Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Mr. Bob Carlson is a 59 year old male who came to Ventura County Medical Center (VCMC) with nausea, upper back pain he rated 7/10, and diaphoretic. His vital signs were BP 156/92, HR 90, RR 22 SpO2 90%, and temperature 99.5. Physical examination revealed clear lung sounds, mild tachypnea, S1 S2 present, and several ulcerations to the right foot. Ordes were given to obtain a 12-lead ECG and labs (CBC, CMP, Coagulations, Cardiac Enzymes, and Lipid Profile). In addition orders were given to start Mr. Carlson on 2L oxygen via nasal cannula and obtain venous access. A 20 gauge IV was started in his left AC.
Mr. Carlson’s medical history revealed that he is a type II diabetic, has hypertension, hyperlipidemia, and smokes 1/2 pack of cigarettes a day for the past 40 years. His diabetes is poorly managed and Mr.Carlson had a left below the knee amputation 2 years ago due to diabetic ulcers that were gangrenous. In addition, Mr. Carlson has a history of IV drug use but now receives a daily dose (90 mg) of Methadone at a local clinic. He is divorced, no children and is currently living with his 85 year old mother.
Mr. Carlson’s ECG results showed ST-segment elevation in leads II, III, and aVf and in V4, V5 and V6 with ST-segment depression V1, V2, and V3. The provider identified this to be an MI occurring in the inferior portion of the heart, likely affecting his right coronary artery (RCA). Lab results confirmed a ST-segment elevation MI (Troponin-I 12.9, CK 520, and CKMB 25.2). A code STEMI was called and Mr. Carlson was immediately prepared for a Percutaneous coronary intervention (PCI). While waiting for transfer to the Catheterization Lab at Community Memorial Hospital (CMH) Mr. Carlson was given 325 mg of Aspirin, 2 mg Morphine, and was started on a 5000 unit bolus of Heparin. Nitroglycerin was not given due to the profound hypotension associated with nitroglycerin and patients experiencing an inferior myocardial infarction.
Mr. Carlson was transferred to the CMH catheterization lab. His vitals were stable and he was able to give informed consent. The cardiac angiography showed a 95% occlusion to the RCA. A stent was placed, the patient tolerated the procedure well. The patient’s right femoral artery was closed successfully with manual pressure.
Mr. Carlson returned to the cardiac care unit where upon assessment his groin was found to be soft and without hepatoma and with minimal drainage from incision site. His peripheral pulses were present, and distal to the incision his skin was warm with capillary refill less than 2 seconds. Mr. Carlson was transferred back to VCMC the following day were he recovered without further incident.
Before discharge Mr. Carlson’s was educated on his new prescriptions and was educated on the importance of taking his daily aspirin. He met with the diabetes educator, dietician, and social worker before discharge. Mr. Carlson was informed of smoking cessation programs in the area but declined to enroll. Case Management found placement in a skilled nursing facility for 20 days, the amount of days 100% covered by Medi-Cal, where he could start a cardiac rehabilitation program. A home health organization was organized to help provide care for Mr. Carlson when he returned to his home.
- What medications do you anticipate Mr. Carlson being prescribed upon discharge? Aspirin, ACE-I or ARB, beta blocker, and a statin
- What nursing interventions are critical prior to the patient being taken to the cath lab? -Assess the client’s and family’s knowledge and understanding of the procedure. – Provide routine preoperative care as ordered. Signed consent is required and maintain patient NPO. -Assess for hypersensitivity to iodine, radiologic contrast media, or seafood. An iodine-based radiologic contrast dye is typically used for angiogram. Iodine or seafood allergy increases the risk for anaphylaxis and requires an alternative dye or special precautions. -Record baseline assessment data, including vital signs, height, and weight. Mark the locations of peripheral pulses; document their equality and amplitude.
- Which risk factors may have contributed to Mr. Carlson’s myocardial infarction? Hyperlipidemia, uncontrolled diabetes, smoking, inactivity, drug use, and diet.
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.

Share This Book
Myocardial Infarction (MI) Case Study (45 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Definition of Myocardial Infarction (MI)
Myocardial infarction, commonly known as a heart attack, is a critical medical event that occurs when the blood supply to the heart muscle is severely reduced or completely blocked. It is a leading cause of death worldwide and a significant public health concern.
Introduction to Myocardial Infarction (MI)
This nursing case study aims to provide a comprehensive understanding of myocardial infarction by delving into its various aspects, including its pathophysiology, risk factors, clinical presentation, diagnostic methods, and management strategies. Through the exploration of a fictional patient’s journey, we will shed light on the intricate nature of this life-threatening condition and highlight the importance of early recognition and intervention.
Background and Significance of Myocardial Infarction
Myocardial infarction is a sudden and often catastrophic event that can have profound consequences on an individual’s health and well-being. Understanding its underlying mechanisms and risk factors is essential for healthcare professionals, as timely intervention can be life-saving. This case study not only serves as a learning tool but also emphasizes the critical role of medical practitioners in identifying and managing myocardial infarctions promptly.
Pathophysiology of Myocardial Infarction
A crucial aspect of comprehending myocardial infarction is exploring its pathophysiology. We will delve into the intricate details of how atherosclerosis, the buildup of plaque in coronary arteries, leads to the formation of blood clots and the subsequent interruption of blood flow to the heart muscle. This disruption in blood supply triggers a cascade of events, ultimately resulting in the death of cardiac cells.
Risk Factors of Myocardial Infarction
Understanding the risk factors associated with myocardial infarction is vital for prevention and early detection. This case study will examine both modifiable and non-modifiable risk factors, including age, gender, family history, smoking, high blood pressure, diabetes, and high cholesterol levels. Recognizing these risk factors is instrumental in developing effective strategies for prevention and risk reduction.
Clinical Presentation Myocardial Infarction
Recognizing the signs and symptoms of myocardial infarction is crucial for timely intervention. We will present a fictional patient’s experience, illustrating the typical clinical presentation, which often includes chest pain or discomfort, shortness of breath, nausea, lightheadedness, and diaphoresis. Through this patient’s journey, we will highlight the importance of accurate symptom assessment and prompt medical attention.
Diagnostic Methods for Myocardial Infarction
Modern medicine offers various diagnostic tools to confirm a myocardial infarction swiftly and accurately. This case study will explore these diagnostic methods, such as electrocardiography (ECG), cardiac biomarkers, and imaging techniques like coronary angiography. By understanding these diagnostic modalities, healthcare professionals can make informed decisions and initiate appropriate treatments promptly.
Management Strategies for Myocardial Infarction
The management of myocardial infarction involves a multidisciplinary approach, including medication, revascularization procedures, and lifestyle modifications. We will discuss the fictional patient’s treatment plan, emphasizing the importance of reestablishing blood flow to the affected heart muscle and preventing further complications.
Nursing Case Study for Myocardial Infarction (MI)
Having established a foundational understanding of myocardial infarction, we will now delve deeper into Mr. Salazar’s case, tracing his journey through diagnosis, treatment, and recovery. This in-depth examination will shed light on the real-world application of the principles discussed in the introduction, providing valuable insights into the clinical management of myocardial infarction and its impact on patient outcomes.
Mr. Salazar, a 57-year-old male, arrives at the Emergency Department (ED) with complaints of chest pain that began approximately one hour after dinner while he was working. He characterizes the discomfort as an intense “crushing pressure” located centrally in his chest, extending down his left arm and towards his back. He rates the pain’s severity as 4/10. Upon examination, Mr. Salazar exhibits diaphoresis and pallor, accompanied by shortness of breath (SOB).
What further nursing assessments need to be performed for Mr. Salazar?
- Heart Rate (HR): The number of heartbeats per minute.
- Blood Pressure (BP): The force of blood against the walls of the arteries, typically measured as systolic (during heartbeats) and diastolic (between heartbeats) pressure.
- Respiratory Rate (RR): The number of breaths a patient takes per minute.
- Body Temperature (Temp): The measurement of a patient’s internal body heat.
- Oxygen Saturation (SpO2): The percentage of oxygen in the blood.
- S1: The first heart sound, often described as “lub,” is caused by the closure of the mitral and tricuspid valves.
- S2: The second heart sound, known as “dub,” results from the closure of the aortic and pulmonic valves.
- These sounds provide important diagnostic information about the condition of the heart.
- Clear: Normal, healthy lung sounds with no added sounds.
- Crackles (Rales): Discontinuous, often high-pitched sounds are heard with conditions like pneumonia or heart failure.
- Wheezes: Whistling, musical sounds often associated with conditions like asthma or chronic obstructive pulmonary disease (COPD).
- Pulses refer to the rhythmic expansion and contraction of arteries with each heartbeat. Common pulse points for assessment include the radial artery (wrist), carotid artery (neck), and femoral artery (groin). Evaluating pulses helps assess the strength, regularity, and rate of blood flow.
- Edema is the abnormal accumulation of fluid in body tissues, leading to swelling. It can occur in various body parts and may indicate underlying conditions such as heart failure, kidney disease, or localized injury. Edema assessment involves evaluating the degree of swelling and its location.
- Skin condition (temperature, color, etc.)
What interventions do you anticipate being ordered by the provider?
- Oxygen therapy involves administering oxygen to a patient to increase the level of oxygen in their blood. It is used to treat conditions such as respiratory distress, and hypoxia (low oxygen levels), and to support patients with breathing difficulties.
- Nitroglycerin is a medication used to treat angina (chest pain) and to relieve symptoms of heart-related conditions. It works by relaxing and widening blood vessels, which improves blood flow to the heart, reducing chest pain.
- Aspirin is a common over-the-counter medication and antiplatelet drug. In the context of myocardial infarction (heart attack), it is often administered to reduce blood clot formation, potentially preventing further blockage in coronary arteries.
- A 12-lead EKG is a diagnostic test that records the electrical activity of the heart from 12 different angles. It provides information about the heart’s rhythm, rate, and any abnormalities, helping diagnose conditions like arrhythmias, heart attacks, and ischemia.
- Cardiac enzymes are proteins released into the bloodstream when heart muscle cells are damaged or die, typically during a heart attack. Measuring these enzymes, such as troponin and creatine kinase-MB (CK-MB), helps confirm a heart attack diagnosis and assess its severity.
- A chest X-ray is a diagnostic imaging procedure that creates images of the chest and its internal structures, including the heart and lungs. It is used to identify issues like lung infections, heart enlargement, fluid accumulation, or fractures in the chest area.
- Possibly an Echocardiogram
Upon conducting a comprehensive assessment, it was observed that the patient exhibited no signs of jugular vein distention (JVD) or edema. Auscultation revealed normal heart sounds with both S1 and S2 present, while the lungs remained clear, albeit with scattered wheezes. The patient’s vital signs were recorded as follows:
- BP 140/90 mmHg SpO 2 90% on Room Air
- HR 92 bpm and regular Ht 173 cm
- RR 32 bpm Wt 104 kg
- Temp 36.9°C
The 12-lead EKG repor t indicated the presence of “Normal sinus rhythm (NSR) with frequent premature ventricular contractions (PVCs) and three- to four-beat runs of ventricular tachycardia (VT).” Additionally, there was ST-segment elevation in leads I, aVL, and V2 through V6 (3-4mm), accompanied by ST-segment depression in leads III and aVF.
Cardiac enzyme levels were collected but were awaiting results at the time of assessment. A chest x-ray was also ordered to provide further diagnostic insights.
In response to the patient’s condition, the healthcare provider prescribed the following interventions:
- Aspirin: 324 mg administered orally once.
- Nitroglycerin: 0.4 mg administered sublingually (SL), with the option of repeating the dose every five minutes for a maximum of three doses.
- Morphine: 4 mg to be administered intravenously (IVP) as needed for unrelieved chest pain.
- Oxygen: To maintain oxygen saturation (SpO2) levels above 92%.
These interventions were implemented to address the patient’s myocardial infarction (heart attack) and alleviate associated symptoms, with a focus on relieving chest pain, improving oxygenation, and closely monitoring vital signs pending further diagnostic results.
What intervention should you, as the nurse, perform right away? Why?
- Apply oxygen – this can be done quickly and easily and can help to prevent further complications from low oxygenation.
- Oxygen helps to improve oxygenation as well as to decrease myocardial oxygen demands.
- Often it takes a few minutes or more for medications to be available from the pharmacy, so it makes sense to take care of this intervention first.
- ABC’s – breathing/O 2 .
What medication should be the first one administered to this patient? Why? How often?
- Nitroglycerin 0.4mg SL – it is a vasodilator and works on the coronary arteries. The goal is to increase blood flow to the myocardium. If this is effective, the patient merely has angina. However, if it is not effective, the patient may have a myocardial infarction.
- Aspirin should also be given, but it is to decrease platelet aggregation and reduce mortality. While it can somewhat help prevent the worsening of the blockage, it does little for the current pain experienced by the patient.
- Morphine should only be given if the nitroglycerin and aspirin do not relieve the patient’s chest pain.
What is the significance of the ST-segment changes on Mr. Salazar's 12-lead EKG?
- ST-segment changes on a 12-lead EKG indicate ischemia (lack of oxygen/blood flow) or infarction (death of the muscle tissue) of the myocardium (heart muscle).
- This indicates an emergent situation. The patient’s coronary arteries are blocked and need to be reopened by pharmacological (thrombolytic) or surgical (PCI) intervention.
- Time is tissue – the longer the coronary arteries stay blocked, the more of the patient’s myocardium that will die. Dead heart tissue doesn’t beat.
Mr. Salazar’s chest pain was unrelieved after three (3) doses of sublingual nitroglycerin (NTG). Morphine 5 mg intravenous push (IVP) was administered, as well as 324 mg chewable baby aspirin. His pain was still unrelieved at this point
Mr. Salazar’s cardiac enzyme results were as follows:
Troponin I 3.5 ng/mL
Based on the results of Mr. Salazar's labs and his response to medications, what is the next intervention you anticipate? Why?
- Mr. Salazar needs intervention. He will either receive thrombolytics or a heart catheterization (PCI).
- Based on the EKG changes, elevated Troponin level, and the fact that his symptoms are not subsiding, it’s possible the patient has a significant blockage in one or more of his coronary arteries.
- It seems as though it may be an Anterior-Lateral MI because ST elevation is occurring in I, aVL, and V 2 -V 6 .
Mr. Salazar was taken immediately to the cath lab for a Percutaneous Coronary Intervention (PCI). The cardiologist found a 90% blockage in his left anterior descending (LAD) artery. A stent was inserted to keep the vessel open.
What is the purpose of Percutaneous Coronary Intervention (PCI), also known as a heart catheterization?
- A PCI serves to open up any coronary arteries that are blocked. First, they use contrast dye to determine where the blockage is, then they use a special balloon catheter to open the blocked vessels.
- If that doesn’t work, they will place a cardiac stent in the vessel to keep it open.[ /faq]
[faq lesson="true" blooms="Application" question="What is the expected outcome of a PCI? What do you expect to see in your patient after they receive a heart catheterization?"]
- Blood flow will be restored to the myocardium with minimal residual damage.
- The patient should have baseline vital signs, relief of chest pain, normal oxygenation status, and absence of heart failure symptoms (above baseline).
- The patient should be able to ambulate without significant chest pain or SOB.
- The patient should be free from bleeding or hematoma at the site of catheterization (often femoral, but can also be radial or (rarely) carotid.
Mr. Salazar tolerated the PCI well and was admitted to the cardiac telemetry unit for observation overnight. Four (4) hours after the procedure, Mr. Salazar reports no chest pain. His vital signs are now as follows:
- BP 128/82 mmHg SpO 2 96% on 2L NC
- HR 76 bpm and regular RR 18 bpm
- Temp 37.1°C
Mr. Salazar will be discharged home 24 hours after his arrival to the ED and will follow up with his cardiologist next week.
What patient education topics would need to be covered with Mr. Salazar?
- He should be taught any dietary and lifestyle changes that should be made.
- Diet – low sodium, low cholesterol, avoid sugar/soda, avoid fried/processed foods.
- Exercise – 30-45 minutes of moderate activity 5-7 days a week, u nless instructed otherwise by a cardiologist. This will be determined by the patient’s activity tolerance – how much can they do and still be able to breathe and be pain-free?
- Stop smoking and avoid caffeine and alcohol.
- Medication Instructions
- Nitroglycerin – take one SL tab at the onset of chest pain. If the pain does not subside after 5 minutes, call 911 and take a second dose. You can take a 3rd dose 5 minutes after the second if the pain does not subside. Do NOT take if you have taken Viagra in the last 24 hours.
- Aspirin – take 81 mg of baby aspirin daily
- Anticoagulant – the patient may be prescribed an anticoagulant if they had a stent placed. They should be taught about bleeding risks.
- When to call the provider – CP unrelieved by nitroglycerin after 5 minutes. Syncope. Evidence of bleeding in stool or urine (if on anticoagulant). Palpitations, shortness of breath, or difficulty tolerating activities of daily living.
Linchpins for Myocardial Infarction Nursing Case Study
In summary, Mr. Salazar’s case highlights the urgency of recognizing and responding to myocardial infarction promptly. The application of vital signs, EKG, cardiac enzymes, and medications like aspirin, nitroglycerin, and morphine played a pivotal role in his care. Diagnostic tools like echocardiography and chest X-rays contributed to a comprehensive evaluation.
Nurses must remain vigilant and compassionate in such emergencies. This case study emphasizes the importance of adhering to best practices in the assessment, diagnosis, and management of myocardial infarction, with the ultimate goal of achieving favorable patient outcomes.
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
- Open access
- Published: 25 March 2024
Fibrinogen to HDL-Cholesterol ratio as a predictor of mortality risk in patients with acute myocardial infarction
- Congzhuo Jia 1 , 2 na1 ,
- Wanying Wu 1 , 2 na1 ,
- Huan Lu 3 , 6 ,
- Jin Liu 1 , 2 ,
- Shiqun Chen 1 , 5 ,
- Guoxiao Liang 4 ,
- Yang Zhou 1 , 2 ,
- Sijia Yu 1 , 2 , 3 ,
- Linfang Qiao 1 , 2 , 3 ,
- Jinming Chen 1 , 2 , 3 ,
- Ning Tan 1 , 2 ,
- Yong Liu 1 , 2 &
- Jiyan Chen 1 , 2
Lipids in Health and Disease volume 23 , Article number: 86 ( 2024 ) Cite this article
153 Accesses
Metrics details
Acute myocardial infarction (AMI) is characterized by inflammation, oxidative stress, and atherosclerosis, contributing to increased mortality risk. High-density lipoprotein (HDL) takes a crucial part in mitigating atherosclerosis and inflammation through its diverse functionalities. Conversely, fibrinogen is implicated in the development of atherosclerotic plaques. However, the mortality risk predictive capacity of fibrinogen to HDL-cholesterol ratio (FHR) in AMI patients remains unexplored. This research aimed to evaluate the effectiveness of FHR for mortality risk prediction in relation to AMI.
