Susan A. McLaughlin 1 ,
- Peng Zheng 1 , 2 ,
- Sarasvati Bahadursingh 3 ,
- Aleksandr Y. Aravkin 1 , 2 , 4 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Hilary R. Lawlor 1 ,
- Erin C. Mullany 1 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 ,
- Sneha I. Nicholson 1 ,
- Jürgen Rehm 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ,
- Gregory A. Roth 1 , 2 , 13 ,
- Reed J. D. Sorensen 1 ,
- Sarah Lewington 3 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Communications volume 15 , Article number: 4082 ( 2024 ) Cite this article
1248 Accesses
1 Altmetric
Metrics details
- Cardiovascular diseases
- Epidemiology
- Risk factors
Cohort and case-control data have suggested an association between low to moderate alcohol consumption and decreased risk of ischemic heart disease (IHD), yet results from Mendelian randomization (MR) studies designed to reduce bias have shown either no or a harmful association. Here we conducted an updated systematic review and re-evaluated existing cohort, case-control, and MR data using the burden of proof meta-analytical framework. Cohort and case-control data show low to moderate alcohol consumption is associated with decreased IHD risk – specifically, intake is inversely related to IHD and myocardial infarction morbidity in both sexes and IHD mortality in males – while pooled MR data show no association, confirming that self-reported versus genetically predicted alcohol use data yield conflicting findings about the alcohol-IHD relationship. Our results highlight the need to advance MR methodologies and emulate randomized trials using large observational databases to obtain more definitive answers to this critical public health question.
Similar content being viewed by others

Alcohol consumption and risks of more than 200 diseases in Chinese men
Alcohol intake and the risk of chronic kidney disease: results from a systematic review and dose–response meta-analysis

Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers
Introduction.
It is well known that alcohol consumption increases the risk of morbidity and mortality due to many health conditions 1 , 2 , with even low levels of consumption increasing the risk for some cancers 3 , 4 . In contrast, a large body of research has suggested that low to moderate alcohol intake – compared to no consumption – is associated with a decreased risk of ischemic heart disease (IHD). This has led to substantial epidemiologic and public health interest in the alcohol-IHD relationship 5 , particularly given the high prevalence of alcohol consumption 6 and the global burden of IHD 7 .
Extensive evidence from experimental studies that vary short-term alcohol exposure suggests that average levels of alcohol intake positively affect biomarkers such as apolipoprotein A1, adiponectin, and fibrinogen levels that lower the risk of IHD 8 . In contrast, heavy episodic drinking (HED) may have an adverse effect on IHD by affecting blood lipids, promoting coagulation and thus thrombosis risk, and increasing blood pressure 9 . With effects likely to vary materially by patterns of drinking, alcohol consumption must be considered a multidimensional factor impacting IHD outcomes.
A recent meta-analysis of the alcohol-IHD relationship using individual participant data from 83 observational studies 4 found, among current drinkers, that – relative to drinking less than 50 g/week – any consumption above this level was associated with a lower risk of myocardial infarction (MI) incidence and consumption between >50 and <100 g/week was associated with lower risk of MI mortality. When evaluating other subtypes of IHD excluding MI, the researchers found that consumption between >100 and <250 g/week was associated with a decreased risk of IHD incidence, whereas consumption greater than 350 g/week was associated with an increased risk of IHD mortality. Roerecke and Rehm further observed that low to moderate drinking was not associated with reduced IHD risk when accompanied by occasional HED 10 .
The cohort studies and case-control studies (hereafter referred to as ‘conventional observational studies’) used in these meta-analyses are known to be subject to various types of bias when used to estimate causal relationships 11 . First, neglecting to separate lifetime abstainers from former drinkers, some of whom may have quit due to developing preclinical symptoms (sometimes labeled ‘sick quitters’ 12 , 13 ), and to account for drinkers who reduce their intake as a result of such symptoms may introduce reverse causation bias 13 . That is, the risk of IHD in, for example, individuals with low to moderate alcohol consumption may be lower when compared to IHD risk in sick quitters, not necessarily because intake at this level causes a reduction in risk but because sick quitters are at higher risk of IHD. Second, estimates can be biased because of measurement error in alcohol exposure resulting from inaccurate reporting, random fluctuation in consumption over time (random error), or intentional misreporting of consumption due, for example, to social desirability effects 14 (systematic error). Third, residual confounding may bias estimates if confounders of the alcohol-IHD relationship, such as diet or physical activity, have not been measured accurately (e.g., only via a self-report questionnaire) or accounted for. Fourth, because alcohol intake is a time-varying exposure, time-varying confounding affected by prior exposure must be accounted for 15 . To date, only one study that used a marginal structural model to appropriately adjust for time-varying confounding found no association between alcohol consumption and MI risk 16 . Lastly, if exposure to a risk factor, such as alcohol consumption, did not happen at random – even if all known confounders of the relationship between alcohol and IHD were perfectly measured and accounted for – the potential for unmeasured confounders persists and may bias estimates 11 .
In recent years, the analytic method of Mendelian randomization (MR) has been widely adopted to quantify the causal effects of risk factors on health outcomes 17 , 18 , 19 . MR uses single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) for the exposure of interest. A valid IV should fulfill the following three assumptions: it must be associated with the risk factor (relevance assumption); there must be no common causes of the IV and the outcome (independence assumption); and the IV must affect the outcome only through the exposure (exclusion restriction or ‘no horizontal pleiotropy’ assumption) 20 , 21 . If all three assumptions are fulfilled, estimates derived from MR are presumed to represent causal effects 22 . Several MR studies have quantified the association between alcohol consumption and cardiovascular disease 23 , including IHD, using genes known to impact alcohol metabolism (e.g., ADH1B/C and ALDH2 24 ) or SNP combinations from genome-wide association studies 25 . In contrast to the inverse associations found in conventional observational studies, MR studies have found either no association or a harmful relationship between alcohol consumption and IHD 26 , 27 , 28 , 29 , 30 , 31 .
To advance the knowledge base underlying our understanding of this major health issue – critical given the worldwide ubiquity of alcohol use and of IHD – there is a need to systematically review and critically re-evaluate all available evidence on the relationship between alcohol consumption and IHD risk from both conventional observational and MR studies.
The burden of proof approach, developed by Zheng et al. 32 , is a six-step meta-analysis framework that provides conservative estimates and interpretations of risk-outcome relationships. The approach systematically tests and adjusts for common sources of bias defined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. The key statistical tool to implement the approach is MR-BRT (meta-regression—Bayesian, regularized, trimmed 33 ), a flexible meta-regression tool that does not impose a log-linear relationship between the risk and outcome, but instead uses a spline ensemble to model non-linear relationships. MR-BRT also algorithmically detects and trims outliers in the input data, takes into account different reference and alternative exposure intervals in the data, and incorporates unexplained between-study heterogeneity in the uncertainty surrounding the mean relative risk (RR) curve (henceforth ‘risk curve’). For those risk-outcome relationships that meet the condition of statistical significance using conventionally estimated uncertainty intervals (i.e., without incorporating unexplained between-study heterogeneity), the burden of proof risk function (BPRF) is derived by calculating the 5th (if harmful) or 95th (if protective) quantile risk curve – inclusive of between-study heterogeneity – closest to the log RR of 0. The resulting BPRF is a conservative interpretation of the risk-outcome relationship based on all available evidence. The BPRF represents the smallest level of excess risk for a harmful risk factor or reduced risk for a protective risk factor that is consistent with the data, accounting for between-study heterogeneity. To quantify the strength of the evidence for the alcohol-IHD relationship, the BPRF can be summarized in a single metric, the risk-outcome score (ROS). The ROS is defined as the signed value of the average log RR of the BPRF across the 15th to 85th percentiles of alcohol consumption levels observed across available studies. The larger a positive ROS value, the stronger the alcohol-IHD association. For ease of interpretation, the ROS is converted into a star rating from one to five. A one-star rating (ROS < 0) indicates a weak alcohol-IHD relationship, and a five-star rating (ROS > 0.62) indicates a large effect size and strong evidence. Publication and reporting bias are evaluated with Egger’s regression and by visual inspection with funnel plots 34 . Further conceptual and technical details of the burden of proof approach are described in detail elsewhere 32 .
Using the burden of proof approach, we systematically re-evaluate all available eligible evidence from cohort, case-control, and MR studies published between 1970 and 2021 to conservatively quantify the dose-response relationship between alcohol consumption and IHD risk, calculated relative to risk at zero alcohol intake (i.e., current non-drinking, including lifetime abstinence or former use). We pool the evidence from all conventional observational studies combined, as well as individually for all three study designs, to estimate mean IHD risk curves. Based on patterns of results established by previous meta-analyses 4 , 35 , we also use data from conventional observational studies to estimate risk curves by IHD endpoint (morbidity or mortality) and further by sex, in addition to estimating risk curves for MI overall and by endpoint. We follow PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines 36 through all stages of this study (Supplementary Information section 1 , Fig. S1 and Tables S1 and S2 ) and comply with GATHER (Guidelines on Accurate and Transparent Health Estimates Reporting) recommendations 37 (Supplementary Information section 2 , Table S3 ). The main findings and research implications of this work are summarized in Table 1 .
We updated the systematic review on the dose-response relationship between alcohol consumption and IHD previously conducted for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 1 . Of 4826 records identified in our updated systematic review (4769 from databases/registers and 57 by citation search and known literature), 11 were eligible based on our inclusion criteria and were included. In total, combined with the results of the previous systematic reviews 1 , 38 , information from 95 cohort studies 26 , 27 , 29 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 27 case-control studies 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , and five MR studies 26 , 27 , 28 , 29 , 31 was included in our meta-analysis (see Supplementary Information section 1 , Fig. S1 , for the PRISMA diagram). Details on the extracted effect sizes, the design of each included study, underlying data sources, number of participants, duration of follow-up, number of cases and controls, and bias covariates that were evaluated and potentially adjusted for can be found in the Supplementary Information Sections 4 , 5 , and 6 .
Table 2 summarizes key metrics of each risk curve modeled, including estimates of mean RR and 95% UI (inclusive of between-study heterogeneity) at select alcohol exposure levels, the exposure level and RR and 95% UI at the nadir (i.e., lowest RR), the 85th percentile of exposure observed in the data and its corresponding RR and 95% UI, the BPRF averaged at the 15th and 85th percentile of exposure, the average excess risk or risk reduction according to the exposure-averaged BPRF, the ROS, the associated star rating, the potential presence of publication or reporting bias, and the number of studies included.
We found large variation in the association between alcohol consumption and IHD by study design. When we pooled the results of cohort and case-control studies, we observed an inverse association between alcohol at average consumption levels and IHD risk; that is, drinking average levels of alcohol was associated with a reduced IHD risk relative to drinking no alcohol. In contrast, we did not find a statistically significant association between alcohol consumption and IHD risk when pooling results from MR studies. When we subset the conventional observational studies to those reporting on IHD by endpoint, we found no association between alcohol consumption and IHD morbidity or mortality due to large unexplained heterogeneity between studies. When we further subset those studies that reported effect size estimates by sex, we found that average alcohol consumption levels were inversely associated with IHD morbidity in males and in females, and with IHD mortality in males but not in females. When we analyzed only the studies that reported on MI, we found significant inverse associations between average consumption levels and MI overall and with MI morbidity. Visualizations of the risk curves for morbidity and mortality of IHD and MI are provided in Supplementary Information Section 9 (Figs. S2a –c, S3a –c, and S4a–c ). Among all modeled risk curves for which a BPRF was calculated, the ROS ranged from −0.40 for MI mortality to 0.20 for MI morbidity. In the Supplementary Information, we also provide details on the RR and 95% UIs with and without between-study heterogeneity associated with each 10 g/day increase in consumption for each risk curve (Table S10 ), the parameter specifications of the model (Tables S11 and S12 ), and each risk curve from the main analysis estimated without trimming 10% of the data (Fig. S5a–l and Table S13 ).
Risk curve derived from conventional observational study data
The mean risk curve and 95% UI were first estimated by combining all evidence from eligible cohort and case-control studies that quantified the association between alcohol consumption and IHD risk. In total, information from 95 cohort studies and 27 case-control studies combining data from 7,059,652 participants were included. In total, 243,357 IHD events were recorded. Thirty-seven studies quantified the association between alcohol consumption and IHD morbidity only, and 44 studies evaluated only IHD mortality. The estimated alcohol-IHD association was adjusted for sex and age in all but one study. Seventy-five studies adjusted the effect sizes for sex, age, smoking, and at least four other covariates. We adjusted our risk curve for whether the study sample was under or over 50 years of age, whether the study outcome was consistent with the definition of IHD (according to the International Classification of Diseases [ICD]−9: 410-414; and ICD-10: I20-I25) or related to specified subtypes of IHD, whether the outcome was ascertained by self-report only or by at least one other measurement method, whether the study accounted for risk for reverse causation, whether the reference group was non-drinkers (including lifetime abstainers and former drinkers), and whether effect sizes were adjusted (1) for sex, age, smoking, and at least four other variables, (2) for apolipoprotein A1, and (3) for cholesterol, as these bias covariates were identified as significant by our algorithm.
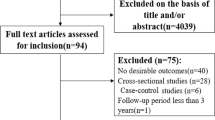
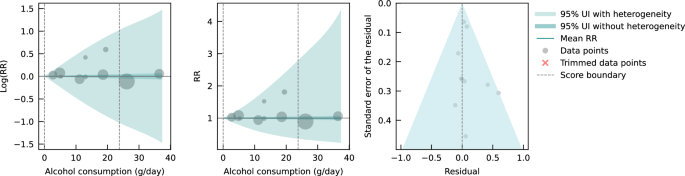
Pooling all data from cohort and case-control studies, we found that alcohol consumption was inversely associated with IHD risk (Fig. 1 ). The risk curve was J-shaped – without crossing the null RR of 1 at high exposure levels – with a nadir of 0.69 (95% UI: 0.48–1.01) at 23 g/day. This means that compared to individuals who do not drink alcohol, the risk of IHD significantly decreases with increasing consumption up to 23 g/day, followed by a risk reduction that becomes less pronounced. The average BPRF calculated between 0 and 45 g/day of alcohol intake (the 15th and 85th percentiles of the exposure range observed in the data) was 0.96. Thus, when between-study heterogeneity is accounted for, a conservative interpretation of the evidence suggests drinking alcohol across the average intake range is associated with an average decrease in the risk of IHD of at least 4% compared to drinking no alcohol. This corresponds to a ROS of 0.04 and a star rating of two, which suggests that the association – on the basis of the available evidence – is weak. Although we algorithmically identified and trimmed 10% of the data to remove outliers, Egger’s regression and visual inspection of the funnel plot still indicated potential publication or reporting bias.
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard error that includes the reported standard error and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from case-control study data
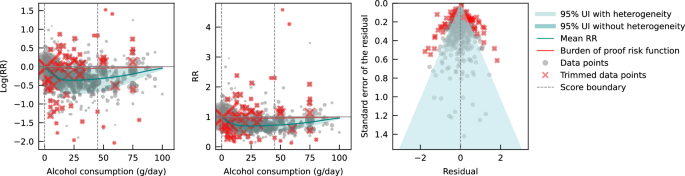
Next, we estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD by subsetting the data to case-control studies only. We included a total of 27 case-control studies (including one nested case-control study) with data from 60,914 participants involving 16,892 IHD cases from Europe ( n = 15), North America ( n = 6), Asia ( n = 4), and Oceania ( n = 2). Effect sizes were adjusted for sex and age in most studies ( n = 25). Seventeen of these studies further adjusted for smoking and at least four other covariates. The majority of case-control studies accounted for the risk of reverse causation ( n = 25). We did not adjust our risk curve for bias covariates, as our algorithm did not identify any as significant.
Evaluating only data from case-control studies, we observed a J-shaped relationship between alcohol consumption and IHD risk, with a nadir of 0.65 (0.50–0.85) at 23 g/day (Fig. 2 ). The inverse association between alcohol consumption and IHD risk reversed at an intake level of 61 g/day. In other words, alcohol consumption between >0 and 60 g/day was associated with a lower risk compared to no consumption, while consumption at higher levels was associated with increased IHD risk. However, the curve above this level is flat, implying that the association between alcohol and increased IHD risk is the same between 61 and 100 g/day, relative to not drinking any alcohol. The BPRF averaged across the exposure range between the 15th and 85th percentiles, or 0–45 g/day, was 0.87, which translates to a 13% average reduction in IHD risk across the average range of consumption. This corresponds to a ROS of 0.14 and a three-star rating. After trimming 10% of the data, no potential publication or reporting bias was found.
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation that includes the reported standard deviation and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from cohort study data
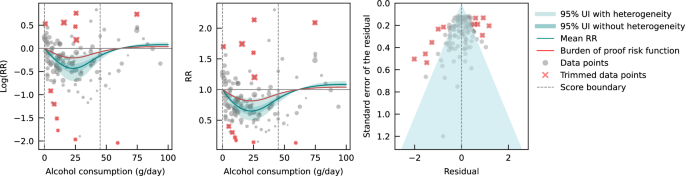
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies – of which one was a retrospective cohort study – with data from 6,998,738 participants were included. Overall, 226,465 IHD events were recorded. Most data were from Europe ( n = 43) and North America ( n = 33), while a small number of studies were conducted in Asia ( n = 14), Oceania ( n = 3), and South America ( n = 2). The majority of studies adjusted effect sizes for sex and age ( n = 76). Fifty-seven of these studies also adjusted for smoking and at least four other covariates. Out of all cohort studies included, 88 accounted for the risk of reverse causation. We adjusted our risk curve for whether the study outcome was consistent with the definition of IHD or related to specified subtypes of IHD, and whether effect sizes were adjusted for apolipoprotein A1, as these bias covariates were identified as significant by our algorithm.
When only data from cohort studies were evaluated, we found a J-shaped relationship between alcohol consumption and IHD risk that did not cross the null RR of 1 at high exposure levels, with a nadir of 0.69 (0.47–1.01) at 23 g/day (Fig. 3 ). The shape of the risk curve was almost identical to the curve estimated with all conventional observational studies (i.e., cohort and case-control studies combined). When we calculated the average BPRF of 0.95 between the 15th and 85th percentiles of observed alcohol exposure (0–50 g/day), we found that alcohol consumption across the average intake range was associated with an average reduction in IHD risk of at least 5%. This corresponds to a ROS of 0.05 and a two-star rating. We identified potential publication or reporting bias after 10% of the data were trimmed.
Risk curve derived from Mendelian randomization study data
Lastly, we pooled evidence on the relationship between genetically predicted alcohol consumption and IHD risk from MR studies. Four MR studies were considered eligible for inclusion in our main analysis, with data from 559,708 participants from China ( n = 2), the Republic of Korea ( n = 1), and the United Kingdom ( n = 1). Overall, 22,134 IHD events were recorded. Three studies used the rs671 ALDH2 genotype found in Asian populations, one study additionally used the rs1229984 ADH1B variant, and one study used the rs1229984 ADH1B Arg47His variant and a combination of 25 SNPs as IVs. All studies used the two-stage least squares (2SLS) method to estimate the association, and one study additionally applied the inverse-variance-weighted (IVW) method and multivariable MR (MVMR). For the study that used multiple methods to estimate effect sizes, we used the 2SLS estimates for our main analysis. Further details on the included studies are provided in Supplementary Information section 4 (Table S6 ). Due to limited input data, we elected not to trim 10% of the observations. We adjusted our risk curve for whether the endpoint of the study outcome was mortality and whether the associations were adjusted for sex and/or age, as these bias covariates were identified as significant by our algorithm.
We did not find any significant association between genetically predicted alcohol consumption and IHD risk using data from MR studies (Fig. 4 ). No potential publication or reporting bias was detected.
As sensitivity analyses, we modeled risk curves with effect sizes estimated from data generated by Lankester et al. 28 using IVW and MVMR methods. We also used effect sizes from Biddinger et al. 31 , obtained using non-linear MR with the residual method, instead of those from Lankester et al. 28 in our main model (both were estimated with UK Biobank data) to estimate a risk curve. Again, we did not find a significant association between genetically predicted alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S6a–c and Table S14 ). To test for consistency with the risk curve we estimated using all included cohort studies, we also pooled the conventionally estimated effect sizes provided in the four MR studies. We did not observe an association between alcohol consumption and IHD risk due to large unexplained heterogeneity between studies (see Supplementary Information Section 10 , Fig. S7, and Table S14 ). Lastly, we pooled cohort studies that included data from China, the Republic of Korea, and the United Kingdom to account for potential geographic influences. Again, we did not find a significant association between alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S8, and Table S14 ).
Conventional observational and MR studies published to date provide conflicting estimates of the relationship between alcohol consumption and IHD. We conducted an updated systematic review and conservatively re-evaluated existing evidence on the alcohol-IHD relationship using the burden of proof approach. We synthesized evidence from cohort and case-control studies combined and separately and from MR studies to assess the dose-response relationship between alcohol consumption and IHD risk and to compare results across different study designs. It is anticipated that the present synthesis of evidence will be incorporated into upcoming iterations of GBD.
Our estimate of the association between genetically predicted alcohol consumption and IHD runs counter to our estimates from the self-report data and those of other previous meta-analyses 4 , 35 , 158 that pooled conventional observational studies. Based on the conservative burden of proof interpretation of the data, our results suggested an inverse association between alcohol and IHD when all conventional observational studies were pooled (alcohol intake was associated with a reduction in IHD risk by an average of at least 4% across average consumption levels; two-star rating). In evaluating only cohort studies, we again found an inverse association between alcohol consumption and IHD (alcohol intake was associated with a reduction in IHD risk by an average of at least 5% at average consumption levels; two-star rating). In contrast, when we pooled only case-control studies, we estimated that average levels of alcohol consumption were associated with at least a 13% average decrease in IHD risk (three-star rating), but the inverse association reversed when consumption exceeded 60 g/day, suggesting that alcohol above this level is associated with a slight increase in IHD risk. Our analysis of the available evidence from MR studies showed no association between genetically predicted alcohol consumption and IHD.
Various potential biases and differences in study designs may have contributed to the conflicting findings. In our introduction, we summarized important sources of bias in conventional observational studies of the association between alcohol consumption and IHD. Of greatest concern are residual and unmeasured confounding and reverse causation, the effects of which are difficult to eliminate in conventional observational studies. By using SNPs within an IV approach to predict exposure, MR – in theory – eliminates these sources of bias and allows for more robust estimates of causal effects. Bias may still occur, however, when using MR to estimate the association between alcohol and IHD 159 , 160 . There is always the risk of horizontal pleiotropy in MR – that is, the genetic variant may affect the outcome via pathways other than exposure 161 . The IV assumption of exclusion restriction is, for example, violated if only a single measurement of alcohol consumption is used in MR 162 ; because alcohol consumption varies over the life course, the gene directly impacts IHD through intake at time points other than that used in the MR analysis. To date, MR studies have not succeeded in separately capturing the multidimensional effects of alcohol intake on IHD risk (i.e., effects of average alcohol consumption measured through frequency-quantity, in addition to the effects of HED) 159 because the genes used to date only target average alcohol consumption that encompasses intake both at average consumption levels and HED. In other words, the instruments used are not able to separate out the individual effects of these two different dimensions of alcohol consumption on IHD risk using MR. Moreover, reverse causation may occur through cross-generational effects 160 , 163 , as the same genetic variants predispose both the individual and at least one of his or her parents to (increased) alcohol consumption. In this situation, IHD risk could be associated with the parents’ genetically predicted alcohol consumption and not with the individual’s own consumption. None of the MR studies included accounted for cross-generational effects, which possibly introduced bias in the effect estimates. It is important to note that bias by ancestry might also occur in conventional observational studies 164 . In summary, estimates of the alcohol-IHD association are prone to bias in all three study designs, limiting inferences of causation.
The large difference in the number of available MR versus conventional observational studies, the substantially divergent results derived from the different study types, and the rapidly developing field of MR clearly argue for further investigation of MR as a means to quantify the association between alcohol consumption and IHD risk. Future studies should investigate non-linearity in the relationship using non-linear MR methods. The residual method, commonly applied in non-linear MR studies such as Biddinger et al. 31 , assumes a constant, linear relationship between the genetic IV and the exposure in the study population; a strong assumption that may result in biased estimates and inflated type I error rates if the relationship varies by population strata 165 . However, by log-transforming the exposure, the relationships between the genetic IV and the exposure as expressed on a logarithmic scale may be more homogeneous across strata, possibly reducing the bias effect of violating the assumption of a constant, linear relationship. Alternatively, or in conjunction, the recently developed doubly ranked method, which obviates the need for this assumption, could be used 166 . Since methodology for non-linear MR is an active field of study 167 , potential limitations of currently available methods should be acknowledged and latest guidelines be followed 168 . Future MR studies should further (i) employ sensitivity analyses such as the MR weighted median method 169 to relax the exclusion restriction assumption that may be violated, as well as applying other methods such as the MR-Egger intercept test; (ii) use methods such as g-estimation of structural mean models 162 to adequately account for temporal variation in alcohol consumption in MR, and (iii) attempt to disaggregate the effects of alcohol on IHD by dimension in MR, potentially through the use of MVMR 164 . General recommendations to overcome common MR limitations are described in greater detail elsewhere 159 , 163 , 170 , 171 and should be carefully considered. With respect to prospective cohort studies used to assess the alcohol-IHD relationship, they should, at a minimum: (i) adjust the association between alcohol consumption and IHD for all potential confounders identified, for example, using a causal directed acyclic graph, and (ii) account for reverse causation introduced by sick quitters and by drinkers who changed their consumption. If possible, they should also (iii) use alcohol biomarkers as objective measures of alcohol consumption instead of or in addition to self-reported consumption to reduce bias through measurement error, (iv) investigate the association between IHD and HED, in addition to average alcohol consumption, and (v) when multiple measures of alcohol consumption and potential confounders are available over time, use g-methods to reduce bias through confounding as fully as possible within the limitations of the study design. However, some bias – due, for instance, to unmeasured confounding in conventional observational and to horizontal pleiotropy in MR studies – is likely inevitable, and the interpretation of estimates should be appropriately cautious, in accordance with the methods used in the study.
With the introduction of the Moderate Alcohol and Cardiovascular Health Trial (MACH15) 172 , randomized controlled trials (RCTs) have been revisited as a way to study the long-term effects of low to moderate alcohol consumption on cardiovascular disease, including IHD. In 2018, soon after the initiation of MACH15, the National Institutes of Health terminated funding 173 , reportedly due to concerns about study design and irregularities in the development of funding opportunities 174 . Although MACH15 was terminated, its initiation represented a previously rarely considered step toward investigating the alcohol-IHD relationship using an RCT 175 . However, while the insights from an RCT are likely to be invaluable, the implementation is fraught with potential issues. Due to the growing number of studies suggesting increased disease risk, including cancer 3 , 4 , associated with alcohol use even at very low levels 176 , the use of RCTs to study alcohol consumption is ethically questionable 177 . A less charged approach could include the emulation of target trials 178 using existing observational data (e.g., from large-scale prospective cohort studies such as the UK Biobank 179 , Atherosclerosis Risk in Communities Study 180 , or the Framingham Heart Study 181 ) in lieu of real trials to gather evidence on the potential cardiovascular effects of alcohol. Trials like MACH15 can be emulated, following the proposed trial protocols as closely as the observational dataset used for the analysis allows. Safety and ethical concerns, such as those related to eligibility criteria, initiation/increase in consumption, and limited follow-up duration, will be eliminated because the data will have already been collected. This framework allows for hypothetical trials investigating ethically challenging or even untenable questions, such as the long-term effects of heavy (episodic) drinking on IHD risk, to be emulated and inferences to broader populations drawn.
There are several limitations that must be considered when interpreting our findings. First, record screening for our systematic review was not conducted in a double-blinded fashion. Second, we did not have sufficient evidence to estimate and examine potential differential associations of alcohol consumption with IHD risk by beverage type or with MI endpoints by sex. Third, despite using a flexible meta-regression tool that overcame several limitations common to meta-analyses, the results of our meta-analysis were only as good as the quality of the studies included. We were able, however, to address the issue of varying quality of input data by adjusting for bias covariates that corresponded to core study characteristics in our analyses. Fourth, because we were only able to include one-sample MR studies that captured genetically predicted alcohol consumption, statistical power may be lower than would have been possible with the inclusion of two-sample MR studies, and studies that directly estimated gene-IHD associations were not considered 23 . Finally, we were not able to account for participants’ HED status when pooling effect size estimates from conventional observational studies. Given established differences in IHD risk for drinkers with and without HED 35 and the fact that more than one in three drinkers reports HED 6 , we would expect that the decreased average risk we found at moderate levels of alcohol consumption would be attenuated (i.e., approach the IHD risk of non-drinkers) if the presence of HED was taken into account.
Using the burden of proof approach 32 , we conservatively re-evaluated the dose-response relationship between alcohol consumption and IHD risk based on existing cohort, case-control, and MR data. Consistent with previous meta-analyses, we found that alcohol at average consumption levels was inversely associated with IHD when we pooled conventional observational studies. This finding was supported when aggregating: (i) all studies, (ii) only cohort studies, (iii) only case-control studies, (iv) studies examining IHD morbidity in females and males, (v) studies examining IHD mortality in males, and (vi) studies examining MI morbidity. In contrast, we found no association between genetically predicted alcohol consumption and IHD risk based on data from MR studies. Our confirmation of the conflicting results derived from self-reported versus genetically predicted alcohol use data highlights the need to advance methodologies that will provide more definitive answers to this critical public health question. Given the limitations of randomized trials, we advocate using advanced MR techniques and emulating target trials using observational data to generate more conclusive evidence on the long-term effects of alcohol consumption on IHD risk.
This study was approved by the University of Washington IRB Committee (study #9060).
The burden of proof approach is a six-step framework for conducting meta-analysis 32 : (1) data from published studies that quantified the dose-response relationship between alcohol consumption and ischemic heart disease (IHD) risk were systematically identified and obtained; (2) the shape of the mean relative risk (RR) curve (henceforth ‘risk curve’) and associated uncertainty was estimated using a quadratic spline and algorithmic trimming of outliers; (3) the risk curve was tested and adjusted for biases due to study attributes; (4) unexplained between-study heterogeneity was quantified, adjusting for within-study correlation and number of studies included; (5) the evidence for small-study effects was evaluated to identify potential risks of publication or reporting bias; and (6) the burden of proof risk function (BPRF) – a conservative interpretation of the average risk across the exposure range found in the data – was estimated relative to IHD risk at zero alcohol intake. The BPRF was converted to a risk-outcome score (ROS) that was mapped to a star rating from one to five to provide an intuitive interpretation of the magnitude and direction of the dose-response relationship between alcohol consumption and IHD risk.
We calculated the mean RR and 95% uncertainty intervals (UIs) for IHD associated with levels of alcohol consumption separately with all evidence available from conventional observational studies and from Mendelian randomization (MR) studies. For the risk curves that met the condition of statistical significance when the conventional 95% UI that does not include unexplained between-study heterogeneity was evaluated, we calculated the BPRF, ROS, and star rating. Based on input data from conventional observational studies, we also estimated these metrics by study design (cohort studies, case-control studies), and by IHD endpoint (morbidity, mortality) for both sexes (females, males) and sex-specific. For sex-stratified analyses, we only considered studies that reported effect sizes for both females and males to allow direct comparison of IHD risk across different exposure levels; however, we did not collect information about the method each study used to determine sex. We also estimated risk curves for myocardial infarction (MI), overall and by endpoint, using data from conventional observational studies. As a comparison, we also estimated each risk curve without trimming 10% of the input data. We did not consider MI as an outcome or disaggregate findings by sex or endpoint for MR studies due to insufficient data.
With respect to MR studies, several statistical methods are typically used to estimate the associations between genetically predicted exposure and health outcomes (e.g., two-stage least squares [2SLS], inverse-variance-weighted [IVW], multivariable Mendelian randomization [MVMR]). For our main analysis synthesizing evidence from MR studies, we included the reported effect sizes estimated using 2SLS if a study applied multiple methods because this method was common to all included studies. In sensitivity analyses, we used the effect sizes obtained by other MR methods (i.e., IVW, MVMR, and non-linear MR) and estimated the mean risk curve and uncertainty. We also pooled conventionally estimated effect sizes from MR studies to allow comparison with the risk curve estimated with cohort studies. Due to limited input data from MR studies, we elected not to trim 10% of the observations. Furthermore, we estimated the risk curve from cohort studies with data from countries that corresponded to those included in MR studies (China, the Republic of Korea, and the United Kingdom). Due to a lack of data, we were unable to estimate a risk curve from case-control studies in these geographic regions.
Conducting the systematic review
In step one of the burden of proof approach, data for the dose-response relationship between alcohol consumption and IHD risk were systematically identified, reviewed, and extracted. We updated a previously published systematic review 1 in PubMed that identified all studies evaluating the dose-response relationship between alcohol consumption and risk of IHD morbidity or mortality from January 1, 1970, to December 31, 2019. In our update, we additionally considered all studies up to and including December 31, 2021, for eligibility. We searched articles in PubMed on March 21, 2022, with the following search string: (alcoholic beverage[MeSH Terms] OR drinking behavior[MeSH Terms] OR “alcohol”[Title/Abstract]) AND (Coronary Artery Disease[Mesh] OR Myocardial Ischemia[Mesh] OR atherosclerosis[Mesh] OR Coronary Artery Disease[TiAb] OR Myocardial Ischemia[TiAb] OR cardiac ischemia[TiAb] OR silent ischemia[TiAb] OR atherosclerosis Outdent [TiAb] OR Ischemic heart disease[TiAb] OR Ischemic heart disease[TiAb] OR coronary heart disease[TiAb] OR myocardial infarction[TiAb] OR heart attack[TiAb] OR heart infarction[TiAb]) AND (Risk[MeSH Terms] OR Odds Ratio[MeSH Terms] OR “risk”[Title/Abstract] OR “odds ratio”[Title/Abstract] OR “cross-product ratio”[Title/Abstract] OR “hazards ratio”[Title/Abstract] OR “hazard ratio”[Title/Abstract]) AND (“1970/01/01”[PDat]: “2021/12/31”[PDat]) AND (English[LA]) NOT (animals[MeSH Terms] NOT Humans[MeSH Terms]). Studies were eligible for inclusion if they met all of the following criteria: were published between January 1, 1970, and December 31, 2021; were a cohort study, case-control study, or MR study; described an association between alcohol consumption and IHD and reported an effect size estimate (relative risk, hazard ratio, odds ratio); and used a continuous dose as exposure of alcohol consumption. Studies were excluded if they met any of the following criteria: were an aggregate study (meta-analysis or pooled cohort); utilized a study design not designated for inclusion in this analysis: not a cohort study, case-control study, or MR study; were a duplicate study: the underlying sample of the study had also been analyzed elsewhere (we always considered the analysis with the longest follow-up for cohort studies or the most recently published analysis for MR studies); did not report on the exposure of interest: reported on combined exposure of alcohol and drug use or reported alcohol consumption in a non-continuous way; reported an outcome that was not IHD or a composite outcome that included but was not limited to IHD, or outcomes lacked specificity, such as cardiovascular disease or all-cause mortality; were not in English; and were animal studies. All screenings of titles and abstracts of identified records, as well as full texts of potentially eligible studies, and extraction of included studies, were done by a single reviewer (SC or HL) independently. If eligible, studies were extracted for study characteristics, exposure, outcome, adjusted confounders, and effect sizes and their uncertainty. While the previous systematic review only considered cohort and case-control studies, our update also included MR studies. We chose to consider only ‘one-sample’ MR studies, i.e., those in which genes, risk factors, and outcomes were measured in the same participants, and not ‘two-sample’ MR studies in which two different samples were used for the MR analysis so that we could fully capture study-specific information. We re-screened previously identified records for MR studies to consider all published MR studies in the defined time period. We also identified and included in our sensitivity analysis an MR study published in 2022 31 which used a non-linear MR method to estimate the association between genetically predicted alcohol consumption and IHD. When eligible studies reported both MR and conventionally estimated effect sizes (i.e., for the association between self-reported alcohol consumption and IHD risk), we extracted both. If studies used the same underlying sample and investigated the same outcome in the same strata, we included the study that had the longest follow-up. This did not apply when the same samples were used in conventional observational and MR studies, because they were treated separately when estimating the risk curve of alcohol consumption and IHD. Continuous exposure of alcohol consumption was defined as a frequency-quantity measure 182 and converted to g/day. IHD was defined according to the International Classification of Diseases (ICD)−9, 410-414, and ICD-10, I20-I25.
The raw data were extracted with a standardized extraction sheet (see Supplementary Information Section 3 , Table S4 ). For conventional observational studies, when multiple effect sizes were estimated from differently adjusted regression models, we used those estimated with the model reported to be fully adjusted or the one with the most covariates. In the majority of studies, alcohol consumption was categorized based on the exposure range available in the data. If the lower end of a categorical exposure range (e.g., <10 g/day) of an effect size was not specified in the input data, we assumed that this was 0 g/day. If the upper end was not specified (e.g., >20 g/day), it was calculated by multiplying the lower end of the categorical exposure range by 1.5. When the association between alcohol and IHD risk was reported as a linear slope, the average consumption level in the sample was multiplied by the logarithm of the effect size to effectively render it categorical. From the MR study which employed non-linear MR 31 , five effect sizes and their uncertainty were extracted at equal intervals across the reported range of alcohol exposure using WebPlotDigitizer. To account for the fact that these effect sizes were derived from the same non-linear risk curve, we adjusted the extracted standard errors by multiplying them by the square root of five (i.e., the number of extracted effect sizes). Details on data sources are provided in Supplementary Information Section 4 .
Estimating the shape of the risk-outcome relationship
In step two, the shape of the dose-response relationship (i.e., ‘signal’) between alcohol consumption and IHD risk was estimated relative to risk at zero alcohol intake. The meta-regression tool MR-BRT (meta-regression—Bayesian, regularized, trimmed), developed by Zheng et al. 33 , was used for modeling. To allow for non-linearity, thus relaxing the common assumption of a log-linear relationship, a quadratic spline with two interior knots was used for estimating the risk curve 33 . We used the following three risk measures from included studies: RRs, odds ratios (ORs), and hazard ratios (HRs). ORs were treated as equivalent to RRs and HRs based on the rare outcome assumption. To counteract the potential influence of knot placement on the shape of the risk curve when using splines, an ensemble model approach was applied. Fifty component models with random knot placements across the exposure domain were computed. These were combined into an ensemble by weighting each model based on model fit and variation (i.e., smoothness of fit to the data). To prevent bias from outliers, a robust likelihood-based approach was applied to trim 10% of the observations. Technical details on estimating the risk curve, use of splines, the trimming procedure, the ensemble model approach, and uncertainty estimation are described elsewhere 32 , 33 . Details on the model specifications for each risk curve are provided in Supplementary Information section 8 . We first estimated each risk curve without trimming input data to visualize the shape of the curve, which informed knot placement and whether to set a left and/or right linear tail when data were sparse at low or high exposure levels (see Supplementary Information Section 10 , Fig. S5a–l ).
Testing and adjusting for biases across study designs and characteristics
In step three, the risk curve was tested and adjusted for systematic biases due to study attributes. According to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria 183 , the following six bias sources were quantified: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. Representativeness was quantified by whether the study sample came from a location that was representative of the underlying geography. Exposure assessment was quantified by whether alcohol consumption was recorded once or more than once in conventional observational studies, or with only one or multiple SNPs in MR studies. Outcome ascertainment was quantified by whether IHD was ascertained by self-report only or by at least one other measurement method. Reverse causation was quantified by whether increased IHD risk among participants who reduced or stopped drinking was accounted for (e.g., by separating former drinkers from lifetime abstainers). Control for confounding factors was quantified by which and how many covariates the effect sizes were adjusted for (i.e., through stratification, matching, weighting, or standardization). Because the most adjusted effect sizes in each study were extracted in the systematic review process and thus may have been adjusted for mediators, we additionally quantified a bias covariate for each of the following potential mediators of the alcohol-IHD relationship: body mass index, blood pressure, cholesterol (excluding high-density lipoprotein cholesterol), fibrinogen, apolipoprotein A1, and adiponectin. Selection bias was quantified by whether study participants were selected and included based on pre-existing disease states. We also quantified and considered as possible bias covariates whether the reference group was non-drinkers, including lifetime abstainers and former drinkers; whether the sample was under or over 50 years of age; whether IHD morbidity, mortality, or both endpoints were used; whether the outcome mapped to IHD or referred only to subtypes of IHD; whether the outcome mapped to MI; and what study design (cohort or case-control) was used when conventional observational studies were pooled. Details on quantified bias covariates for all included studies are provided in Supplementary Information section 5 (Tables S7 and S8 ). Using a Lasso approach 184 , the bias covariates were first ranked. They were then included sequentially, based on their ranking, as effect modifiers of the ‘signal’ obtained in step two in a linear meta-regression. Significant bias covariates were included in modeling the final risk curve. Technical details of the Lasso procedure are described elsewhere 32 .
Quantifying between-study heterogeneity, accounting for heterogeneity, uncertainty, and small number of studies
In step four, the between-study heterogeneity was quantified, accounting for heterogeneity, uncertainty, and small number of studies. In a final linear mixed-effects model, the log RRs were regressed against the ‘signal’ and selected bias covariates, with a random intercept to account for within-study correlation and a study-specific random slope with respect to the ‘signal’ to account for between-study heterogeneity. A Fisher information matrix was used to estimate the uncertainty associated with between-study heterogeneity 185 because heterogeneity is easily underestimated or may be zero when only a small number of studies are available. We estimated the mean risk curve with a 95% UI that incorporated between-study heterogeneity, and we additionally estimated a 95% UI without between-study heterogeneity as done in conventional meta-regressions (see Supplementary Information Section 7 , Table S10 ). The 95% UI incorporating between-study heterogeneity was calculated from the posterior uncertainty of the fixed effects (i.e., the ‘signal’ and selected bias covariates) and the 95% quantile of the between-study heterogeneity. The estimate of between-study heterogeneity and the estimate of the uncertainty of the between-study heterogeneity were used to determine the 95% quantile of the between-study heterogeneity. Technical details of quantifying uncertainty of between-study heterogeneity are described elsewhere 32 .
Evaluating potential for publication or reporting bias
In step five, the potential for publication or reporting bias was evaluated. The trimming algorithm used in step two helps protect against these biases, so risk curves found to have publication or reporting bias using the following methods were derived from data that still had bias even after trimming. Publication or reporting bias was evaluated using Egger’s regression 34 and visual inspection using funnel plots. Egger’s regression tested for a significant correlation between residuals of the RR estimates and their standard errors. Funnel plots showed the residuals of the risk curve against their standard errors. We reported publication or reporting bias when identified.
Estimating the burden of proof risk function
In step six, the BPRF was calculated for risk-outcome relationships that were statistically significant when evaluating the conventional 95% UI without between-study heterogeneity. The BPRF is either the 5th (if harmful) or the 95th (if protective) quantile curve inclusive of between-study heterogeneity that is closest to the RR line at 1 (i.e., null); it indicates a conservative estimate of a harmful or protective association at each exposure level, based on the available evidence. The mean risk curve, 95% UIs (with and without between-study heterogeneity), and BPRF (where applicable) are visualized along with included effect sizes using the midpoint of each alternative exposure range (trimmed data points are marked with a red x), with alcohol consumption in g/day on the x-axis and (log)RR on the y-axis.
We calculated the ROS as the average log RR of the BPRF between the 15th and 85th percentiles of alcohol exposure observed in the study data. The ROS summarizes the association of the exposure with the health outcome in a single measure. A higher, positive ROS indicates a larger association, while a negative ROS indicates a weak association. The ROS is identical for protective and harmful risks since it is based on the magnitude of the log RR. For example, a mean log BPRF between the 15th and 85th percentiles of exposure of −0.6 (protective association) and a mean log BPRF of 0.6 (harmful association) would both correspond to a ROS of 0.6. The ROS was then translated into a star rating, representing a conservative interpretation of all available evidence. A star rating of 1 (ROS: <0) indicates weak evidence of an association, a star rating of 2 (ROS: 0–0.14) indicates a >0–15% increased or >0–13% decreased risk, a star rating of 3 (ROS: >0.14–0.41) indicates a >15–50% increased or >13–34% decreased risk, a star rating of 4 (ROS: >0.41–0.62) indicates a >50–85% increased or >34–46% decreased risk, and a star rating of 5 (ROS: >0.62) indicates a >85% increased or >46% decreased risk.
Statistics & reproducibility
The statistical analyses conducted in this study are described above in detail. No statistical method was used to predetermine the sample size. When analyzing data from cohort and case-control studies, we excluded 10% of observations using a trimming algorithm; when analyzing data from MR studies, we did not exclude any observations. As all data used in this meta-analysis were from observational studies, no experiments were conducted, and no randomization or blinding took place.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The findings from this study were produced using data extracted from published literature. The relevant studies were identified through a systematic literature review and can all be accessed online as referenced in the current paper 26 , 27 , 28 , 29 , 31 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 . Further details on the relevant studies can be found on the GHDx website ( https://ghdx.healthdata.org/record/ihme-data/gbd-alcohol-ihd-bop-risk-outcome-scores ). Study characteristics of all relevant studies included in the analyses are also provided in Supplementary Information Section 4 (Tables S5 and S6 ). The template of the data collection form is provided in Supplementary Information section 3 (Table S4 ). The source data includes processed data from these studies that underlie our estimates. Source data are provided with this paper.
Code availability
Analyses were carried out using R version 4.0.5 and Python version 3.10.9. All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Bryazka, D. et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 400 , 185–235 (2022).
Article Google Scholar
World Health Organization. Global Status Report on Alcohol and Health 2018 . (World Health Organization, Geneva, Switzerland, 2019).
Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br. J. Cancer 112 , 580–593 (2015).
Article CAS PubMed Google Scholar
Wood, A. M. et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391 , 1513–1523 (2018).
Article PubMed PubMed Central Google Scholar
Goel, S., Sharma, A. & Garg, A. Effect of alcohol consumption on cardiovascular health. Curr. Cardiol. Rep. 20 , 19 (2018).
Article PubMed Google Scholar
Manthey, J. et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393 , 2493–2502 (2019).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Brien, S. E., Ronksley, P. E., Turner, B. J., Mukamal, K. J. & Ghali, W. A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342 , d636 (2011).
Rehm, J. et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 112 , 968–1001 (2017).
Roerecke, M. & Rehm, J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am. J. Epidemiol. 171 , 633–644 (2010).
Hernan, M. A. & Robin, J. M. Causal Inference: What If . (CRC Press, 2023).
Marmot, M. Alcohol and coronary heart disease. Int. J. Epidemiol. 13 , 160–167 (1984).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 332 , 1267–1273 (1988).
Davis, C. G., Thake, J. & Vilhena, N. Social desirability biases in self-reported alcohol consumption and harms. Addict. Behav. 35 , 302–311 (2010).
Mansournia, M. A., Etminan, M., Danaei, G., Kaufman, J. S. & Collins, G. Handling time varying confounding in observational research. BMJ 359 , j4587 (2017).
Ilomäki, J. et al. Relationship between alcohol consumption and myocardial infarction among ageing men using a marginal structural model. Eur. J. Public Health 22 , 825–830 (2012).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 , 1133–1163 (2008).
Article MathSciNet PubMed Google Scholar
Burgess, S., Timpson, N. J., Ebrahim, S. & Davey Smith, G. Mendelian randomization: where are we now and where are we going? Int. J. Epidemiol. 44 , 379–388 (2015).
Sleiman, P. M. & Grant, S. F. Mendelian randomization in the era of genomewide association studies. Clin. Chem. 56 , 723–728 (2010).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362 , k601 (2018).
de Leeuw, C., Savage, J., Bucur, I. G., Heskes, T. & Posthuma, D. Understanding the assumptions underlying Mendelian randomization. Eur. J. Hum. Genet. 30 , 653–660 (2022).
Sheehan, N. A., Didelez, V., Burton, P. R. & Tobin, M. D. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 5 , e177 (2008).
Van de Luitgaarden, I. A. et al. Alcohol consumption in relation to cardiovascular diseases and mortality: a systematic review of Mendelian randomization studies. Eur. J. Epidemiol. 1–15 (2021).
Edenberg, H. J. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 30 , 5–13 (2007).
PubMed PubMed Central Google Scholar
Gelernter, J. et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol. Psychiatry 86 , 365–376 (2019).
Article CAS PubMed PubMed Central Google Scholar
Millwood, I. Y. et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393 , 1831–1842 (2019).
Au Yeung, S. L. et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS ONE 8 , e68054 (2013).
Article ADS PubMed PubMed Central Google Scholar
Lankester, J., Zanetti, D., Ingelsson, E. & Assimes, T. L. Alcohol use and cardiometabolic risk in the UK Biobank: a Mendelian randomization study. PLoS ONE 16 , e0255801 (2021).
Cho, Y. et al. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci. Rep. 5 , 18422 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349 , g4164 (2014).
Biddinger, K. J. et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 5 , e223849–e223849 (2022).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. 28 , 2038–2044 (2022).
Zheng, P., Barber, R., Sorensen, R. J., Murray, C. J. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph. Stat. 30 , 544–556 (2021).
Article MathSciNet Google Scholar
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315 , 629–634 (1997).
Roerecke, M. & Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 12 , 182 (2014).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10 , 89 (2021).
Stevens, G. A. et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 13 , e1002056 (2016).
Griswold, M. G. et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 , 1015–1035 (2018).
Albert, C. M. et al. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation 100 , 944–950 (1999).
Arriola, L. et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart 96 , 124–130 (2010).
Bazzano, L. A. et al. Alcohol consumption and risk of coronary heart disease among Chinese men. Int. J. Cardiol. 135 , 78–85 (2009).
Bell, S. et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 356 , j909 (2017).
Bergmann, M. M. et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int. J. Epidemiol. 42 , 1772–1790 (2013).
Beulens, J. W. J. et al. Alcohol consumption and risk for coronary heart disease among men with hypertension. Ann. Intern. Med. 146 , 10–19 (2007).
Bobak, M. et al. Alcohol, drinking pattern and all-cause, cardiovascular and alcohol-related mortality in Eastern Europe. Eur. J. Epidemiol. 31 , 21–30 (2016).
Boffetta, P. & Garfinkel, L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology 1 , 342–348 (1990).
Britton, A. & Marmot, M. Different measures of alcohol consumption and risk of coronary heart disease and all-cause mortality: 11-year follow-up of the Whitehall II Cohort Study. Addiction 99 , 109–116 (2004).
Camargo, C. A. et al. Moderate alcohol consumption and risk for angina pectoris or myocardial infarction in U.S. male physicians. Ann. Intern. Med. 126 , 372–375 (1997).
Chang, J. Y., Choi, S. & Park, S. M. Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers. Sci. Rep. 10 , 13419 (2020).
Chiuve, S. E. et al. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm 7 , 1374–1380 (2010).
Colditz, G. A. et al. Moderate alcohol and decreased cardiovascular mortality in an elderly cohort. Am. Heart J. 109 , 886–889 (1985).
Dai, J., Mukamal, K. J., Krasnow, R. E., Swan, G. E. & Reed, T. Higher usual alcohol consumption was associated with a lower 41-y mortality risk from coronary artery disease in men independent of genetic and common environmental factors: the prospective NHLBI Twin Study. Am. J. Clin. Nutr. 102 , 31–39 (2015).
Dam, M. K. et al. Five year change in alcohol intake and risk of breast cancer and coronary heart disease among postmenopausal women: prospective cohort study. BMJ 353 , i2314 (2016).
Degerud, E. et al. Associations of binge drinking with the risks of ischemic heart disease and stroke: a study of pooled Norwegian Health Surveys. Am. J. Epidemiol. 190 , 1592–1603 (2021).
de Labry, L. O. et al. Alcohol consumption and mortality in an American male population: recovering the U-shaped curve–findings from the normative Aging Study. J. Stud. Alcohol 53 , 25–32 (1992).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int. J. Epidemiol. 34 , 199–204 (2005).
Dyer, A. R. et al. Alcohol consumption and 17-year mortality in the Chicago Western Electric Company study. Prev. Med. 9 , 78–90 (1980).
Ebbert, J. O., Janney, C. A., Sellers, T. A., Folsom, A. R. & Cerhan, J. R. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J. Gen. Intern. Med. 20 , 14–20 (2005).
Ebrahim, S. et al. Alcohol dehydrogenase type 1 C (ADH1C) variants, alcohol consumption traits, HDL-cholesterol and risk of coronary heart disease in women and men: British Women’s Heart and Health Study and Caerphilly cohorts. Atherosclerosis 196 , 871–878 (2008).
Friedman, L. A. & Kimball, A. W. Coronary heart disease mortality and alcohol consumption in Framingham. Am. J. Epidemiol. 124 , 481–489 (1986).
Fuchs, F. D. et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 160 , 466–474 (2004).
Garfinkel, L., Boffetta, P. & Stellman, S. D. Alcohol and breast cancer: a cohort study. Prev. Med. 17 , 686–693 (1988).
Gémes, K. et al. Alcohol consumption is associated with a lower incidence of acute myocardial infarction: results from a large prospective population-based study in Norway. J. Intern. Med. 279 , 365–375 (2016).
Gigleux, I. et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J. Nutr. 136 , 3027–3032 (2006).
Goldberg, R. J., Burchfiel, C. M., Reed, D. M., Wergowske, G. & Chiu, D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation 89 , 651–659 (1994).
Goldberg, R. J. et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch. Intern. Med. 155 , 686–694 (1995).
Gordon, T. & Doyle, J. T. Drinking and coronary heart disease: the Albany Study. Am. Heart J. 110 , 331–334 (1985).
Gun, R. T., Pratt, N., Ryan, P., Gordon, I. & Roder, D. Tobacco and alcohol-related mortality in men: estimates from the Australian cohort of petroleum industry workers. Aust. N.Z. J. Public Health 30 , 318–324 (2006).
Harriss, L. R. et al. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction 102 , 1574–1585 (2007).
Hart, C. L. & Smith, G. D. Alcohol consumption and mortality and hospital admissions in men from the Midspan collaborative cohort study. Addiction 103 , 1979–1986 (2008).
Henderson, S. O. et al. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS ONE 2 , e377 (2007).
Hippe, M. et al. Familial predisposition and susceptibility to the effect of other risk factors for myocardial infarction. J. Epidemiol. Community Health 53 , 269–276 (1999).
Ikehara, S. et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan collaborative cohort study. Stroke 39 , 2936–2942 (2008).
Ikehara, S. et al. Alcohol consumption, social support, and risk of stroke and coronary heart disease among Japanese men: the JPHC Study. Alcohol. Clin. Exp. Res. 33 , 1025–1032 (2009).
Iso, H. et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke 26 , 767–773 (1995).
Jakovljević, B., Stojanov, V., Paunović, K., Belojević, G. & Milić, N. Alcohol consumption and mortality in Serbia: twenty-year follow-up study. Croat. Med. J. 45 , 764–768 (2004).
PubMed Google Scholar
Keil, U., Chambless, L. E., Döring, A., Filipiak, B. & Stieber, J. The relation of alcohol intake to coronary heart disease and all-cause mortality in a beer-drinking population. Epidemiology 8 , 150–156 (1997).
Key, T. J. et al. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 89 , 1613S–1619S (2009).
Kitamura, A. et al. Alcohol intake and premature coronary heart disease in urban Japanese men. Am. J. Epidemiol. 147 , 59–65 (1998).
Kivelä, S. L. et al. Alcohol consumption and mortality in aging or aged Finnish men. J. Clin. Epidemiol. 42 , 61–68 (1989).
Klatsky, A. L. et al. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am. J. Cardiol. 96 , 346–351 (2005).
Kono, S., Ikeda, M., Tokudome, S., Nishizumi, M. & Kuratsune, M. Alcohol and mortality: a cohort study of male Japanese physicians. Int. J. Epidemiol. 15 , 527–532 (1986).
Kunutsor, S. K. et al. Self-reported alcohol consumption, carbohydrate deficient transferrin and risk of cardiovascular disease: the PREVEND prospective cohort study. Clin. Chim. Acta 520 , 1–7 (2021).
Kurl, S., Jae, S. Y., Voutilainen, A. & Laukkanen, J. A. The combined effect of blood pressure and C-reactive protein with the risk of mortality from coronary heart and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 31 , 2051–2057 (2021).
Larsson, S. C., Wallin, A. & Wolk, A. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: two prospective cohorts. Int. J. Cardiol. 231 , 207–210 (2017).
Lazarus, N. B., Kaplan, G. A., Cohen, R. D. & Leu, D. J. Change in alcohol consumption and risk of death from all causes and from ischaemic heart disease. BMJ 303 , 553–556 (1991).
Lee, D.-H., Folsom, A. R. & Jacobs, D. R. Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia 47 , 185–194 (2004).
Liao, Y., McGee, D. L., Cao, G. & Cooper, R. S. Alcohol intake and mortality: findings from the National Health Interview Surveys (1988 and 1990). Am. J. Epidemiol. 151 , 651–659 (2000).
Licaj, I. et al. Alcohol consumption over time and mortality in the Swedish Women’s Lifestyle and Health cohort. BMJ Open 6 , e012862 (2016).
Lindschou Hansen, J. et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur. J. Epidemiol. 26 , 439–447 (2011).
Makelä, P., Paljärvi, T. & Poikolainen, K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: a follow-up study in a population with a low consumption level. J. Stud. Alcohol 66 , 722–728 (2005).
Malyutina, S. et al. Relation between heavy and binge drinking and all-cause and cardiovascular mortality in Novosibirsk, Russia: a prospective cohort study. Lancet 360 , 1448–1454 (2002).
Maraldi, C. et al. Impact of inflammation on the relationship among alcohol consumption, mortality, and cardiac events: the health, aging, and body composition study. Arch. Intern. Med. 166 , 1490–1497 (2006).
Marques-Vidal, P. et al. Alcohol consumption and cardiovascular disease: differential effects in France and Northern Ireland. The PRIME study. Eur. J. Cardiovasc. Prev. Rehabil. 11 , 336–343 (2004).
Meisinger, C., Döring, A., Schneider, A., Löwel, H. & KORA Study Group. Serum gamma-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis 189 , 297–302 (2006).
Merry, A. H. H. et al. Smoking, alcohol consumption, physical activity, and family history and the risks of acute myocardial infarction and unstable angina pectoris: a prospective cohort study. BMC Cardiovasc. Disord. 11 , 13 (2011).
Miller, G. J., Beckles, G. L., Maude, G. H. & Carson, D. C. Alcohol consumption: protection against coronary heart disease and risks to health. Int. J. Epidemiol. 19 , 923–930 (1990).
Mukamal, K. J., Chiuve, S. E. & Rimm, E. B. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Arch. Intern. Med. 166 , 2145–2150 (2006).
Ng, R., Sutradhar, R., Yao, Z., Wodchis, W. P. & Rosella, L. C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 49 , 113–130 (2020).
Onat, A. et al. Moderate and heavy alcohol consumption among Turks: long-term impact on mortality and cardiometabolic risk. Arch. Turkish Soc. Cardiol. 37 , 83–90 (2009).
Google Scholar
Pedersen, J. Ø., Heitmann, B. L., Schnohr, P. & Grønbaek, M. The combined influence of leisure-time physical activity and weekly alcohol intake on fatal ischaemic heart disease and all-cause mortality. Eur. Heart J. 29 , 204–212 (2008).
Reddiess, P. et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. Can. Med. Assoc. J. 193 , E117–E123 (2021).
Article CAS Google Scholar
Rehm, J. T., Bondy, S. J., Sempos, C. T. & Vuong, C. V. Alcohol consumption and coronary heart disease morbidity and mortality. Am. J. Epidemiol. 146 , 495–501 (1997).
Renaud, S. C., Guéguen, R., Schenker, J. & d’Houtaud, A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology 9 , 184–188 (1998).
Ricci, C. et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ 361 , k934 (2018).
Rimm, E. B. et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 338 , 464–468 (1991).
Roerecke, M. et al. Heavy drinking occasions in relation to ischaemic heart disease mortality– an 11-22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. Int. J. Epidemiol. 40 , 1401–1410 (2011).
Romelsjö, A., Allebeck, P., Andréasson, S. & Leifman, A. Alcohol, mortality and cardiovascular events in a 35 year follow-up of a nationwide representative cohort of 50,000 Swedish conscripts up to age 55. Alcohol Alcohol. 47 , 322–327 (2012).
Rostron, B. Alcohol consumption and mortality risks in the USA. Alcohol Alcohol. 47 , 334–339 (2012).
Ruidavets, J.-B. et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 341 , c6077 (2010).
Schooling, C. M. et al. Moderate alcohol use and mortality from ischaemic heart disease: a prospective study in older Chinese people. PLoS ONE 3 , e2370 (2008).
Schutte, R., Smith, L. & Wannamethee, G. Alcohol - The myth of cardiovascular protection. Clin. Nutr. 41 , 348–355 (2022).
Sempos, C., Rehm, J., Crespo, C. & Trevisan, M. No protective effect of alcohol consumption on coronary heart disease (CHD) in African Americans: average volume of drinking over the life course and CHD morbidity and mortality in a U.S. national cohort. Contemp. Drug Probl. 29 , 805–820 (2002).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and coronary heart disease: a perspective from the British Regional Heart Study. Int. J. Epidemiol. 23 , 482–494 (1994).
Simons, L. A., McCallum, J., Friedlander, Y. & Simons, J. Alcohol intake and survival in the elderly: a 77 month follow-up in the Dubbo study. Aust. N.Z. J. Med. 26 , 662–670 (1996).
Skov-Ettrup, L. S., Eliasen, M., Ekholm, O., Grønbæk, M. & Tolstrup, J. S. Binge drinking, drinking frequency, and risk of ischaemic heart disease: a population-based cohort study. Scand. J. Public Health 39 , 880–887 (2011).
Snow, W. M., Murray, R., Ekuma, O., Tyas, S. L. & Barnes, G. E. Alcohol use and cardiovascular health outcomes: a comparison across age and gender in the Winnipeg Health and Drinking Survey Cohort. Age Ageing 38 , 206–212 (2009).
Song, R. J. et al. Alcohol consumption and risk of coronary artery disease (from the Million Veteran Program). Am. J. Cardiol. 121 , 1162–1168 (2018).
Streppel, M. T., Ocké, M. C., Boshuizen, H. C., Kok, F. J. & Kromhout, D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J. Epidemiol. Community Health 63 , 534–540 (2009).
Suhonen, O., Aromaa, A., Reunanen, A. & Knekt, P. Alcohol consumption and sudden coronary death in middle-aged Finnish men. Acta Med. Scand. 221 , 335–341 (1987).
Thun, M. J. et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N. Engl. J. Med. 337 , 1705–1714 (1997).
Tolstrup, J. et al. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ 332 , 1244–1248 (2006).
Wannamethee, G. & Shaper, A. G. Alcohol and sudden cardiac death. Br. Heart J. 68 , 443–448 (1992).
Wannamethee, S. G. & Shaper, A. G. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am. J. Public Health 89 , 685–690 (1999).
Wilkins, K. Moderate alcohol consumption and heart disease. Health Rep. 14 , 9–24 (2002).
Yang, L. et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int. J. Epidemiol. 41 , 1101–1113 (2012).
Yi, S. W., Yoo, S. H., Sull, J. W. & Ohrr, H. Association between alcohol drinking and cardiovascular disease mortality and all-cause mortality: Kangwha Cohort Study. J. Prev. Med. Public Health 37 , 120–126 (2004).
Younis, J., Cooper, J. A., Miller, G. J., Humphries, S. E. & Talmud, P. J. Genetic variation in alcohol dehydrogenase 1C and the beneficial effect of alcohol intake on coronary heart disease risk in the Second Northwick Park Heart Study. Atherosclerosis 180 , 225–232 (2005).
Yusuf, S. et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395 , 795–808 (2020).
Zhang, Y. et al. Association of drinking pattern with risk of coronary heart disease incidence in the middle-aged and older Chinese men: results from the Dongfeng-Tongji cohort. PLoS ONE 12 , e0178070 (2017).
Augustin, L. S. A. et al. Alcohol consumption and acute myocardial infarction: a benefit of alcohol consumed with meals? Epidemiology 15 , 767–769 (2004).
Bianchi, C., Negri, E., La Vecchia, C. & Franceschi, S. Alcohol consumption and the risk of acute myocardial infarction in women. J. Epidemiol. Community Health 47 , 308–311 (1993).
Brenner, H. et al. Coronary heart disease risk reduction in a predominantly beer-drinking population. Epidemiology 12 , 390–395 (2001).
Dorn, J. M. et al. Alcohol drinking pattern and non-fatal myocardial infarction in women. Addiction 102 , 730–739 (2007).
Fan, A. Z., Ruan, W. J. & Chou, S. P. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev. Med. 118 , 336–343 (2019).
Fumeron, F. et al. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Investig. 96 , 1664–1671 (1995).
Gaziano, J. M. et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 329 , 1829–1834 (1993).
Genchev, G. D., Georgieva, L. M., Weijenberg, M. P. & Powles, J. W. Does alcohol protect against ischaemic heart disease in Bulgaria? A case-control study of non-fatal myocardial infarction in Sofia. Cent. Eur. J. Public Health 9 , 83–86 (2001).
CAS PubMed Google Scholar
Hammar, N., Romelsjö, A. & Alfredsson, L. Alcohol consumption, drinking pattern and acute myocardial infarction. A case referent study based on the Swedish Twin Register. J. Intern. Med. 241 , 125–131 (1997).
Hines, L. M. et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N. Engl. J. Med. 344 , 549–555 (2001).
Ilic, M., Grujicic Sipetic, S., Ristic, B. & Ilic, I. Myocardial infarction and alcohol consumption: a case-control study. PLoS ONE 13 , e0198129 (2018).
Jackson, R., Scragg, R. & Beaglehole, R. Alcohol consumption and risk of coronary heart disease. BMJ 303 , 211–216 (1991).
Kabagambe, E. K., Baylin, A., Ruiz-Narvaez, E., Rimm, E. B. & Campos, H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am. J. Clin. Nutr. 82 , 1336–1345 (2005).
Kalandidi, A. et al. A case-control study of coronary heart disease in Athens, Greece. Int. J. Epidemiol. 21 , 1074–1080 (1992).
Kaufman, D. W., Rosenberg, L., Helmrich, S. P. & Shapiro, S. Alcoholic beverages and myocardial infarction in young men. Am. J. Epidemiol. 121 , 548–554 (1985).
Kawanishi, M., Nakamoto, A., Konemori, G., Horiuchi, I. & Kajiyama, G. Coronary sclerosis risk factors in males with special reference to lipoproteins and apoproteins: establishing an index. Hiroshima J. Med. Sci. 39 , 61–64 (1990).
Kono, S. et al. Alcohol intake and nonfatal acute myocardial infarction in Japan. Am. J. Cardiol. 68 , 1011–1014 (1991).
Mehlig, K. et al. CETP TaqIB genotype modifies the association between alcohol and coronary heart disease: the INTERGENE case-control study. Alcohol 48 , 695–700 (2014).
Miyake, Y. Risk factors for non-fatal acute myocardial infarction in middle-aged and older Japanese. Fukuoka Heart Study Group. Jpn. Circ. J. 64 , 103–109 (2000).
Oliveira, A., Barros, H., Azevedo, A., Bastos, J. & Lopes, C. Impact of risk factors for non-fatal acute myocardial infarction. Eur. J. Epidemiol. 24 , 425–432 (2009).
Oliveira, A., Barros, H. & Lopes, C. Gender heterogeneity in the association between lifestyles and non-fatal acute myocardial infarction. Public Health Nutr. 12 , 1799–1806 (2009).
Romelsjö, A. et al. Abstention, alcohol use and risk of myocardial infarction in men and women taking account of social support and working conditions: the SHEEP case-control study. Addiction 98 , 1453–1462 (2003).
Schröder, H. et al. Myocardial infarction and alcohol consumption: a population-based case-control study. Nutr. Metab. Cardiovasc. Dis. 17 , 609–615 (2007).
Scragg, R., Stewart, A., Jackson, R. & Beaglehole, R. Alcohol and exercise in myocardial infarction and sudden coronary death in men and women. Am. J. Epidemiol. 126 , 77–85 (1987).
Tavani, A., Bertuzzi, M., Gallus, S., Negri, E. & La Vecchia, C. Risk factors for non-fatal acute myocardial infarction in Italian women. Prev. Med. 39 , 128–134 (2004).
Tavani, A. et al. Intake of specific flavonoids and risk of acute myocardial infarction in Italy. Public Health Nutr. 9 , 369–374 (2006).
Zhou, X., Li, C., Xu, W., Hong, X. & Chen, J. Relation of alcohol consumption to angiographically proved coronary artery disease in chinese men. Am. J. Cardiol. 106 , 1101–1103 (2010).
Yang, Y. et al. Alcohol consumption and risk of coronary artery disease: a dose-response meta-analysis of prospective studies. Nutrition 32 , 637–644 (2016).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4 , 330–345 (2017).
Mukamal, K. J., Stampfer, M. J. & Rimm, E. B. Genetic instrumental variable analysis: time to call Mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur. J. Epidemiol. 35 , 93–97 (2020).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 , 693–698 (2018).
Shi, J. et al. Mendelian randomization with repeated measures of a time-varying exposure: an application of structural mean models. Epidemiology 33 , 84–94 (2022).
Burgess, S., Swanson, S. A. & Labrecque, J. A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 36 , 253–257 (2021).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur. J. Epidemiol. 35 , 99–111 (2020).
Burgess, S. Violation of the constant genetic effect assumption can result in biased estimates for non-linear mendelian randomization. Hum. Hered. 88 , 79–90 (2023).
Tian, H., Mason, A. M., Liu, C. & Burgess, S. Relaxing parametric assumptions for non-linear Mendelian randomization using a doubly-ranked stratification method. PLoS Genet. 19 , e1010823 (2023).
Levin, M. G. & Burgess, S. Mendelian randomization as a tool for cardiovascular research: a review. JAMA Cardiol. 9 , 79–89 (2024).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 4 , 186 (2019).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 , 304–314 (2016).
Holmes, M. V., Ala-Korpela, M. & Smith, G. D. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 14 , 577–590 (2017).
Labrecque, J. A. & Swanson, S. A. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am. J. Epidemiol. 188 , 231–238 (2019).
Spiegelman, D. et al. The Moderate Alcohol and Cardiovascular Health Trial (MACH15): design and methods for a randomized trial of moderate alcohol consumption and cardiometabolic risk. Eur. J. Prev. Cardiol. 27 , 1967–1982 (2020).
DeJong, W. The Moderate Alcohol and Cardiovascular Health Trial: public health advocates should support good science, not undermine it. Eur. J. Prev. Cardiol. 28 , e22–e24 (2021).
National Institutes of Health. NIH to End Funding for Moderate Alcohol and Cardiovascular Health Trial https://www.nih.gov/news-events/news-releases/nih-end-funding-moderate-alcohol-cardiovascular-health-trial (2018).
Miller, L. M., Anderson, C. A. M. & Ix, J. H. Editorial: from MACH15 to MACH0 – a missed opportunity to understand the health effects of moderate alcohol intake. Eur. J. Prev. Cardiol. 28 , e23–e24 (2021).
Anderson, B. O. et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 8 , e6–e7 (2023).
Au Yeung, S. L. & Lam, T. H. Unite for a framework convention for alcohol control. Lancet 393 , 1778–1779 (2019).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183 , 758–764 (2016).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
The ARIC Investigators. The Atherosclerosis Risk in Communit (ARIC) study: design and objectives. Am. J. Epidemiol. 129 , 687–702 (1989).
Mahmood, S. S., Levy, D., Vasan, R. S. & Wang, T. J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383 , 999–1008 (2014).
Gmel, G. & Rehm, J. Measuring alcohol consumption. Contemp. Drug Probl. 31 , 467–540 (2004).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 , 924–926 (2008).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 58 , 267–288 (1996).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta‐analysis. Stat. Med. 16 , 753–768 (1997).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the final report, or the decision to publish.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Sinclair Carr, Dana Bryazka, Susan A. McLaughlin, Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Hilary R. Lawlor, Erin C. Mullany, Christopher J. L. Murray, Sneha I. Nicholson, Gregory A. Roth, Reed J. D. Sorensen & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Christopher J. L. Murray, Gregory A. Roth & Emmanuela Gakidou
Clinical Trial Service Unit & Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, Oxfordshire, UK
Sarasvati Bahadursingh & Sarah Lewington
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
Institute for Mental Health Policy Research, Centre for Addiction and Mental Health, Toronto, ON, Canada
Jürgen Rehm
Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada
Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Faculty of Medicine, Institute of Medical Science (IMS), University of Toronto, Toronto, ON, Canada
World Health Organization / Pan American Health Organization Collaborating Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
Center for Interdisciplinary Addiction Research (ZIS), Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany
Institute of Clinical Psychology and Psychotherapy, Technische Universität Dresden, Dresden, Germany
Division of Cardiology, Department of Medicine, University of Washington, Seattle, WA, USA
Gregory A. Roth
You can also search for this author in PubMed Google Scholar
Contributions
S.C., S.A.M., S.I.H., and E.C.M. managed the estimation or publications process. S.C. wrote the first draft of the manuscript. S.C. had primary responsibility for applying analytical methods to produce estimates. S.C. and H.R.L. had primary responsibility for seeking, cataloging, extracting, or cleaning data; designing or coding figures and tables. S.C., D.B., S.B., E.C.M., S.I.N., J.R., and R.J.D.S. provided data or critical feedback on data sources. S.C., D.B., P.Z., A.Y.A., S.I.N., and R.J.D.S. developed methods or computational machinery. S.C., D.B., P.Z., S.B., S.I.H., E.C.M., C.J.L.M., S.I.N., J.R., R.J.D.S., S.L., and E.G. provided critical feedback on methods or results. S.C., D.B., S.A.M., S.B., S.I.H., C.J.L.M., J.R., G.A.R., S.L., and E.G. drafted the work or revised it critically for important intellectual content. S.C., S.I.H., E.C.M., and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Sinclair Carr .
Ethics declarations
Competing interests.
G.A.R. has received support for this manuscript from the Bill and Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Shiu Lun Au Yeung, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Carr, S., Bryazka, D., McLaughlin, S.A. et al. A burden of proof study on alcohol consumption and ischemic heart disease. Nat Commun 15 , 4082 (2024). https://doi.org/10.1038/s41467-024-47632-7
Download citation
Received : 14 June 2023
Accepted : 08 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41467-024-47632-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