A retrospective study involving 13,221 AMI patients from the Cardiorenal ImprovemeNt II cohort (NCT05050877) was conducted. Baseline FHR levels were used to categorize patients into quartiles. The assessment of survival disparities among various groups was conducted by employing Kaplan‒Meier diagram. Cox regression was performed for investigating the correlation between FHR and adverse clinical outcomes, while the Fine-Gray model was applied to evaluate the subdistribution hazard ratios for cardiovascular death.
Over a median follow-up of 4.66 years, 2309 patients experienced all-cause death, with 1007 deaths attributed to cardiovascular disease (CVD). The hazard ratio (HR) and its 95% confidence interval (CI) for cardiac and all-cause death among individuals in the top quartile of FHR were 2.70 (1.99–3.65) and 1.48 (1.26–1.75), respectively, in comparison to ones in the first quartile, after covariate adjustment. Restricted cubic spline analysis revealed that FHR was linearly correlated with all-cause mortality, irrespective of whether models were adjusted or unadjusted (all P for nonlinearity > 0.05).
AMI patients with increased baseline FHR values had higher all-cause and cardiovascular mortality, regardless of established CVD risk factors. FHR holds promise as a valuable tool for evaluating mortality risk in AMI patients.
Trial registration
The Cardiorenal ImprovemeNt II registry NCT05050877.
Introduction
Acute myocardial infarction (AMI), a significant thrombotic complication of atherosclerosis, remains a pivotal risk factor for morbidity and mortality. In-depth research underscores the involvement of lipoproteins, blood lipids, endothelial injury, and inflammation in the onset and advancement of atherosclerosis [ 1 , 2 ]. Given the high prevalence of AMI, there is an imperative need for a better understanding of its causative factors to develop effective prognosis and treatment strategies [ 3 ].
Fibrinogen (FIB) has emerged as a key participant in atherosclerotic plaque development, influencing the function of endothelial cells [ 4 , 5 ]. Besides, it is of critical significance in the coagulation cascade, profoundly influencing blood viscosity and platelet aggregation [ 6 ]. As an acute-phase protein, FIB exhibits enhanced biosynthesis, reaching plasma concentrations of several folds during inflammation [ 7 ]. Prior studies have proposed FIB as a prospective indicator for predicting the risk of cardiovascular outcomes [ 8 ]. Recent evidence has also indicated that FIB independently influences the progression of cardiovascular disease (CVD) and serves as a biomarker for inflammation and coagulation [ 9 ].
Conversely, high-density lipoprotein (HDL) cholesterol has been acknowledged as “good cholesterol” from early observational studies demonstrating its inverse relationship with the risk of cardiovascular disease [ 10 , 11 ]. Recent advances have highlighted atheroprotective roles of HDL by reducing cell proliferation and inflammatory signaling pathways [ 12 , 13 ]. HDL provides defense against endothelial injury and suppresses the expression of adhesion molecules during atherosclerosis development [ 14 ]. Furthermore, HDL plays a multifaceted role in regulating the coagulation cascade, positively correlating with anticoagulant responses and neutralizing the procoagulant effects of anionic phospholipids [ 15 ]. Although HDL has been linked with potential atheroprotective properties, efforts on raising HDL cholesterol levels through pharmacological interventions have failed to translate into reduced cardiovascular disease risk [ 16 ]. Emerging findings from genetic studies have further revealed that mutations leading to lower plasma HDL cholesterol levels are not correlated with higher risk of ischemic heart disease [ 17 ]. Additionally, a recent mendelian randomization study indicated genetic mechanisms that elevating plasma HDL cholesterol levels do not appear to decrease the risk of MI [ 18 ].
Thus, despite the contrasting significance of FIB and HDL in relation to coagulation and inflammatory alterations, their combined role in mortality prediction among patients with AMI remains largely unexplored. To date, limited studies have explored the coexistence of elevated FIB values and decreased HDL that were associated with recurrent cerebral venous thrombosis (CVT) among those previously diagnosed with CVT [ 19 ]. Additionally, this combination was closely correlated with the onset of CVD among diabetes patients [ 20 ].
Therefore, the research sought to explore the feasibility of utilizing the fibrinogen to HDL-cholesterol ratio (FHR) as an indicative marker for predicting adverse clinical outcomes in AMI population. The primary goal of this analysis was to evaluate potential biomarkers for better risk stratification and patient management in patients with high-risk AMI.
Study design and participants
Data for this retrospective study were sourced from the multicenter Cardiorenal ImprovemeNt II (CIN-II, NCT05050877) cohort registry, covering the period from January 2007 to December 2020. The research was carried out across five central tertiary teaching hospitals located in different regions of southern China. The study included patients who met the following requirements for enrollment: 1) meeting the diagnostic criteria of AMI; 2) aged ≥ 18 years; 3) not presenting with any concomitant malignancy, pregnancy, autoimmune disease, liver disease or infectious disease; 4) including only the initial admission for patients admitted twice or more; 5) possessing complete baseline, discharge status, and follow-up data; and 6) not demonstrating an outlier baseline FHR (Fig. 1 ). The present study enrolled 13,221 patients for the final analysis. This study adhered to the Declaration of Helsinki and was endorsed by the Ethics Committee of Guangdong Provincial People’s Hospital.
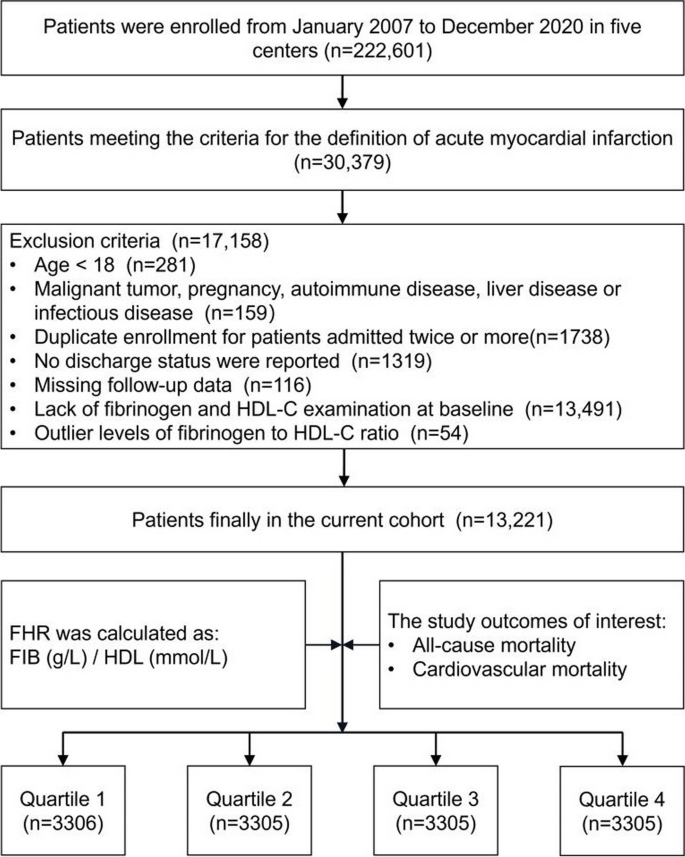
Study flowchart. FHR fibrinogen to HDL-cholesterol ratio
Data collection
This study collected data from the electronic clinical management system, which includes baseline information such as demographic features, coexisting conditions, laboratory tests, treatments during hospitalization, and discharge medications. Prior to blood sample extraction, participants were required to undergo a fasting period (> 8 h). Routine blood tests, fibrinogen, high-sensitivity troponin T (hs-TnT), total cholesterol (TC), triglyceride HDL and low-density lipoprotein (LDL) were tested by standard laboratory methods. Fibrinogen levels were assessed using the STA-R Evolution R System (Beijing Stago Diagnosis Trading Co., Ltd., Beijing, China) along with supplied reagents of the instrument (Diagnostica Stago, Taverny, France). HDL, TC, triglyceride and LDL levels were measured using an automatic biochemistry analyzer (Hitachi 7600, Tokyo, Japan) and assayed by an enzymatic method according to the manufacturer’s instructions. Plasma hs-TnT levels were quantified utilizing an electrochemiluminescence immunoassay (IT3000, Roche, Switzerland). Given the heightened sensitivity, this assay is reported with units of picograms/milliliter (pg/mL). The determination of comorbidities relied on preadmission diagnoses or diagnoses established during hospitalization. To gather follow-up information, survival data from the Centers for Disease Control and Prevention were cross-referenced. Senior cardiologists provided oversight for quality control and conducted periodic data verification procedures.
Outcome and definition
The study endpoints were cardiovascular and all-cause mortality. FHR was calculated as the plasma FIB concentration (g/L) divided by the plasma HDL-cholesterol level (mmol/L). Diagnoses of AMI, diabetes mellitus (DM), atrial fibrillation (AF) and hypertension were ascertained in accordance with the International Classification of Diseases, the tenth revision (ICD-10). Cardiovascular mortality was primarily identified by ICD-10 codes: I00–I99, Q20–Q28.
Statistical analysis
The study stratified participants into four groups based on quartiles of baseline FHR values. Continuous variates were summarized as means (SD) or medians (IQR), while categorical variates were presented as counts and percentages. The variances among groups were evaluated utilizing one-way ANOVA, the Kruskal‒Wallis test and the Pearson chi-square test for continuous variates with normal or nonnormal distributions and categorical variates, respectively. The hazards of endpoints across different subsets were presented by Kaplan‒Meier methods. The correlation between baseline FHR levels and outcomes was illustrated by hazard ratio (HR, 95% CI) employed by Cox proportional hazard model. The established risk factors known to influence outcomes were selected as potential confounding covariates. Subsequently, multivariate stepwise Cox regression models were utilized to calculate the influencing variables of FHR (α in = 0.05, α out = 0.10). Three models were established sequentially: 1) without adjustment; 2) with adjustment for age and sex; 3) with further adjustment for covariates in Model 2, including smoking, monocyte count, TC, serum creatinine, LDL, triglycerides, use of antiplatelets, chronic kidney disease, congestive heart failure, stroke, hypertension, and DM. Restricted cubic spline (RCS) analyses were conducted to assess the potential nonlinear association between FHR and both cardiac and all-cause death, adjusting for the same covariates as mentioned above. Additionally, subgroup analyses were carried out, stratified by various demographic characteristics, comorbidities as well as laboratory examinations including age, sex, smoking status, DM, hypertension, stroke, LDL, TC and triglycerides. The study employed Youden’s index (sensitivity + specificity-1) and conducted an analysis of the area under the curve (AUC) for identifying the optimal cut-off value of mortality prediction. To evaluate whether incorporating the combination of FIB and HDL improved mortality prediction, the integrated discrimination improvement (IDI) and net reclassification improvement (NRI) were assessed. R software (version 4.2.1) was used for all analyses. In this analysis, if a two-tailed P value was below 0.05, statistical significance was present.
Baseline characteristics
Figure 1 displays the study flowchart illustrating the process of selecting patients. In total, 13,221 patients were enrolled, and their baseline characteristics were comprehensively summarized in Table 1 . Among the AMI population involved in the current study, the average age was 61.7 ± 12.0 years, with 81.9% of them being male. To ensure sufficient variability across the subgroups, the participants were divided into four subgroups, stratified by the quartiles of baseline FHR values which ranged from 0.002 to 0.998 and were distributed as follows in each subgroup: Quartile 1 ( N = 3306), Quartile 2 ( N = 3305), Quartile 3 ( N = 3305), and Quartile 4 ( N = 3305). The defined cutoff values for FHR were Q1 (< 3.11), Q2 (3.11–4.50), Q3 (4.50–6.35), and Q4 (> 6.35). Male and smoking participants were more prevalent in higher FHR quartiles compared with control group. Additionally, they exhibited a higher prevalence of coexisting conditions such as high blood pressure, congestive heart failure, DM, chronic kidney dysfunction, hyperlipidemia, and stroke. Conversely, the frequency of AF and anemia tended to be lower in the higher FHR quartiles. Among the laboratory parameters, individuals belonging to the upper quartiles of FHR exhibited higher levels of serum creatinine, monocytes and platelets. In contrast, patients in the higher levels of FHR in this study exhibited significantly lower TC, LDL, creatinine kinase MB, hemoglobin and albumin levels.
Predictive value of FHR on all-cause mortality
A total of 2,309 (17.5%) patients encountered mortality throughout the 10-year follow-up. The occurrence of all-cause mortality across FHR quartiles was as follows: Q1-11.6% (384/3306), Q2-16.5% (544/3305), Q3-18.8% (620/3305), and Q4-23.0% (761/3305). Kaplan‒Meier analysis curves demonstrated progressively adverse outcomes with elevated FHR levels ( P < 0.001, Fig. 2 A). Furthermore, data for the enrolled patients were subjected to Cox regression analysis to assess the prognostic relevance of various FHR values (Table 2 ). The unadjusted model showed that individuals in higher quartiles exhibited a greater likelihood of mortality due to all causes than individuals in the first quartile (the reference group) (HR 1.18, 95% CI 1.04–1.35, P = 0.013; HR 1.28, 95% CI 1.23–1.45, P < 0.001; HR 1.55, 95% CI 1.37–1.76, P < 0.001; respectively). These findings remained statistically significant even after comprehensive adjustment for covariates in the fully adjusted analysis, including age, sex, smoking, LDL, triglyceride, TC, serum creatinine, monocyte count, use of antiplatelets, hypertension, chronic kidney disease, congestive heart failure, DM and stroke (Table 2 ; HR 1.16, 95% CI 0.98–1.37, P = 0.09; HR 1.25, 95% CI 1.06–1.47, P = 0.008; HR 1.48, 95% CI 1.26–1.75, P < 0.001; respectively). Moreover, RCS models revealed that FHR was linearly correlated with all-cause mortality, as evidenced by both the unadjusted and adjusted models (Fig. 3 A & Figure S 1 : P value for nonlinearity > 0.05).
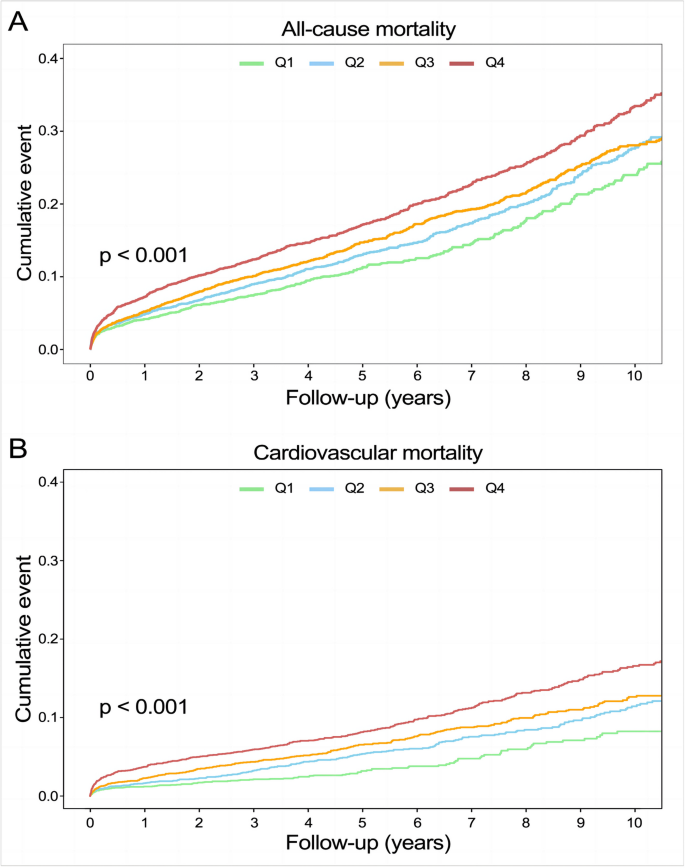
Kaplan–Meier analysis for all-cause ( A ) and cardiovascular mortality ( B ) according to different FHR levels
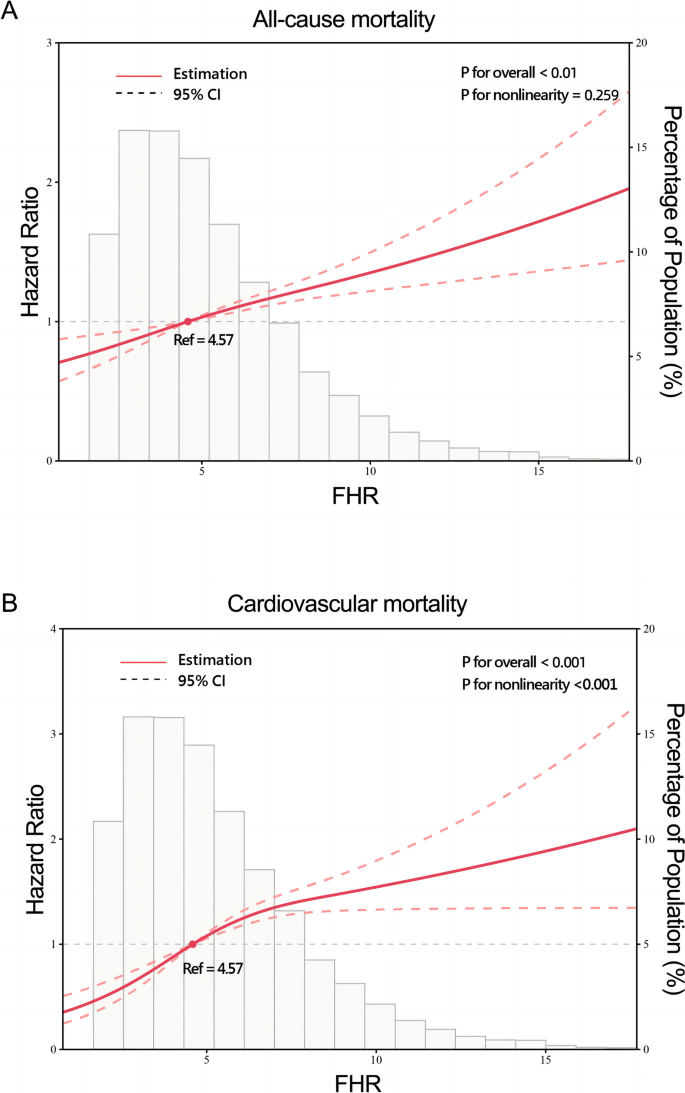
Restricted cubic splines for the relationship between FHR and all-cause ( A ) and cardiovascular mortality. B Adjusted for age, sex, smoking, LDL, triglyceride, total cholesterol, serum creatinine, monocyte count, use of antiplatelets, hypertension, chronic kidney disease, congestive heart failure, diabetes mellitus and stroke
Association of FHR with cardiovascular mortality
Over a median follow-up of 4.66 (2.48–7.48) years, 2309 patients experienced all-cause mortality, with 1007 deaths attributed to cardiovascular causes. The incidence of cardiovascular mortality across the FHR quartiles was presented as follows: Q1-3.6% (120/3306), Q2-6.8% (226/3305), Q3-8.5% (280/3305), and Q4-11.5% (381/3305). The Kaplan‒Meier plot illustrated a statistically significant association between elevated FHR values and diminished survival in AMI patients (Fig. 2 B; P < 0.001). Based on the adjustment for potential confounders (Table 2 ), a higher FHR was consistently correlated with a higher likelihood of mortality due to CVD. The HRs and 95% CIs were as follows: Q2 – HR 1.84, 95% CI 1.35–2.50, P < 0.001; Q3 – HR 2.15, 95% CI 1.59–2.91, P < 0.001; Q4 – HR 2.70, 95% CI 1.99–3.65, P < 0.001. To further explore the correlation between FHR and cardiac mortality, RCS models were utilized, revealing a significant nonlinear association between FHR and cardiovascular death in patients diagnosed with AMI ( P for nonlinearity < 0.001) (Fig. 3 B).