The NIAAA is the lead agency for U.S. research on the causes, consequences, prevention and treatment of alcohol use disorder and alcohol-related problems.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Major research initiatives.
Addressing alcohol-related issues—from basic science to clinical studies.
Extramural Research: Research at Grantee Institutions
Primary areas of research, funding opportunities, and staff listings for Extramural Research Divisions.
Intramural Research: Research in NIAAA Labs
Organization, primary areas of research, and staff listings for Intramural Research Labs.

Guidance and policies for scientists pursuing alcohol research.

NIAAA’s research priorities for the next five years.

NIAAA’s peer-reviewed scientific journal.
Research on factors that compel youth to begin and continue drinking.
Research on medications in development for treatment of alcohol use disorder.
The NIAAA data archive is a data repository that houses and shares human subjects data generated by NIAAA-funded research.
Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner.
Current and potential alcohol research investigators and trainees are encouraged to subscribe to our new email list to receive NIAAA information and updates relevant to the research community. To sign up, enter your contact information below.

Sign Up for Email Updates
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism
- Search Menu
- Author Guidelines
- Submission Site
- Open Access
- About Alcohol and Alcoholism
- About the Medical Council on Alcohol
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the MCA
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, the heritage of the 19th century — a concept of addiction, temperance and degeneration, trying to eradicate alcoholism — different approaches, after prohibition — the creation of a modern disease concept, one or many types of alcoholism — genetic findings and potential subtypes, the core of alcohol dependence — tolerance and withdrawal or sensitization and reward craving, the disease concept revisited, new treatment options and future directions.
- < Previous
ONE HUNDRED YEARS OF ALCOHOLISM: THE TWENTIETH CENTURY
- Article contents
- Figures & tables
- Supplementary Data
Karl Mann, Derik Hermann, Andreas Heinz, ONE HUNDRED YEARS OF ALCOHOLISM: THE TWENTIETH CENTURY, Alcohol and Alcoholism , Volume 35, Issue 1, January 2000, Pages 10–15, https://doi.org/10.1093/alcalc/35.1.10
- Permissions Icon Permissions
The past 100 years witnessed the formation of a disease concept of alcoholism and a rapid increase in the knowledge of its aetiopathology and treatment options. In the first half of the century, public sanctions aimed at the abolition of alcoholism. In the United States, alcohol prohibition was revoked in the economic turmoil of the Great Depression. In Germany, proposed medical procedures to reduce the fertility of alcoholics had catastrophic consequences during the fascist dictatorship. A revived focus on alcoholics as patients with a right to medical treatment came out of self-organized groups, such as Alcoholics Anonymous. The current disease concept includes the psychosocial and neurobiological foundations and consequences of alcoholism. Neurobiological research points to the dispositional factor of monoaminergic dysfunction and indicates that neuroadaptation and sensitization may play a role in the maintenance of addictive behaviour. New treatment options include pharmacological approaches and indicate that behaviour and motivational therapy and the attendance of patient groups may equally reduce the relapse risk. The task of the future will be to apply scientific discoveries in the best interest of the patients and to support their efforts to be respected like subjects suffering from other diseases.
Alcoholism research and treatment underwent significant changes in the 20th century. Within the last 100 years, a disease concept was formed, which is now widely accepted, the psychosocial and neurobiological consequences of alcoholism have been characterized and treatment programmes have been established and continuously refined. First attempts were made to formulate models of the disposition and development of alcohol dependence that integrate both neurobiological and psychosocial findings. In this essay, we will highlight some of the cornerstones of our present understanding of alcoholism and reflect on some of the organizations and research traditions whose activities were crucial in the development of current concepts. Given the scope of the subject, this review will be both incomplete and subjective, and we will be unable to mention many subjects and institutions whose contributions to current alcoholism concepts were as important and fundamental as the ones we are able to discuss.
An uncontrollable, overwhelming and irresistible desire to consume alcohol was described by Benjamin Rush in 1784, and delirium tremens was independently described by both Pearson and Sutton in 1813 ( Kielhorn, 1988 ). Alcohol craving and withdrawal symptoms were integral parts of the concept of addiction and of the destructive effects of alcohol consumption promoted by the temperance movement in the 19th century ( Levine, 1984 ). In several European countries and in the United States, temperance movements were stimulated by the excessive consumption of liquor and other highly distilled alcoholic beverages, which was uninhibited by cultural traditions and appeared especially problematic among poor working class families during industrialization ( Levine, 1984 ; Henkel, 1998 ). There was, however, a fundamental difference to current concepts of alcoholism: the temperance movement suggested that anyone who consumed excessive amounts of alcohol would suffer from alcohol-related problems and did not suggest that alcoholism could affect certain specifically vulnerable individuals primarily ( Levine, 1984 ; Heather, 1992 ).
A focus on the individual was promoted by degenerationism, the theory that biological factors, toxic environmental influences or moral vices may trigger a cascade of social, moral and medical problems, which increase in each generation and will finally lead to the extinction of that family ( Bynum, 1984 ). The theory of degeneration was based on the pre-Darwinian concept that acquired character traits were passed on to the offspring and assumed that an array of different symptoms and diseases, such as impulsivity, alcoholism, strokes, dementia, microcephaly and epilepsy, were all expressions of one underlying pathology — degeneration ( Hermle, 1986 ).
Degenerationism thus offered a medical explanation for the social problems which were so visible at the end of the 19th century, and excessive alcohol consumption played a crucial role in the concept, as it was seen as a vice which also affects the next generation. In the early 20th century, the degeneration theory suffered from an increasing knowledge about modes of transmission of heritable traits, which pointed to the separate inheritance of different mental and physical diseases, and distinguished between heritable traits and toxic effects on the germ plasm or embryo, thus fundamentally questioning the postulate of the inheritance of acquired traits ( Hermle, 1986 ). However, degenerationism substantially contributed to the concerns about the specific alcohol-related problems of certain individuals.
In the first 30 years of the 20th century, degenerationism and the successors of the temperance movement sparked widespread political activities in the field of alcohol addiction. In the United States, the Anti-Saloon League followed the approach of the temperance movement and focused on the general problems of alcohol consumption. It succeeded in the implementation of alcohol prohibition, which was legally enforced from 1919 to 1933. Prohibition was initially successful in reducing alcohol intake; however, illegal alcohol consumption slowly increased in the late 1920s ( Tyrrell, 1997 ). Prohibition was finally abolished not so much because it failed to abolish alcohol intake, but because of shifting priorities in the Great Depression, when it was argued that liquor production would create jobs and that alcohol taxes might help to reduce income taxes ( Levine, 1984 ).
In Germany, the focus on the individual and their heritable vulnerability to alcohol addiction was imbued with alarmist concerns about the proliferation of the mentally ill, which was supposed to threaten the survival of the nation or ‘race.' Consequently, compulsory sterilization of ‘severe alcoholics' was already advocated by some medical doctors before it was legalized during the Nazi dictatorship. The number of alcohol-dependent patients murdered during the Nazi regime is unknown ( Henkel, 1998 ).
It was in the wake of the failure of prohibition that the current concept of alcoholism was formed, and the worldwide shock about the cruelty and inhumanity of Nazi politics may have promoted the modern disease concept with its focus on individual therapy and its emphasis that alcohol addiction is a disease just like any other physical or mental malady ( Levine, 1984 ; Henkel, 1998 ). A decisive point was the foundation of Alcoholics Anonymous (AA) in the late 1930s. Similar to previous temperance movements, Alcoholics Anonymous displayed a sympathetic and supporting attitude towards the addicted person, but unlike previous groups, AA was only for alcoholics and was not concerned with the general level of alcohol consumption in the population. In fact, the view that all it would take to create an alcohol addict would be his excessive alcohol consumption was no longer persuasive after the end of prohibition ( Levine, 1984 ). Likewise, the existence of alcohol tolerance and withdrawal was widely neglected in the 1930s and early 1940s, although delirium tremens due to alcohol withdrawal had clearly been described by Hare 1910 in the British Journal of Inebriety ( Edwards, 1990 ). Jellinek (1942) and the Yale Summer School on Alcohol Studies agreed with AA that alcoholism would be a disease with a progressive character and not a moral failing. The 1954 report of the World Health Organization (WHO) reflected this new focus on the individual and stated that ‘the personal make-up is the determining factor, but the pharmacological action (of alcohol) plays a significant role’ ( Edwards, 1990 ). However, it was not until the mid-1950s that convulsions and delirium tremens regained public attention as symptoms of alcohol withdrawal, largely due to the detailed reports of Victor and Adams (1953) and Isbell et al . (1955). In 1955, the WHO acknowledged that ‘very serious withdrawal symptoms’, such as convulsions or delirium, may follow the discontinuation of a prolonged period of very heavy alcohol intake ( Edwards, 1990 ). In his famous book on the disease concept of alcoholism, Jellinek (1960) referred repeatedly to the WHO reports and placed the adaptation of cell metabolism, tolerance and the withdrawal symptoms at the heart of his alcoholism concept, because they would ‘bring about ‘craving’ and a loss of control or inability to abstain.’; In his review of the perception of alcohol withdrawal symptoms in the scientific literature, Edwards (1990) noted that Jellinek's new focus on withdrawal symptoms was ‘in very sharp contrast to the earlier stance of the Yale school.’ It is possible that it was easier to rediscover the physical complications of alcohol withdrawal, because the new disease concept allowed attribution of these complications to an individual disposition rather than to some general effect that prolonged alcohol intake would have on every consumer.
In Germany, the modern disease concept of alcoholism was promoted by Feuerlein (1967, 1996) and others who emphasized that alcohol-dependent patients should have the same entitlement to medical treatment as other patients. It was not until 1968 that a German federal court formally confirmed full insurance coverage of alcoholism-related medical treatment costs, although alcoholism had already been considered a disease since 1915 ( Jellinek, 1960 ).
While it had long been observed that the familial risk for alcoholism is increased, it was only because of twin and adoption studies that a genetic contribution to alcoholism was confirmed ( Kaji, 1960 ; Cadoret and Gath, 1978 ). The observation that family members who share half of their genes are not more likely to develop alcoholism compared with family members who share only a quarter of their genes was incompatible with the simple genetic mechanism of inheritance ( Bleuler, 1955 ; Schuckit et al ., 1972 ).
Based on adoption studies, Cloninger et al . (1981) suggested the existence of two types of alcoholism, a mostly environmentally triggered, late-onset type 1 and a male-limited type 2 with a high genetic loading, legal problems and moderate alcohol consumption. The attempt to distinguish between two subtypes of alcoholism stimulated considerable research efforts. Many authors, however, questioned the dichotomy and argued that once patients suffering from comorbid antisocial personality disorder were excluded, the distinction between type 1 and type 2 alcoholics no longer offered clinical subtypes with distinct severity ( Irwin et al ., 1990 ). Instead, subgrouping was suggested to be based on age of onset, the presence of childhood risk factors such as hyperactivity, and severity of alcoholism ( Schuckit et al ., 1995 ; Johnson et al ., 1996 ). Alcoholism types may thus vary on a continuum of severity, rather than represent distinctly different disease entities ( Bucholz et al ., 1996 ). The genetic disposition to alcoholism may manifest in such unsuspicious forms as a low level of response to alcohol intake in subjects not yet accustomed to chronic alcohol intoxication ( Schuckit and Smith, 1996 ). A low level of alcohol response has recently been associated with an increased availability of raphe serotonin transporters and a low central serotonin turnover rate ( Heinz et al ., 1998 ; Schuckit et al ., 1999 ). A low serotonin turnover rate is a potential marker of early-onset alcoholism ( Fils-Aime et al ., 1996 ) and may be caused or aggravated by early social stress experiences ( Higley et al ., 1996 a , b ). These findings may help to link the clinical disposition to alcoholism with the growing literature on neurobiological alterations that precede and follow the manifestation of alcohol dependence.
The last three decades of the twentieth century witnessed a rapidly increasing knowledge of the neurobiological correlates of alcohol dependence. Edwards focused on the development of alcohol tolerance and the manifestation of withdrawal when chronic alcohol intake is terminated ( Edwards et al ., 1977 ). His groundbreaking work was used by the WHO in the International Classification of Diseases (ICD-9) and operationalized in the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) as criteria of dependence ( Jurd, 1992 ).
Neurobiological research pointed to alcohol-induced stimulation of inhibitory GABAergic, and the inhibition of excitatory glutamatergic, neurotransmission ( Koob, 1992 ; Tsai et al ., 1995 ). To ensure homeostatic regulation, GABA A receptors may be down-regulated and, indeed, brain imaging studies observed reduced cortical GABA A receptors among alcoholics ( Abi-Dargham et al ., 1998 ). When the sedative effects of alcohol are suddenly withdrawn during early abstinence, reduced GABAergic inhibition and increased glutamatergic excitatory neurotransmission may manifest as anxiety, seizures and autonomic dysregulation ( Tsai et al ., 1995 ). Alcohol consumption may then be reinstated to reduce withdrawal, thus acting as a negative reinforcer ( Edwards, 1990 ). Associative learning may transform neutral emotional or environmental stimuli into alcohol-associated cues that induce a conditioned compensatory response to alcohol, ‘conditioned withdrawal’, and craving ( Ludwig et al ., 1974 ; McCusker and Brown, 1990 ). Acamprosate, a drug used to reduce craving in abstinent alcoholics, blocks glutamatergic N -methyl-d-aspartate receptors and may exert its therapeutic effects by decreasing conditioned withdrawal ( Verheul et al ., 1999 ).
However, cue-induced craving is only moderately associated with the severity of physical reactions such as changes in heart rate and skin conductance to cue presentation ( Niaura et al ., 1988 ). A secondary, potentially independent pathway has been suggested that may induce alcohol craving due to the mood-enhancing, positive-reinforcing effects of alcohol consumption ( Wise, 1988 ; Koob and Le Moal, 1997 ). This pathway seems to involve the so-called dopaminergic reward system and its opioidergic stimulation via μ-opiate receptors ( Spanagel et al ., 1992 ; Di Chiara, 1995 ). The role of the dopaminergic system may lie in the direction of attention towards reward-indicating stimuli, rather than in the induction of euphoria or positive mood states ( Schultz et al ., 1995 ; Berridge and Robinson, 1998), which are associated with alcohol consumption and may be mediated by opioidergic neurotransmission ( Volpicelli et al ., 1995 ). Stimulus-dependent dopamine release may be specifically vulnerable to sensitization, thus mediating a stronger behavioural response upon re-exposure to the drug-associated cue ( Robinson and Berridge, 1993 ). These observations may have important implications for our understanding of the ‘addiction memory’ and for therapeutic strategies: systematic cue exposure and response prevention might help to extinguish conditioned craving, although therapeutic study results so far are ambiguous ( O'Brien et al ., 1998 ), and naltrexone medication may prevent cue-induced reinstatement of alcohol craving (Katner, 1999).
The focus on cue-induced craving and the underlying learning mechanisms ( Glautier et al ., 1994 ; Carter and Tiffany, 1999 ) has revived the discussion on whether the disease concept of alcoholism should be replaced by a social learning perspective ( Heather, 1992 ). What was not being denied are the organic consequences of chronic alcohol intake, such as brain atrophy ( Mann et al ., 1995 ) or neuroadaptive processes such as a reduction of central dopamine D2 receptors ( Volkow et al ., 1996 ). Rather, it is argued that cigarette smoking similarly causes physical dependence or neuroadaptation without therefore being considered a disease. The disease concept may label patients and promote apathy associated with the ‘sick role’ ( Heather, 1992 ).
A response to these concerns rests on several arguments. Firstly, it is argued that the sick role per se does not stigmatize patients and that the stigma associated with specific diseases such as ‘consumption’ never promoted similar attempts to deny its disease status, and instead promoted relabelling as tuberculosis ( Keller, 1976 ). Secondly, it is argued that a state may be called a disease even in the absence of abnormalities of anatomic structure. A case in point may be essential hypertension, which is commonly understood as a disease, although the aetiology and pathogenesis are currently unknown. Keller (1976) suggested calling alcoholism a disease, because its behavioural manifestations represent a disablement. This argument resembles the concept of a mental disorder given by the American Psychiatric Association (1987), which argued that a mental disorder is characterized by present distress, disability, or a significantly increased risk of suffering death, pain, disability, or an important loss of freedom. Culver and Gert (1982) added that the state must exist ‘in the absence of a distinct (external) sustaining cause’, so that distress due to political oppression may be distinguished from a mental malady. Applying this definition to cigarette smoking indicates that smoking should be considered a mental disorder, as it is associated with the increased risk of suffering death, and it would thus be considered a malady or disease by Culver and Gert (1982). This brings up the question of whether fast driving then must be called a disease, as it increases the risk of dying in a traffic accident. It could be answered that the association between fast driving and traffic accidents is rather low and that the habit of driving fast might be terminated without experiencing the distress associated with drug withdrawal symptoms.
As aloof as these discussions sometimes appear, they have important implications for the treatment of alcoholism. In 1956, a Board of the American Medical Association (AMA) passed a resolution that urged hospitals to admit patients with alcoholism equally with patients treated for other diseases. This act is usually seen as the moment when alcoholism was formally recognized as a disease in the United States; however, alcoholism was already listed as a disease in 1933 in the Standard Classified Nomenclature of Diseases, which was approved by the AMA and the American Psychiatric Association ( Keller, 1976 ). Yet the 1956 resolution highlights the important legal issues that are associated with the disease status of alcoholism, not least being the question of whether treatment costs should be covered by health insurances ( Jurd, 1992 ). Research in the field of costs and benefits of alcoholism therapy supported the demand to treat alcoholism within the medical system ( Holder, 1998 ).
The last decade of the 20th century witnessed substantial progress in treatment options and strategies. Of special importance is the general practitioner, who sees the vast majority of patients with alcohol problems, while fewer than 10% actually enter specialized treatment programmes ( Wienberg, 1992 ). Brief interventions in primary health care institutions are very often effective in reducing alcohol consumption ( Bien et al ., 1993 ). For those patients who need more extensive treatment, primary health care services have a gatekeeper function. Motivational enhancement in primary health care ( Miller and Rollnick, 1991 ) can effectively increase the participation in treatment programmes and was associated with reduced subsequent relapse rates ( Bien et al ., 1993 ). Specialized treatment programmes were evaluated in project MATCH. Project MATCH examined three treatment options, cognitive behaviour therapy, twelve-step facilitation according to the AA programme and motivational enhancement therapy, and found them similarly effective ( Project Match Research Group, 1998 ). As disappointing as this result may be for the discovery of prospective indicators of treatment response, it shows that the major treatment options available to alcoholics worldwide work successfully and that the eclectic combination of behaviour therapy and the attendance of self-help groups may indeed combine two powerful treatment strategies. With naltrexone and acamprosate, two pharmaceuticals are available that successfully reduce the relapse risk during early abstinence ( O'Malley et al ., 1996 ; Sass et al ., 1996 ). However, even with an accompanying medical treatment, most alcoholics relapse. The goal of the future will therefore be to describe subgroups of patients that may respond positively to specific medications. As acamprosate and naltrexone affect different neurotransmitter systems, neurobiological screening of alcoholics may help to discover predictors of treatment response. Preliminary results indicate that sleep disorders, EEG activity and delayed recovery of dopamine receptor sensitivity during early abstinence are associated with the relapse risk and may help to identify patients who require specific treatment strategies ( Bauer, 1994 ; Heinz et al ., 1996 ; Brower et al ., 1998 ; Winterer et al ., 1998 ).
Basic research has profoundly helped to understand alcohol effects at the level of signal transduction. We now know that drugs affect neurotransmitter release, receptor sensitivity, post-synaptic second-messenger mechanisms and, perhaps most importantly, gene expression ( Koob, 1992 ; Nestler, 1994 ). These observations indicate that human fate is not passively determined by the genetic constitution, but rather that biological and ultimately environmental stimuli regulate gene expression. Increasing knowledge of the molecular mechanisms of dependence may enable us to target these pathological conditions more specifically than we are able today.
Finally, the history of the last 100 years warns us that ‘ethics are not an option,’ as Edwards stated in a 1999 conference at the Central Institute of Mental Health, Mannheim. That alcoholism had been considered a disease in Germany since 1915 ( Jellinek, 1960 ) did not prevent the dehumanizing treatment of patients with alcohol dependence during the Nazi era. It is an integral part of the professional mission to assist patients in their effort to be treated equally inside and outside of medical therapy. Our increasing knowledge about the disposition towards alcohol dependence and a high relapse risk can help to identify patients with demands for special therapeutic efforts, it should never be used to stigmatize these subjects. To monitor the consequences of our research is part of the professional duty.
Author to whom correspondence should be addressed.
Abi-Dargham, A., Krystal, J. H., Anjivel, S., Scanley, B. E., Zoghbi, S., Baldwin, R. M., Rajeevan, N., Ellis, S., Petrakis, I. L., Seibyl, J. P., Charney, D. S., Laruelle, M. and Innis, R. B. ( 1998 ) Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123]Iomezanil. American Journal of Psychiatry 155 , 1550 –1555.
American Psychiatric Association (1987) Diagnostic and Statistical Manual of Mental Disorders , 3rd edn, revised. American Psychiatric Association, Washington, DC.
Bauer, L. O. ( 1994 ) Electroencephalographic and autonomic predictors of relapse in alcohol-dependent patients. Alcoholism: Clinical and Experimental Research 18 , 755 –760.
Berridge, K. C. and Robinson, T. E. ( 1988 ) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews 28 , 309 –369.
Bien, T. H., Miller, W. R. and Tonigan, S. J. ( 1993 ) Brief interventions for alcohol problems: a review. Addiction 88 , 315 –336.
Bleuler, M. (1955) Familial and personal background of chronic alcoholics. In Etiology of Chronic Alcoholism , Dietholm, O. ed., pp. 110–166. Charles C. Thomas, Springfield, IL.
Brower, K. J., Aldrich, M. S. and Hall, J. M. ( 1998 ) Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcoholism: Clinical and Experimental Research 22 , 1864 –1871.
Bucholz, K. K., Heath, A. C., Reich, T., Hesselbrock, V. M., Kramer, J. R., Nurnberger, J. I., Jr. and Schuckit, M. A. ( 1996 ) Can we subtype alcoholism? A latent class analysis of data from relatives of alcoholics in a multicenter family study of alcoholism. Alcoholism: Clinical and Experimental Research 20 , 1462 –1471.
Bynum, W. F. ( 1984 ) Alcoholism and degeneration in 19th century European medicine and psychiatry. British Journal of Addiction 79 , 59 –70.
Cadoret, R. J. and Gath, A. ( 1978 ) Inheritance of alcoholism in adoptees. British Journal of Psychiatry 132 , 252 –258.
Carter, B. L. and Tiffany, S. T. ( 1999 ) Meta-analysis of cue-reactivity in addiction research. Addiction 94 , 327 –340.
Cloninger, C. R., Bohman, M. and Sigvardsson, S. ( 1981 ) Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry 38 , 861 –868.
Culver, C. and Gert, B. (1982) Philosophy in Medicine . Oxford University Press, Oxford.
Di Chiara, G. ( 1995 ) The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug and Alcohol Dependence 38 , 95 –137.
Edwards, G. ( 1990 ) Withdrawal symptoms and alcohol dependence: fruitful mysteries. British Journal of Addiction 85 , 447 –461.
Edwards, G., Gross, M. M., Keller, M. et al . (1977) Alcohol-related Disabilities . WHO Offset Publication No. 32. World Health Organization, Geneva.
Feuerlein, W. ( 1967 ) Der Alkoholismus in sozialpsychiatrischer Sicht. Medizinische Klinik 62 , 922 –926.
Feuerlein, W. ( 1996 ) Alkoholismus als Krankheit. Herz 21 , 213 –216.
Fils-Aime, M. L., Eckhardt, M. J., George, D. T., Brown, G. L., Mefford, I. and Linnoila, M. ( 1996 ) Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindolacetic acid levels than late-onset alcoholics. Archives of General Psychiatry 53 , 211 –216.
Glautier, S., Drummond, C. and Remington, B. ( 1994 ) Alcohol as an unconditioned stimulus in human classical conditioning. Psychopharmacology (Berlin) 116 , 360 –368.
Heather, N. ( 1992 ) Why alcoholism is not a disease. The Medical Journal of Australia 156 , 212 –215.
Heinz, A., Dufeu, P., Kuhn, S., Dettling, M., Gräf, K. J., Kürten, I., Rommelspacher, H. and Schmidt, L. G. ( 1996 ) Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Archives of General Psychiatry 53 , 1123 –1128.
Heinz, A., Higley, J. D., Gorey, J. G., Saunders, R. C., Jones, D. W., Hommer, D., Zajicek, K., Suomi, S. J., Lesch, K. P., Weinberger, D. R. and Linnoila, M. ( 1998 ) In vivo association between alcohol intoxication, aggression and serotonin transporter availability in non-human primates. American Journal of Psychiatry 155 , 1023 –1028.
Henkel, D. (1998) ‘Die Trunksucht ist die Mutter der Armut.’ In Sucht und Armut, Henkel, D. and Vogt, L. eds, pp. 13–79. Leske and Budrich, Opladen.
Hermle, L. ( 1986 ) Die Degenerationslehre in der Psychiatrie. Fortschritte der Neurologie und Psychiatrie 54 , 69 –79.
Higley, J. D., Suomi, S. J. and Linnoila, M. ( 1996 ) A non-human primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcoholism: Clinical and Experimental Research 20 , 629 –642.
Higley, J. D., Suomi, S. J. and Linnoila, M. ( 1996 ) A non-human primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism: Clinical and Experimental Research 20 , 643 –650.
Holder, H. D. ( 1998 ) The cost offsets of alcoholism treatment. Recent Developments in Alcoholism 14 , 361 –374.
Irwin, M., Schuckit, M. and Smith, T. L. ( 1990 ) Clinical importance of age of onset in type 1 and type 2 primary alcoholics. Archives of General Psychiatry 47 , 320 –324.
Isbell, H., Fraser, H. F., Wikler, A., Belleville, R. E. and Eiserman, A. J. ( 1955 ) An experimental study of the etiology of ‘rum fits' and delirium tremens. Quarterly Journal of Studies on Alcohol 16 , 1 –33.
Jellinek, E. M. (1942) Alcohol Addiction and Chronic Alcoholism . Yale University Press, New Haven.
Jellinek, E. M. (1960) The Disease Concept of Alcoholism . Hillhouse , New Brunswick.
Johnson, E. O., van den Bree, M. B. M. and Pickens, R. W. ( 1996 ) Subtypes of alcohol-dependent men: a typology based on relative genetic and environmental loading. Alcoholism: Clinical and Experimental Research 20 , 1472 –1480.
Jurd, S. M. ( 1992 ) Why alcoholism is a disease. The Medical Journal of Australia 156 , 215 –217.
Kaji, L. (1960) Alcoholism in Twins: Studies on the Etiology and Sequels of Abuse of Alcohol . Almqvist and Wiksell, Stockholm.
Katner, S. N., Magalong, J. G. and Weiss, F. ( 1999 ) Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20 , 471 –479.
Keller, M. ( 1976 ) The disease concept of alcoholism revisited. Journal of Studies on Alcohol 37 , 1694 –1717.
Kielhorn, F. W. ( 1988 ) Zur Geschichte des Alkoholismus: Pearson, Sutton und das Delirium tremens. Suchtgefahren 34 , 111 –114.
Koob, G. F. ( 1992 ) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences 13 , 177 –184.
Koob, G. F. and Le Moal, M. ( 1997 ) Drug abuse: hedonic homeostatic dysregulation. Science 278 , 52 –58.
Levine, H. G. ( 1984 ) The alcohol problem in America: from temperance to alcoholism. British Journal of Addiction 79 , 109 –119.
Ludwig, A. M., Wikler, A. and Stark, L. H. ( 1974 ) The first drink: psychobiological aspects of craving. Archives of General Psychiatry 30 , 539 –547.
Mann, K., Mundle, G., Strayle, M. and Wakat, P. ( 1995 ) Neuroimaging in alcoholism: CT and MRI results and clinical correlates. Journal of Neural Transmission 99 , 145 –155.
McCusker, C. G. and Brown, K. ( 1990 ) Alcohol-predictive cues enhance tolerance and precipitate ‘craving’ for alcohol in social drinkers. Journal of Studies on Alcoholism 51 , 494 –499.
Miller, W. R. and Rollnick, S. (1991) Motivational Interviewing: Preparing People to Change Addictive Behavior . Guilford Press, New York.
Nestler, E. J. ( 1994 ) Molecular neurobiology of drug addiction. Neuropsychopharmacology 11 , 77 –87.
Niaura, R. S., Rohsenow, D. J., Binkoff, J. A., Monti, P. M., Pedrazza, M. and Abrams, D. B. ( 1988 ) Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology 97 , 133 –152.
O'Brien, C., Childress, A. R., Ehrman, R. and Robbins, S. J. ( 1998 ) Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology 12 , 15 –22.
O'Malley, S. S., Jaffe, A. J., Chang, G., Rode, S., Schottenfeld, R., Meyer, R. E. and Rounsaville, B. ( 1996 ) Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry 53 , 217 –224.
Project MATCH Research Group ( 1998 ) Matching alcoholism treatment to client heterogeneity: project MATCH three-year drinking outcomes. Alcoholism: Clinical and Experimental Research 22 , 1300 –1311.
Robinson, T. E. and Berridge, K. C. ( 1993 ) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews 18 , 247 –291.
Sass, H., Soyka, M., Mann, K. and Zieglgänsberger, W. ( 1996 ) Relapse prevention by acamprosate: results from a placebo-controlled study on alcohol dependence. Archives of General Psychiatry 53 , 673 –680.
Schuckit, M. A. and Smith, T. L. ( 1996 ) An 8-year follow-up of 450 sons of alcoholics and control subjects. Archives of General Psychiatry 45 , 211 –216.
Schuckit, M. A., Goodwin, D. A. and Winokur, G. ( 1972 ) A study of alcoholism in half-siblings. American Journal of Psychiatry 129 , 1132 –1136.
Schuckit, M. A., Tipp, J. E., Smith, T. L., Shapiro, E., Hesselbrock, V. M., Buchholz, K. K., Reich, T. and Nurnberger, J. I. ( 1995 ) An evaluation of type A and B alcoholics. Addiction 90 , 1189 –1203.
Schuckit, M. A., Mazzanti, C., Smith, T. L., Ahmed, U., Radel, M., Iwata, N. and Goldman, D. ( 1999 ) Selective genotyping for the role of 5-HT 2A , 5-HT 2C , and GABAα 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biological Psychiatry 45 , 647 –651.
Schultz, W., Dayan, P. and Montague, P. R. ( 1995 ) A neural substrate of prediction and reward. Science 275 , 1593 –1599.
Spanagel, R., Herz, A. and Shippenberg, T. S. ( 1992 ) Opposing tonically active endogeneous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences of the USA 89 , 2046 –2050.
Tsai, G., Gastfriend, D. R. and Coyle, J. T. ( 1995 ) The glutamatergic basis of human alcoholism. American Journal of Psychiatry 152 , 332 –340.
Tyrrell, I. ( 1997 ) The US prohibition experiment: myths, history and implications. Addiction 92 , 1405 –1409.
Verheul, R., Van den Brink, W. and Geerlings, P. ( 1999 ) A three-pathway psychobiological model of craving for alcohol. Alcohol and Alcoholism 34 , 197 –222.
Victor, M. and Adams, R. E. ( 1953 ) The effect of alcohol on the nervous system. Research Publications of the Association for Research on Nervous and Mental Disease 32 , 526 –573.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Hitzemann, R., Ding, Y. S., Pappas, N., Shea, C. and Piscani, K. ( 1996 ) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism: Clinical and Experimental Research 20 , 1594 –1598.
Volpicelli, J. R., Watson, N. T., King, A. C., Sherman, C. E. and O'Brien, C. P. ( 1995 ) Effect of naltrexone on alcohol ‘high’ in alcoholics. American Journal of Psychiatry 152 , 613 –615.
Wienberg, G. (1992) Die vergessene Mehrheit . Zur Realität der Versorgung alkohol- und medikamentenabhängiger Patienten . Psychiatrie Verlag, Berlin.
Winterer, G., Klöppel, B., Heinz, A., Schmidt, L. G., Frick, K. and Herrmann, W. M. ( 1998 ) Quantitative EEG (QEEG) analysed with artificial neural networks predicts relapse in patients with chronic alcoholism and points to a frontally pronounced disturbance. Psychiatry Research 78 , 101 –113.
Wise, R. A. ( 1988 ) The neurobiology of craving: implications for the understanding and treatment of addiction. Journal of Abnormal Psychology 97 , 118 –132.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3502
- Copyright © 2024 Medical Council on Alcohol and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Binge drinking is a growing public health crisis − a neurobiologist explains how research on alcohol use disorder has shifted
Assistant Professor of Biology, Biomedical Engineering and Pharmacology, Penn State
Disclosure statement
Nikki Crowley receives funding from The National Institutes of Health, The Brain and Behavior Research Foundation, and the Penn State Huck Institutes of the Life Sciences endowment funds.
Penn State provides funding as a founding partner of The Conversation US.
View all partners
With the new Amy Winehouse biopic “Back to Black ” in U.S. theaters as of May 17, 2024, the late singer’s relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from “death by misadventure” at the age of 27. That’s the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include “alcohol toxicity” following a second coroner’s inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of “heavy drinking” increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists’ and physicians’ understanding of the relationship between women’s health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
The biology of addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn’t until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse’s death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to “ hangxiety ,” the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain’s key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we’ve found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don’t return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don’t yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Women are forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there’s one population that’s been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn’t until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don’t know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women’s health and propensity for alcohol addiction.

Looking ahead
Researchers and lawmakers are recognizing the vital need for increased research on women’s health. Major federal investments into women’s health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
- Amy Winehouse
- Binge drinking
- Neurobiology
- Intoxication
- Alcohol consumption
- Alcohol use
- Alcohol use disorder
- COVID-19 pandemic

Senior Research Fellow - Women's Health Services

Cybersecurity Program Architect

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Alcohol and Your Brain: The Latest Scientific Insights
Want to protect your brain here's what you need to know about alcohol consumption..
Posted March 18, 2024 | Reviewed by Devon Frye
- What Is Alcoholism?
- Find a therapist to overcome addiction
- Transient memory loss, “blackouts,” and hangovers related to alcohol consumption are brain health risks.
- Alcohol use disorder (alcoholism) is a risk factor for developing dementia.
- Heavy or excessive alcohol consumption is dangerous to the brain for a number of reasons.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear.

Depending on who you ask, you might be told to drink a few glasses of red wine a day or to avoid alcohol altogether. The reasons for such recommendations are many, but, by and large, they tend to stem from a study someone read about or saw reported in the news.
So why is it so hard to know whether alcohol is good or bad for us—especially for our brains? In this post, we’ll explore the current science and some practical ideas on how to approach the topic.
What Is Alcohol Anyway?
When people talk about drinking “alcohol,” they’re almost always referring to the consumption of ethanol. Ethanol is a natural product that is formed from the fermentation of grains, fruits, and other sources of sugar. It’s found in a wide range of alcoholic beverages including beer, wine, and spirits like vodka, whiskey, rum, and gin.
Evidence for human consumption of alcohol dates back over 10,000 years. Consumption of alcohol has and continues to serve major roles in religious and cultural ceremonies around the world. But unlike most food products, in the last century, alcohol has been wrapped up in nearly perpetual controversy over its moral effects and health implications.
How Does Alcohol Impact the Brain?
As anyone who’s consumed alcohol knows, ethanol can directly influence brain function. Ethanol is classified as a “depressant” because it has a generally slowing effect on brain activity through activation of γ-aminobutyric acid (GABA) pathways.
In an acute sense, consumption of alcohol can lead to uninhibited behavior, sedation, lapses in judgment, and impairments in motor function. At higher levels, the effects can progress to coma and even death.
The Known Brain-Damaging Effects of Excess Alcohol
There is no debate here: Excessively high levels of alcohol consumption over short periods of time are toxic and potentially deadly, specifically because of its effects on the brain.
One critical fact to understand about the overall and brain-specific effects of alcohol is that the entirety of the debate around the risk/benefit ratio concerns mild to moderate alcohol consumption. As it relates to the effects of high amounts of alcohol on the body and brain, the research is consistent: It’s a very bad choice.
High amounts of alcohol use are causal risk factors in the development of disease in the heart, liver, pancreas, and brain (including the brains of children in utero). In fact, 1 in 8 deaths in Americans aged 20-64 is attributable to alcohol use. When it comes to adults, excessive alcohol use can cause multiple well-defined brain issues ranging from short-term confusion to dementia .
What Is “Excessive” or “High” Alcohol Use?
Key to the nuance in the conversation about alcohol use are definitions. Across the board, “excessive” or “high” alcohol use is linked to worse overall and brain health outcomes. So what does that mean?
While definitions can be variable, one way to look at this is the consumption of 4 or more drinks on an occasion (for women) and 5 or more for men. Additionally, excess alcohol is defined as drinking more than 8 drinks a week (women) and 15 a week (men), or consuming alcohol if you are pregnant or younger than age 21.
Beyond this, by definition, consuming enough alcohol to cause a “brownout,” “blackout,” hangover, or other overt brain symptomatology is evidence that the alcohol you’ve consumed is creating problems in your brain. Alcohol use disorder (or alcoholism ) is also a clear issue for the brain. It has been linked to a higher risk for dementia, especially early-onset dementia in a study of 262,000 adults, as well as to smaller brain size .
Is There a “Safe” Amount of Alcohol for the Brain?
In a highly publicized article from Nature Communications , researchers looked at brain imaging data from nearly 37,000 middle-aged to older adults and cross-referenced their brain scans with their reported alcohol consumption. The findings were profound: People who drank more alcohol had smaller brains, even in people drinking only one or two alcoholic beverages a day.

Conversely, other recent data suggest a lower risk for dementia in people consuming a few alcoholic beverages a day. This includes a 2022 study showing that in around 27,000 people, consuming up to 40 grams of alcohol (around 2.5 drinks) a day was linked to a lower risk for dementia versus abstinence in adults over age 60. A much larger study of almost 4 million people in Korea noted that mild to moderate alcohol consumption was linked to a lower risk for dementia compared to non-drinking.
How Do We Make Sense of This Data?
When it comes to the bottom line as it relates to alcohol consumption and brain health, the data are rather solid on some fronts, and a bit less so on others. There’s also the potential for confounding variables, including the fact that many people like to drink alcohol to enjoy and enhance social bonds (which we know are beneficial for the brain). Here’s a summary of what the most recent research is telling us.
- Experiencing transient memory loss, “blackouts,” or hangovers related to alcohol consumption is overt evidence of threats to brain health.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear, but it seems unreasonable to start alcohol use for brain health.

Austin Perlmutter, M.D. , is a board-certified internal medicine physician and the co-author of Brain Wash .
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Introduction
- Conclusions
- Article Information
a Adjusted for age at survey, sex, race and ethnicity, marital status, educational level, annual household income, insurance status, smoking status, cancer type, age at cancer diagnosis, and currently prescribed medication and/or receiving treatment.
b Included individuals reporting races or ethnicities other than Hispanic, non-Hispanic Black, or non-Hispanic White and individuals with more than 1 race or ethnicity.
c Included breast, colon and rectum, and head and neck cancer. Esophageal cancer was not included because the association with alcohol drinking is confined largely to squamous cell carcinoma, whereas most cases of esophageal cancer were adenocarcinoma in the US. Liver cancer was not included as it was not specifically included in the All of Us Research Program survey.
d Self-reported current medication prescription and/or treatment in the Personal Medical History survey.
a Non-Hispanic White was used as the reference group.
c Included breast, colon and rectum, and head and neck cancer. Esophageal cancer was not included because the association with alcohol drinking is confined largely to squamous cell carcinoma whereas most cases of esophageal cancer were adenocarcinoma in the US. Liver cancer was not included as it was not specifically included in the All of Us Research Program survey.
e Adjusted for age at survey, sex, race and ethnicity, marital status, educational level, annual household income, insurance status, smoking status, cancer type, age at cancer diagnosis, and currently prescribed medication and/or receiving treatment.
eTable 1. Cancer Characteristics According to Sex, All of Us Research Program
eTable 2. Alcohol Use Disorders Identification Test–Consumption (AUDIT-C)
eTable 3. Characteristics of Cancer Survivors Who Underwent Cancer Treatment Within 1 Year Before the Baseline Survey, All of Us Research Program
eTable 4. Adjusted Odds Ratios of Current Drinking Among Cancer Survivors, All of Us Research Program
eTable 5. Adjusted Odds Ratios of Risky Drinking Behaviors Among Current Drinking Cancer Survivors, All of Us Research Program
eTable 6. Prevalence of Alcohol Consumption Patterns Among Survey Participants Without Prior Cancer Diagnosis According to Sex, All of Us Research Program
eFigure 1. Flow Chart of the Study Population
eFigure 2. (A) Mean AUDIT-C Score Among Cancer Survivors According to Sex; (B) Venn Diagram Showing Cancer Survivors Engaged in Exceeding Moderate Drinking, Binge Drinking and Hazardous Drinking Among 11815 Current Drinkers, All of Us Research Program
eFigure 3. Mean AUDIT-C Score Among Cancer Survivors According to Age at Cancer Diagnosis and Smoking Status, All of Us Research Program
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Shi M , Luo C , Oduyale OK , Zong X , LoConte NK , Cao Y. Alcohol Consumption Among Adults With a Cancer Diagnosis in the All of Us Research Program. JAMA Netw Open. 2023;6(8):e2328328. doi:10.1001/jamanetworkopen.2023.28328
Manage citations:
© 2024
- Permissions
Alcohol Consumption Among Adults With a Cancer Diagnosis in the All of Us Research Program
- 1 Division of Public Health Sciences, Department of Surgery, Washington University in St Louis School of Medicine, St Louis, Missouri
- 2 Division of Hematology, Medical Oncology and Palliative Care, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison
- 3 University of Wisconsin Carbone Cancer Center, Madison
- 4 Alvin J. Siteman Cancer Center, Washington University in St Louis School of Medicine, St Louis, Missouri
- 5 Division of Gastroenterology, Department of Medicine, Washington University in St Louis School of Medicine, St Louis, Missouri
Question What is the prevalence of current alcohol consumption and of risky alcohol consumption among cancer survivors in the US?
Findings In this cross-sectional study of 15 199 adults with a cancer diagnosis from the All of Us Research Program, 77.7% self-reported as current drinkers, and among these, 13.0% exceeded moderate drinking, 23.8% reported binge drinking, and 38.3% engaged in hazardous drinking. Among 1839 survivors receiving cancer treatment, the prevalence of current drinking and risky drinking were similar to the overall cohort and across treatment types.
Meaning This study suggests that current drinking and risky drinking are common among US cancer survivors even during cancer treatment.
Importance Alcohol consumption is associated with adverse oncologic and treatment outcomes among individuals with a diagnosis of cancer. As a key modifiable behavioral factor, alcohol consumption patterns among cancer survivors, especially during treatment, remain underexplored in the United States.
Objective To comprehensively characterize alcohol consumption patterns among US cancer survivors.
Design, Setting, and Participants This cross-sectional study used data from May 6, 2018, to January 1, 2022, from the National Institutes of Health All of Us Research Program, a diverse US cohort with electronic health record (EHR) linkage, and included 15 199 participants who reported a cancer diagnosis and 1839 patients among a subset with EHR data who underwent treatment within the past year of the baseline survey. Data analysis was performed from October 1, 2022, to January 31, 2023.
Main Outcomes and Measures Prevalence of current drinking and of risky drinking behaviors, including exceeding moderate drinking (>2 drinks on a typical drinking day), binge drinking (≥6 drinks on 1 occasion), and hazardous drinking (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C] score ≥3 for women or ≥4 for men).
Results This study included 15 199 adults (mean [SD] age at baseline, 63.1 [13.0] years; 9508 women [62.6%]) with a cancer diagnosis. Overall, 11 815 cancer survivors (77.7%) were current drinkers. Among current drinkers, 1541 (13.0%) exceeded moderate drinking, 2812 (23.8%) reported binge drinking, and 4527 (38.3%) engaged in hazardous drinking. After multivariable adjustment, survivors who were younger than 65 years, men, or of Hispanic ethnicity or who received a diagnosis before 18 years of age or ever smoked were more likely to exceed moderate drinking (aged <50 years: odds ratio [OR], 2.90 [95% CI, 2.41-3.48]; aged 50-64 years: OR, 1.84 [95% CI, 1.58-2.15]; men: OR, 2.38 [95% CI, 2.09-2.72]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.04-1.64]; aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.04-2.24]; former smokers: OR, 2.46 [95% CI, 2.16-2.79]; current smokers: OR, 4.14 [95% CI, 3.40-5.04]) or binge drink (aged <50 years: OR, 4.46 [95% CI, 3.85-5.15]; aged 50-64 years: OR, 2.15 [95% CI, 1.90-2.43]; men: OR, 2.10 [95% CI, 1.89-2.34]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.09-1.58]; aged <18 years at diagnosis: OR, 1.71 [95% CI, 1.24-2.35]; former smokers: OR, 1.69 [95% CI, 1.53-1.87]; current smokers: OR, 2.27 [95% CI, 1.91-2.71]). Survivors with cancer diagnosed before 18 years of age or who ever smoked were more likely to be hazardous drinkers (aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.11-2.08]; former smokers: OR, 1.83 [95% CI, 1.68-1.99]; current smokers: OR, 2.13 [95% CI, 1.79-2.53]). Of 1839 survivors receiving treatment as captured in the EHR, 1405 (76.4%) were current drinkers, and among these, 170 (12.1%) exceeded moderate drinking, 329 (23.4%) reported binge drinking, and 540 (38.4%) engaged in hazardous drinking, with similar prevalence across different types of cancer treatment.
Conclusions and Relevance This cross-sectional study of a diverse US cohort suggests that alcohol consumption and risky drinking behaviors were common among cancer survivors, even among individuals receiving treatment. Given the adverse treatment and oncologic outcomes associated with alcohol consumption, additional research and implementation studies are critical in addressing this emerging concern among cancer survivors.
With more than 18 million cancer survivors in the United States as of 2022, 1 identifying modifiable behavioral factors that could improve survivorship and quality of life is a clinical and public health priority. Alcohol consumption, which is ubiquitous in the US and causally linked with multiple types of cancer (oral cavity, pharynx, larynx, esophagus, colorectum, liver, and female breast cancer), 2 , 3 is also associated with adverse health outcomes among individuals with a diagnosis of cancer, including higher risks of recurrence 4 , 5 or onset of new primary cancers 5 - 7 as well as death. 4 , 5 , 8 - 12 In addition, alcohol is associated with worsened treatment outcomes, such as decreased effectiveness and increased risk of complications. 13 - 17 Despite these findings, currently, no specific surveillance and counseling guidelines are in place for cancer survivors. Cancer survivors are advised to adhere to the American Cancer Society guideline on nutrition and physical activity for cancer prevention, including (1) that it is best not to drink alcohol and (2) that individuals who choose to drink alcohol should limit alcohol intake to 1 drink or fewer per day for women and 2 drinks or fewer per day for men. 18
A 2018 statement from the American Society of Clinical Oncology (ASCO) reinforces the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda. 19 However, our understanding of alcohol drinking patterns among cancer survivors in the US is limited. Using the National Health Interview Survey (2000-2017), Sanford et al 20 reported that 35% of cancer survivors who were current drinkers exceeded moderate drinking limits (>1 drink for women and >2 drinks for men) and 21% engaged in binge drinking (≥5 drinks during at least 1 day over the past year). However, to our knowledge, patterns of drinking, including frequency as well as the co-occurrence of multiple risky drinking behaviors, have not been described. 21 , 22 The Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) score, a validated score that incorporates frequency of drinking, quantity of drinking, and binge drinking, has been used in primary care and other settings to identify individuals engaging in hazardous drinking. 23 - 26 One study in 17 European countries and Israel reported that 20% of cancer survivors aged 50 years or older engaged in hazardous drinking, 27 yet such analyses have not been conducted in the US, to our knowledge. More important, although we recently began to recognize the potential adverse effects of drinking during cancer treatment, alcohol consumption patterns during such a critical time window for cancer survivors remain underexplored. To address these knowledge gaps that are critical for short- and long-term survivorship for US cancer survivors, we aimed to comprehensively characterize alcohol consumption patterns among cancer survivors overall and during cancer treatment, using data collected from the All of Us Research Program, a diverse US cohort with electronic health record (EHR) linkage.
We identified cancer survivors enrolled in the National Institutes of Health All of Us Research Program, one of the largest, diverse biomedical cohorts within the US. 28 , 29 The All of Us Research Program collects data using survey responses, EHR data, biospecimen collection, and physical measurements. 28 , 30 The All of Us Research Program institutional review board approved all study procedures. All participants provided written informed consent to share EHRs, surveys, and other study data with qualified investigators for broad-based research. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Among 142 100 participants who completed the Basics, Overall Health, Lifestyle, and Personal Medical History surveys, we identified 15 297 cancer survivors who self-reported a cancer diagnosis (excluding individuals with skin cancer and multiple cancers) from May 6, 2018, to January 1, 2022 (eFigure 1 in Supplement 1 ). We categorized the cancers as alcohol-related cancers (breast, colon and rectum, and head and neck) 2 and nonalcohol-related cancers (eTable 1 in Supplement 1 ). Esophageal cancer was categorized as nonalcohol related because the association with alcohol drinking is confined largely to squamous cell carcinoma, 2 whereas most cases of esophageal cancer in the US were adenocarcinoma. 31 Liver cancer was not included in alcohol-related cancers because it was not specifically included in the survey. We also retrieved information on age at cancer diagnosis (child [≤11 years], adolescent [12-17 years], adult [18-64 years], older adult [65-74 years], or elderly adult [≥75 years]) and current treatment status (“Are you currently prescribed medications and/or receiving treatment for this condition?” with an answer of yes or no).
Current alcohol consumption status (never, former, and current drinkers) was defined based on the questions in the Lifestyle survey. Participants were asked “In your entire life, have you had at least 1 drink of any kind of alcohol, not counting small tastes or sips?” which was adapted from the National Epidemiologic Survey on Alcohol and Related Conditions. We defined participants who reported not having at least 1 drink of any kind of alcohol as never drinkers, those who had at least 1 drink in their entire life but never had a drink in the past year as former drinkers, and those who had at least 1 drink in the past year as current drinkers. After excluding 98 participants without adequate information to define their current alcohol consumption status, 15 199 cancer survivors were retained in the analyses.
Among current drinkers, we further characterized risky drinking behaviors based on 3 questions: (1) frequency of drinking: “How often did you have a drink containing alcohol in the past year?” with options of never, monthly or less, 2 to 4 times a month, 2 to 3 times a week, or 4 or more times a week; (2) quantity of drinking: “On a typical day when you drink, how many drinks do you have?” with options of 1 or 2, 3 or 4, 5 or 6, 7 to 9, or 10 or more; and (3) binge drinking: “How often did you have 6 or more drinks on 1 occasion in the past year?” with options of never, less than monthly, monthly, weekly, or daily or almost daily. Exceeding moderate drinking was defined from answers about quantity of drinking as participants who drink more than 2 drinks on a typical day when they drink. Binge drinking was defined from the question about binge drinking as participants who ever had 6 or more drinks on 1 occasion. To create the AUDIT-C score (range, 0-12), we added scores of 3 questions with 5 possible answers, which were scored from 0 (less alcohol use) to 4 points (more alcohol use) (eTable 2 in Supplement 1 ). 24 Hazardous drinkers included women with AUDIT-C scores of 3 or higher and men with scores of 4 or higher. 24 , 32 , 33
We included information on age, sex, race and ethnicity, marital status, educational level, annual household income, and insurance status from the Basics survey and general health condition from the Overall Health survey. Sex was categorized based on the question “What was your biological sex assigned at birth?” as women, men, and other sex (including participants who selected “intersex,” “prefer not to answer,” “none of these,” and “skip”). Data on race and ethnicity were collected because prior research has demonstrated different drinking patterns according to racial and ethnic groups. 34 , 35 Race and ethnicity were categorized as Hispanic, non-Hispanic Black, non-Hispanic White, and other according to participant self-report. Other race included individuals reporting races other than Hispanic, non-Hispanic Black, or non-Hispanic White (Asian, Middle Eastern or North African, Native Hawaiian or Other Pacific Islander, and participants who responded that none of the provided options fully describe them) and individuals with more than 1 race and ethnicity. Smoking status was assessed in the Lifestyle survey: participants who reported not smoking at least 100 cigarettes in their entire life were categorized as never smokers, those smoking at least 100 cigarettes in their entire life but now do not smoke at all were categorized as former smokers, and those smoking at least 100 cigarettes in the entire life and now smoke every day or some days were categorized as current smokers.
After linking with the EHR, 36 we identified 10 892 cancer survivors with a first medical encounter 1 year or more before the baseline surveys and a subset of 1839 patients who underwent treatment within the past year of the baseline survey. Treatment was retrieved based on prior studies, using the Current Procedural Terminology , 4th Edition; Healthcare Common Procedure Coding System; Systematized Nomenclature of Medicine Clinical Terms; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Procedure Coding System ; and RxNorm. 37 - 39 We further classified the treatment as surgery, chemotherapy, hormone therapy, radiotherapy, and immunotherapy. We identified treatment modalities that aligned with self-reported cancer type. For surgery, we ensured to include only procedures that matched the specific cancers for which patients received a diagnosis. For instance, we did not count colectomies for any patient without a diagnosis of colorectal cancer.
Statistical analysis was performed from October 1, 2022, to January 31, 2023. We estimated the crude prevalence of current drinking among cancer survivors as well as the crude prevalence of risky drinking behaviors (including exceeding moderate drinking, binge drinking, and hazardous drinking) among current drinkers. Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% CIs of current drinking and risky drinking behaviors among current drinkers, adjusting for age at survey (<50, 50-64, or ≥65 years), sex (women, men, or other), race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, or other), marital status (never or ever), educational level (<high school, high school or General Educational Development certification, some college, or college), annual household income (<$34 999, $35 000-$74 999, $75 000-$149 999, or ≥$150 000), insurance status (yes or no), smoking status (never, former, or current), cancer type (nonalcohol-related cancers or alcohol-related cancers), age at cancer diagnosis (<18, 18-64, or ≥65 years), and medication and/or receiving treatment (yes or no).
Among the subset of cancer survivors with EHR data who underwent treatment, we estimated the crude prevalence of current drinking and risky drinking behaviors overall and according to type of cancer treatment. To compare with the general population, we conducted secondary analyses to estimate the crude prevalence of current and risky drinking behaviors among survey participants without a prior cancer diagnosis. Data were analyzed in the All of Us Research Workbench (R, version 4.0.2 [R Group For Statistical Computing]).
In the overall cohort of 15 199 cancer survivors, the mean (SD) age at baseline was 63.1 (13.0) years, 9508 survivors (62.6%) were women, and 11 633 survivors (76.5%) were non-Hispanic White ( Table 1 ). Most cancers (11 515 [75.8%]) were diagnosed when the patient was between 18 and 64 years of age. Most cancer survivors had a college degree (9291 [61.1%]) and a high annual household income, 5333 (35.1%) were former smokers, and 997 (6.6%) were current smokers. Among 1839 cancer survivors who underwent cancer treatment within the past year of the baseline survey, their characteristics were similar to those in the overall cohort (eTable 3 in Supplement 1 ).
Of 15 199 cancer survivors, 11 815 (77.7%) were current drinkers (women, 7344 of 9508 [77.2%]; men, 3971 of 5049 [78.6%]) ( Table 2 ). After multivariable adjustment, survivors who were non-Hispanic White, with alcohol-related cancers, without self-reported current medication prescription and/or treatment, and who were ever smokers were more likely to be current drinkers ( Figure 1 ; eTable 4 in Supplement 1 ). Compared with non-Hispanic White individuals, survivors who were Hispanic (OR, 0.65; 95% CI, 0.56-0.76), non-Hispanic Black (OR, 0.71; 95% CI, 0.61-0.82), and of other race and ethnicity (OR, 0.49; 95% CI 0.41-0.58) were less likely to be current drinkers. Survivors with alcohol-related cancers were 16% more likely (OR, 1.16; 95% CI, 1.06-1.27) to be current drinkers. Compared with survivors who self-reported they were not currently receiving prescription medication or treatment, those who underwent treatment were less likely to be current drinkers (OR, 0.87; 95% CI, 0.80-0.94). Former smokers (OR, 1.27; 95% CI, 1.16-1.39) and current smokers (OR, 1.44; 95% CI, 1.22-1.70) were also more likely to be current drinkers compared with never smokers.
Of 11 815 survivors who were current drinkers, 1541 (13.0%) exceeded moderate drinking (women, 777 of 7344 [10.6%]; men, 696 of 3971 [17.5%]), and 2812 (23.8%) reported binge drinking (women, 1560 of 7344 [21.2%]; men, 1119 of 3971 [28.2%]) ( Table 2 ; eFigure 2 in Supplement 1 ). After multivariable adjustment, survivors who were younger than 65 years, who were men, who were Hispanic, with cancer diagnosed before 18 years of age, or who ever smoked were more likely to exceed moderate drinking (aged <50 years: odds ratio [OR], 2.90 [95% CI, 2.41-3.48]; aged 50-64 years: OR, 1.84 [95% CI, 1.58-2.15]; men: OR, 2.38 [95% CI, 2.09-2.72]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.04-1.64]; aged <18 years at diagnosis: OR, 1.52 [95% CI, 1.04-2.24]; former smokers: OR, 2.46 [95% CI, 2.16-2.79]; current smokers: OR, 4.14 [95% CI, 3.40-5.04]) and engage in binge drinking (aged <50 years: OR, 4.46 [95% CI, 3.85-5.15]; aged 50-64 years: OR, 2.15 [95% CI, 1.90-2.43]; men: OR, 2.10 [95% CI, 1.89-2.34]; Hispanic ethnicity: OR, 1.31 [95% CI, 1.09-1.58]; aged <18 years at diagnosis: OR, 1.71 [95% CI, 1.24-2.35]; former smokers: OR, 1.69 [95% CI, 1.53-1.87]; current smokers: OR, 2.27 [95% CI, 1.91-2.71]) ( Figure 2 ; eTable 5 in Supplement 1 ). The odds of engaging in more than moderate drinking or binge drinking were similar among current drinkers who reported receiving medication and/or undergoing treatment and those who did not.
A total of 4527 current drinkers (38.3%) engaged in hazardous drinking, defined by an AUDIT-C score of 3 or higher for women and 4 or higher for men, with similar prevalences among women and men. After multivariable adjustment, survivors with cancer diagnosed before 18 years of age were more likely to be hazardous drinkers (OR, 1.52; 95% CI, 1.11-2.08) compared with those diagnosed at 65 years of age or older (eTable 5 in Supplement 1 ). Compared with never smokers, former smokers were 83% more likely (OR, 1.83; 95% CI, 1.68-1.99) to be hazardous drinkers, and current smokers had more than 2-fold the odds (OR, 2.13; 95% CI, 1.79-2.53) of engaging in hazardous drinking. For survivors with the highest risk of hazardous drinking (current smokers who received a cancer diagnosis before 18 years of age), their risky drinking behaviors were associated with more frequent, heavy drinking as well as binge drinking (eFigure 3 in Supplement 1 ). No association was observed between self-reported receipt of medication or treatment and hazardous drinking. Of 119 977 survey participants without a prior cancer diagnosis, 96 058 (80.1%) were current drinkers; among these, 19 949 (20.8%) exceeded moderate drinking, 34 135 (35.5%) reported binge drinking, and 48 090 (50.1%) engaged in hazardous drinking (eTable 6 in Supplement 1 ).
Of 1839 cancer survivors who received treatment within the past year of the baseline survey, 1405 (76.4%) self-reported as current drinkers ( Table 3 ), similar to the prevalence in the overall cohort of patients who self-reported receiving medication and/or treatment and being current drinkers (4211 of 5531 [76.1%]). This prevalence was largely similar for each cancer treatment, with the highest for patients who underwent surgery (329 of 409 [80.4%]) ( Table 3 ). Of 1405 current drinkers who received treatment within the past year of the baseline survey, 170 (12.1%) exceeded moderate drinking, 329 (23.4%) reported binge drinking, and 540 (38.4%) engaged in hazardous drinking.
Our study extends the scope of prior understanding through using a diverse US cohort to characterize risky drinking behaviors comprehensively among cancer survivors. We again highlight that alcohol consumption and risky drinking behaviors are common among cancer survivors, and we found that, among current drinkers, men, Hispanic individuals, those with cancer diagnosed before 18 years of age, and smokers are more likely to engage in risky drinking behaviors. More important, by linking with EHR data to annotate treatment information, we found that drinking and risky drinking behaviors are prevalent even among individuals concurrently receiving treatment for cancer.
Similar to a prior study using a nationally representative survey, 20 we found that most cancer survivors were current drinkers, and non-Hispanic White individuals or ever smokers were more likely to be current drinkers. In addition, we found that survivors with alcohol-related cancers or without self-reported current treatment were more likely to be current drinkers. Also in line with the previous study, 20 we found that, among current drinkers, survivors who were younger, men, Hispanic, and ever smokers were more likely to exceed moderate drinking or binge drink. Comparable with previous findings, 40 our study also suggested that Hispanic individuals are less likely to drink compared with non-Hispanic White individuals, but Hispanic individuals who choose to drink are more likely to consume higher volumes of alcohol, possibly due in part to acculturation. 41 Although adolescent or young adult cancer survivors were reported to be more likely than peers without cancer to drink alcohol, 42 our study found that survivors with cancer diagnosed before 18 years of age were more likely to engage in both heavy and binge drinking. Using validated AUDIT-C scores that incorporate frequency of drinking, quantity of drinking, and binge drinking, we reported for the first time, to our knowledge, that 38.3% of cancer survivors in this diverse US cohort engaged in hazardous drinking. This higher prevalence compared with those reported in Europe by Bosque-Prous et al 27 might be explained in part by using lower cutoff points to define hazardous drinking in our study (AUDIT-C scores of ≥3 for women and ≥4 for men) vs those used by Bosque-Prous et al 27 (AUDIT-C scores of ≥4 for women and ≥5 for men). Although more studies are warranted, the high prevalence of cancer survivors engaged in hazardous drinking highlights the need for immediate interventions to reduce alcohol intake among US cancer survivors.
Alcohol consumption and risky drinking behaviors among cancer survivors are associated with various adverse long-term outcomes, including higher risk of recurrence, 4 , 5 secondary primary tumors, 5 - 7 and increased mortality. 4 , 5 , 8 - 12 In a meta-analysis involving 209 597 cancer survivors, alcohol consumption was associated with a 17% increased risk of cancer recurrence and an 8% increased risk of overall mortality. 4 More studies are warranted to elucidate the role of each risky drinking behavior and the overall pattern in long-term outcomes. Survivors with cancer diagnosed before 18 years of age or ever smokers were more likely to be hazardous drinkers. Because of the persistent excess risks for second primary cancers throughout the life course for childhood cancer survivors 43 - 45 and the elevated risks for alcohol- and tobacco-related secondary primary cancers among drinkers who ever smoke, 6 targeted efforts for alcohol reduction are needed for these 2 groups of survivors who are more susceptible.
As highlighted in the 2018 ASCO statement, 19 in addition to long-term survivorship, accumulating data support the associations between alcohol drinking and treatment outcomes among cancer survivors. For instance, alcohol use worsens postsurgical outcomes, including increased risk of surgical complications, longer hospitalizations, more surgical procedures, prolonged recovery, higher health care costs, 46 - 48 and higher mortality. 19 , 49 Alcohol use during and after radiotherapy is associated with a higher risk of osteonecrosis of the jaw among patients with head and neck cancers. 50 - 53 In addition, alcohol is well known to have neurotoxic, cardiotoxic, and hepatotoxic effects. 54 - 56 Among patients undergoing chemotherapy, alcohol has been suggested to worsen cognition and cardiotoxicity. 57 , 58 Furthermore, alcohol use is associated with hepatic dysfunction and regulates cytochrome enzymatic activity, 54 which is important for the metabolism of chemotherapeutic agents and possibly alters their effectiveness or toxic effects. Although the association of alcohol use with immunotherapy for cancer is unclear, the treatment outcomes may be somewhat affected due to alcohol-induced immune dysfunction. 59
Our understanding of alcohol consumption patterns among cancer survivors receiving treatment has just begun to emerge. In a recent pilot study of 69 patients in Wisconsin, 30% of cancer survivors reported drinking alcohol while receiving chemotherapy, and 38% of these drinkers reported at least some complications. 60 To date, the All of Us Research Program is the only national cohort that allows us to capture alcohol consumption patterns in the context of cancer treatment. Unexpectedly, a large proportion of cancer survivors undergoing cancer treatment were current drinkers (76.4%) or were engaged in risky drinking (exceeding moderate drinking, 12.1%; binge drinking, 23.4%; hazardous drinking, 38.4%); these proportions were similar across different types of cancer treatment as well as in the overall cohort. Taken together, our findings point to the immediate and unmet need to intervene on the behalf of individuals with risky drinking behaviors in oncologic care settings. Clinicians should collect alcohol consumption information while also informing survivors of the potential harms in an effort to reduce risky alcohol use. Given that drinking is deeply ingrained in societal norms and rituals, and considering the limited awareness of how alcohol consumption is associated with cancer outcomes, it is imperative to provide support to patients who are identified as alcohol users and offer them guidance. Our findings also call for large-scale epidemiologic studies to further evaluate the association of alcohol with therapeutic efficacy and treatment outcomes among cancer survivors.
This study has some strengths, including the use of a large and diverse national cohort to comprehensively characterize risky drinking behaviors, including hazardous drinking, whereas previous studies focused on exceeding moderate drinking and binge drinking only. More important, we used the EHR linkages to retrieve information on cancer treatment.
Our study also has several limitations. First, per the Dietary Guidelines for Americans 2020-2025, exceeding moderate drinking was defined as having more than 1 drink per day for women. 61 However, the All of Us Research Program survey only allowed us to define exceeding moderate drinking among women as having more than 2 drinks. Similarly, we characterized patients who consumed 6 or more drinks on 1 occasion as binge drinkers, instead of those who consumed 4 or more drinks for women or 5 or more drinks for men per the National Institute on Alcohol Abuse and Alcoholism guideline. 62 However, with these underestimates, the prevalence of women exceeding moderate drinking was high, as was the prevalence of binge drinking among both women and men, which further highlight the pressing need for reduction of alcohol consumption. Second, because the All of Us Research Program survey asked about average alcohol consumption in the past year, we retrieved cancer treatment information during the same time in the EHR. However, the exact timing of alcohol consumption in association with cancer treatment was not clear. Additional studies are required to validate and refine our findings.
This cross-sectional study found that current and risky drinking (exceeding moderate drinking, binge drinking, and hazardous drinking) were common among US cancer survivors even during cancer treatment. Given the short- and long-term adverse treatment and oncologic outcomes associated with alcohol consumption, additional research and implementation studies are critical to address this emerging concern among cancer survivors.
Accepted for Publication: June 30, 2023.
Published: August 10, 2023. doi:10.1001/jamanetworkopen.2023.28328
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Shi M et al. JAMA Network Open .
Corresponding Author: Yin Cao, ScD, MPH, Division of Public Health Sciences, Department of Surgery, Washington University in St Louis School of Medicine, 660 S Euclid Ave, Campus Box 8100, St Louis, MO 63110 ( [email protected] ).
Author Contributions: Ms Shi and Dr Cao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Ms Shi and Dr Luo contributed equally.
Concept and design: Shi, Cao.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Shi, Oduyale, Cao.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Luo, Zong, Cao.
Obtained funding: Cao.
Administrative, technical, or material support: LoConte, Cao.
Supervision: Cao.
Conflict of Interest Disclosures: Dr LoConte reported receiving personal fees from AbbVie and PDGX; and grants from Exact Sciences outside the submitted work. Dr Cao reported receiving personal fees from Geneoscopy outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by grants P30 CA091842 and R21 AA027608 from the US National Institutes of Health (Dr Cao). Dr Oduyale was supported by the Foundation for Barnes-Jewish Hospital.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