Subgroup analysis
To assess potential interactions between the four FHR subsets and various covariates (age, sex, smoking status, DM, hypertension, stroke, LDL, triglycerides and total cholesterol) in relation to all-cause mortality, post-hoc subgroup analysis was performed (Fig. 4 ). Interestingly, patients in Q2, Q3, and Q4 exhibited consistent characteristics in certain subsets (male, non-smoker, DM, non-hypertension, non-stroke, LDL ≥ 2.95 mmol/L, triglyceride ≥ 1.35 mmol/L and total cholesterol ≥ 4.57 mmol/L), compared to ones in quartile 1 ( P for interaction > 0.05).
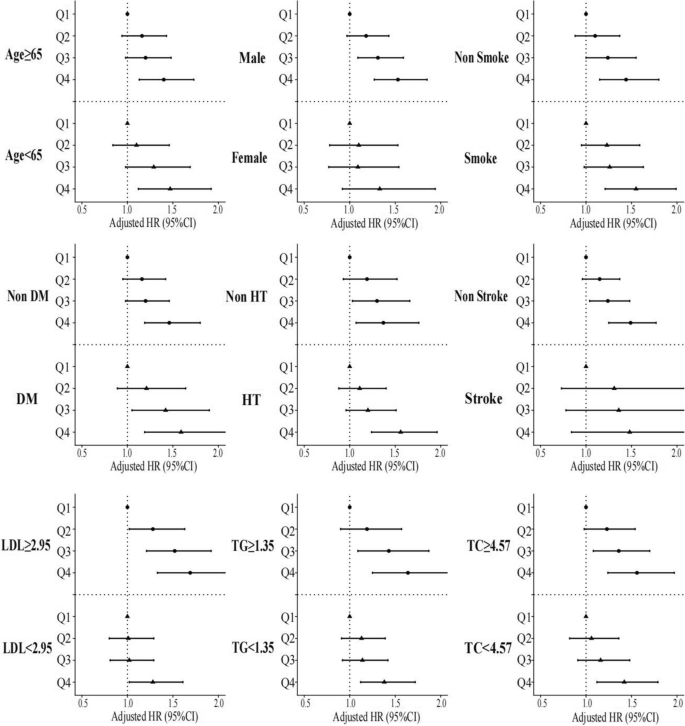
Forest plot of all-cause mortality according to different subgroups. Adjusted for age, sex, smoking, LDL, triglyceride, total cholesterol, serum creatinine, monocyte count, use of antiplatelets, hypertension, chronic kidney disease, congestive heart failure, diabetes mellitus and stroke
Association of FHR with all-cause and cardiovascular mortality
The predictive value of FHR, FIB, HDL, hs-TnT as well as the combination of FHR and hs-TnT for cardiovascular mortality risk assessment among AMI patients was conducted through Receiver operating characteristic curve analysis (Figure S 2 - 5 ). For FHR, the AUC was 0.624 (95% CI 0.607–0.642) and the optimal cut-off value was 4.38, with sensitivity and specificity recorded at 68% and 50%, respectively (Figure S 3 ). As depicted in Figure S 2 , the cut-off value for hs-TnT was 1208.50 pg/mL, with a sensitivity of 64% and specificity of 51%. Notably, in both scenarios, the AUC for FHR, at 0.624, exceeded that of hs-TnT, which measured 0.592 (95% CI 0.564–0.621), as well as FIB (AUC 0.613, 95% CI 0.596–0.630) and HDL (AUC 0.558, 95% CI 0.539–0.576) (Figure S 5 ). Additionally, the combination of FHR and hs-TnT for predicting cardiovascular mortality achieved the utmost AUC at 0.627 (95% CI 0.598–0.656) (Figure S 4 ). Similar results were observed in predicting all-cause mortality. Further, the improved predictive capacity of the combination of FIB and HDL was assessed by IDI and NRI as shown in Table S 1 . Obviously, both IDI and NRI indicated that FHR led to a slight but significant improvement in all-cause and cardiovascular mortality prediction.
This retrospective real-world study was conducted on a substantial cohort from China, with a period of 10-year follow-up. The current study investigated the joint effect of plasma HDL and FIB levels in predicting adverse outcomes among AMI patients. The findings demonstrated that the integrated categorization of HDL and FIB enhanced the predictive value for adverse outcomes, incrementally increasing the likelihood of death owing to cardiac and all-cause events. Multivariate Cox analysis also indicated a higher risk of mortality among individuals within the highest FHR quartile than others. Even after adjusting for confounding variables, patients in Q4 exhibited a 48% increased risk of all-cause death as well as a 1.7-fold increase in cardiovascular mortality in comparison to ones in reference group. Additionally, the RCS curve demonstrated elevated FHR was linearly correlated to all-cause mortality. Furthermore, receiver operating characteristic curve analysis indicated that FHR, with an AUC of 0.624, outperformed hs-TnT (AUC: 0.592) individually, and the combined use of both biomarkers yielded the highest AUC (0.627) in predicting cardiac and all-cause mortality. Moreover, the IDI and NRI analysis for FHR in predicting mortality exhibited significant improvement compared to those for FIB and HDL. These results highlight the potential of combining FIB, an indicator of inflammation and coagulation state, with HDL, a complex circulating lipoprotein, which could enhance the predictive capacity in subsequent risk stratification of AMI patients.
FIB, a liver-synthesized serum glycoprotein, is of great importance in both the inflammatory and coagulation cascades, making it a key factor in the formation and progression of coronary atherosclerosis [ 7 ]. Several studies have revealed its significance in various aspects of CVD previously [ 21 , 22 ]. For instance, a case–control study [ 23 ] revealed significantly elevated FIB levels 3 to 6 months after hospitalization for CVD in comparison to healthy controls. Individuals in the top quartile of FIB exhibited an odds ratio of 6.0 (95% CI 3.5–10.4) in comparison to ones in the lowest group after age adjustment. Similarly, in a prospective investigation [ 24 ], FIB levels were assessed 6 months prior to study entry, and differences in FIB levels were observed between survivors and those who died. Moreover, another study explored the association of FIB with cardiac adverse events following AMI, reporting significantly higher FIB levels in individuals with a prior history of AMI or peripheral artery disease in comparison to those without prevalent CVD [ 25 ]. In addition, FIB levels were found to correlate with early alterations in the carotid artery due to atherosclerosis, even among individuals with minimal CVD risk [ 26 ]. On the other hand, HDL has exhibited numerous protective benefits in cardiac disease mainly attributed to its ability to exert cholesterol efflux, anti-inflammatory and anti-oxidant [ 27 ] properties on endothelial cells and macrophages, etc. [ 28 ]. Cockerill et al. illustrated that physiological concentrations of HDLs isolated from healthy donors decreased the expression of endothelial adhesion molecules induced by cytokines [ 29 ]. Additionally, within the coagulation cascade, HDL plays a multifaceted regulatory function, as indicated by its positive correlation with anticoagulant responses and its ability to counteract the procoagulant characteristics of anionic phospholipids [ 15 ]. Considering that plasma FIB and HDL hold significance in coagulation and inflammatory alterations and are closely linked to cardiovascular incidents, further investigations were essential to assess whether their interplay, like FHR, might assist in identifying individuals at high risk within the CVD population.
A prior study by Ma et al. [ 19 ] demonstrated a correlation between concurrent increases in FIB and declines in HDL levels and an increased risk of recurrent cerebral thrombosis, whereas the separate evaluation of FIB and HDL levels did not yield significant results. According to a study by Kowalski et al. [ 30 ], the co-occurrence of elevated D-dimer, a breakdown product of FIB, and lower HDL levels appeared to contribute to the progression of acute pulmonary emboli. Moreover, other studies indicated a strong association between simultaneous elevation of FIB and reduction in HDL levels with recurrent CVT in previously diagnosed CVT patients, as well as the onset of CVD in the DM cohort [ 20 ]. In addition, Sung et al. observed a significant higher FIB and lower HDL levels in patients enrolled from the outpatient department who experienced major adverse cardiovascular events than in those who did not. Notably, an inverse relationship was observed between FIB and HDL levels, which implies an interaction between FIB and HDL may exacerbate atherosclerosis and thrombosis [ 31 ]. Moreover, another study revealed a relationship between FHR and idiopathic sudden sensorineural hearing loss, a condition with a higher prevalence among individuals with underlying inflammatory and systemic vascular diseases such as atherosclerosis and diabetes mellitus [ 32 ]. Treatment outcomes were classified into four groups in accordance with the degree of hearing recovery, and FHR was found to be significantly lower in groups associated with better outcomes. This study suggested FHR might be a valuable prognostic indicator for hearing recovery among those patients [ 33 ].
In various cardiological studies, the monocyte/HDL ratio (MHR) and neutrophil/HDL ratio (NHR) have been explored as useful inflammatory biomarkers for predicting adverse cardiac outcomes. Lütfü Aşkın et al. evaluated MHR in 99 consecutive STEMI patients, classifying them into two subsets based on the median of QRS score. The results indicated a correlation between elevated MHR and a higher QRS score, suggesting its potential as an independent predictor for high QRS scores in STEMI patients [ 34 ]. In another research conducted by Huang et al., NHR was evaluated among 528 elderly AMI patients. They found NHR was linked to long-term mortality and recurrent MI, which might serve as a predictor for worse clinical outcomes of elderly AMI patients [ 35 ]. In addition, other studies have illustrated the potential role of von Willebrand factor (vWF) as a pro-atherogenic biomarker predicting adverse cardiac outcomes [ 36 ]. For instance, Mario et al. have shown that shear-induced platelet aggregation correlated with enhanced vWF concentration among AMI patients [ 37 ]. Rutten et al. observed a substantial increase in active vWF levels among individuals experiencing ST-elevation MI for the first time compared to controls ( P < 0.0001), emphasizing the central role of vWF in the progression of thrombosis [ 38 ]. Moreover, Sergio et al. investigated the prognostic role of hemoglobin decline among patients with acute coronary syndrome (ACS). Their study involved 7,781 invasively managed ACS patients, categorized based on in-hospital hemoglobin decline, and further subdivided according to the presence of adjudicated in-hospital bleeding. The findings revealed a decline in hemoglobin of ≥ 3 g/dL during hospitalization, irrespective of overt bleeding, was independently linked to a higher risk of 1-year mortality [ 39 ].
In our study, we compared FHR with hs-TnT for mortality prediction. Cardiac troponin T, a biomarker widely recommended for diagnosing AMI [ 40 ], has been found independently associated with adverse outcomes following acute coronary syndrome [ 41 ]. James et al. assessed the role of hs-TnT in 3,546 individuals from the Dallas Heart Study, revealing that baseline hs-TnT concentrations were linked to the presence of structural heart disease and subsequent risk of total mortality [ 42 ].
The current study is the first to evaluate the concurrent presence of both parameters in individuals with AMI using FHR. FIB and HDL levels have previously been assessed separately, showing implications in inflammatory and atherothrombotic processes [ 43 ]. The research revealed a statistically significant association between elevated FHR values and worse outcomes among AMI patients. These results highlight that the joint effect of HDL and FIB could augment mortality prediction in AMI population. This enhanced predictive value could facilitate future risk stratification for the AMI population. Thus, incorporating these two fundamental markers in clinical practice could be advantageous.
Study strengths and limitations
The current study illustrated elevated FHR values were strongly correlated with enhanced all-cause as well as cardiac mortality among AMI patients, for the first time. Considering the clinical burden of complications associated with CVD, the assessment of FHR holds the potential to serve as a powerful indicator of long-term mortality among AMI patients. However, this research exists a few limitations. Firstly, the predominance of male participants in this study (4.5:1) may introduce bias and limit the generalizability of our findings. It is essential to acknowledge that this study was conducted on a population from southern China hospitalized with AMI, which may not fully represent the broader Chinese population. Additionally, the inverse association between FHR and TC/LDL-cholesterol may indicate different clinical phases and baseline status of inflammation or malnutrition among this population, which might potentially bias the study results. Further investigations are requested to assess if those outcomes could be extrapolated to other populations. Secondly, the measurements of HDL and FIB concentrations were only conducted at baseline, but changes in these biomarkers during the follow-up period may also hold clinical significance. Thirdly, despite the comprehensive adjustment for potential risk factors in the analysis, certain variables could not be measured or acquired, potentially resulting in residual confounding which might be unavoidable. Fourthly, the CIN-II cohort lacks data regarding GRACE scores, a risk assessment instrument utilized in patients with ACS as well as left ventricular ejection fractions. Consequently, we opted to employ hs-TnT as an alternative to predict long-term mortality among AMI patients for comparison. Although there were studies demonstrating association between hs-TnT and adverse cardiovascular outcomes, it is better representing acute mortality after the ischemic event. Lastly, as this was an observational study, the underlying mechanisms behind the association require further investigation.
This real-world cohort study revealed that higher FHR values were independently related to adverse clinical outcomes among AMI patients, suggesting FHR holds potential as a prognostic indicator to identify individuals at higher risk of mortality in the context of AMI. Since FHR can be easily and inexpensively measured, it might contribute to improved clinical decision-making and patient management for AMI patients.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- Acute myocardial infarction
High density lipoprotein
Fibrinogen to HDL-cholesterol ratio
Cardiovascular disease
Atrial fibrillation
Cerebral venous thrombosis
Diabetes mellitus
Low density lipoprotein
Total cholesterol
High-sensitivity troponin T
Von Willebrand factor
Acute coronary syndrome
Area under the curve
Restricted cubic spline
Integrated discrimination improvement
Net reclassification improvement
Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, et al. Inflammation, immunity, and infection in Atherothrombosis: JACC Review topic of the Week. J Am Coll Cardiol. 2018;72(17):2071–81.
Article CAS PubMed PubMed Central Google Scholar
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95.
Article CAS PubMed Google Scholar
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Article PubMed PubMed Central Google Scholar
Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost. 2003;89(4):601–9.
Paraskevas KI, Baker DM, Vrentzos GE, Mikhailidis DP. The role of fibrinogen and fibrinolysis in peripheral arterial disease. Thromb Res. 2008;122(1):1–12.
Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in Hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2017;37(3):e13–21.
Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62.
Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Schnabel R, Munzel T, et al. Inflammation, atherosclerotic burden and cardiovascular prognosis. Atherosclerosis. 2007;195(2):e126–34.
DeFilippis AP, Trainor PJ, Thanassoulis G, Brumback LC, Post WS, Tsai MY, et al. Atherothrombotic factors and atherosclerotic cardiovascular events: the multi-ethnic study of atherosclerosis. Eur Heart J. 2022;43(10):971–81.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62(5):707–14.
Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000.
Article PubMed Google Scholar
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72.
Ruiz M, Frej C, Holmér A, Guo LJ, Tran S, Dahlbäck B. High-density lipoprotein-Associated apolipoprotein M limits endothelial inflammation by delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37(1):118–29.
Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(9):1450–5.
van der Stoep M, Korporaal SJA, Van Eck M. High-density lipoprotein as a modulator of platelet and coagulation re sponses. Cardiovasc Res. 2014;103(3):362–71.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–32.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet (London England). 2012;380(9841):572–80.
Ma X, Ji XM, Fu P, Ding YC, Xue Q, Huang Y. Coexistence of high fibrinogen and low high-density lipoprotein cholesterol levels predicts recurrent cerebral venous thrombosis. Chin Med J (Engl). 2015;128(13):1732–7.
Pacilli A, De Cosmo S, Trischitta V, Bacci S. Role of relationship between HbA1c, fibrinogen and HDL-cholesterol on cardiovascular disease in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;228(1):247–8.
Surma S, Banach M. Fibrinogen and atherosclerotic Cardiovascular diseases-review of the literature and clinical studies. Int J Mol Sci. 2021;23(1):193.
Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham offspring Population. Circulation. 2000;102(14):1634–8.
van der Bom JG, de Maat MP, Bots ML, Haverkate F, de Jong PT, Hofman A, et al. Elevated plasma fibrinogen: cause or consequence of cardiovascular disease? Arterioscler Thromb Vasc Biol. 1998;18(4):621–5.
Bodrova VV, Shustova ON, Khaspekova SG, Mazurov AV. Laboratory markers of platelet production and turnover. Biochem (Mosc). 2023;88(Suppl 1):S39–51.
Article CAS Google Scholar
Scarabin PY, Aillaud MF, Amouyel P, Evans A, Luc G, Ferrières J, et al. Associations of fibrinogen, factor VII and PAI-1 with baseline findings among 10,500 male participants in a prospective study of myocardial infarction–the PRIME study. Prospective epidemiological study of myocardial infarction. Thromb Haemost. 1998;80(5):749–56.
CAS PubMed Google Scholar
Grebe MT, Luu B, Sedding D, Heidt MC, Kemkes-Matthes B, Schaefer CA, et al. Fibrinogen promotes early atherosclerotic changes of the carotid artery in young, healthy adults. J Atheroscler Thromb. 2010;17(10):1003–8.
Jia C, Anderson JLC, Gruppen EG, Lei Y, Bakker SJL, Dullaart RPF, et al. High-density lipoprotein anti-inflammatory capacity and Incident Cardiovascular events. Circulation. 2021;143(20):1935–45.
Darabi M, Kontush A. High-density lipoproteins (HDL): novel function and therapeutic applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(1):159058.
Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15(11):1987–94.
Kowalski J, Jędrzejczyk JT, Barylski M, Ciećwierz J, Sienkiewicz M, Kowalczyk E. Value of D-dimer and HDL cholesterol concentrations in predicting the occurrence of acute pulmonary embolism. Pol Merkur Lekarski. 2016;40(239):283–7.