How Binge Drinking Shifted Research On Alcohol Use Disorders
W ith the new Amy Winehouse biopic "Back to Black " in U.S. theaters as of May 17, 2024, the late singer's relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from "death by misadventure" at the age of 27. That's the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include "alcohol toxicity" following a second coroner's inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of "heavy drinking" increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists' and physicians' understanding of the relationship between women's health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
Signaling molecules in the prefrontal cortex are altered by repeated exposure to excessive alcohol consumption in animal models. jambojam/iStock via Getty Images
The Biology Of Addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn't until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse's death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to " hangxiety ," the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain's key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we've found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don't return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don't yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Work in the author's lab explores how alcohol use can alter the way neurons communicate in the prefrontal cortex brain region. Estrogen receptors are labeled in purple and receptors for somatostatin, a key regulatory hormone, in blue. Victora Nudell
Women are Forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there's one population that's been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn't until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don't know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women's health and propensity for alcohol addiction.
Middle-aged women are at the highest risk for some of the most severe health consequences of binge drinking. Peter Dazeley/The Image Bank via Getty Images
Looking Ahead
Researchers and lawmakers are recognizing the vital need for increased research on women's health. Major federal investments into women's health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
Nikki Crowley is an Assistant Professor of Biology, Biomedical Engineering and Pharmacology at Penn State. This article is republished from The Conversation under a Creative Commons license . Read the original article .


- Entertainment

This is how many of us are drinking alcohol to ‘cope’

Levels of low mental wellbeing have risen dramatically compared to those reported pre-Covid, national alcohol charity Drinkaware has claimed.
The number of people suffering has tripled from 11% pre-Covid in 2018, to 34% in 2023. More than half of adults (56%) said they drank alcohol over the past 30 days for ‘coping’ reasons.
Today's top videos
Story continues below.
Drinkaware chief Dearbhla O’Brien said: ‘While we recognise and welcome an increase in the number of people choosing not to drink, it’s clear that the impact of Covid-19 is still evident when it comes to adults and their mental health. With 56% of adults citing that they drink for coping reasons, Drinkaware wants to highlight that those prone to anxiety and low mood should be vigilant when it comes to alcohol consumption.’
She added: ‘Alcohol is an anxiolytic, which means it may help reduce feelings of anxiety or depression at the time of consumption, but once the effects have worn off, it can increase the feelings of anxiety or depression both short and long-term.’

The research found long-term trends of mental wellbeing plummeted when the pandemic hit in 2020, with more than a third (37%) of adults reporting low mental wellbeing compared with just 11% in 2018. The level then stabilised from 35% in 2021 to 30% in 2022.
However, prevalence of low mental wellbeing increased again last year to 34% of adults, the research for Mental Health Awareness Week found.
An ‘intrinsic’ link between harmful drinking and low mental wellbeing was uncovered, with 39% of adults who reported binge drinking suffering from the problem. The research also found 44% of adults who reported increased drinking in the past year had a low mental wellbeing score.
It was also revealed that almost a third (31%) cited mental health as a motivation to reduce their alcohol intake, with 41% citing small positive changes already made to their consumption habits in the past 30 days.
Ireland has the third-highest number of pubs per capita in the world
Huge cultural shift as ireland’s young are swapping alcohol for the gym, teenage girls consuming more alcohol than teenage boys, new research shows, must read irish news.

More: Trending Irish News

A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Handwashing
- Hand Hygiene as a Family Activity
- Hand Hygiene FAQs
- Handwashing Facts
- Publications, Data, & Statistics
- Health Promotion Materials
- Global Handwashing Day
- Life is Better with Clean Hands Campaign
- Clinical Safety
- Healthcare Training
- Clean Hands Count Materials
About Handwashing
- Many diseases and conditions are spread by not washing hands with soap and clean, running water.
- Handwashing with soap is one of the best ways to stay healthy.
- If soap and water are not readily available, use a hand sanitizer with at least 60% alcohol to clean your hands.

Why it's important
Washing hands can keep you healthy and prevent the spread of respiratory and diarrheal infections. Germs can spread from person to person or from surfaces to people when you:
- Touch your eyes, nose, and mouth with unwashed hands
- Prepare or eat food and drinks with unwashed hands
- Touch surfaces or objects that have germs on them
- Blow your nose, cough, or sneeze into hands and then touch other people's hands or common objects
Key times to wash hands
You can help yourself and your loved ones stay healthy by washing your hands often, especially during these key times when you are likely to get and spread germs:
- Before, during, and after preparing food
- Before and after eating food
- Before and after caring for someone at home who is sick with vomiting or diarrhea
- Before and after treating a cut or wound
- After using the toilet
- After changing diapers or cleaning up a child who has used the toilet
- After blowing your nose, coughing, or sneezing
- After touching an animal, animal feed, or animal waste
- After handling pet food or pet treats
- After touching garbage
How it works
Washing your hands is easy, and it’s one of the most effective ways to prevent the spread of germs. Follow these five steps every time.
- Wet your hands with clean, running water (warm or cold), turn off the tap, and apply soap.
- Lather your hands by rubbing them together with the soap. Lather the backs of your hands, between your fingers, and under your nails.
- Scrub your hands for at least 20 seconds . Need a timer? Hum the “Happy Birthday” song from beginning to end twice.
- Rinse your hands well under clean, running water.
- Dry your hands using a clean towel or an air dryer.
Use hand sanitizer when you can't use soap and water
Washing hands with soap and water is the best way to get rid of germs in most situations. If soap and water are not readily available, you can use an alcohol-based hand sanitizer that contains at least 60% alcohol. You can tell if the sanitizer contains at least 60% alcohol by looking at the product label.
What you can do
CDC has health promotion materials to encourage kids and adults to make handwashing part of their everyday lives.
- Share social media graphics and messages.
- Print stickers and place clings on bathroom mirrors.
- Promote handwashing on or around Global Handwashing Day , celebrated each year on October 15.
- Distribute fact sheets to share information about hand hygiene for specific audiences.
- Frequent Questions About Hand Hygiene
- Hand Hygiene in Healthcare Settings
- The Life is Better with Clean Hands Campaign
Clean Hands
Having clean hands is one of the best ways to avoid getting sick and prevent the spread of germs to others.
For Everyone
Health care providers.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Alcohol Res Health
- v.33(1-2); 2010

Alcohol’s Effects on Brain and Behavior
Over the past 40 years, rigorous examination of brain function, structure, and attending factors through multidisciplinary research has helped identify the substrates of alcohol-related damage in the brain. One main area of this research has focused on the neuropsychological sequelae of alcoholism, which has resulted in the description of a pattern of sparing and impairment that provided an essential understanding of the functional deficits as well as of spared capabilities that could be useful in recovery. These studies have elucidated the component processes of memory, problem solving, and cognitive control, as well as visuospatial, and motor processes and their interactions with cognitive control processes. Another large area of research has focused on observable brain pathology, using increasingly sophisticated imaging technologies—progressing from pneumoencephalography to computed tomography, magnetic resonance imaging (MRI), diffusion tensor imaging, and functional MRI—that have enabled ever more detailed insight into brain structure and function. These advancements also have allowed analysis of the course of brain structural changes through periods of drinking, abstinence, and relapse.
Lingering and accruing untoward consequences of alcohol use disorders (also referred to as chronic alcoholism and alcohol dependence and abuse) on cognitive and motor functions, recognized for centuries, commonly have been attributed to generalized toxic effects of alcohol on the brain. (For more information, see the sidebar “History of Neurobiological Studies in Alcohol Research.” ) This depiction has the patina of a complete understanding of alcohol-induced problems but actually has required rigorous examination of brain function, structure, and attending factors through multidisciplinary experimentation to determine the substrates of alcohol-related damage to the brain. Advancement of this knowledge has been underwritten by 40 years of intramural and extramural funding by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Achievement of a mechanistic understanding of this complex behavioral and medical condition has required numerous innovations on many levels of neuroscience investigation. These have included the development of quantitative neuroimaging approaches for safe, in vivo interrogation of brain structure, tissue quality, and neurochemistry, as well as of assessment tools for characterizing the patterns of sparing and impairment of the constellation of functions and their component processes affected by alcoholism. This brief history recounts the state of knowledge in the early days of alcoholism research and highlights progress achieved in the application and development of neuroscience methods directed toward an empirical and mechanistic understanding of the effects of the “alcohol dependence syndrome” on human brain and behavior. The focus of this review is on human studies of brain structure and function, and the imaging approaches are limited to structural and magnetic resonance (MR) 1 -based functional methods.
History of Neurobiological Studies in Alcohol Research
Looking at publications from the early 1970s, one is struck by the lack of research on alcohol’s actions on the brain. However, closer consideration shows that there also was a lack of neurobiology research in general; moreover, most of the techniques critical to modern neuroscience were not available in 1970. Behavioral genetics and electrophysiological recording from slices of brain tissue were in their infancy, and other tools (e.g., recombinant receptors, patch-clamp recording, single-channel analysis, microdialysis, gene expression measurement, and recombinant inbred mice) that commonly are used today simply did not exist. What research areas were emerging in the 1970s and how have they contributed to the success of alcohol research over the past 40 years?
The Role of Acetaldehyde
One prescient idea was that the primary breakdown product of alcohol, acetaldehyde, rather than the alcohol itself (i.e., ethanol), may have a key role in brain changes produced by chronic alcohol consumption. The observation that opiates in the poppy plant are produced in a chemical reaction called condensation from dopamine and acetaldehyde led to the hypothesis that excessive alcohol consumption might generate sufficient acetaldehyde in the brain to allow condensation with biogenic amines including dopamine, serotonin, and norepinephrine to produce psychoactive alkaloids such as salsolinol. These ideas first were developed in a series of articles from the laboratory of Virginia Davis, including articles published in Science and Nature ( Davis and Walsh 1970 ; Yamanaka et al. 1970 ). The idea that alcohol is only a “pro-drug” and that acetaldehyde is the effective agent has a boomerang quality because it is discarded every few years, only to return later. In fact, evidence continues to accumulate that alcohol consumption can result in brain acetaldehyde levels that may be pharmacologically important ( Deng and Deitrich 2008 ). However, the role of acetaldehyde as a precursor of alkaloid condensation products is less compelling. Lee and colleagues (2010) concluded that alcohol consumption does not result in production of salsolinol; however, initial studies by other researchers have provided some evidence that another alkaloid, tetrahydropapavroline, may be formed in the brain from ethanol and has important pharmacological properties—bringing the discussion full circle to Davis’ proposal of 40 years ago.
Alcohol’s Actions on Neurotransmitters
Alcohol’s actions on synaptic transmission essentially were unknown in 1970 and only have been slowly (and sometimes painfully) established during the past decades. One of the first studies showed that ethanol inhibited the release of the signaling molecule (i.e., neurotransmitter) acetylcholine from the cortex ( Phillis and Jhamandas 1970 ); these studies subsequently were extended to show ethanol-related inhibition of release of other neurotransmitters. One of the mechanisms responsible was an inhibition of voltage-dependent ion channels ( Harris and Hood 1980 ). These studies initiated exploration of ethanol’s actions on ion channels, which has become central to the neurobiology of alcohol. One prescient study by Davidoff (1973) found that ethanol enhanced neurotransmission using the neurotransmitter γ-aminobutyric acid (GABA) in the spinal cord. This was ignored until the mid-1980s (e.g., Allan and Harris 1986), but since then, GABA receptors have emerged as a major target of ethanol’s actions and continue to be an area of intense research interest ( Kumar et al. 2009 ).
Another receptor now recognized as central to alcohol’s actions is the N -methyl- d -aspartic acid (NMDA) subtype of glutamate receptors. This receptor forms a channel through the cell membrane that upon activation allows the flow of positively charged ions (e.g., Na + , K + , or Ca 2+ into and out of the cell). Remarkably, the inhibitory action of alcohol on these key receptors was not identified until 1989 ( Lovinger et al. 1989 ). Another type of channel affected by alcohol is known as calcium-activated potassium channels. These channels now are known to be very sensitive to ethanol and important for alcohol’s actions in animal models, such as the fruit fly Drosophila and round worm Caenorhabditis, as well as in the mammalian nervous system ( Treistman and Martin 2009 ). This was first noted by Yamamoto and Harris (1983) using biochemical measurements, but further progress required development of electro-physiological techniques to measure currents from these channels as well as cloning of the cDNAs encoding a family of channels known as big-conductance K + (BK) channels. Ethanol’s actions on these channels were not defined until the mid 1990s (e.g., Dopico et al. 1996 ).
The neurotransmitter dopamine now occupies a place of prominence in the neurobiology of alcoholism because acute alcohol exposure activates dopaminergic reward pathways and chronic treatment produces a hypodopaminergic state associated with dysphoria and, perhaps, relapse ( Koob and Volkow 2010 ). However, dopamine is a relative newcomer to neuropharmacology, and interest in alcohol’s actions on dopaminergic systems developed slowly. A pioneering study ( Black et al. 1980 ) noted decreased dopaminergic function during alcohol withdrawal in mice. Only much later (e.g., Samson et al. 1992 ) was alcohol self-administration linked to release of dopamine in the nucleus accumbens.
Other Research Directions
It also is informative to consider ideas that have not contributed markedly to current science. One research theme of the 1970s was ethanol interactions with membrane lipids. The rationale was that ethanol is such a small nondescript molecule that it is unlikely to have specific binding sites on proteins and is likely to nonspecifically enter the cell membranes and alter the physical properties of the lipids found in these membranes. Indeed, evidence emerged that ethanol could disorder brain membranes and that chronic alcohol treatment resulted in tolerance to this action ( Chin and Goldstein 1977 ). This was an exciting development—a neurochemical action of alcohol that resulted in tolerance! However, rather large concentrations of alcohol were required to produce small changes in membrane structure. Moreover, it was difficult (perhaps impossible) to show a link between the lipid changes and changes in the functions of one or more proteins that could account for altered neuronal excitability. These considerations lead to a paradigm shift and the search for alcohol-responsive sites on brain proteins ( Franks and Lieb 1987 ; Harris et al. 2008 ). Nevertheless, emerging evidence shows a role for lipids in the regulation of many ion channels, and there still is interest in the possibility that alcohol can alter these lipid– protein interactions and thus alter protein function ( Yuan et al. 2008 ).
Conclusions
In summary, the technology for neurobiological studies was remarkably primitive in 1970, and few laboratories were applying even these limited approaches to understanding neuronal actions of ethanol. However, several prescient ideas emerged quite early, including a role for acetaldehyde and its condensation products in alcohol’s action, as well as the identification of GABAergic synapses and ion channels as sensitive targets of alcohol in the brain.
—R. Adron Harris, Ph.D.
- Black RF, Hoffman PL, Tabakoff B. Receptor-mediated dopaminergic function after ethanol withdrawal. Alcoholism: Clinical and Experimental Research. 1980; 4 :294–297. [ PubMed ] [ Google Scholar ]
- Chin JH, Goldstein DB. Drug tolerance in biomembranes: A spin label study of the effects of ethanol. Science. 1977; 196 :684–685. [ PubMed ] [ Google Scholar ]
- Davidoff RA. Alcohol and presynaptic inhibition in an isolated spinal cord preparation. Archives of Neurology. 1973; 28 :60–63. [ PubMed ] [ Google Scholar ]
- Davis VE, Walsh MJ. Alcohol, amines, and alkaloids: A possible biochemical basis for alcohol addiction. Science. 1970; 167 :1005–1007. [ PubMed ] [ Google Scholar ]
- Deng XS, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Current Drug Abuse Review. 2008; 1 :3–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dopiko AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca(2+)-activated K+ channels in isolated neurohypophysial terminals. Molecular Pharmacology. 1996; 49 :40–48. [ PubMed ] [ Google Scholar ]
- Franks NP, Lieb WR. Are the biological effects of ethanol due to primary interactions with lipids or with proteins? Alcohol and Alcoholism. 1987; 1 (Suppl. 1):139–145. [ PubMed ] [ Google Scholar ]
- Harris RA, Allan AM. Functional coupling of gamma-aminobutyric acid receptors to chloride channels in brain membranes. Science. 1985; 228 :1108–1110. [ PubMed ] [ Google Scholar ]
- Harris RA, Hood WF. Inhibition of synaptosomal calcium uptake by ethanol. The Journal of Pharmacology and Experimental Therapeutics. 1980; 213 :562–568. [ PubMed ] [ Google Scholar ]
- Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Science Signaling. 2008; 1 :re7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010; 35 :217–238. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kumar S, Porcu P, Werner DF, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology. 2009; 205 :529–564. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee J, Ramchandani VA, Hamazaki K, et al. A critical evaluation of influence of ethanol and diet on salsolinol enantiomers in humans and rats. Alcoholism: Clinical and Experimental Research. 2010; 34 :242–250. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989; 243 :1721–1724. [ PubMed ] [ Google Scholar ]
- Phillis JW, Jhamandas K. The effects of chlorpromazine and ethanol on in vivo release of acetylcholine from the cerebral cortex. Comparative and General Pharmacology. 1971; 7 :306–310. [ PubMed ] [ Google Scholar ]
- Samson HH, Tolliver GA, Haraguchi M, Hodge CW. Alcohol self-administration: Role of mesolimbic dopamine. Annals of the New York Academy of Sciences. 1992; 654 :242–253. [ PubMed ] [ Google Scholar ]
- Treistman SN, Martin GE. BK channels: Mediators and models for alcohol tolerance. Trends in Neuroscience. 2009; 32 :629–637. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yamamoto HA, Harris RA. Calcium– dependent 86 Rb efflux and ethanol intoxication: Studies of human red blood cells and rodent brain synaptosomes. European Journal of Pharmacology. 1983; 88 (4):357–363. [ PubMed ] [ Google Scholar ]
- Yamanaka Y, Walsh MJ, Davis VE. Salsolinol, an alkaloid derivative of dopamine formed in vitro during alcohol metabolism. Nature. 1970; 227 :1143–1144. [ PubMed ] [ Google Scholar ]
- Yuan C, O’Connell RJ, Wilson A, et al. Acute alcohol tolerance is intrinsic to the BKCa protein, but is modulated by the lipid environment. The Journal of Biological Chemistry. 2008; 283 :5090–5098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Neuropsychological Sequelae of Alcoholism
Cognitive psychology in the early 1970s was ripe with newly evolving theories about the complexities of cognition, and scientists had developed paradigms useful for testing hypotheses about the new theories. These model-driven tests provided the basis for recognizing that 33 to 50 percent of people with alcohol use disorders exhibit detectable cognitive or motor impairments ( Arciniegas and Beresford 2001 ). Many of the early theorists initiated application of test paradigms in acute alcohol consumption ( Tamerin et al. 1971 ; Weingartner and Faillace 1971 ), blackouts ( Goodwin et al. 1969 ), alcoholism detoxification ( Eckardt et al. 1978 ; Parsons 1983 ; Ryan and Butters 1983 ; Ryback 1971 ; Tarter 1975 ), and alcoholism complicated by the amnesia marking Korsakoff’s syndrome (KS), a result of Wernicke’s encephalopathy (WE) ( Lishman 1990 ; Talland 1965 ; Victor et al. 1971 ). Application of information processing theories, cognitive models, and paradigms useful in testing selective components of complex functions ultimately provided adequate tools for examination and detection of the mild to severe impairments that alcoholics without KS (i.e., “uncomplicated” alcoholics) sustain. Taken together, this extensive body of literature resulted in the careful description of the pattern of sparing and impairment characteristic of the typical recovering chronic alcoholic, thus providing an essential understanding of the functional deficits suffered in the context of those spared and useful in recovery. On a basic science level, these patterns directed neuroimaging, neuropathology, cell physiology, and neurochemistry efforts in seeking neural substrates of the identified deficits. (For more information on the development of these technologies, see the sidebar “History of Neurobiological Studies in Alcohol Research.” )
Component Processes of Memory: Then and Now
Alcoholics with KS were of special value to memory theorists ( Butters and Cermak 1980 ; Oscar-Berman and Ellis 1987 ; Squire et al. 1993 ; Warrington and Weiskrantz 1970 ). Their innovative test paradigms resulted in data contributing substantially to current knowledge about component processes of memory applicable to alcoholism complicated with KS and to milder forms of memory impairment found in uncomplicated alcoholism. These theorists found that memory comprises multiple, dissociable functions supported by different brain regions and systems ( Squire and Butters 1992 ). KS amnesia is characterized by severe and relatively circumscribed deficits in remembering new information (i.e., forming new memories), regardless of type of memoranda material (e.g., words, pictures, odors, touches). The capacity for “remembering” can be tested with paradigms for explicit memory and implicit memory. Paradigms for explicit memory include approaches such as free or cued recall tests (e.g., asking people to repeat elements of a story they heard an hour ago) or recognition tests (e.g., asking people to select from a series of items the ones that were presented on a test). Implicit memory tests assess, for example, improved performance on a motor skill or ability to select a word infrequently used to complete a word stem (e.g., when asked to complete “STR _ _ _,” answer “STR AIT ” instead of the more commonly used “STR EET ”). Alcoholic KS patients show notable impairment on tests of explicit memory, especially those requiring open-ended recall without cues, but are relatively spared on verbal (i.e., word stem completion [ Verfaellie and Keane 2002 ]) and non-verbal (i.e., picture completion [ Fama et al. 2006 ]) tests of implicit memory. That cueing can enhance remembering of new explicitly learned information by KS patients suggested that retrieval processes are more affected than encoding or consolidation processes.
On a practical level, this depiction of memory abilities could mean that when provided with adequate aids, patients with KS may be able to enhance their otherwise fragile memory. Combined with evidence that alcoholic KS amnesia can range from mild to profound ( Pitel et al. 2008 ), this possibility suggested that the brain substrate for amnesia could be different from another type of amnesia resistant to memory enhancement cueing ( Milner 2005 ). Such differences support a distinction between behavioral types and neural causes of amnesia and provide further evidence for the nonunitary concept of memory. 2 Moreover, the retrieval deficit foundation of KS led memory theorists to seek nonmnemonic bases for fragile memory performance and in doing so to place KS amnesia in the context of the additional cognitive deficits characteristic of uncomplicated alcoholism.
Problem Solving and Cognitive Control Processes: Then and Now
A striking feature of alcoholics is their continued drinking despite their knowledge of the untoward physiological or psychological consequences of their behavior. This characteristic became one of the diagnostic criteria for alcohol dependence specified in the Diagnostic and Statistical Manual of Mental Disorders, 4 th Edition (DSM–IV) ( American Psychiatric Association 1994 ). It also fits the description of people with lesions of the frontal lobes, who are characterized as “impulsive, inconsiderate, uninhibited, inflexible, or ill-mannered....” ( Brewer 1974 , p. 41). As a group, alcoholics share this constellation of behaviors characteristic of frontal lobe dysfunction, which also can include impaired judgment, blunted affect, poor insight, distractibility, cognitive rigidity, and reduced motivation.
Originally described clinically, most of these behaviors now have received empirical support through creative behavioral testing and currently through functional imaging studies. A subgroup of these behaviors are considered “executive functions” ( Oscar-Berman et al. 2004 ). These include processes such as working memory (i.e., the ability to keep a number of items in a short-term memory store for evaluation or modification, such as remembering a string of numbers to perform mental arithmetic), problem solving, attentional focus (i.e., the ability to attend to one focus and exclude extraneous information from distracting from focus), and sequencing and temporal ordering (i.e., putting items into a logical order or prioritizing tasks to accomplish throughout the day) ( Salmon et al. 1986 ; Sullivan et al. 1997 ).
Vulnerability to distraction by irrelevant information ( Hada et al. 2000 ) and engagement in risky behavior ( Bjork et al. 2004 ; Fein et al. 2006 ) each may contribute to difficulty in establishing and maintaining mental set (that is, a cognitive strategy) when solving a problem ( Fabian and Parsons 1983 ; Tarter and Parsons 1971 ). Therefore, rather than being hampered by perseverative responding—that is, giving the same response that was correct for a previous question to a new question requiring a different response—alcoholics are more prone to failure in finding a theme when solving a problem ( Sullivan et al. 1993 ).
It may be of little surprise that alcoholics are particularly challenged in reordering their everyday living and work activities considering these deficits in working memory, maintenance of mental set, distractibility, and sequencing. Together, these difficulties could result in “learned helplessness” and dampened motivation to face the challenge of change. Not all alcoholics, however, exhibit impairment in all of these functions, thereby adding to the heterogeneity of the expression of the alcohol dependence syndrome. Recognition of which of these processes are spared and which are impaired in a given patient could provide an empirical basis for targeted behavioral therapy during periods of recovery.
Visuospatial Processes: Then and Now
Early neuropsychological studies of alcoholism often focused on KS and used test batteries (e.g., the Wechsler-Bellevue, Halstead-Reitan, Luria-Nebraska tests) that were quantitative and standardized but not necessarily selective to specific components of cognitive functions. Nonetheless, difficulties in performing tests of visuospatial ability were commonly identified with the Wechsler tests of intelligence ( Victor et al. 1989 ). These tests were found to be reliably sensitive to alcoholism-related dysfunction, including the block design test, in which patients are timed while copying two-dimensional designs using three-dimensional blocks, and the object assembly test, in which patients are timed while constructing a common object from puzzle pieces ( Parsons and Nixon 1993 ). Longitudinal assessment identified enduring impairments in visuospatial perception (e.g., seeing a figure embedded in a complex drawing) ( Fama et al. 2004 ) and construction (e.g., copying a complex line drawing) ( Sullivan et al. 1992 ) in both uncomplicated alcoholics ( Beatty et al. 1996 ; Brandt et al. 1983 ) and alcoholics with KS ( Victor et al. 1989 ).
Recognizing the complexity of visuospatial processing, later studies employed new paradigms to parse its components. An example demonstrating the interaction of perceiving complex visual information and the ability to focus attention without distraction comes from the global–local test. This test requires subjects to attend and respond to either a large letter or tiny letters presented in the form of the large letter. A large letter is a considered a global stimulus, which usually is processed by the right cerebral hemisphere; conversely, a tiny letter is considered a local stimulus, which usually is processed by the left cerebral hemisphere. When the large (global stimulus) and tiny (local stimulus) letters both contain target letters, responses are fast. However, when global and local information are contradictory, alcoholics find it difficult to disengage from one level of processing to the other. Moreover, the degree of difficulty in disengaging correlates with the integrity of the corpus callosum, the brain structure that connects the two cerebral hemispheres and enables transfer and integration of information (like global and local features) between the hemispheres ( Müller-Oehring et al. 2009 ). Such disruption of information sharing between the hemispheres in alcoholics was predicted by experiments predating quantitative brain-imaging methods that provided behavioral evidence for callosal dysfunction long before it was demonstrated with behavior-neuroimaging studies ( Oscar-Berman 1992 ). Similarly, another brain region that had been implicated in visuospatial processing deficits in alcoholics was the parietal lobes, assumed from studies of focal lesions; however, only recently was this association confirmed with MRI and visuospatial testing in alcoholics ( Fein et al. 2009 ).
Motor Systems, Speed of Movement, and Interaction with Cognitive Control Processes: Then and Now
Dramatic improvement occurs from acute alcohol intoxication to sobriety in eye–hand coordination, stability in gait and balance, and speeded performance. This clinically obvious improvement may have diminished the recognition of residual impairment in upper- and lower-limb motor control, which alcoholics can sustain even with prolonged sobriety. Thus, relative to cognitive studies, this area may have received short-shrift in formal testing. Nonetheless, a common theme did emerge when formal studies of motor performance were included in neuropsychological assessment—namely, that alcoholics can perform eye-hand–coordinated tasks at normal levels but do so at slower speed ( Johnson-Greene et al. 1997 ; Sullivan et al. 2002 ). This speed–accuracy trade off may underlie performance deficits noted on timed tests, whether of a cognitive or motor nature.
Caricatures depict “drunkards” as stumbling and uncoordinated, yet these motor signs are, for the most part, quelled with sobriety. More detailed quantitative assessment of gait and balance using walk-a-line testing or force platform technology, however, has revealed an enduring instability in alcoholic men and women even after prolonged abstinence. Thus, even with sobriety, recovering alcoholics are at a heightened risk of falling. Although the severity of this instability has been found to relate to the condition of the brain (especially the cerebellum, which is a brain structural substrate of gait and balance), alcoholics often are able to overcome this impairment by use of simple aids from vision, touch, and broad-based stance ( Sullivan et al. 2006 ) (see figure 1 ).