PubMed Google Scholar
Cho SW, Kim BG, Kim BO, Byun YS, Goh CW, Rhee KJ, et al. Hemorheological and glycemic parameters and HDL cholesterol for the Prediction of Cardiovascular events. Arq Bras Cardiol. 2016;106(1):56–61.
PubMed PubMed Central Google Scholar
Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol. 2010;15(2):111–5.
Hıra İ, Yaşar M, Kaya A, Bayram A, Özcan İ. Prognostic value of Fibrinogen/HDL ratio in Idiopathic Sudden Sensorineural hearing loss. J Int Adv Otol. 2021;17(2):91–5.
Aşkın L, Çetin M, Türkmen S, Taşolar H, Aktürk E. The relationship between monocyte/high-density lipoprotein ratio and selvester QRS score in patients with STEMI. Turk Kardiyol Dern Ars. 2018;46(4):260–7.
Huang JB, Chen YS, Ji HY, Xie WM, Jiang J, Ran LS, et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: a comparison study. Lipids Health Dis. 2020;19(1):59.
Rutten B, Maseri A, Cianflone D, Laricchia A, Cristell NA, Durante A, et al. Plasma levels of active Von Willebrand factor are increased in patients with first ST-segment elevation myocardial infarction: a multicenter and multiethnic study. Eur Heart J Acute Cardiovasc Care. 2015;4(1):64–74.
Mazzucato M, Cozzi MR, Pradella P, Ruggeri ZM, De Marco L. Distinct roles of ADP receptors in Von Willebrand factor-mediated platelet signaling and activation under high flow. Blood. 2004;104(10):3221–7.
Gragnano F, Golia E, Natale F, Bianchi R, Pariggiano I, Crisci M, et al. Von Willebrand factor and Cardiovascular Disease: from a biochemical marker to an attractive therapeutic target. Curr Vasc Pharmacol. 2017;15(5):404–15.
Leonardi S, Gragnano F, Carrara G, Gargiulo G, Frigoli E, Vranckx P, et al. Prognostic implications of declining hemoglobin content in patients hospitalized with Acute Coronary syndromes. J Am Coll Cardiol. 2021;77(4):375–88.
Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53.
Ang DS, Kao MP, Dow E, Lang C, Struthers A. The prognostic value of high sensitivity troponin T 7 weeks after an acute coronary syndrome. Heart. 2012;98(15):1160–5.
de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–12.
Yuan D, Jiang P, Zhu P, Jia S, Zhang C, Liu Y, et al. Prognostic value of fibrinogen in patients with coronary artery disease and prediabetes or diabetes following percutaneous coronary intervention: 5-year findings from a large cohort study. Cardiovasc Diabetol. 2021;20(1):143.
Download references
Acknowledgements
We express our sincere gratitude to all the participants for their valuable contributions to this study.
This research was supported by grants from Guangdong Provincial Science and Technology Project (2020B1111170011); Guangdong Provincial People’s Hospital (BY012022066); Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No. Y0120220151); and Guangdong Provincial Science and Technology Project (KJ022021049).
Author information
Congzhuo Jia and Wanying Wu contributed equally to this work and share first authorship.
Authors and Affiliations
Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, China
Congzhuo Jia, Wanying Wu, Jin Liu, Shiqun Chen, Yang Zhou, Sijia Yu, Linfang Qiao, Jinming Chen, Ning Tan, Yong Liu & Jiyan Chen
Department of Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, China
Congzhuo Jia, Wanying Wu, Jin Liu, Yang Zhou, Sijia Yu, Linfang Qiao, Jinming Chen, Ning Tan, Yong Liu & Jiyan Chen
The Second School of Clinical Medicine, Southern Medical University, Guangzhou, 510515, China
Huan Lu, Sijia Yu, Linfang Qiao & Jinming Chen
The School of Pharmacy, Guangdong Medical University, Dongguan, 523000, China
Guoxiao Liang
Global Health Research Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Science, Guangzhou, 510100, China
Shiqun Chen
Department of Cardiology, Yangjiang People’s Hospital, Yangjiang, 529500, China
You can also search for this author in PubMed Google Scholar
Contributions
CZJ, WYW contributed to the conception and design. CZJ, WYW, JYC, NT and YL were involved in acquiring the data. HL, JL, SQC, GXL, YZ, SJY, LFQ and JMC performed the analysis and interpretation of the findings. CZJ and WYW were responsible for drafting the article. The final version of the manuscript underwent revision, review, and endorsement by all authors.
Corresponding authors
Correspondence to Ning Tan , Yong Liu or Jiyan Chen .
Ethics declarations
Ethics approval and consent to participate.
The research obtained ethics approval from the Ethics Committee of the Guangdong Provincial People’s Hospital (No. GDREC2019-555 H-2) and each related study party by the institutional review boards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jia, C., Wu, W., Lu, H. et al. Fibrinogen to HDL-Cholesterol ratio as a predictor of mortality risk in patients with acute myocardial infarction. Lipids Health Dis 23 , 86 (2024). https://doi.org/10.1186/s12944-024-02071-7
Download citation
Received : 28 November 2023
Accepted : 05 March 2024
Published : 25 March 2024
DOI : https://doi.org/10.1186/s12944-024-02071-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- HDL cholesterol
- Inflammation
Lipids in Health and Disease
ISSN: 1476-511X
- General enquiries: [email protected]
Emergent Coronary Thrombectomy for Acute Myocardial Infarction Immediately Following Craniotomy with Tumor Resection
- Case Report
- Open access
- Published: 27 March 2024

Cite this article
You have full access to this open access article
- Curtis R. Ginder ORCID: orcid.org/0000-0001-8507-9624 1 ,
- Giselle A. Suero-Abreu 2 ,
- Saad S. Ghumman 3 ,
- Brian A. Bergmark 4 ,
- Omar Arnaout 5 &
- Robert P. Giugliano 4
88 Accesses
1 Altmetric
Explore all metrics
The management of perioperative acute myocardial infarction (AMI) following oncologic neurosurgery requires balancing competing risks of myocardial ischemia and postoperative bleeding. There are limited human data to establish the safest timing of antiplatelet or anticoagulation therapy following neurosurgical procedures. For patients with malignancy experiencing AMI in the acute postoperative period, staged percutaneous coronary intervention (PCI) with upfront coronary aspiration thrombectomy followed by delayed completion PCI may offer an opportunity for myocardial salvage while minimizing postoperative bleeding risks. CYP2C19 genotyping and platelet aggregation studies can help confirm adequate platelet inhibition once antiplatelet therapy is resumed.
Similar content being viewed by others
High and intermediate risk pulmonary embolism in the ICU
Scott J. Millington, Nadia Aissaoui, … Antoine Vieillard-Baron

Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage
Jennifer A. Frontera, John J. Lewin III, … Cindy L. Zerfoss
Cost-effectiveness of CT perfusion for the detection of large vessel occlusion acute ischemic stroke followed by endovascular treatment: a model-based health economic evaluation study
Henk van Voorst, Jan W. Hoving, … for the MR CLEAN Registry Investigators
Avoid common mistakes on your manuscript.
Introduction
Perioperative myocardial infarction is an important complication associated with an increased mortality risk following noncardiac surgery [ 1 ]. The risk of perioperative cardiac events is more significant for patients undergoing neurosurgical procedures who have chronic antiplatelet and antithrombotic therapy interrupted perioperatively [ 2 ]. Utilizing postoperative high-sensitivity troponin levels, a prospective cohort study identified perioperative myocardial injury in 20.5% of patients undergoing elective neurosurgery [ 3 ]. Patients undergoing oncologic surgery may face even greater perioperative risks, with one population-based study identifying a more than twofold increase in the incidence of arterial thromboembolism and myocardial infarction in the first 6 months following a new diagnosis of cancer [ 4 ].
We present the management of a patient who suffered an ST-segment elevation myocardial infarction immediately following craniotomy with tumor resection and who was managed with a staged coronary interventional procedure to achieve myocardial salvage while minimizing intraparenchymal brain hemorrhage. Written informed consent was obtained from the patient prior to manuscript preparation.
Case Presentation
The patient is a 70-year-old man, non-smoker, with a history of stage IIIA non-small cell lung cancer diagnosed 10 years ago treated with neoadjuvant cisplatin and etoposide, lobectomy with radical mediastinal lymphadenectomy, and adjuvant carboplatin and pemetrexed followed by a 5-year course of adjuvant erlotinib. He had a prior anterior myocardial infarction (MI) complicated by cardiac arrest treated with percutaneous coronary intervention (PCI) with drug-eluting stents (DES) to the proximal and mid-left anterior descending (LAD) artery 3 years ago at an outside institution. He completed a course of dual-antiplatelet therapy with aspirin and ticagrelor and had since been on aspirin monotherapy. After several months of increasing forgetfulness, he presented to the hospital and cross-sectional imaging revealed a 5 cm by 7 cm frontal lobe mass with surrounding edema concerning recurrent metastatic disease from lung cancer (Fig. 1 ). A pre-operative medical evaluation revealed no symptoms at a moderate level of functional capacity and a normal transthoracic echocardiogram. Aspirin was held for 5 days before the planned craniotomy, and he received no other antiplatelet or anticoagulation medications in the preoperative period. A stereotactic left frontal craniotomy with gross total resection of the tumor lasted 5 h and concluded with adequate hemostasis and without intraoperative complication. Postoperative imaging revealed expected postsurgical changes without postoperative bleeding (Fig. 1 ).
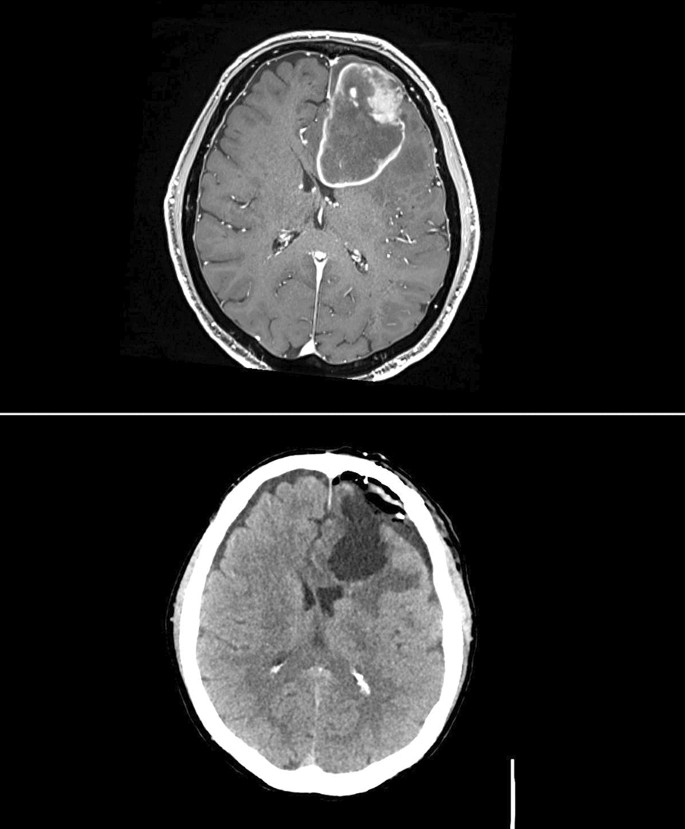
Frontal lobe mass pre and post resection . (Upper panel) Preoperative magnetic resonance imaging (MRI) revealed a large left frontal lobe lesion measuring 5 cm by 7 cm with significant surrounding edema and left-to-right midline shift. (Lower panel) Immediate postoperative computed tomography (CT) revealed expected postsurgical changes without postoperative bleeding
Two hours postoperatively, the patient developed substernal chest discomfort. Serial electrocardiograms (ECGs) were obtained and demonstrated an evolving anterior wall ST-segment elevation myocardial infarction (Fig. 2 ). A bedside ultrasound revealed new anterior and septal hypokinesis with moderately reduced left ventricular function. He began to develop an increasing burden of non-sustained ventricular tachycardia prompting the addition of intravenously administered lidocaine.
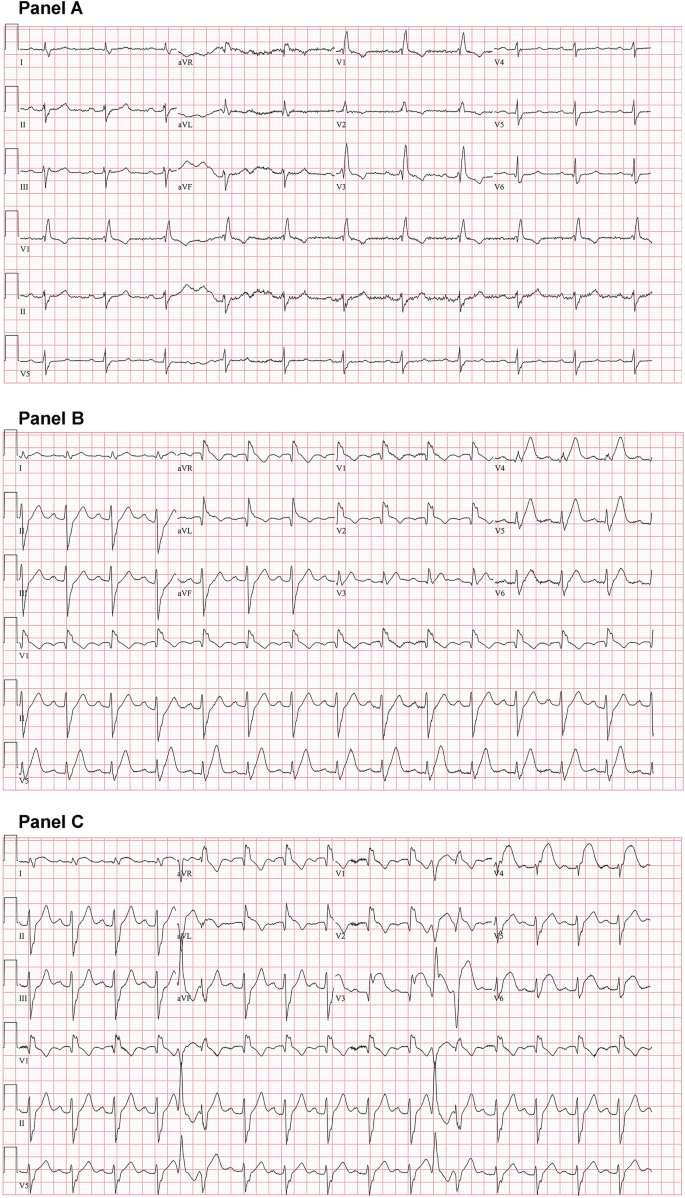
Baseline and postoperative electrocardiograms (ECGs). Baseline ECG (panel A) showed sinus rhythm with a right bundle branch block. A postoperative ECG 1 h after symptom onset demonstrated a right bundle branch block with hyperacute T waves of the anterior and apical leads (panel B) which evolved into ST segment elevation of the anterior, apical, and lateral leads (panel C)
Treatment Plan/Decision-Making
A prompt multidisciplinary conversation was held between the critical care, neurosurgical, and cardiovascular medicine teams. It was concluded that the risk of mortality from an acute anterior wall myocardial infarction with increasing ventricular ectopy outweighed the risks of postoperative bleeding associated with single antiplatelet therapy and the use of heparin during coronary angiography. It was considered that intravenous unfractionated heparin and aspirin therapy offered the lowest risk of operative site bleeding, with a preference to avoid P2Y 12 inhibitor therapy for at least 10 days following the craniotomy.
A 600-mg aspirin suppository was immediately administered and coronary angiography via femoral artery access was performed with the goal of deferring stent placement, if possible. Diagnostic angiography revealed a dual-LAD system with an acute thrombotic occlusion of the lateral branch of the LAD at the origin of a previously placed stent in the proximal segment of the vessel with extension into the septal branch of the LAD (Fig. 3 ). After a discussion between the interventional cardiology, cardiac intensive care unit, and neurosurgical teams, there was a consensus to proceed with PCI. A lower-than-normal activated clotting time (ACT) target of 150–200 s was planned to balance the competing risks of catheter or wire-related thrombosis and intracerebral hemorrhage. The lesion was crossed with a guidewire and was dilated with a 2.5-mm balloon. Aspiration thrombectomy was then performed using the Indigo CAT Rx catheter (Penumbra, Alameda, CA) with subsequent dilation with a 3.0-mm non-compliant (NC) balloon resulting in resolution of the thrombus within the prior LAD stent (Fig. 4 ). Considering high risk of intraparenchymal brain hemorrhage associated with P2Y 12 inhibitor use in the immediate postoperative setting, no further intervention was performed with a plan for a staged completion of LAD PCI. The time from wire insertion in the LAD to equipment removal was 36 min, and actual ACT values ranged from 142 to 166 s.