Relationship between alcoholism, balance with and without use of stabilizing aids, and the cerebellar vermis. Balance testing is conducted using a force platform, which detects sway as people attempt to stand still. Study participants try to maintain quiet balance for 30 seconds under different experimental conditions. When no stabilizing aids can be used, the sway paths are quite long, especially in alcoholics (see stabilograms on the left). With sensory (i.e., vision or light touch) or stance (feet apart) aids, the sway paths are short, even in alcoholics. In alcoholics, longer sway path length correlated with smaller volumes of the anterior vermis of the cerebellum, circled in turquoise on magnetic resonance images (correlation plot).
Brain Pathology
Postmortem studies: then and now.
Both postmortem and in vivo studies of the brains of alcoholics have contributed to understanding the permanent central nervous system damage inflicted by chronic alcoholism. Evolving methods have enabled study of brain tissue at different levels of analysis. Initial studies focusing on larger structures (i.e., gross morphology) revealed shrinkage of total brain size, with disproportionately greater volume deficits in frontal superior cortex in uncomplicated alcoholics ( Courville 1955 ; Kril et al. 1997 ). Cases with medical comorbidities common to chronic alcoholism exhibited additional focal pathology. For example, alcoholics with WE, which is caused by severe deficiency of thiamine (vitamin B1) associated with poor eating habits of some chronic alcoholics, typically showed shrinkage of the mammillary bodies, 3 thalamus, and cerebellar vermis. Cases with Marchiafava-Bignami disease showed thinning or lesions of the corpus callosum; with central pontine myelinolysis show degradation of myelin sheathing of the white matter in the central pons, and alcoholic cerebellar degeneration is marked by shrinkage of the cerebellar hemispheres and vermis ( Victor et al. 1989 ).
Because of the high prevalence of WE pathology seen in autopsy cases in Australia ( Harper et al. 1995 )—upwards of 80 percent of WE cases had been overlooked in clinical neuropathology examination and about 90 percent of them were associated with alcoholism—Harper et al. campaigned to have bread and rice products enriched with thiamine (for a personal recounting by Dr. Harper, go to http://www.rsoa.org/profileharper.htm ). Later neuropathological studies reported a significant decrease of WE lesions detectable postmortem ( Harper 2006 ). Regardless of whether the improved condition of the brains of chronic alcoholics was solely attributable to thiamine-enriched food, this public health precaution may well have saved lives and reduced the debilitating effects of WE, whether related to alcoholism or other causes of thiamine deficiency.
An outcome of this series of pathological studies was the development the New South Wales Tissue Resource Centre ( Sheedy et al. 2008 ) at the University of Sydney, Australia, funded in part by the NIAAA. More than 2,000 cases of alcoholism and other neuropsychiatric conditions and controls are being obtained prospectively, with extensive antemortem characterization. Postmortem brains undergo standardized preservation procedures, enabling studies, for example, of neurochemical and genetic markers of alcoholism, by researchers throughout the world.
In Vivo Neuroimaging Studies: Then and Now
Pneumoencephalography.
Initial in vivo studies of the brains of alcoholics were conducted using pneumoencephalography (PEG). To obtain images of the brain, the ventricular system was drained of cerebrospinal fluid (CSF), which was then replaced with air, usually resulting in severe headache. The images obtained with PEG were two dimensional only and provided tissue contrast of little use for quantification; however, they did provide initial in vivo evidence for ventricular enlargement in detoxifying alcoholics (see figure 2A ) ( Brewer and Perrett 1971 ).

Examples of different neuroimaging modalities. A) Pneumoencephalogram—the air in the ventricles shows up white. Adapted from ( Brewer 1974 ). B) Early-generation computed tomography (CT)—the cerebrospinal fluid (CSF) in the large sulci shows up black. C) Second-generation CT—bone shows up white, brain tissue is gray, CSF is black. D) T1-weighted magnetic resonance (MR)—gray matter shows up gray, white matter is white, CSF is black. E) Diffusion tensor fractional anisotropy image—white matter tracts show up white. F) Regions showing activation on functional MR imaging (fMRI) (yellow) are superimposed on a T1-weighted MRI.
Computed Tomography
With the advent of computed tomography (CT), significant progress was made in indexing the severity of brain shrinkage in terms of enlargement of the ventricles and regional cortical sulci (see figure 2B and C ). The expansion of the fluid-filled spaces of the brain was interpreted as a sign of local tissue shrinkage rather than as irreversible tissue loss (i.e., atrophy) ( Ron et al. 1982 ). The distinction between permanent and transient brain tissue damage was made in light of the landmark longitudinal imaging study of Carlen and colleagues (1978) , who reported at least partial reversal of ventricular and sulcal enlargement in alcoholics who had remained sober for about 1 month to 2 years compared with an initial CT taken a few weeks after detoxification.
Although imperfect (see Hill and Mikhael 1979 ), this seminal longitudinal study was an impetus for developing quantitative methods for deriving regional volumes of CSF in alcoholics and for employing adequate control groups to adjust volume measurements for variation attributable to sex differences, normal aging, and measurement error (e.g., resulting from differences in head placement in the scanner). Later controlled studies generated objective evidence for an age–alcoholism interaction, in which older alcoholics had more enlarged ventricles than would be expected for their age ( Jernigan et al. 1982 ; Pfefferbaum et al. 1986 , 1988 ).
A quantum leap for in vivo image resolution and differentiation of tissue type and quality came with MRI (for a review of methods and findings, see Rosenbloom and Pfefferbaum 2008 ). Some of the quantitative methods developed for CT also were applicable to MRI (see figure 2D ), but additional ones needed to be developed to differentiate gray matter from white matter ( Lim and Pfefferbaum 1989 ). Application of semiautomated segmentation methods to measure volumes of gray matter (which contains cell bodies of neurons) and white matter (which contain the fiber bundles and extension of neurons that connect brain regions) revealed profiles of regional differences between alcoholics and control subjects that were modulated by age. In particular, among adults, older, but not younger, alcoholics showed a disproportionate deficit in both gray matter and white matter cortical volume when the volumes were statistically adjusted for brain tissue declines associated with normal aging in adulthood ( Pfefferbaum et al. 1997 ). This age– alcoholism interaction also was present in other brain structures, including the corpus callosum ( Pfefferbaum et al. 1996 ), hippocampus ( Sullivan et al. 1995 ), and cerebellum ( Sullivan et al. 2000 a ). Although it is likely that older alcoholics could have consumed more alcohol in their lifetimes than younger ones, differences in amount drunk over a lifetime was not the only reason for the age–alcohol interaction.
In vivo neuroimaging using conventional MRI has provided convergent validity for the gross white matter structural abnormalities (i.e., dysmorphology) observed postmortem by showing evidence for white matter volume shrinkage with chronic heavy drinking ( Estruch et al. 1997 ; Hommer et al. 1996 , 2001 ; Pfefferbaum et al. 1992 , 1996 ; Symonds et al. 1999 ). Although postmortem studies have been essential in identifying sources of microstructural abnormalities in alcoholism, the process of preparing brain samples for analysis (i.e., fixation) and postmortem collapse of fluid-filled spaces (e.g., ventricles, sulci, and blood vessels) alter brain morphology from the living state; thus, postmortem results do not necessarily reflect all of the alcohol-related effects on the living brain ( Pfefferbaum et al. 2004 ) (see figure 3 ). Therefore, depiction of the gross anatomy of the living alcoholic brain was a critical initial step for verifying alcoholism-associated untoward effect on brain structure; however, the characterization of the microstructural integrity of the residual white matter volume in vivo required further innovations in neuroimaging.

Comparison of magnetic resonance imaging (MRI) data obtained postmortem or in vivo. Top, left: Views from the side and the top of a formalin-fixed whole-brain specimen. This brain was then set in a gel-like material (i.e., agar) for MRI studies (middle top figure). All MRIs are coronal sections showing the lateral ventricles, which are seen as bright or light gray. In the living brain, the lateral ventricles expand with age, as is evident when comparing the 25-year-old with the 60- and 61-year-old brains. With postmortem fixation, the ventricles of the brain collapse, making the fixed brain from a 61-year-old case look like the in vivo brain of a 25-year-old control subject.
SOURCE: Adapted from Pfefferbaum et al. 2004 .
MR Diffusion Tensor Imaging
The development of MR diffusion tensor imaging (DTI) provided a noninvasive approach for in vivo examination of the microstructure of brain tissue, particularly white matter (for a review of the method, see Rosenbloom and Pfefferbaum 2008 ). White matter pathology is a consistent finding in the brains of alcohol-dependent people. Postmortem study of alcoholics had identified pathology in white matter constituents and noted demyelination ( Lewohl et al. 2000 ; Tarnowska-Dziduszko et al. 1995 ), microtubule disruption ( Paula-Barbosa and Tavares 1985 ; Putzke et al. 1998 ), and axonal deletion. Other studies detected morphological distortion of cell extensions ( Harper et al. 1987 ; Pentney 1991 ) and volume reduction arising from shrinkage or deletion of cell bodies ( Alling and Bostrom 1980 ; Badsberg-Jensen and Pakkenberg 1993 ; De la Monte 1988 ; Harper and Kril 1991 , 1993 ; Lancaster 1993 ).
DTI permits assessment of water diffusion orientational coherence, measured as anisotropy, by quantifying the magnitude and orientation of water mobility on a voxel-by-voxel basis in a structure of interest. Tissue with high anisotropy is indicative of restricted diffusion that typically is found in a regularly organized region, such as a white matter fiber (see figure 2E ). Evidence for microstructural degradation of white matter integrity that evades detection with conventional structural MRI is detectable with DTI. In alcoholics, such disruption of white matter microstructure is especially prominent in frontal brain regions, such as the genu of the corpus callosum. The alcohol-related deficits in white matter anisotropy exceed those observed in normal aging ( Pfefferbaum et al. 2000 b ; Sullivan et al. 2001 ), cannot be accounted for by shrinkage in the underlying tissue mass ( Pfefferbaum and Sullivan 2003 ), and occur in both men ( Pfefferbaum et al. 2000 a ) and women ( Pfefferbaum and Sullivan 2002 ). These findings are functionally meaningful because the degree of abnormality detected in certain fiber tracts correlated with compromised performance on tests of attention and working memory ( Pfefferbaum et al. 2000 a ), cognitive flexibility ( Chanraud et al. 2009 ), and speeded performance and postural stability ( Pfefferbaum et al. 2010 ). (For more information on ways to establish an association between changes in brain structures and functional alterations, see the sidebar “Double Dissociation.” )
The Double Dissociation Model
One benefit of the development of technologies for quantitative analysis of brain structure and neuropsychological test performance was the introduction of a new way to establish associations and dissociations between brain structures and function using a modified version of the “double dissociation” model (Teuber 1955) (see figure 1 ). According to the classical double dissociation model, to be able to draw the conclusion that a certain brain structure or network is the neural source of a particular cognitive or motor function, it is essential to demonstrate first an association between the two. This can be done by demonstrating that compromised performance on a test assessing the function (e.g., on the matrix reasoning test, which assesses nonverbal intelligence) occurs with a brain lesion in the hypothesized neural source (e.g., the parietal cortex). Then, the next crucial step is to demonstrate a double dissociation using tests for two different functions (e.g., the matrix reasoning test and a test of spatial working memory) and assessing lesions in two different brain regions (e.g., the parietal cortex and the prefrontal cortex). Double dissociation exists if compromised performance on test 1 (i.e., matrix reasoning) occurs with a brain lesion in site 1 (i.e., parietal cortex) but not site 2 (i.e., prefrontal cortex), whereas compromised performance on test 2 (i.e., spatial working memory test) only occurs with a brain lesion in site 2 (i.e., prefrontal cortex). However, uncomplicated alcoholics normally do not endure discrete and complete structural brain lesions, per se. Therefore, the traditional double dissociation approach would require identification of two subject groups—one group with a brain lesion in one location and another group with a lesion in a different location—and tests of two functions, one related to the brain lesion in one subject group and the other function related to the brain lesion in the other subject group.

Overview of the double dissociation model. The “classical” approach assesses lesions in a specific brain region that are associated with circumscribed deficits. The incomplete model allows assessment of associations between dynamic lesions (e.g., lesions that may decrease and increase with sobriety and relapse) and deficits in performance that can vary in severity along a continuum.
Instead of two separate groups of alcoholics, however, Pefferbaum and colleagues (2006) studied a group of alcoholics who were heterogeneous with respect to both behavior and brain integrity. Some exhibited behavioral deficits on tests of spatial working memory and others on matrix reasoning testing (see figure 2 ). In addition, some of the alcoholics showed compromise (i.e., abnormally high diffusivity) of the genu and the splenium of the corpus callosum. Correlational analysis indicated a double dissociation: Poor working memory performance correlated with greater diffusivity in the genu but not the splenium, whereas poor matrix reasoning performance correlated with greater diffusivity in the splenium but not the genu.

Example of a demonstration of double dissociation establishing the association between lesions in different regions of the corpus callosum and specific cognitive deficits. Thus, deficits in working memory were associated with compromised structure of the frontal end (i.e., genu) of the corpus callosum, whereas deficits in matrix reasoning were associated with compromised structure of the posterior end (i.e., splenium) of the corpus callosum.
The development of quantitative measures of brain structure (e.g., regional tissue volume) joined with quantitative measures of cognitive or motor performance enabled quantification of the relationship on a continuum (see figure 1 ). Establishment of double dissociation indicates that significant variability is present in brain structural and functional measures of alcoholics and provides evidence that the cognitive and motor deficits of alcoholics are not simply the result of generalized brain insult but rather are related to compromise of specific brain systems.
— Edith V. Sullivan, Ph.D., and Adolf Pfefferbaum, M.D
One of the most appealing applications of DTI is fiber tracking and the quantification of the exquisite visual modeling of fiber systems (see figure 4 ). Quantitative fiber tracking has revealed degradation of selective fiber systems in alcoholics that are greater in anterior and superior than posterior and inferior fiber bundles ( Pfefferbaum et al. 2009 , 2010 ). Although the pattern of disruption can be different in alcoholic men and women, both sexes are affected ( Pfefferbaum et al. 2009 ).

An example of fiber tracking. Midsagittal view of a diffusion tensor image (DTI) of fractional anisotropy in gray tones, where brighter intensities in white matter reflect a more highly and linearly organized microstructure. Superimposed are three-dimensional, bilateral depictions of fiber bundles identified with fiber tracking of DTI data: mustard = superior cingulate bundle; green = inferior cingulate bundle; blue = corticospinal tracts; orange = fornix; red = pontocerebellar tracts.
Analyses of individual components of DTI metrics have provided novel in vivo information about myelin integrity (measured as radial diffusivity) and axonal integrity (measured as axial diffusivity). In general, DTI findings in alcoholism indicate a greater role for demyelination than axonal degeneration in the compromise of white matter integrity. This distinction provides convergent validity with postmortem findings, establishing DTI metrics as in vivo markers of white matter neuropathology.
Functional MRI
Whereas MRI and DTI provide visual and quantitative information about brain structure, functional MRI (fMRI) can detect changes in blood oxygenation that occur when a subject performs cognitive or motor tasks while in the scanner (see figure 2F ). In short, fMRI is a safe, noninvasive method that can detect the small but consistent changes in blood oxygenation when a specific brain region is activated (i.e., the blood oxygen level–dependent or BOLD response) ( Adalsteinsson et al. 2002 ). It has enabled detection of how alcoholics and control subjects may differ in the brain systems that are recruited to perform a task. For example, fMRI studies performed in recovering alcoholics have revealed that in test situations in which alcoholics are adequately practiced to perform cognitive tasks on which they usually show impairment, the brain systems activated during task performance differ from those activated by control subjects. A theme emerging from these studies has been that alcoholics can show performance compensation at the price of cortical processing inefficiency. For instance, when engaging spatial working memory and attention, control subjects activate the dorsal neural stream and dorsolateral prefrontal cortex. By contrast, alcoholics activate the ventral neural stream and ventrolateral prefrontal cortex ( Pfefferbaum et al. 2001 ). In a verbal working memory setting, alcoholics recruit more widely spread areas of frontal and cerebellar brain regions than do control subjects to achieve normal levels of performance ( Desmond et al. 2003 ). Finally, in a task requiring resolution of proactive interference (that is, interference resulting from previously encountered information), alcoholics activate a frontally based brain system associated with high-level executive function rather than the basal forebrain system that is activated in control subjects and which is adequate for completing this low-level function ( De Rosa et al. 2004 ).
Degradation of brain structure appears to underlie alcoholism-related alterations in the selection of cognitive strategies to execute a task, and the new neural pathways taken can be identified with fMRI. These analyses found that a change in processing strategy occurs, where alcoholics use inefficient neural systems to complete a task at hand because the preferred neural nodes or connecting fiber tracks are compromised. Such compensatory activation may be crucial for adequately completing a task but curtails available capacity to carry out multiple activities in parallel. Ultimately, structural abnormalities impose a fundamental change in the choice of cognitive operations possible for the alcoholic (see figure 5 ). In this way, alcohol-induced insult to the brain that limits higher-order cognitive capacity may sustain the propensity to engage in harmful drinking and enable the alcohol dependence syndrome. These compensatory brain mechanisms identified with fMRI are consistent with earlier theories about processing inefficiency based on cognitive testing only ( Nixon et al. 1995 ; Ryback 1971 ).

Different patterns of brain activation exist in alcoholics and control subjects. The figure is a composite of images from several functional magnetic resonance imaging (fMRI) studies. Brain regions showing greater activation in controls than alcoholics to accomplish a given task are highlighted in yellow and brain regions showing greater activation in alcoholics than in controls are shown in turquoise. On a functional level, the shift in functional anatomy (as determined by fMRI) combined with incomplete brain lesions (indicated by diffusion tensor imaging) can result in apparently normal performance, but at the price of usurping reserves that reduce processing capacity for conducting multiple tasks simultaneously or efficiently.
SOURCE: Oscar-Berman and Marinkovic 2007 .
Another theme of fMRI studies has been the identification of reward, emotional control, and oversight systems in recovering alcoholics; youth with low versus high risk for developing alcohol use disorders; or in craving paradigms. In discerning emotional information suggested by pictures focusing on facial features, high-risk youth displayed less brain activation compared with low-risk youth, suggesting a predisposition for attenuated ability to interpret facial emotion ( Hill et al. 2007 ). Craving paradigms use alcohol beverage stimuli (e.g., a chilled glass of foaming beer) to examine differences between alcoholics and control subjects in brain activation in response to alcohol-relevant stimuli ( Myrick et al. 2004 ; Tapert et al. 2003 ). These studies have resulted in the identification of alcohol reward brain systems ( Makris et al. 2008 ) (see figure 6 ). Brain regions commonly invoked in rewarding conditions are the nucleus accumbens and ventral tegmental area. As a point of translation, these brain regions identified in humans also are implicated in animal models of alcohol dependence and craving ( Koob 2009 ).

The reward model developed by Oscar-Berman. Using evidence from structural and functional magnetic resonance imaging (MRI), Oscar-Berman and colleagues proposed this model of brain regions involved in what they termed is the extended reward and oversight system. The arrows indicate known directional connections between brain structures of the extended reward and oversight system. SLEA, sublenticular extended amygdala.
SOURCE: Adapted from Makris et al. 2008 .
Course of Brain Structural Changes in Alcoholism
Alcoholism follows a dynamic course, with alternating periods of excessive drinking and sobriety. Concomitant with this course, measurable decline and improvement occurs in selective functions of cognitive and motor abilities ( Brandt et al. 1983 ; Parsons 1983 ). But only with the advent of in vivo longitudinal neuroimaging have researchers been able to document changes in brain structure in parallel with drinking behavior and functional changes (e.g., Rosenbloom et al. 2007 ; Sullivan et al. 2000 b ). These studies began with the landmark study of Carlen and colleagues (1978) , who used CT to show recovery of brain tissue with sobriety.
Longitudinal MRI studies of alcoholics have found that following about 1 month of abstinence from alcohol, cortical gray matter ( Pfefferbaum et al. 1995 ), overall brain tissue ( Gazdzinski et al. 2005 ), and hippocampal tissue ( Gazdzinski et al. 2008 ) increase in volume. With longer-term follow-up, alcoholics who maintain sobriety may show shrinkage of the third ventricular volume ( Pfefferbaum et al. 1995 ) or a general increase in brain volume ( Gazdzinski et al. 2005 ) notable in frontal and temporal regions ( Cardenas et al. 2007 ). Alcoholics who relapse into drinking, in contrast, show expansion of the third ventricle and shrinkage of white matter ( Pfefferbaum et al. 1995 ) or loss of overall brain tissue relative to that seen at study entry ( Cardenas et al. 2007 ; Gazdzinski et al. 2005 ). Cortical white matter volume may be particularly amenable to recovery with prolonged sobriety ( Agartz et al. 2003 ; Meyerhoff 2005 ; O’Neill et al. 2001 ; Shear et al. 1994 ) or vulnerable to further decline with continued drinking ( Pfefferbaum et al. 1995 ). Over a 5-year longitudinal study, prolonged sobriety was associated with improvement or stabilization of measures of brain tissue volume. By contrast, a return to drinking was associated with ventricular enlargement and cortical gray matter loss, especially in the frontal lobes, and the extent of cortical volume shrinkage correlated with the amount drunk over the 5 years ( Pfefferbaum et al. 1998 ).
Several factors can diminish the likelihood of recovery of brain structure with sobriety, including older age, heavier alcohol consumption, concurrent hepatic disease, history of withdrawal seizures, malnutrition, and concurrent smoking ( Yeh et al. 2007 ). Inability to ethically enforce control over drinking and other factors in human alcoholism limits these studies to naturalistic designs. By contrast, animal studies afford control over factors contributing to change for the better or the worse with continued or discontinued alcohol exposure. Animal models of alcoholism may also advance our understanding of the brain volume changes documented in the course of human alcoholism (see figures 7 and and8 8 ).