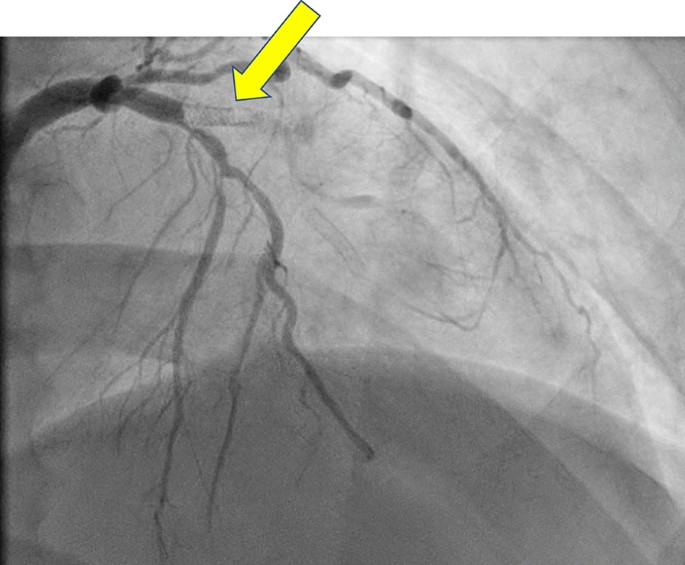
Diagnostic coronary angiography. Initial diagnostic coronary angiogram showing a dual-LAD (left anterior descending) system with a chronic total occlusion of the left circumflex artery and an acute thrombotic lesion at the origin of a previously placed stent in the proximal portion of the lateral LAD branch. The thrombus burden extended into the septal branch of the LAD which was jailed by the previously placed proximal LAD stent struts
Figure 3 Cine (MP4 8325 kb)
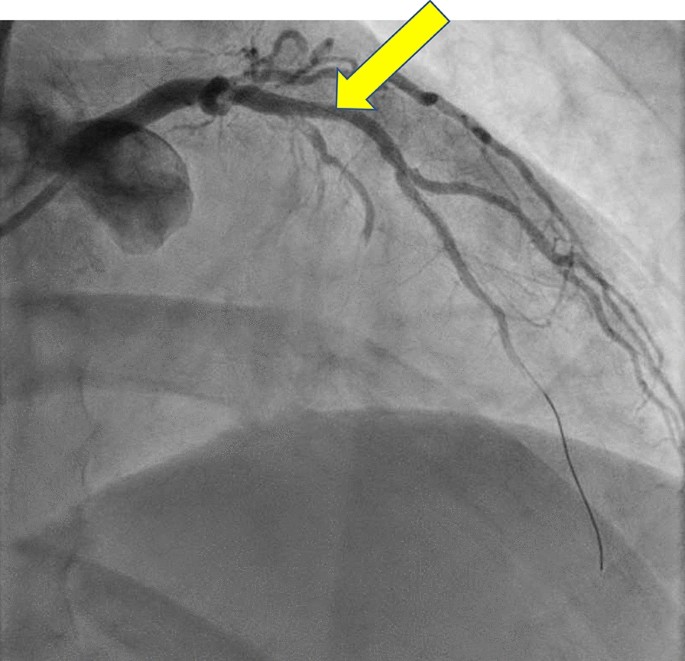
Coronary angiography following thrombectomy. Resolution of the acute thrombus following dilation, aspiration thrombectomy, and post-dilation. The no-reflow phenomenon was observed in the apical portion of the lateral left anterior descending (LAD) branch. Further intervention of the septal LAD branch was deferred because of the jailed origin and potential need for bifurcation stent placement in the immediate postoperative period
Figure 4 Cine (MP4 4098 kb)
Hospital Course
The patient returned to the cardiac intensive care unit for close neurologic and cardiac monitoring. Aspirin 81 mg daily monotherapy was continued. Interval cross-sectional computed tomography (CT) head imaging was performed every 8 h for the first day to screen for subclinical or preclinical bleeding that would require surgical evacuation. These studies demonstrated minimal bleeding in the surgical bed that remained stable on daily follow-up imaging over the next 2 days. He had no focal deficits on neurologic examination.
CYP2C19 genetic testing revealed a *1/*2 genotype which is an “intermediate metabolizer” phenotype. Baseline platelet aggregation studies were obtained on aspirin but before initiation of P2Y 12 inhibitor therapy. On day 10 postoperatively, clopidogrel 75 mg daily was initiated without a loading dose. To confirm adequate platelet inhibition prior to staged completion PCI, repeat platelet aggregation studies were performed after 5 days of clopidogrel 75 mg daily.
With adequate platelet inhibition confirmed (73% inhibition of platelet aggregation using ADP 20 μmol/L, Table 1 ), he underwent repeat coronary angiography with PCI on postoperative day 15. The previously placed LAD stent had remained patent following the initial aspiration thrombectomy and balloon dilation intervention (Fig. 5 ). There was TIMI 3 flow in the LAD. Intravascular ultrasound (IVUS) revealed underexpanded proximal and distal stents within the LAD as well as significant atherosclerosis in the intervening segments between the stents (Fig. 6 ). A 3.5 × 28 mm DES was placed to the mid-LAD and post-dilated with a 3.5-mm NC distally and a 4.0-mm NC at high pressure proximally. Post-intervention IVUS revealed a well-expanded and apposed stent without edge dissection (Fig. 7 ). There was TIMI 3 flow and no residual stenosis (Fig. 8 ). He was discharged home on postoperative day 17 and completed cardiac rehabilitation as an outpatient. The final pathology from the tumor resection revealed metastatic lung adenocarcinoma.
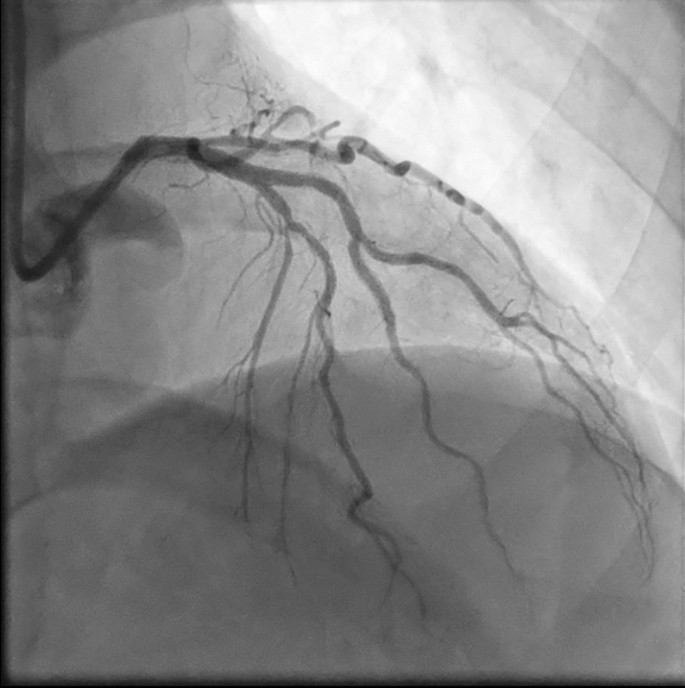
Follow-up diagnostic angiography. Repeat coronary angiography on postoperative day 15 revealed a patent left anterior descending (LAD) following aspiration thrombectomy and balloon angioplasty performed during the index angiography
Figure 5 Cine (MP4 5283 kb)
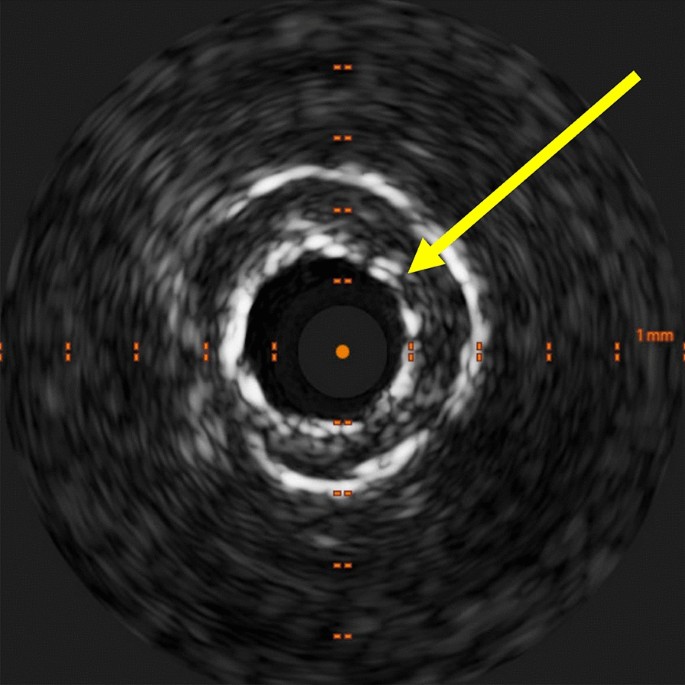
Intravascular ultrasound (IVUS) of underexpanded stent. IVUS at the time of the second procedure showed an underexpanded stent in the mid-LAD (left anterior descending). The prior stent (yellow arrow) had a diameter of 2.4 mm as compared to a vessel diameter of 4.0 mm
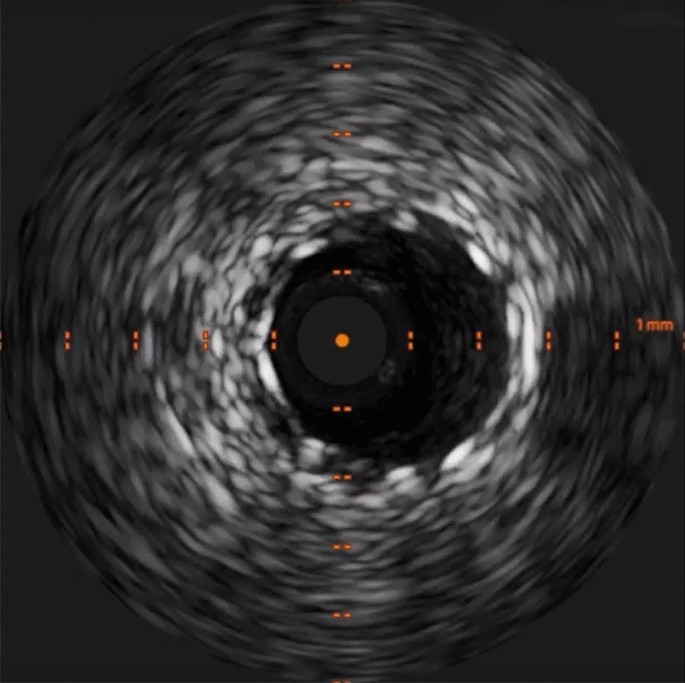
Post-intervention Intravascular ultrasound (IVUS). Post-intervention IVUS demonstrated a well-apposed and expanded stent without edge dissection
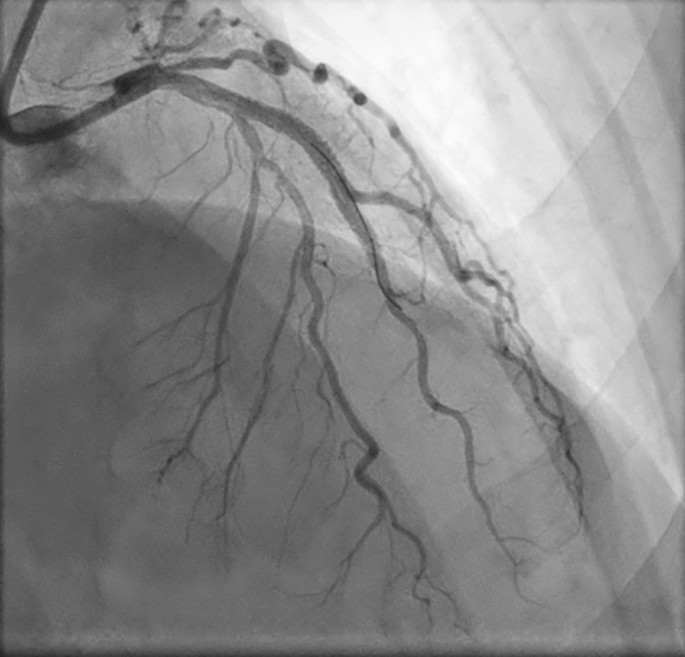
Coronary angiography following percutaneous coronary intervention (PCI) with drug-eluting stent (DES) placement. Staged PCI performed 15 days postoperatively revealed underexpanded proximal and distal stents within the left anterior descending (LAD). The proximal and distal stents were dilated and a new 3.5 × 28 mm Synergy DES was placed from the distal to proximal stents, overlapping both
Figure 8 Cine (MP4 4907 kb)
Discussion and Clinical Implications
We describe a clinical scenario of a postoperative ST-elevation myocardial infarction 2 h following craniotomy with tumor resection treated with aspiration thrombectomy and balloon dilation followed by a staged completion PCI 15 days later following the gradual introduction of P2Y 12 inhibitor. To our knowledge, this staged approach has not been previously described, and it may provide temporizing therapy to patients with postoperative acute coronary syndrome at a high risk of bleeding until definitive revascularization can be completed.
Clopidogrel was selected as the preferred P2Y 12 agent in this case to minimize the risk of intracranial bleeding [ 5 ]. The risk of bleeding was balanced against the risk of stent thrombosis which is associated with carriage of a reduced-function CYP2C19 allele [ 6 ]. CYP2C19 genetic testing was performed before the initiation of clopidogrel, identifying an intermediate metabolizer phenotype. Follow-up platelet aggregation studies were then performed to determine if sufficient platelet inhibition could be achieved with clopidogrel and aspirin therapy prior to proceeding with staged PCI. While CYP2C19 genotyping is not routinely performed in all patients with acute coronary syndromes undergoing PCI, selective testing—as performed in this case—may allow better risk quantification and stratification when competing risks are present.
There are conflicting data on the risks of postoperative intracranial bleeding in patients who continue aspirin in the perioperative period. One multicenter retrospective study reported a 2.6-fold increase in the rates of postoperative bleeding in patients undergoing open craniotomy for the repair of unruptured aneurysms who continued to take aspirin perioperatively [ 2 ]. A smaller single-center retrospective study showed no difference in bleeding complications for patients undergoing craniotomy with tumor resection who continued aspirin perioperatively [ 7 ].
The management of patients who do experience cardiovascular complications following neurosurgical procedures requires individualized risk–benefit analysis. There are limited human data for patients experiencing an acute thrombotic event to guide the safest timing of therapeutic anticoagulation resumption or initiation. An experimental animal model examining rats undergoing craniotomy and corticectomy identified a 30% rate of intracerebral hemorrhage in rats receiving therapeutic heparin (1.5–3 times control-activated partial thromboplastin time) on postoperative day 1 compared to a rate of 80% in rats receiving supratherapeutic (> 3 times control) doses of heparin [ 8 ]. A prior review of perioperative anticoagulation management in neurosurgery concluded that therapeutic anticoagulation can be initiated 3–5 days following neurosurgery in patients at the highest risk of thromboembolic events [ 9 ].
Patients with cancer face an elevated risk of developing cardiovascular complications, including higher morbidity and mortality associated with coronary artery disease [ 10 , 11 , 12 ]. Specifically, managing acute coronary syndrome (ACS) in patients with cancer and in the perioperative setting presents unique challenges due to their multiple comorbidities and increased bleeding risk. Additionally, the prognosis of these patients when undergoing invasive procedures for ACS is influenced by factors such as cancer type, staging, time since diagnosis, and ongoing oncological treatment [ 13 , 14 , 15 ]. Growing evidence shows that patients with cancer have worse in-hospital outcomes post-PCI with increased rates of major adverse cardiovascular events, 90-day readmission, and bleeding complications [ 11 , 13 , 16 , 17 , 18 ]. Long-term data showed a twofold higher rate of MI and repeat vascularization and a nearly threefold higher rate of stent thrombosis over 5 years post-PCI in patients with cancer [ 19 ].
The decision to pursue conservative medical management or an invasive strategy for patients with cancer and ACS is complex. It requires an individualized approach weighing the inherent risks of coronary revascularization procedures and the use of antiplatelets, guided by genetic testing when feasible. In 2016, the Society for Cardiovascular Angiography and Interventions (SCAI) issued a consensus document addressing special considerations of cardio-oncology patients in the cardiac catheterization laboratory, with respect to thrombocytopenia, coagulopathies, bleeding tendencies, vascular access complications, and increased stent thrombosis risk [ 20 ]. However, the management of ACS in this high-risk population needs more specific evidence-based guidelines calling for multidisciplinary collaboration and further research efforts to improve outcomes in cardio-oncology patients.
Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–32.
Article PubMed Google Scholar
Han HJ, Kim J, Jang CK, et al. Perioperative low-dose aspirin management for planned clipping surgery: when, how long, and with what precautions? Neurosurgery. 2023. https://doi.org/10.1227/neu.0000000000002710 .
Saka E, Canbaz M, Abdullah T, et al. Perioperative myocardial injury after elective neurosurgery: incidence, risk factors, and effects on mortality. Neurosurg Rev. 2022;45:2151–9.
Navi Babak B, Reiner Anne S, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–38.
Article CAS PubMed PubMed Central Google Scholar
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Article CAS PubMed Google Scholar
Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62.
Rahman M, Donnangelo LL, Neal D, Mogali K, Decker M, Ahmed MM. Effects of perioperative acetyl salicylic acid on clinical outcomes in patients undergoing craniotomy for brain tumor. World Neurosurgery. 2015;84:41–7.
Laohaprasit V, Mayberg MR. Risks of anticoagulation therapy after experimental corticectomy in the rat. Neurosurgery. 1993;32:625–9.
Lazio BE, Simard JM. Anticoagulation in neurosurgical patients. Neurosurgery. 1999;45:838.
Bharadwaj A, Potts J, Mohamed MO, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–93.
Landes U, Kornowski R, Bental T, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28:5–10.
Nakatsuma K, Shiomi H, Morimoto T, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur Heart J Qual Care Clin Outcomes. 2018;4:200–7.
PubMed Google Scholar
Potts JE, Iliescu CA, Lopez Mattei JC, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. 2019;40:1790–800.
Roule V, Verdier L, Blanchart K, et al. Systematic review and meta-analysis of the prognostic impact of cancer among patients with acute coronary syndrome and/or percutaneous coronary intervention. BMC Cardiovasc Disord. 2020;20:38.
Article PubMed PubMed Central Google Scholar
Hess CN, Roe MT, Clare RM, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015. https://doi.org/10.1161/JAHA.115.001779 .
Ueki Y, Vögeli B, Karagiannis A, et al. Ischemia and bleeding in cancer patients undergoing percutaneous coronary intervention. JACC CardioOncol. 2019;1:145–55.
Kwok CS, Wong CW, Kontopantelis E, et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur Heart J. 2021;42:1019–34.
Tabata N, Sueta D, Yamamoto E, et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur Heart J Qual Care Clin Outcomes. 2018;4:290–300.
Guo W, Fan X, Lewis BR, et al. Cancer patients have a higher risk of thrombotic and ischemic events after percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14:1094–105.
Iliescu CA, Grines CL, Herrmann J, et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2016;87:E202–23.
Download references
No funding was received for the writing of this manuscript and the publication fee was waived. Dr. Ginder receives funding from the National Heart Lung and Blood Institute under Award Number T32HL007604.
Author information
Authors and affiliations.
Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Curtis R. Ginder
Division of Cardiovascular Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Giselle A. Suero-Abreu
Ballad Health CVA Heart Institute, Bristol, TN, USA
Saad S. Ghumman
Thrombolysis in Myocardial Infarction (TIMI) Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Hale BTM, Suite 7022, 60 Fenwood Road, Boston, MA, 02115, USA
Brian A. Bergmark & Robert P. Giugliano
Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Omar Arnaout
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Curtis Ginder, Dr. Giselle Suero-Abreu, and Dr. Robert Giugliano created the concept and design of the manuscript. Dr. Curtis Ginder, Dr. Giselle Suero-Abreu, Dr. Saad Ghumman, Dr. Brian Bergmark, Dr. Omar Arnaout, and Dr. Robert Giugliano drafted, edited, and revised the manuscript.
Corresponding author
Correspondence to Robert P. Giugliano .
Ethics declarations
Conflict of interest.
Dr. Bergmark has received institutional research grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, Philips, Abbott Vascular, SpectraWAVE, and Inari. Dr. Bergmark reports consulting fees from Abiomed, Abbott Vascular, Bain Life Sciences, Endovascular Engineering, Terumo, and SpectraWAVE. Dr. Giugliano receives clinical trial and research support from Amgen, Anthos Therapeutics, Daiichi-Sankyo, and Ionis; honoraria for lectures or the continuing medical education program from Amgen, Centrix, Daiichi-Sankyo, Dr. Reddy’s Laboratories, Medical Education Resources, Medscape, Menarini, Merck, Pfizer, SAJA Pharmaceuticals, Servier, Shanghai Medical Telescope, and Voxmedia; and is a consultant for Amarin, Amgen, Bayer, Boston Scientific, Caladrius, CryoLife, CSL Behring, CVS Caremark, Daiichi Sankyo, Esperion, Gilead, Hen-grui, Inari, Janssen, Novartis, Paratek, Pfizer, PhaseBio Pharmaceuticals, and Samsung. Dr. Giugliano is an Editor-in-Chief of Cardiology and Therapy. Dr. Giugliano was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Dr. Curtis Ginder, Dr. Giselle Suero-Abreu, Dr. Saad Ghumman, and Dr. Omar Arnaout have no disclosures.
Ethical Approval
The patient presented in this case report granted written informed consent and permission for publication of his clinical course.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Ginder, C.R., Suero-Abreu, G.A., Ghumman, S.S. et al. Emergent Coronary Thrombectomy for Acute Myocardial Infarction Immediately Following Craniotomy with Tumor Resection. Cardiol Ther (2024). https://doi.org/10.1007/s40119-024-00356-7
Download citation
Received : 28 October 2023
Accepted : 17 January 2024
Published : 27 March 2024
DOI : https://doi.org/10.1007/s40119-024-00356-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Perioperative ACS
- Aspiration thrombectomy
- CYP2C19 genotyping
- Platelet aggregation
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 30 March 2024
Development and validation of a prognostic model for predicting post-discharge mortality risk in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI)
- Lingling Zhang ORCID: orcid.org/0009-0005-6902-3475 1 na1 ,
- Zhican Liu ORCID: orcid.org/0000-0002-5532-1632 1 , 2 na1 ,
- Yunlong Zhu 1 , 2 , 3 ,
- Mingxin Wu 1 , 2 ,
- Haobo Huang 1 ,
- Wenbin Yang 5 ,
- Ke Peng 4 &
- Jianping Zeng ORCID: orcid.org/0000-0002-4485-6164 1 , 2
Journal of Cardiothoracic Surgery volume 19 , Article number: 163 ( 2024 ) Cite this article
Metrics details
Accurately predicting post-discharge mortality risk in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI) remains a complex and critical challenge. The primary objective of this study was to develop and validate a robust risk prediction model to assess the 12-month and 24-month mortality risk in STEMI patients after hospital discharge.
A retrospective study was conducted on 664 STEMI patients who underwent PPCI at Xiangtan Central Hospital Chest Pain Center between 2020 and 2022. The dataset was randomly divided into a training cohort ( n = 464) and a validation cohort ( n = 200) using a 7:3 ratio. The primary outcome was all-cause mortality following hospital discharge. The least absolute shrinkage and selection operator (LASSO) regression model was employed to identify the optimal predictive variables. Based on these variables, a regression model was constructed to determine the significant predictors of mortality. The performance of the model was evaluated using receiver operating characteristic (ROC) curve analysis and decision curve analysis (DCA).
The prognostic model was developed based on the LASSO regression results and further validated using the independent validation cohort. LASSO regression identified five important predictors: age, Killip classification, B-type natriuretic peptide precursor (NTpro-BNP), left ventricular ejection fraction (LVEF), and the usage of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitors (ACEI/ARB/ARNI). The Harrell's concordance index (C-index) for the training and validation cohorts were 0.863 (95% CI: 0.792–0.934) and 0.888 (95% CI: 0.821–0.955), respectively. The area under the curve (AUC) for the training cohort at 12 months and 24 months was 0.785 (95% CI: 0.771–0.948) and 0.812 (95% CI: 0.772–0.940), respectively, while the corresponding values for the validation cohort were 0.864 (95% CI: 0.604–0.965) and 0.845 (95% CI: 0.705–0.951). These results confirm the stability and predictive accuracy of our model, demonstrating its reliable discriminative ability for post-discharge all-cause mortality risk. DCA analysis exhibited favorable net benefit of the nomogram.
The developed nomogram shows potential as a tool for predicting post-discharge mortality in STEMI patients undergoing PPCI. However, its full utility awaits confirmation through broader external and temporal validation.
Peer Review reports
Introduction
In light of the escalating global prevalence of coronary artery disease, ST-segment elevation myocardial infarction (STEMI) has been identified as a predominant contributor to cardiovascular mortality [ 1 ]. While recent advancements in primary percutaneous coronary intervention (PPCI) have significantly improved short-term therapeutic outcomes for STEMI patients, a pronounced long-term mortality risk remains post-discharge [ 2 ]. Therefore, the need for detailed risk stratification for these patients is paramount, guiding therapeutic interventions and optimizing long-term clinical outcomes [ 3 , 4 ].
Numerous evaluative instruments and risk-assessment algorithms have been developed to gauge post-discharge mortality risk among STEMI cohorts [ 5 , 6 , 7 , 8 ]. However, many of these models draw upon data from extensive clinical trials primarily conducted in European and North American populations, raising questions regarding their applicability and precision for patients outside these regions. Moreover, a majority of these models lean towards conventional statistical methods for variable selection and model development, potentially overlooking crucial predictive factors.
Emerging prominently in both statistical and machine learning disciplines, the Least Absolute Shrinkage and Selection Operator (LASSO) regression technique has positioned itself as an effective tool for feature selection and data dimensionality reduction [ 9 ]. By strategically penalizing regression coefficients, LASSO adeptly identifies variables closely associated with prognostic outcomes, leading to a model that is both concise and rigorous [ 10 ]. With this perspective, this study aims to employ the LASSO regression approach to develop and validate a new predictive algorithm for post-discharge mortality risk in STEMI patients, using data gathered from the Chest Pain Center at Xiangtan Central Hospital over a three-year period. We anticipate that this novel model will enhance clinical decision-making, refine treatment approaches, and improve long-term survival rates for patients.
Study design and participants
In this retrospective cohort study, we enrolled 664 ST-segment elevation myocardial infarction (STEMI) patients who underwent percutaneous coronary intervention (PCI) at Xiangtan Central Hospital Chest Pain Center between January 1, 2020, and July 31, 2022 (Fig. 1 ). The inclusion criteria were: 1) first-time STEMI patients as defined by the guidelines [ 2 ]; 2) receipt of emergency PCI treatment. Exclusion criteria included: 1) age under 18 years; 2) missing essential data; 3) in-hospital death; 4) STEMI patients who did not undergo PCI; 5) expected survival of fewer than six months due to malignant tumors or other non-cardiac diseases. The dataset was randomly divided in a 7:3 ratio into training ( n = 464) and validation ( n = 200) cohorts.
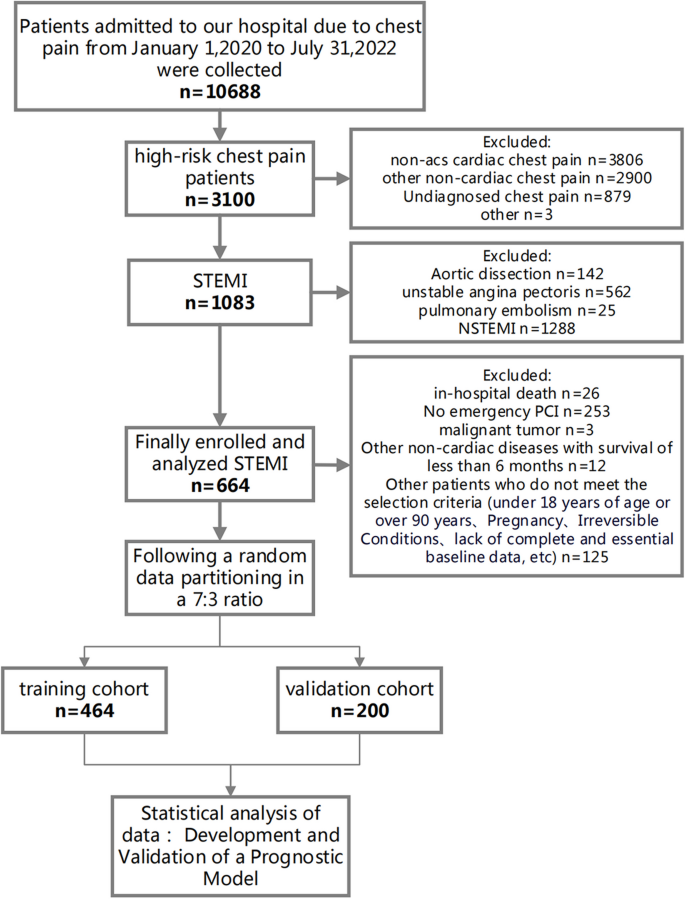
Flow diagram for participant screening, eligibility, and analysis. (Note: The flow diagram in Fig. 1 outlines the process of participant screening, eligibility assessment, and data analysis in the study. The diagram illustrates the sequential steps followed from the initial screening of participants to the final analysis of the collected data)
Data collection and variable definitions
Patient records were retrieved from the hospital's electronic medical record system and the China Chest Pain Center (CCPC) Data platform. These records comprised demographic information, pre-admission medical history, biochemical indicators upon admission, medication usage, and PCI treatment-related details.
A 'current smoker' is defined as an individual who has regularly smoked tobacco within the past year. Regular smoking is characterized as smoking at least once per week, regardless of the quantity smoked.In parallel, a 'current drinker' is defined as an individual who, over the past year, has consumed an average of at least a certain number of alcohol units per week. One alcohol unit is equivalent to approximately 10 ml or 8 g of pure alcohol, roughly corresponding to one bottle of standard strength (5%) beer, a small glass (125 ml) of wine, or a small shot (25 ml) of spirits.
Cardiogenic shock was defined as a state of critical end-organ hypoperfusion due to primary cardiac dysfunction. This was clinically diagnosed based on a combination of hemodynamic parameters and clinical signs, including sustained hypotension (systolic blood pressure < 90 mmHg for at least 30 min or the need for supportive measures to maintain systolic blood pressure above 90 mmHg), evidence of pulmonary congestion, and signs of impaired organ perfusion.
Follow-up and outcome measures
We followed up with all study participants until January 31, 2023. A dedicated team of five experienced cardiovascular physicians and two nurses collected outcome events through outpatient visits, telephone follow-ups, and community check-ins. The primary follow-up end point event was the all-cause death risk.
Ethics and informed consent
This study was approved by the Ethics Committee of Xiangtan Central Hospital (Xiangtan, China) (Ethics Approval No. 2023–02-001) and adhered to the principles outlined in the Helsinki Declaration. As a retrospective study that only collected clinical data without intervening in patient treatment, informed consent was waived.
Statistical analysis
All data was normalized through z-score transformation, resulting in a mean of 0 and a standard deviation of 1. For the selection of predictors, we deployed the Least Absolute Shrinkage and Selection Operator (LASSO) regression technique to identify variables exhibiting a significant correlation with all-cause mortality. A regression model was subsequently developed incorporating these selected variables using the glmnet package in R for LASSO regression modeling. Each patient's mortality risk score was computed through a linear combination of the chosen predictive variables and their respective coefficients. The optimal lambda (λ) parameter, minimizing cross-validation error, was selected. The model was refitted using the selected λ and all available observations, causing most covariate coefficients to shrink to zero while retaining only those non-zero coefficients identified by the LASSO procedure. These non-zero coefficients were classified as mortality risk predictors. A mortality risk prediction nomogram was then constructed using the "rms" package. Model performance was assessed via discrimination and calibration analyses. Discrimination was quantified using the area under the Receiver Operating Characteristic (ROC) curve, while calibration was assessed by examining calibration plots. We utilized Decision Curve Analysis (DCA) to assess the clinical utility of our predictive model. DCA quantifies the net benefits of a model at various threshold probabilities, balancing the true positives against the false positives [ 11 ]. This approach helps in identifying clinically relevant threshold ranges where the model provides significant decision-making advantages. The predictive accuracy of the risk model was evaluated via the C-statistics for discrimination and the Hosmer–Lemeshow chi-square test for calibration.
Group differences were assessed using independent samples t-tests, chi-square tests, or Mann–Whitney U tests as appropriate. Normality was tested with the Kolmogorov–Smirnov test. Normally distributed continuous variables were reported as mean ± standard deviation, while non-normally distributed continuous variables were expressed as medians with interquartile ranges. Categorical variables were presented as n (%). All statistical tests were two-sided, with a p -value of < 0.05 deemed statistically significant. Model development, discrimination, and calibration performance were evaluated using similar methods. All statistical analyses were conducted using R software, version 4.2.0 ( http://www.R-project.org ).
Table 1 delineates the baseline characteristics of patients with STEMI following PPCI intervention, categorized into the training cohort ( N = 464) and the validation cohort ( N = 200). The male constituent in both cohorts was 78.2% and 76.0%, respectively, with a P -value of 0.595. The mean age was reported at 63.0 years; the age distribution for the training cohort ranged between 55.0 and 71.0 years, while the validation cohort exhibited a similar range from 54.0 to 71.0 years ( P = 0.855). Concerning historical medical data, both the training and validation cohorts demonstrated smoking prevalences of 56.9% and 57.0%, respectively. Therapeutically, β-blocker administration was observed in 88.8% of the training cohort and 89.0% of the validation cohort. In the context of PPCI procedural specifics, the Radial artery technique was the preferred method, with adoption rates of 93.3% in the training cohort and 92.5% in the validation cohort. Mortality indices for the training and validation cohorts were 5.82% and 4.00%, respectively, yielding a P -value of 0.439. A comprehensive data set, including statistical figures, P -values, and relevant terminologies, is tabulated in Table 1 .
Table 2 offers an incisive univariate Cox regression analysis elucidating the mortality risk post-discharge in STEMI patients who underwent PPCI intervention. A one-year increment in age emerged as a salient factor, correlating with an amplified mortality risk (HR = 1.062, 95% CI: 1.026–1.099, P = 0.001). History of cerebrovascular events, notably stroke, signaled a heightened death risk (HR = 2.45, 95% CI: 1.035–5.8, P = 0.042). Paradoxically, current smokers exhibited a relative attenuation in mortality risk (HR = 0.439, 95% CI: 0.201–0.959, P = 0.039). Renal compromise underscored a conspicuous escalation in mortality risk (HR = 3.775, 95% CI: 1.751–8.136, P = 0.001). The presence of atrial fibrillation corresponded with a marked surge in mortality risk (HR = 2.818, 95% CI: 1.067–7.447, P = 0.037). Significantly, mortality risk metrics within the Killip classification groups II-VI superseded that of the Killip classification group I (HR = 2.05, 95% CI: 1.529–2.747, P < 0.001). From a biochemical perspective, an increment of 100 units in NT-proBNP subtly paralleled with an augmented mortality risk (HR = 1.009, 95% CI: 1.005–1.013, P < 0.001). Every 1% reduction in left ventricular ejection fraction portended an elevated mortality risk (HR = 0.921, 95% CI: 0.887–0.956, P < 0.001). Medicinally, the administration of Beta-blockers (HR = 0.275, 95% CI: 0.12–0.629, P = 0.002) and agents from the ACEI/ARB/ARNI spectrum (HR = 0.16, 95% CI: 0.075–0.342, P < 0.001) resonated with a conspicuous decrement in mortality risk. The onset of hemodynamic shock was identified as a pivotal exacerbator of mortality risk (HR = 3.655, 95% CI: 1.384–9.657, P = 0.009). A more granular inspection of the data can be ascertained in Table 2 .
Through the application of the LASSO regression model, we discerned five pivotal prognostic factors robustly correlated with mortality outcomes: age, the Killip classification, NT-proBNP levels, LVEF, and the administration of ACEI/ARB/ARNI therapies, as illustrated in Fig. 2 .
LASSO Regression Coefficient Path and CV LASSO Regression Coefficient Path. A LASSO Regression Coefficient Path. B CV LASSO Regression Coefficient Path. (Note:The LASSO regression coefficient path displays how the coefficients of each variable change with increasing regularization parameter λ.The CV LASSO regression coefficient path illustrates the coefficients' behavior with λ tuned through cross-validation. Both paths provide insights into the impact of regularization on variable selection and coefficient estimation in the LASSO regression model)
Detailed outcomes from the COX multivariable regression analysis, derived from the quintet of predictors elicited by LASSO regression, are tabulated in Table 3 :
Incremental age, specified as each advancing year, was linked to a pronounced escalation in mortality risk (HR = 1.047, 95% CI: 1.012–1.083, P = 0.008). A heightened Killip classification substantially correlated with augmented mortality risk (HR = 1.515, 95% CI: 1.094–2.098, P = 0.012). For every centesimal augmentation in NT-proBNP levels, a discernible amplification in mortality risk was evident (HR = 1.005, 95% CI: 1.001–1.009, P = 0.045). In contrast, each percentage point elevation in the left ventricular ejection fraction (LVEF) was significantly allied with a decrement in death risk (HR = 0.952, 95% CI: 0.911–0.995, P = 0.028). Notably, patients undergoing ACEI/ARB/ARNI therapeutic regimens manifested a marked diminution in mortality susceptibility (HR = 0.200, 95% CI: 0.089–0.450, P < 0.001).
Employing time-dependent ROC curves, we elucidated the model's discriminative prowess. A C-index of 0.863 was observed in the training cohort, with a 95% CI ranging from 0.792 to 0.934. This corresponded to AUC values of 0.864 and 0.845 at the 12-month and 24-month intervals, respectively. A C-index of 0.888 was evident for the validation set, enveloped by a 95% CI of 0.821–0.955. This translated to AUC metrics of 0.785 and 0.812 for the 12 and 24 months, respectively (Fig. 3 A and B). Post the execution of 500 bootstrap resampling iterations, the model's intrinsic stability was emphatically confirmed (Fig. 3 C). Temporal calibration curves ratified impeccable model alignment across the 12- and 24-month benchmarks for the training and validation sets, thereby underscoring the model's robustness (Fig. 4 ).