Ventricular size in alcoholic and nonalcoholic humans and in alcohol-exposed and nonexposed rats. A) A 61-year-old control man. B) A 51-year-old alcoholic man. Note the markedly enlarged lateral ventricles and temporal horns in the alcoholic man. C) Wistar rat before alcohol exposure. D) Wistar rat after 16 weeks of chronic exposure to alcohol vapor. Note the markedly enlarged lateral ventricles, similar to those seen in the alcoholic man.
SOURCE: A and B were adapted from Rosenbloom and Pefferbaum 2008 ; C and D were adapted from Pfefferbaum et al. 2008 .

Changes in ventricular size in humans and rats after resumption of drinking or continued sobriety. A) A 41-year-old alcoholic woman when sober (left) and 1 year later after resuming drinking (right). Note the ventricular expansion (red circle). B) A 48-year-old woman before (left) and after (right) 1 year’s continued sobriety. Note the ventricular contraction (red circle). C) Wistar rat before (left) and after (right) acute binge alcohol gavage for 4 days. Note the ventricular and pericollicular expansion of cerebrospinal fluid (CSF) (red arrows). D) The same animal after 1 week recovery (right), showing return to pre-exposure CSF-filled spaces.
Advances in Neuroscience
The advances made over these first 40 years have enriched understanding of alcoholism from a neuroscience perspective and have expanded concepts of neuroplasticity in the human brain. The innovations enabling discoveries also have generalized to other areas of neuroscience, exemplified by our understanding of neural degradation with chronic alcoholism and repair with sobriety. Original concepts of brain structure modification were unidirectional—that is, degradation occurred with age or disease without the chance of neuronal regeneration. Now, evidence supports the possibility of neurogenesis as part of a repair process ( Nixon and Crews 2004 ) or at least for creating a milieu for repair of cell bodies and their processes. Repair of white matter constituents, including myelin, also can transpire. A greater understanding of this process is emerging following the identification, for example, of altered myelin repair gene expression in the frontal cortex of alcoholics ( Liu et al. 2006 ). The fate of cortical volume in chronic alcoholism also may be related to genetic regulation that selectively affects gray but not white matter ( Srivastava et al. 2010 ).
Although the neuropsychological impairments attendant to alcoholism have existed through the centuries, understanding of their neural mechanisms has required identification of selective functional components and brain integrity affected and not affected, together with the knowledge of the course, extent, and loci of disruption and repair. What researchers found 40 years ago is a likely reflection of the disorder seen today, but a mechanistic understanding of the full constellation of effects and the scope and limit of improvement with sobriety has evolved from being considered widespread and nonspecific to being specific in terms of brain circuitry and systems. Environmental, genetic, metabolic, and behavioral factors that influence restitution of neurofunction have yet to be identified but are amenable to study with neuroimaging. With systematic longitudinal study and rigorous characterization of people with alcohol use disorders, neuroimaging in conjunction with neuropsychology can enable in vivo detection and tracking of brain systems affected by alcoholism, the functional relevance of identified neuropathology, the scope and limit of the brain’s plasticity at different ages of alcohol exposure and withdrawal, and insight into neural mechanisms of insult and recovery. Still on the neuroscience research horizon are acknowledgment of the heterogeneity of expression of alcoholism’s untoward effects, delineation of substrates of neural change with addiction and further change with alternating periods of drinking and sobriety, and viable approaches for curtailing drinking in alcohol abusers.
Acknowledgments
This work was support by NIAAA grants AA010723, AA017168, and AA017923 to E.V.S.; AA06399 and AA012404 to R.A.H.; and AA005965, AA012388, AA013521-INIA, and AA017347 to A.P.
1 For a definition of this and other technical terms, see the Glossary, pp. 161–164..
2 The nonunitary concept of memory posits that different types of memory exist (e.g., short term versus long term; episodic versus implicit) that represent either different mnemonic systems or different component processes of a system. Each system and component requires different brain regions for processing, and disruption of local brain regions or systems are the foundation of different types of memory impairment or amnesia.
3 Shrinkage of the mammillary bodies is observed only after chronic alcohol consumption, whereas swelling can be observed with acute consumption ( Sheedy et al. 1999 ).
F inancial D isclosure
The authors declare that they have no competing financial interests.
- Adalsteinsson E, Sullivan EV, Pfefferbaum A. Biochemical, functional and microstructural magnetic resonance imaging (MRI) In: Liu Y, Lovinger DM, editors. Methods in Alcohol-Related Neuroscience Research. Boca Raton, FL: CRC Press; 2002. pp. 345–372. [ Google Scholar ]
- Agartz I, Brag S, Franck J, et al. MR volumetry during acute alcohol withdrawal and abstinence: A descriptive study. Alcohol and Alcoholism. 2003; 38 :71–78. [ PubMed ] [ Google Scholar ]
- Alling C, Bostrom K. Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathologica (Berl) 1980; 50 :77–80. [ PubMed ] [ Google Scholar ]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorder. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [ Google Scholar ]
- Arciniegas DB, Beresford TP. Neuropsychiatry: An Introductory Approach. Cambridge: Cambridge University Press; 2001. [ Google Scholar ]
- Badsberg-Jensen G, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993; 342 :1201–1204. [ PubMed ] [ Google Scholar ]
- Beatty WW, Hames KA, Blanco CR, et al. Visuospatial perception, construction and memory in alcoholism. Journal of Studies on Alcohol. 1996; 57 :136–143. [ PubMed ] [ Google Scholar ]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: Relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004; 34 :133–150. [ PubMed ] [ Google Scholar ]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Archives of General Psychiatry. 1983; 40 :435–442. [ PubMed ] [ Google Scholar ]
- Brewer C. Alcoholic brain damage: Implications for sentencing policy (with a note on the air-encephalogram) Medicine, Science, and the Law. 1974; 14 :40–43. [ PubMed ] [ Google Scholar ]
- Brewer C, Perrett L. Brain damage due to alcohol consumption: An air-encephalographic, psychometric and electroencephalographic study. British Journal of Addiction to Alcohol and Other Drugs. 1971; 66 :170–182. [ PubMed ] [ Google Scholar ]
- Butters N, Cermak LS. Alcoholic Korsakoff’s Syndrome: An Information Processing Approach to Amnesia. New York: Academic Press, Inc.; 1980. [ Google Scholar ]
- Cardenas VA, Studholme C, Gazdzinski S, et al. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007; 34 :879–887. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carlen PL, Wortzman G, Holgate RC, et al. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978; 200 :1076–1078. [ PubMed ] [ Google Scholar ]
- Chanraud S, Reynaud M, Wessa M, et al. Diffusion tensor tractography in mesencephalic bundles: Relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009; 34 :1223–1232. [ PubMed ] [ Google Scholar ]
- Courville CB. Effects of Alcohol on the Nervous System of Man. Los Angeles: San Lucas Press; 1955. [ Google Scholar ]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Archives of Neurology. 1988; 45 :990–992. [ PubMed ] [ Google Scholar ]
- De Rosa E, Desmond JE, Anderson AK, et al. The human basal forebrain integrates old and the new. Neuron. 2004; 41 :825–837. [ PubMed ] [ Google Scholar ]
- Desmond JE, Chen SH, De Rosa E, et al. Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. NeuroImage. 2003; 19 :1510–1520. [ PubMed ] [ Google Scholar ]
- Eckardt MJ, Parker ES, Noble EP, et al. Relationship between neuropsychological performance and alcohol consumption in alcoholics. Biological Psychiatry. 1978; 13 :551–565. [ PubMed ] [ Google Scholar ]
- Estruch R, Nicolas JM, Salamero M, et al. Atrophy of the corpus callosum in chronic alcoholism. Journal of the Neurological Sciences. 1997; 146 :145–151. [ PubMed ] [ Google Scholar ]
- Fabian MS, Parsons OA. Differential improvement of functions in recovering alcoholic women. Journal of Abnormal Psychology. 1983; 92 :87–95. [ PubMed ] [ Google Scholar ]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcoholism: Clinical and Experimental Research. 2004; 28 :1657–1665. [ PubMed ] [ Google Scholar ]
- Fama R, Pfefferbaum A, Sullivan EV. Visuoperceptual learning in alcoholic Korsakoff Syndrome. Alcoholism: Clinical and Experimental Research. 2006; 30 :680–687. [ PubMed ] [ Google Scholar ]
- Fein G, McGillivray S, Finn P. Normal performance on a simulated gambling task in treatment-naive alcohol-dependent individuals. Alcoholism: Clinical and Experimental Research. 2006; 30 :959–966. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcoholism Clinical and Experimental Research. 2009; 33 :1806–1814. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug and Alcohol Dependence. 2005; 78 :263–273. [ PubMed ] [ Google Scholar ]
- Gazdzinski S, Durazzo TC, Yeh PH, et al. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Research. 2008; 162 :133–145. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goodwin DW, Crane JB, Guze SB. Phenomenological aspects of the alcoholic “blackout.” British Journal of Psychiatry. 1969; 115 :1033–1038. [ PubMed ] [ Google Scholar ]
- Hada M, Porjesz B, Begleiter H, Polic J. Auditory P3a assessment of male alcoholics. Biological Psychiatry. 2000; 48 :276–286. [ PubMed ] [ Google Scholar ]
- Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! European Journal of Neurology. 2006; 13 :1078–1082. [ PubMed ] [ Google Scholar ]
- Harper C, Fornes P, Duyckaerts C, et al. An international perspective on the prevalence of the Wernicke- Korsakoff syndrome. Metabolic Brain Disease. 1995; 10 :17–24. [ PubMed ] [ Google Scholar ]
- Harper C, Kril J. If you drink your brain will shrink: Neuropathological considerations. Alcohol and Alcoholism. 1991;(Supplement 1):375–380. [ PubMed ] [ Google Scholar ]
- Harper CG, Kril JJ. Neuropathological changes in alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol Induced Brain Damage: NIAAA Research Monograph No. 22. Rockville, MD: National Institute of Health; 1993. pp. 39–69. [ Google Scholar ]
- Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? British Medical Journal. 1987; 294 :534–536. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hill SY, Kostelnik B, Holmes B, et al. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism: Clinical and Experimental Research. 2007; 31 :2028–2035. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hill SY, Mikhael M. Computed tomography scans of alcoholics: Cerebral atrophy? Science. 1979; 204 :1237–1238. [ PubMed ] [ Google Scholar ]
- Hommer D, Momenan R, Rawlings R, et al. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996; 53 :359–363. [ PubMed ] [ Google Scholar ]
- Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001; 158 :198–204. [ PubMed ] [ Google Scholar ]
- Jernigan TL, Zatz LM, Ahumada AJ, et al. CT measures of cerebrospinal fluid volume in alcoholics and normal volunteers. Psychiatry Research. 1982; 7 :9–17. [ PubMed ] [ Google Scholar ]
- Johnson-Greene D, Adams KM, Gilman S, et al. Impaired upper limb coordination in alcoholic cerebellar degeneration. Archives of Neurology. 1997; 54 :436–439. [ PubMed ] [ Google Scholar ]
- Koob GF. Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry. 2009; 42 (Suppl 1):S32–S41. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997; 79 :983–998. [ PubMed ] [ Google Scholar ]
- Lancaster FE. Ethanol and white matter damage in the brain. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage: NIAAA Research Monograph No. 22. Rockville, MD: National Institute of Health; 1993. pp. 387–399. [ Google Scholar ]
- Lewohl JM, Wang L, Miles MF, et al. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcoholism: Clinical and Experimental Research. 2000; 24 :1873–1882. [ PubMed ] [ Google Scholar ]
- Lim KO, Pfefferbaum A. Segmentation of MR brain images into cerebrospinal fluid spaces, white and gray matter. Journal of Computer Assisted Tomography. 1989; 13 :588–593. [ PubMed ] [ Google Scholar ]
- Lishman WA. Alcohol and the brain. British Journal of Psychiatry. 1990; 156 :635–644. [ PubMed ] [ Google Scholar ]
- Liu J, Lewohl JM, Harris RA, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006; 31 :1574–1582. [ PubMed ] [ Google Scholar ]
- Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008; 64 :192–202. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meyerhoff DJ. Brain spectroscopic imaging, morphometry, and cognition in social drinkers and recovering alcoholics. Alcoholism: Clinical and Experimental Research. 2005; 29 :153–154. PMID: [ Google Scholar ]
- Milner B. The medial temporal-lobe amnesic syndrome. Psychiatric Clinics of North America. 2005; 28 :599–611. [ PubMed ] [ Google Scholar ]
- Müller-Öehring EM, Schulte T, Fama R, et al. Global-local interference is related to callosal compromise in alcoholism: A behavior-DTI association study. Alcoholism: Clinical and Experimental Research. 2009; 33 :477–489. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004; 29 :393–402. [ PubMed ] [ Google Scholar ]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004; 24 :9714–9722. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nixon SJ, Tivis R, Parsons OA. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcoholism: Clinical and Experimental Research. 1995; 19 :577–581. [ PubMed ] [ Google Scholar ]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: Quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001; 25 :1673–1682. [ PubMed ] [ Google Scholar ]
- Oscar-Berman M. Alcoholism and asymmetries of brain function. Alcohol Health and Research World. 1992; 16 :273–279. [ Google Scholar ]
- Oscar-Berman M, Ellis RJ. Cognitive deficits related to memory impairments in alcoholism. Recent Developments in Alcoholism. 1987; 5 :59–80. [ PubMed ] [ Google Scholar ]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004; 28 :667–675. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Oscar-Berman M, Marinkovic K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007; 17 :239–257. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parsons OA. Cognitive dysfunction and recovery in alcoholics. Substance and Alcohol Actions/Misuse. 1983; 4 :175–190. [ PubMed ] [ Google Scholar ]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurologic Clinics. 1993; 11 :205–218. [ PubMed ] [ Google Scholar ]
- Paula-Barbosa MM, Tavares MA. Long term alcohol consumption induces microtubular changes in the adult rat cerebellar cortex. Brain Research. 1985; 339 :195–199. [ PubMed ] [ Google Scholar ]
- Pentney RJ. Remodeling of neuronal dendritic networks with aging and alcohol. Alcohol and Alcoholism. 1991;(Supplement 1):393–397. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Desmond JE, Galloway C, et al. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. NeuroImage. 2001; 14 :7–20. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996; 20 :752–757. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992; 16 :1078–1089. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Rosenbloom MJ, Crusan K, Jernigan TL. Brain CT changes in alcoholics: Effects of age and alcohol consumption. Alcoholism: Clinical and Experimental Research. 1988; 12 :81–87. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Rosenbloom MJ, Fama R, et al. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: Selective relations to dissociable functions. Alcoholism Clinical and Experimental Research. 2010. In press. [ PMC free article ] [ PubMed ]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological Psychiatry. 2009; 65 :680–690. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002; 15 :708–718. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003; 49 :953–961. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Adalsteinsson E, et al. Postmortem MR imaging of formalin-fixed human brain. NeuroImage. 2004; 21 :1585–1595. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Hedehus M, et al. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 24 :1214–1221. 2000a. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Hedehus M, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echoplanar diffusion tensor imaging. Magnetic Resonance in Medicine. 44 :259–268. 2000b. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997; 21 :521–529. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995; 19 :1177–1191. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998; 55 :905–912. [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Zahr NM, Mayer D, et al. Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcoholism: Clinical and Experimental Research. 2008; 32 :1459–1467. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pfefferbaum A, Zatz LM, Jernigan TL. Computer-interactive method for quantifying cerebrospinal fluid and tissue in brain CT scans: Effects of aging. Journal of Computer Assisted Tomography. 1986; 10 :571–578. [ PubMed ] [ Google Scholar ]
- Pitel AL, Beaunieux H, Witkowski T, et al. Episodic and working memory deficits in alcoholic Korsakoff patients: The continuum theory revisited. Alcoholism: Clinical and Experimental Research. 2008; 32 :1229–1241. [ PubMed ] [ Google Scholar ]
- Putzke J, De Beun R, Schreiber R, et al. Long-term alcohol self-administration and alcohol withdrawal differentially modulate microtubule-associated protein 2 (MAP2) gene expression in the rat brain. Brain Research Molecular Brain Research. 1998; 62 :196–205. [ PubMed ] [ Google Scholar ]
- Ron MA, Acker RW, Shaw GK, Lishman WA. Computerized tomography of the brain in chronic alcoholism: A survey and follow-up study. Brain. 1982; 105 :497–514. [ PubMed ] [ Google Scholar ]
- Rosenbloom MJ, Pfefferbaum A. Magnetic resonance imaging of the living brain: Evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Research and Health. 2008; 31 :362–376. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenbloom MJ, Rohlfing T, O’Reilley A, et al. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with change in brain structure. Psychiatry Research. 2007; 155 :91–102. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ryan C, Butters N. Cognitive deficits in alcoholics. In: Kissin B, Begleiter H, editors. The Pathogenesis of Alcoholism. New York: Plenum Publishing Corporation; 1983. pp. 485–538. [ Google Scholar ]
- Ryback RS. The continuum and specificity of the effects of alcohol on memory: A review. Quarterly Journal of Studies on Alcohol. 1971; 32 :995–1016. [ PubMed ] [ Google Scholar ]
- Salmon DP, Butters N, Schuckit M. Memory for temporal order and frequency of occurrence in detoxified alcoholics. Alcohol. 1986; 3 :323–329. [ PubMed ] [ Google Scholar ]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1994; 18 :172–176. [ PubMed ] [ Google Scholar ]
- Sheedy D, Garrick T, Dedova I, et al. An Australian Brain Bank: A critical investment with a high return! Cell Tissue Bank. 2008; 9 :205–216. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sheedy D, Lara A, Garrick T, Harper C. Size of mamillary bodies in health and disease: Useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcoholism: Clinical and Experimental Research. 1999; 23 :1624–1628. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Squire L, Butters N, editors. Neuropsychology of Memory. 2nd Edition. New York: Guilford Press; 1992. [ Google Scholar ]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993; 44 :453–495. [ PubMed ] [ Google Scholar ]
- Srivastava V, Buzas B, Momenan R, et al. Association of SOD2, a mitochondrial antioxidant enzyme, with gray matter volume shrinkage in alcoholics. Neuropsychopharmacology. 2010; 35 :1120–1128. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sullivan EV, Adalsteinsson E, Hedehus M, et al. Equivalent disruption of regional white matter microstructure in aging healthy men and women. Neuroreport. 2001; 12 :99–104. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Deshmukh A, Desmond JE, et al. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 14 :341–352. 2000a. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcoholism: Clinical and Experimental Research. 2002; 26 :705–713. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Marsh L, Mathalon DH, et al. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995; 19 :110–122. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Mathalon DH, Ha CN, et al. The contribution of constructional accuracy and organizational strategy to nonverbal recall in schizophrenia and chronic alcoholism. Biological Psychiatry. 1992; 32 :312–333. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Mathalon DH, Zipursky RB, et al. Factors of the Wisconsin Card Sorting Test as measures of frontal lobe function in schizophrenia and in chronic alcoholism. Psychiatry Research. 1993; 46 :175–199. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch, and stance on cerebellar vermian-related sway and tremor: A quantitative physiological and MRI study. Cerebral Cortex. 2006; 16 :1077–1086. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 14 :178–188. 2000b. [ PubMed ] [ Google Scholar ]
- Sullivan EV, Shear PK, Zipursky RB, et al. Patterns of content, contextual, and working memory impairments in schizophrenia and nonamnesic alcoholism. Neuropsychology. 1997; 11 :195–206. [ PubMed ] [ Google Scholar ]
- Symonds LL, Archibald SL, Grant I, et al. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? Journal of Neuroimaging. 1999; 9 :201–209. [ PubMed ] [ Google Scholar ]
- Talland GA. Deranged Memory: A Psychonomic Study of the Amnesic Syndrome. New York: Academic Press; 1965. [ Google Scholar ]
- Tamerin JS, Weiner S, Poppen R, et al. Alcohol and memory: Amnesia and short-term memory function during experimentally induced intoxication. American Journal of Psychiatry. 1971; 127 :1659–1664. [ PubMed ] [ Google Scholar ]
- Tapert SF, Cheung EH, Brown GG, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003; 60 :727–735. [ PubMed ] [ Google Scholar ]
- Tarnowska-Dzidusko E, Bertrand E, Szpak G. Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathologica. 1995; 33 :25–29. [ PubMed ] [ Google Scholar ]
- Tarter RE. Psychological deficit in chronic alcoholics: A review. International Journal of the Addictions. 1975; 10 :327–368. [ Google Scholar ]
- Tarter RE, Parsons OA. Conceptural shifting in chronic alcoholics. Journal of Abnormal Psychology. 1971; 77 :71–75. [ PubMed ] [ Google Scholar ]
- Verfaellie M, Keane MM. Scope and limits of implicit memory in amnesia. In: De Gelder B, De Haan E, Heywood C, editors. Out of Mind: Varieties of Unconscious Processes. New York: Oxford Univeristy Press; 2002. pp. 151–162. [ Google Scholar ]
- Victor RM, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome. Philadelphia: F.A. Davis Co; 1971. [ PubMed ] [ Google Scholar ]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd Edition. Philadelphia: F.A. Davis Co; 1989. [ Google Scholar ]
- Warrington EK, Weiskrantz L. The amnesic syndrome: Consolidation or retrieval? Nature. 1970; 228 :628–630. [ PubMed ] [ Google Scholar ]
- Weingartner H, Faillace LA. Alcohol state-dependent learning in man. Journal of Nervous and Mental Disorders. 1971; 153 :395–406. [ PubMed ] [ Google Scholar ]
- Yeh PH, Gazdzinski S, Durazzo TC, et al. Hierarchical linear modeling (HLM) of longitudinal brain structural and cognitive changes in alcohol-dependent individuals during sobriety. Drug and Alcohol Dependence. 2007; 91 :195–204. [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
Review. Impact of alcohol on the central nervous system (CNS) Alcohol exerts various effects on our CNS in various ways, the common ones being depression of the CNS, destruction of the brain cells, contraction of the tissues of the brain, suppression of the excitatory nerve pathway activity, neuronal injury, etc [].Alcohol's impact on the functioning of the brain ranges from mild and ...
Alcohol and Alcoholism welcomes submissions, publishing papers on the biomedical, psychological, and sociological aspects of alcoholism and alcohol research. To gain more information please see the Instructions to Authors page. Recommend to your library.
Alcohol consumption, particularly heavier drinking, is an important risk factor for many health problems and, thus, is a major contributor to the global burden of disease. In fact, alcohol is a necessary underlying cause for more than 30 conditions and a contributing factor to many more. ... Alcohol Research & Health. 2007; 30 (1):38-47. [PMC ...
The growing scale of alcoholism, as well as its multidimensional consequences, justify the need for an in--depth analysis of this phenomenon. Harmful alcohol use and dependence is a risk factor ...
About the Journal. Alcohol, Clinical and Experimental Research provides direct access to the most significant and current research findings on the nature and management of alcoholism and alcohol-related disorders. Increase your chance of being published through our unaccepted manuscript Refer & Transfer program.
Only a small percent of individuals with alcohol use disorder contribute to the greatest societal and economic costs ().For example, in the 2015 National Survey on Drug Use and Health survey (total n = 43,561), a household survey conducted across the United States, 11.8% met criteria for an alcohol use disorder (n = 5124) ().Of these 5124 individuals, 67.4% (n = 3455) met criteria for a mild ...
Alcohol Research: Current Reviews (ARCR) ARCR, a peer-reviewed scientific journal published by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, marks its 50th anniversary in 2024. Explore our "News & Notes" webpage for more on this historic accomplishment.
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain ...
By use of methodological enhancements of previous iterations,1 the systematic analysis from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2016 for 195 countries and territories, 1990-2016,2 is the most comprehensive estimate of the global burden of alcohol use to date. The GBD 2016 Alcohol Collaborators clearly demonstrate the substantial, and larger than previously ...
Alcohol consumption is a major risk factor for poor physical and mental health, accounting for about 3 million deaths and over 130 million disability-adjusted life years worldwide in 2016 (ref. 1
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [].The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4-2.0) of total DALYs globally among females ...
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies - of which one was a ...
Alcohol Research Resource (R24 and R28) Awards. Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner. Current and potential alcohol research investigators and trainees are encouraged to subscribe to our ...
The authors are entirely responsible for the research and results reported in this paper, and their position or opinions do not necessarily represent those of the University of Miami, the National Institute on Alcohol Abuse and Alcoholism, or the National Institute on Drug Abuse. ... Alcohol Research and Health. 2003; 27 (2):181-185. [PMC ...
INTRODUCTION. Alcoholism research and treatment underwent significant changes in the 20th century. Within the last 100 years, a disease concept was formed, which is now widely accepted, the psychosocial and neurobiological consequences of alcoholism have been characterized and treatment programmes have been established and continuously refined.
Alcohol as a mediator. Inspired by the Actor Network Theory (ANT), we draw attention to how nonhuman objects - in this case alcohol - act on users, engage in practices, and operate in networks (assemblages) (Latour, Citation 2005, p. 68).The actor-network refers to the relations between human and non-human actors (Latour, Citation 1994), and in the context of this study, the relations ...
Alcohol and drug use can lead to poor decision making, like breaking the law, sexual abuse, getting in fights, etc. Of the respondents, 92.4% were white and the average age was 22.3 years. This study found that a little more than 68% reported using alcohol and/or drugs during the past year.
Aim: While there is considerable research on the efficacy of interventions designed to reduce alcohol consumption and related harms among college students, there is limited research on students' own perspectives on such interventions. This qualitative study aimed to address this gap by examining college students' perspectives in the context of an alcohol prevention programme for college ...
Abstract. From the perspective of the developmental psychopathologist, the understanding of alcoholism (or alcohol abuse and dependence, if one uses the most recent diagnostic parlance of the DSM ...
Abstract. Alcohol consumption is known to be an addiction that provides negative outcomes mainly on health, excessive drinking of alcohol brings adverse effects on human health, also on activities ...
When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
High amounts of alcohol use are causal risk factors in the development of disease in the heart, liver, pancreas, and brain (including the brains of children in utero). In fact, 1 in 8 deaths in ...
Research on the treatment of alcoholism has gained significant ground over the past 40 years. Studies such as the National Institute on Alcohol Abuse and Alcoholism's Project MATCH, which examined the prospect of tailoring treatments for particular people to better suit their needs, and Project COMBINE, which examined in-depth, cognitive-behavioral therapy and medical management, helped ...
In a recent pilot study of 69 patients in Wisconsin, 30% of cancer survivors reported drinking alcohol while receiving chemotherapy, and 38% of these drinkers reported at least some complications. 60 To date, the All of Us Research Program is the only national cohort that allows us to capture alcohol consumption patterns in the context of ...
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode. This is well over the amount of alcohol thought to produce legal ...
The research reviewed here represents a wide spectrum of approaches to understanding the risks and benefits of alcohol consumption. These research findings can help shape the efforts of communities to reduce the negative consequences of alcohol consumption, assist health practitioners in advising consumers, and help individuals make informed ...
20/05/2024. Levels of low mental wellbeing have risen dramatically compared to those reported pre-Covid, national alcohol charity Drinkaware has claimed. The number of people suffering has tripled from 11% pre-Covid in 2018, to 34% in 2023. More than half of adults (56%) said they drank alcohol over the past 30 days for 'coping' reasons.
Washing your hands is easy, and it's one of the most effective ways to prevent the spread of germs. Follow these five steps every time. Wet your hands with clean, running water (warm or cold), turn off the tap, and apply soap. Lather your hands by rubbing them together with the soap. Lather the backs of your hands, between your fingers, and ...
History of Neurobiological Studies in Alcohol Research. Looking at publications from the early 1970s, one is struck by the lack of research on alcohol's actions on the brain. However, closer consideration shows that there also was a lack of neurobiology research in general; moreover, most of the techniques critical to modern neuroscience were ...