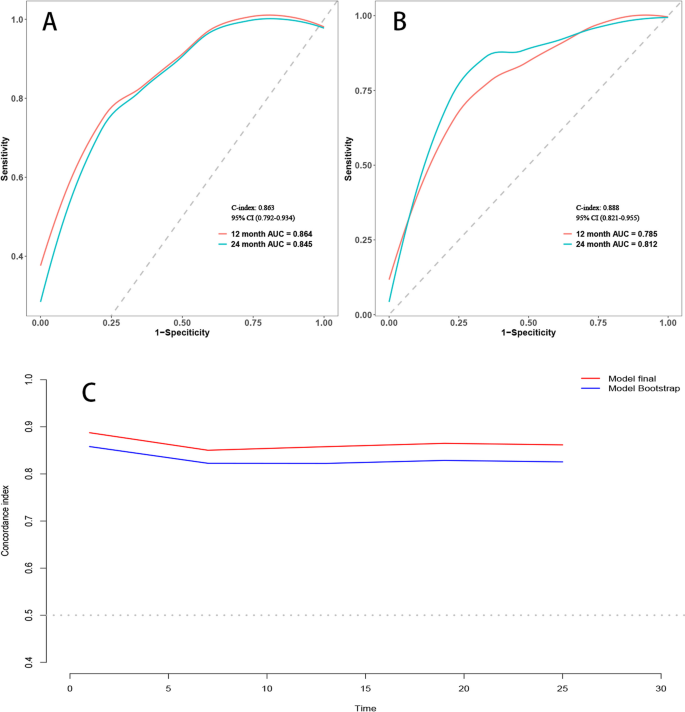
Area under the Receiver Operating Characteristic (ROC) curve and Bootstrap validation. A ROC curves for the training set at 12 months and 24 months. B ROC curves for the validation set at 12 months and 24 months. C Comparison of model stability between the original model and 500 rounds of Bootstrap validation on the training set
Calibration curves at different time points. A Calibration curves for the training set at 12 months and 24 months. B Calibration curves for the validation set at 12 months and 24 months. (Note: The calibration curves depict the agreement between the predicted probabilities and the observed outcomes at different time points. The curves represent the performance of the predictive model in terms of calibration, indicating how well the model's predicted probabilities align with the actual probabilities)
The delineated time-dependent DCA and DCA nomogram across both cohorts unequivocally showcased the net clinical benefit, with the nomogram rendition distinctly surpassing the individual performance of the five discrete subsets (Fig. 5 ). Figure 6 portrays a delineative chart encapsulating the risk scores ascribed to each predictive variable. Elevated scores inherently resonate with an accentuated prospective mortality threat. Leveraging this schematic, patients were accorded scores and stratified into high and low-risk echelons. The Kaplan–Meier survival trajectories were then harnessed to evaluate the congruence across these cohorts. Indubitably, the risk quotient for mortality was attenuated in the low-risk segment compared to its high-risk counterpart across both data partitions (Fig. 7 ).
Decision Curve Analysis (DCA) with Time and DCA Nomogram. A1: DCA with Time for the Training Set. B1: DCA with Time for the Validation Set. A2: DCA Nomogram for the Training Set. B2: DCA Nomogram for the Validation Set. (Note: The DCA curves in A1 and B1 illustrate the net benefit of the predictive model over a range of threshold probabilities at different time points for the training and validation sets. These curves provide insights into the clinical usefulness and added value of the model compared to alternative decision strategies. Additionally, the DCA nomograms in A2 and B2 provide a graphical representation of the decision curves, allowing for a more intuitive interpretation and application of the model's results)
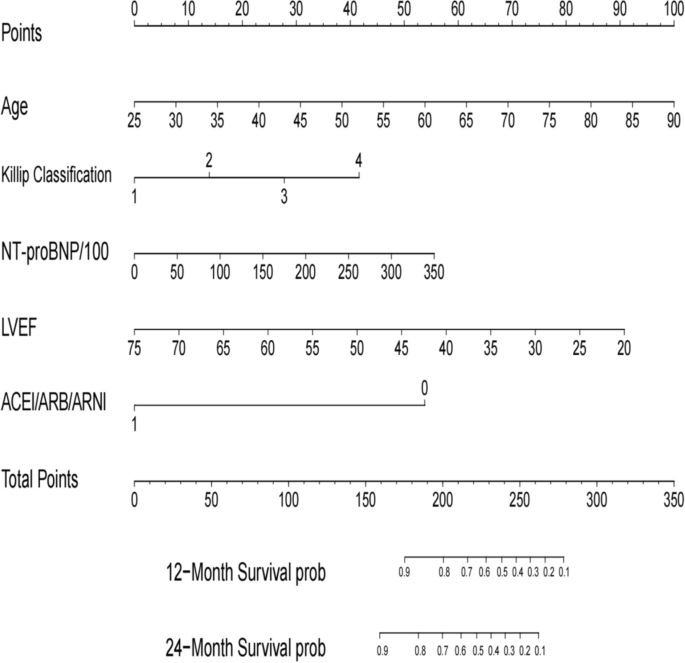
Nomogram for all-cause mortality risk prediction. (Note: The nomogram presents a visual tool for predicting the risk of all-cause mortality. It combines various predictors or risk factors into a comprehensive model that provides an individualized risk assessment. The nomogram allows for a simple and intuitive estimation of the probability of mortality based on the values assigned to each predictor. Clinicians can use this nomogram as a practical aid in risk assessment and shared decision-making with patients regarding appropriate interventions and management strategies)
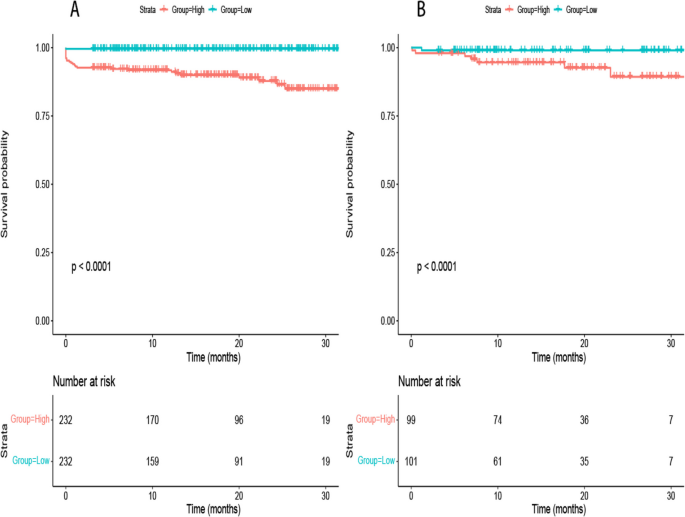
Rationality Analysis: Kaplan–Meier Survival Curves of High-Score and Low-Score Groups. A Rationality Analysis for the Training Set. B Rationality Analysis for the Validation Set. (Note: The Kaplan–Meier survival curves depicted in A and B demonstrate the differences in survival outcomes between the high-score and low-score groups. These curves serve as a rationality analysis to evaluate the predictive performance of the scoring system or model. The separation of the survival curves indicates the ability of the scoring system to stratify patients into distinct risk groups. This analysis provides insights into the reliability and validity of the scoring system in predicting survival outcomes and aids in assessing its clinical utility)
In our analysis presented in Supplementary Table 1 , we observed a significant reduction in mortality among STEMI patients using ACE inhibitors (ACEI), angiotensin receptor blockers (ARB), or angiotensin receptor-neprilysin inhibitors (ARNI). This effect was evident in both groups classified by left ventricular ejection fraction (LVEF), with hazard ratios indicating a substantial protective effect of these medications on survival.
Temporal validation
To address the potential for overfitting and to assess the temporal generalizability of our model, a temporal validation was performed. Supplementary Fig. 1 illustrates the receiver operating characteristic (ROC) curves derived from the predictive model. Panel A presents the ROC curves for mortality predictions at 12 and 24 months post-discharge in the training cohort, which included 480 patients from the 2020–2021 dataset. The model demonstrated good predictive ability with an AUC of 0.819 (95% CI: 0.724–0.914) for 12-month mortality and an AUC of 0.836 (95% CI: 0.761–0.911) for 24-month mortality.
Due to the limited follow-up time available for the validation cohort, which consisted of 184 patients from the 2022 dataset, the model's performance was assessed using shorter-term outcomes. Panel B therefore shows the ROC curves for 6-month and 12-month mortality, yielding AUC values of 0.796 (95% CI: 0.603–0.988) and 0.877 (95% CI: 0.642–1.112), respectively. The shortened follow-up period for the validation cohort necessitated the use of these interim time points for model assessment.
Comparison of two models
Supplementary Fig. 2 compares the predictive accuracy of two models developed via LASSO regression, using ROC curves for the training set (Panel A) and the validation set (Panel B). Model A, defined by the '1se' criterion, demonstrated an AUC of 0.875 in the training set and 0.763 in the validation set, indicating robustness across both datasets with essential predictors: age, Killip classification, ACEI/ARB/ARNI, ntpro-BNP/100, and LVEF. Model B, the 'min' full model, showed comparable AUCs in the training (0.867) and validation (0.765) sets. The performance similarity in both datasets suggests Model A's parsimony is effective for clinical application without compromising predictive ability.
Model evaluation metrics
Supplementary Table 2 in our manuscript details critical model evaluation metrics on both training and validation sets. Notably, the model shows a strong Area Under the Receiver Operating Characteristic Curve (AUC) with 0.88 on the training set and 0.795 on the validation set, indicating its robust predictive ability. The accuracy rates of 0.909 (training) and 0.85 (validation) further affirm the model's effectiveness. Additionally, Sensitivity and Specificity values demonstrate balanced performance in identifying positive and negative cases. The Positive and Negative Likelihood Ratios (PLR and NLR) along with Predictive Values (PPV and NPV) underscore the model's precision in predicting outcomes. These metrics collectively highlight the model's reliability and potential applicability in practical scenarios.
In the present investigation, we meticulously developed and validated a predictive model that quantifies the 12-month and 24-month post-discharge mortality risks for STEMI patients. The primary predictors integrated into this model include age, the Killip classification, NTpro-BNP concentrations, LVEF values, and the therapeutic use of ACEI/ARB/ARNI (Central Illustration) (Fig. 8 ). The model's discriminating capability, as evidenced by its C-index and the area under the ROC curve, underscores its reliability and predictive accuracy.
Central Illustration
The Killip and Kimball classification has historically been a foundational tool in the early studies of post-STEMI mortality patterns. In their landmark 1967 study, Killip and Kimball thoroughly assessed a cohort of 250 patients and proposed an evaluation framework based on clinical manifestations [ 12 ]. This evaluative method remains strongly correlated with mortality outcomes in many contemporary cardiovascular studies despite over five decades. The classification system devised by Killip and Kimball is consistent with findings from recent research [ 13 , 14 ], highlighting the central role of the Killip classification in prognosticating STEMI outcomes.
In recent years, research centered on NTpro-BNP has gained prominence. In his landmark study, De Lemos delineated a distinct correlation between NTpro-BNP concentrations and the prognostic outcomes of acute coronary syndrome [ 15 ]. Our results echo this assertion, endorsing NTpro-BNP as a pivotal prognostic marker for post-STEMI mortality. Contemporary cardiovascular literature further reaffirms the critical role of NTpro-BNP in gauging outcomes among STEMI patients [ 16 , 17 ].
The therapeutic application of ACEI/ARB remains a cornerstone in the management strategies for myocardial infarction. Pfeffer's pioneering work illuminated the significant role of ACEIs in reducing mortality following myocardial infarction [ 18 ]. Our findings align with this perspective, underscoring the beneficial impact of ACEI/ARB in diminishing post-STEMI mortality risks. Beyond Pfeffer's foundational research, recent studies also validate the efficacy of the ACEI/ARB/ARNI ensemble in mitigating cardiovascular event risks [ 19 , 20 ].
Left Ventricular Ejection Fraction (LVEF) and age are quintessential prognostic indicators for heart failure and coronary artery disease. Solomon's study established a pronounced association between diminishing LVEF and heightened mortality risk [ 21 ]. Concurrently, Avezum elucidated that STEMI-associated mortality increases with advancing age [ 22 ].
While historical literature consistently underscores the salient roles of age, the Killip classification, and LVEF in stratifying post-myocardial infarction mortality risks, our model introduces a novel integration. It synergistically incorporates these traditional markers with NTpro-BNP concentrations and the therapeutic regimen of ACEI/ARB/ARNI, offering a more encompassing predictive paradigm. Including ACEI/ARB/ARNI in our model reveals a marked reduction in mortality risk, an insight less emphasized in previous predictive frameworks. By harmonizing these determinants, our findings present a comprehensive and updated perspective on post-STEMI mortality trajectories. Moreover, while prior investigations laid the foundational groundwork, our refined insights are poised to enhance the precision of clinical decision-making.
Explanation of methodology and findings
We employed LASSO regression, an analytical technique that selectively reduces certain regression coefficients to zero, emphasizing the most relevant predictive variables. One of the key advantages of this method is its resistance to overfitting, especially when confronted with a plethora of potential predictors. Accordingly, the five predictors we identified are arguably the most closely correlated with post-discharge mortality risks in STEMI patients.
Novelty of findings
Our model provides a approach to estimating mortality risk in STEMI patients, blending a range of clinical, demographic, and treatment aspects. It potentially helps in identifying patients who might benefit from customized care after discharge, influencing therapeutic decisions such as medication adjustments and lifestyle considerations. We recommend adaptable steps for healthcare professionals to integrate this model into their practice, potentially enhancing patient care and outcomes.
Study limitations
It is crucial to note that our research, being retrospective in nature, may be susceptible to selection bias. Moreover, given that our patient cohort was exclusively sourced from Xiangtan Central Hospital, caution should be exercised when extrapolating our findings to broader populations. Additionally, external validation in more diverse and larger populations is essential to confirm the applicability of our findings. Our study potentially overlooked specific covariates, such as dietary habits and physical activity of patients. Future research should be broader, incorporating multiple centers and considering a more diverse array of covariates.
Directions for future research
In light of our findings, subsequent studies should delve deeper into the mechanistic roles of ACEI/ARB/ARNI in mitigating mortality risks for STEMI patients. A comparison of our model with other well-established models could also provide insightful results. Furthermore, it's imperative to assess the applicability and accuracy of our model across varied populations and geographical locations.
In this study, a model was developed to predict the 12 and 24-month post-discharge mortality risks for STEMI patients post-PCI. Using LASSO regression, we identified five key predictors. While the model shows promise in aiding risk stratification and decision-making for post-PCI STEMI patients, its broader applicability and effectiveness require confirmation through further external and temporal validation.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due the database owner is reluctant to make them public but are available from the corresponding author upon reasonable request.
Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28(1):6–14.
Article PubMed Google Scholar
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio A, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–7.
Article CAS PubMed Google Scholar
Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–42.
Fang C, Chen Z, Zhang J, Jin X, Yang M. Construction and evaluation of nomogram model for individualized prediction of risk of major adverse cardiovascular events during hospitalization after percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Front Cardiovasc Med. 2022;9:1050785.
Article CAS PubMed PubMed Central Google Scholar
Gao N, Qi X, Dang Y, Li Y, Wang G, Liu X, Zhu N, Fu J. Establishment and validation of a risk model for prediction of in-hospital mortality in patients with acute ST-elevation myocardial infarction after primary PCI. BMC Cardiovasc Disor. 2020;20(1):513.
Article CAS Google Scholar
Sherazi SWA, Zheng H, Lee JY. A machine learning-based applied prediction model for identification of Acute Coronary Syndrome (ACS) Outcomes and Mortality in Patients during the Hospital Stay. Sensors-Basel. 2023;23(3):1351.
Article PubMed PubMed Central Google Scholar
Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53.
Tibshirani R. The lasso method for variable selection in the cox model. Stat Med. 1997;16(4):385–95.
Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–74.
Killip TR, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–64.
Sathvik M, Kalva ECSS, Suma G. A study on acute myocardial infarction and its prognostic predictors. Cureus. 2023;15:e34775.
PubMed PubMed Central Google Scholar
Sasaki K, Koeda Y, Yoshizawa R, Ishikawa Y, Ishida M, Itoh T, Morino Y, Saitoh H, Onodera H, Nozaki T, Maegawa Y, Nishiyama O, Ozawa M, Osaki T, Nakamura A. Comparing in-hospital outcomes for acute myocardial infarction patients in high-volume hospitals performing primary percutaneous coronary intervention vs. regional general hospitals. Circ J. 2023;87:1347.
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345(14):1014–21.
Jering KS, Claggett BL, Pfeffer MA, Granger CB, Køber L, Lewis EF, Maggioni AP, Mann DL, McMurray JJV, Prescott MF, Rouleau JL, Solomon SD, Steg PG, von Lewinski D, Braunwald E. Prognostic importance of NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) Following High-Risk Myocardial Infarction in the PARADISE-MI Trial. Circ Heart Fail. 2023;16(5):e010259.
Almeida I, Chin J, Santos H, Miranda H, Santos M, Sá C, Almeida S, Sousa C, Almeida L. Prognostic value of brain natriuretic peptide in ST-elevation myocardial infarction patients: a Portuguese registry. Rev Port Cardiol. 2022;41(2):87–95.
Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77.
She J, Lou B, Liu H, Zhou B, Jiang GT, Luo Y, Wu H, Wang C, Yuan Z. ARNI versus ACEI/ARB in reducing cardiovascular outcomes after myocardial infarction. ESC Heart Failure. 2021;8(6):4607–16.
Ahn WJ, Rha S, Choi BG, Jeong MH, Ahn TH, Yoon J, Kim HS, Seung KB, Gwon HC, Chae SC, Kim CJ, Cha KS, Lee JH, Chae JK, Joo SJ, Yoon CH, Hur SH, Seong IW, Hwang KK, Kim DI, Oh SK, Hwang JY. The impact of angiotensin-converting-enzyme inhibitors versus angiotensin receptor blockers on 3-year clinical outcomes in elderly (≥ 65) patients with acute myocardial infarction without hypertension. Heart Vessels. 2023;38(7):898–908.
Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–44.
Avezum A, Makdisse M, Spencer F, Gore JM, Fox KAA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, Philippe CJ. Impact of age on management and outcome of acute coronary syndrome: observations from the global registry of acute coronary events (GRACE). Am Heart J. 2005;149(1):67–73.
Download references
Acknowledgements
We appreciate the assistance provided by the Chest Pain Center and Department of Scientific Research of Xiangtan Central Hospital in ethical review and data collection.
This study is supported by Scientific Bureau of Xiangtan City (SF-YB20201023), Xiangtan City, Hunan Province, China and Committee of Development and Reform of Hunan Province (2019–875), Changsha, Hunan Province, China.
Author information
Lingling Zhang and Zhican Liu contributed equally to this work.
Authors and Affiliations
Department of Cardiology, Xiangtan Central Hospital, Xiangtan, 411100, China
Lingling Zhang, Zhican Liu, Yunlong Zhu, Mingxin Wu, Haobo Huang & Jianping Zeng
Graduate Collaborative Training Base of Xiangtan Central Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, China
Zhican Liu, Yunlong Zhu, Mingxin Wu & Jianping Zeng
Department of Cardiology, the Second Xiangya Hospital of Central South University, Changsha, Hunan, 410011, China
Yunlong Zhu
Department of Scientific Research, Xiangtan Central Hospital, Xiangtan, 411100, China
Medical Department, Xiangtan Central Hospital, Xiangtan, 411100, China
Wenbin Yang
You can also search for this author in PubMed Google Scholar
Contributions
Lingling Zhang, Zhican Liu, Yunlong Zhu: established the hypothesis, performed the statistical analysis, wrote the manuscript. Jianping Zeng, Haobo Huang, and Wenbin Yang: interpreted statistical analysis and conducted multivariate analysis. Zhican Liu and Lingling Zhang: data collection and participated follow-up. Mingxin Wu and Ke Peng: initiated the study hypothesis, edited the manuscript.
Corresponding authors
Correspondence to Ke Peng or Jianping Zeng .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the Ethics Committee of Xiangtan Central Hospital (Xiangtan, China, No.2023–02-001) and conformed to the principles outlined in the Declaration of Helsinki.The need for informed consent was waived by the ethics committee Review Board of Xiangtan Central Hospital, because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., supplementary material 4., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, L., Liu, Z., Zhu, Y. et al. Development and validation of a prognostic model for predicting post-discharge mortality risk in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI). J Cardiothorac Surg 19 , 163 (2024). https://doi.org/10.1186/s13019-024-02665-3
Download citation
Received : 13 August 2023
Accepted : 20 March 2024
Published : 30 March 2024
DOI : https://doi.org/10.1186/s13019-024-02665-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- ST-segment elevation myocardial infarction (STEMI)
- All-cause mortality risk
- Predictive model
- Least Absolute Shrinkage and Selection Operator (LASSO)
- Decision Curve Analysis (DCA)
Journal of Cardiothoracic Surgery
ISSN: 1749-8090
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Myocardial infarction.
Niranjan Ojha ; Amit S. Dhamoon .
Affiliations
Last Update: August 8, 2023 .
- Continuing Education Activity
Myocardial infarction (MI), colloquially known as "heart attack," is caused by decreased or complete cessation of blood flow to a portion of the myocardium. Myocardial infarction may be"silent," and go undetected, or it could be a catastrophic event leading to hemodynamic deterioration and sudden death. Most myocardial infarctions are due to underlying coronary artery disease, the leading cause of death in the United States. With coronary artery occlusion, the myocardium is deprived of oxygen. Prolonged deprivation of oxygen supply to the myocardium can lead to myocardial cell death and necrosis. Patients can present with chest discomfort or pressure that can radiate to the neck, jaw, shoulder, or arm. In addition to the history and physical exam, myocardial ischemia may be associated with ECG changes and elevated biochemical markers such as cardiac troponins. This activity describes the pathophysiology, evaluation, and management of myocardial infarction and highlights the role of the interprofessional team in improving care for affected patients.
- Review the basic pathophysiology of myocardial infarction.
- Explain the management protocol when presented with acute myocardial infarction, including all necessary laboratory and other diagnostic testing.
- Summarize the long-term management and rehabilitation for a patient post-MI.
- Explain interprofessional team strategies for improving care coordination and communication to advance the prevention and management of myocardial infarction leading to improved outcomes.
- Introduction
Myocardial infarction (MI), colloquially known as “heart attack,” is caused by decreased or complete cessation of blood flow to a portion of the myocardium. Myocardial infarction may be “silent” and go undetected, or it could be a catastrophic event leading to hemodynamic deterioration and sudden death. [1] Most myocardial infarctions are due to underlying coronary artery disease, the leading cause of death in the United States. With coronary artery occlusion, the myocardium is deprived of oxygen. Prolonged deprivation of oxygen supply to the myocardium can lead to myocardial cell death and necrosis. [2] Patients can present with chest discomfort or pressure that can radiate to the neck, jaw, shoulder, or arm. In addition to the history and physical exam, myocardial ischemia may be associated with ECG changes and elevated biochemical markers such as cardiac troponins. [3] [4]
As stated above, myocardial infarction is closely associated with coronary artery disease. INTERHEART is an international multi-center case-control study which delineated the following modifiable risk factors for coronary artery disease: [5] [6]
- Abnormal lipid profile/blood apolipoprotein (raised ApoB/ApoA1)
- Hypertension
- Diabetes mellitus
- Abdominal obesity (waist/hip ratio) (greater than 0.90 for males and greater than 0.85 for females)
- Psychosocial factors such as depression, loss of the locus of control, global stress, financial stress, and life events including marital separation, job loss, and family conflicts
- Lack of daily consumption of fruits or vegetables
- Lack of physical activity
- Alcohol consumption (weaker association, protective)
The INTERHEART study showed that all the above risk factors were significantly associated with acute myocardial infarction except for alcohol consumption, which showed a weaker association. Smoking and abnormal apolipoprotein ratio showed the strongest association with acute myocardial infarction. The increased risk associated with diabetes and hypertension were found to be higher in women, and the protective effect of exercise and alcohol was also found to be higher in women. [5]
Other risk factors include a moderately high level of plasma homocysteine, which is an independent risk factor of MI. Elevated plasma homocysteine is potentially modifiable and can be treated with folic acid, vitamin B6, and vitamin B12. [7]
Some non-modifiable risk factors for myocardial infarction include advanced age, male gender (males tend to have myocardial infarction earlier in life), genetics (there is an increased risk of MI if a first-degree relative has a history of cardiovascular events before the age of 50). [6] [8] The role of genetic loci that increase the risk for MI is under active investigation. [9] [10]
- Epidemiology
The most common cause of death and disability in the western world and worldwide is coronary artery disease. [11] Based on 2015 mortality data from the National Health Interview Survey (NHIS-CDC), MI mortality was 114,023, and MI any-mention mortality (i.e., MI is mentioned as a contributing factor in the death certificate) was 151,863.
As per the National Health and Nutrition Examination Survey (NHANES)-CDC data from 2011 to 2014, an estimated 16.5 million Americans older than 20 years of age have coronary artery disease, and the prevalence was higher in males than females for all ages. As per the NHANES 2011 through 2014, the overall prevalence of MI is 3.0% in US adults older than 20 years of age.
Prevalence of MI in the US Sub-populations
Non-Hispanic Whites
- 4.0% (Male)
- 2.4% (Female)
Non-Hispanic Blacks
- 3.3% (Male)
- 2.2% (Female)
- 2.9% (Male)
- 2.1% (Female)
Non-Hispanic Asians
- 2.6% (Male)
- 0.7% (Female)
Based on the Atherosclerosis Risk in Communities Study (ARIC) performed by National Heart, Lung, and Blood Institute (NHLBI) collected between 2005 and 2014, the estimated annual incidence is 605,000 new MIs and 200,000 recurrent MIs. [12]
The ARIC study also found that the average age at first MI is 65.6 years for males and 72.0 years for females. In the past decades, several studies have shown a declining incidence of MI in the United States. [12]
- Pathophysiology
The acute occlusion of one or multiple large epicardial coronary arteries for more than 20 to 40 minutes can lead to acute myocardial infarction. The occlusion is usually thrombotic and due to the rupture of a plaque formed in the coronary arteries. The occlusion leads to a lack of oxygen in the myocardium, which results in sarcolemmal disruption and myofibril relaxation. [2] These changes are one of the first ultrastructural changes in the process of MI, which are followed by mitochondrial alterations. The prolonged ischemia ultimately results in liquefactive necrosis of myocardial tissue. The necrosis spreads from sub-endocardium to sub-epicardium. The subepicardium is believed to have increased collateral circulation, which delays its death. [2] Depending on the territory affected by the infarction, the cardiac function is compromised. Due to the negligible regeneration capacity of the myocardium, the infarcted area heals by scar formation, and often, the heart is remodeled characterized by dilation, segmental hypertrophy of remaining viable tissue, and cardiac dysfunction. [13]
- History and Physical
The imbalance between oxygen supply and the demand leads to myocardial ischemia and can sometimes lead to myocardial infarction. The patient’s history, electrocardiographic findings, and elevated serum biomarkers help identify ischemic symptoms. Myocardial ischemia can present as chest pain, upper extremity pain, mandibular, or epigastric discomfort that occurs during exertion or at rest. Myocardial ischemia can also present as dyspnea or fatigue, which are known to be ischemic equivalents. [14] The chest pain is usually retrosternal and is sometimes described as the sensation of pressure or heaviness. The pain often radiates to the left shoulder, neck, or arms with no obvious precipitating factors, and it may be intermittent or persistent. The pain usually lasts for more than 20 minutes. [15] It is usually not affected by positional changes or active movement of the region. Additional symptoms, such as sweating, nausea, abdominal pain, dyspnea, and syncope, may also be present. [14] [16] [17] The MI can also present atypically with subtle findings such as palpitations, or more dramatic manifestations, such as cardiac arrest. The MI can sometimes present with no symptoms. [18]
The three components in the evaluation of the MI are clinical features, ECG findings, and cardiac biomarkers.
The resting 12 lead ECG is the first-line diagnostic tool for the diagnosis of acute coronary syndrome (ACS). It should be obtained within 10 minutes of the patient’s arrival in the emergency department. [17] Acute MI is often associated with dynamic changes in the ECG waveform. Serial ECG monitoring can provide important clues to the diagnosis if the initial EKG is non-diagnostic at initial presentation. [14] Serial or continuous ECG recordings may help determine reperfusion or re-occlusion status. A large and prompt reduction in ST-segment elevation is usually seen in reperfusion. [14]
ECG findings suggestive of ongoing coronary artery occlusion (in the absence of left ventricular hypertrophy and bundle branch block): [19]
ST-segment elevation in two contiguous lead (measured at J-point) of
- Greater than 5 mm in men younger than 40 years, greater than 2 mm in men older than 40 years, or greater than 1.5 mm in women in leads V2-V3 and/or
- Greater than 1 mm in all other leads
ST-segment depression and T-wave changes
- New horizontal or down-sloping ST-segment depression greater than 5 mm in 2 contiguous leads and/or T inversion greater than 1 mm in two contiguous leads with prominent R waves or R/S ratio of greater than 1
The hyperacute T-wave amplitude, with prominent symmetrical T waves in two contiguous leads, maybe an early sign of acute MI that may precede the ST-segment elevation. Other ECG findings associated with myocardial ischemia include cardiac arrhythmias, intraventricular blocks, atrioventricular conduction delays, and loss of precordial R-wave amplitude (less specific finding). [14]
ECG findings alone are not sufficient to diagnose acute myocardial ischemia or acute MI as other conditions such as acute pericarditis, left ventricular hypertrophy (LVH), left bundle branch block (LBBB), Brugada syndrome, Takatsubo syndrome (TTS), and early repolarization patterns also present with ST deviation.
ECG changes associated with prior MI (in the absence of left ventricular hypertrophy and left bundle branch block):
- Any Q wave in lead V2-V3 greater than 0.02 s or QS complex in leads V2-V3
- Q wave > 03 s and greater than 1 mm deep or QS complex in leads I, II, aVL, aVF or V4-V6 in any two leads of contiguous lead grouping (I, aVL; V1-V6; II, III, aVF)
- R wave > 0.04 s in V1-V2 and R/S greater than 1 with a concordant positive T wave in the absence of conduction defect.
Biomarker Detection of MI
Cardiac troponins (I and T) are components of the contractile apparatus of myocardial cells and expressed almost exclusively in the heart. Elevated serum levels of cardiac troponin are not specific to the underlying mode of injury (ischemic vs. tension) [14] [20] . The rising and/or falling pattern of cardiac troponins (cTn) values with at least one value above the 99 percentile of upper reference limit (URL) associated with symptoms of myocardial ischemia would indicate an acute MI. Serial testing of cTn values at 0 hours, 3 hours, and 6 hours would give a better perspective on the severity and time course of the myocardial injury. Depending on the baseline cTn value, the rising/falling pattern is interpreted. If the cTn baseline value is markedly elevated, a minimum change of greater than 20% in follow up testing is significant for myocardial ischemia. Creatine kinase MB isoform can also be used in the diagnosis of MI, but it is less sensitive and specific than cTn level. [4] [21]
Different imaging techniques are used to assess myocardial perfusion, myocardial viability, myocardial thickness, thickening and motion, and the effect of myocyte loss on the kinetics of para-magnetic or radio-opaque contrast agents indicating myocardial fibrosis or scars. [14] Some imaging modalities that can be used are echocardiography, radionuclide imaging, and cardiac magnetic resonance imaging (cardiac MRI). Regional wall motion abnormalities induced by ischemia can be detected by echocardiography almost immediately after the onset of ischemia when greater than 20% transmural myocardial thickness is affected. Cardiac MRI provides an accurate assessment of myocardial structure and function. [14]
- Treatment / Management
Acute Management
Reperfusion therapy is indicated in all patients with symptoms of ischemia of less than 12-hours duration and persistent ST-segment elevation. Primary percutaneous coronary intervention (PCI) is preferred to fibrinolysis if the procedure can be performed <120 minutes of ECG diagnosis. If there is no immediate option of PCI (>120 minutes), fibrinolysis should be started within 10 minutes of STEMI after ruling out contraindications. If transfer to a PCI center is possible in 60 to 90 minutes after a bolus of the fibrinolytic agent and patient meets reperfusion criteria, a routine PCI can be done, or a rescue PCI can be planned. [19] [17] If fibrinolysis is planned, it should be carried out with fibrin-specific agents such as tenecteplase, alteplase, or reteplase (class I). [19]
Relief of pain, breathlessness, and anxiety: The chest pain due to myocardial infarction is associated with sympathetic arousal, which causes vasoconstriction and increased workload for the ischemic heart. Intravenous opioids (e.g., morphine) are the analgesics most commonly used for pain relief (Class IIa). [19] The results from CRUSADE quality improvement initiative have shown that the use of morphine may be associated with a higher risk of death and adverse clinical outcomes. [22] The study was done from the CIRCUS (Does Cyclosporine Improve outcome in STEMI patients) database, which showed no significant adverse events associated with morphine use in a case of anterior ST-segment elevation MI. [23] A mild anxiolytic (usually a benzodiazepine) may be considered in very anxious patients (class IIa). Supplemental oxygen is indicated in patients with hypoxemia (SaO2 <90% or PaO2 <60mm Hg) (Class I). [19]
Nitrates: Intravenous nitrates are more effective than sublingual nitrates with regard to symptom relief and regression of ST depression (NSTEMI). The dose is titrated upward until symptoms are relieved, blood pressure is normalized in hypertensive patients, or side effects such as a headache and hypotension are noted. [17]
Beta-blockers: This group of drugs reduces myocardial oxygen consumption by lowering heart rate, blood pressure, and myocardial contractility. They block beta receptors in the body, including the heart, and reduce the effects of circulating catecholamines. Beta-blockers should not be used in suspected coronary vasospasm.
Platelet inhibition: Aspirin is recommended in both STEMI and NSTEMI in an oral loading dose of 150 to 300 mg (non-enteric coated formulation) and a maintenance dose of 75 to 100 mg per day long-term regardless of treatment strategy (class I). [17] Aspirin inhibits thromboxane A2 production throughout the lifespan of the platelet. [24]
Most P2Y12 inhibitors are inactive prodrugs (except for ticagrelor, which is an orally active drug that does not require activation) that require oxidation by hepatic cytochrome P450 system to generate an active metabolite which selectively inhibits P2Y12 receptors irreversibly. Inhibition of P2Y12 receptors leads to inhibition of ATP induced platelet aggregation. The commonly used P2Y12 inhibitors are clopidogrel, prasugrel, and ticagrelor.
The loading dose for clopidogrel is 300 to 600 mg loading dose followed by 75 mg per day.
Prasugrel, 60 mg loading dose, and 10 mg per day of a maintenance dose have a faster onset when compared to clopidogrel. [19]
Patients undergoing PCI should be treated with dual antiplatelet therapy (DAPT) with aspirin + P2Y12 inhibitor and a parenteral anticoagulant. In PCI, the use of prasugrel or ticagrelor is found to be superior to clopidogrel. Aspirin and clopidogrel are also found to decrease the number of ischemic events in NSTEMI and UA. [17]
The anticoagulants used during PCI are unfractionated heparin, enoxaparin, and bivalirudin. The bivalirudin is recommended during primary PCI if the patient has heparin-induced thrombocytopenia. [19]
Long-Term Management
Lipid-lowering treatment: It is recommended to start high-intensity statins that reduce low-density lipoproteins (LDLs) and stabilize atherosclerotic plaques. High-density lipoproteins are found to be protective. [19]
Antithrombotic therapy: Aspirin is recommended lifelong, and the addition of another agent depends on the therapeutic procedure done, such as PCI with stent placement.
ACE inhibitors are recommended in patients with systolic left ventricular dysfunction, or heart failure, hypertension, or diabetes.
Beta-blockers are recommended in patients with LVEF less than 40% if no other contraindications are present.
Antihypertensive therapy can maintain a blood pressure goal of less than 140/90 mm Hg.
Mineralocorticoid receptor antagonist therapy is recommended in a patient with left ventricular dysfunction (LVEF less than 40%).
Glucose lowering therapy in people with diabetes to achieve current blood sugar goals. [19]
Lifestyle Modifications
Smoking cessation is the most cost-effective secondary measure to prevent MI. Smoking has a pro-thrombotic effect, which has a strong association with atherosclerosis and myocardial infarction. [6]
Diet, alcohol, and weight control: A diet low in saturated fat with a focus on whole grain products, vegetables, fruits, and the fish is considered cardioprotective. The target level for bodyweight is body mass index of 20 to 25 kg/m2 and waist circumference of <94 cm for the men and <80 cm for the female. [25]
- Differential Diagnosis
- Angina pectoris
- Non-ST segment elevation myocardial infarction (NSTEMI)
- ST-segment elevation myocardial infarction (STEMI)
- Pulmonary embolism
- Pneumothorax
Despite many advances in treatment, acute MI still carries a mortality rate of 5-30%; the majority of deaths occur prior to arrival to the hospital. In addition, within the first year after an MI, there is an additional mortality rate of 5% to 12%. The overall prognosis depends on the extent of heart muscle damage and ejection fraction. Patients with preserved left ventricular function tend to have good outcomes. Factors that worsen prognosis include:
- Advanced age
- Delayed reperfusion
- Low ejection fraction
- Presence of congestive heart failure
- Elevations in C-reactive protein and B-type natriuretic peptide ( BNP ) levels
- Complications
Type and Manifestation
I: Ischemic
- Reinfarction
- Extension of infarction
II: Arrhythmias
- Supraventricular or ventricular arrhythmia
- Sinus bradycardia and atrioventricular block
III: Mechanical
- Myocardial dysfunction
- Cardiac failure
- Cardiogenic shock
- Cardiac rupture (Free wall rupture, ventricular septal rupture, papillary muscle rupture)
IV: Embolic
- Left ventricular mural thrombus,
- Peripheral embolus
V: Inflammatory
- Pericarditis (infarct associated pericarditis, late pericarditis, or post-cardiac injury pericarditis)
- Pericardial effusion
- Enhancing Healthcare Team Outcomes
The diagnosis and management of patients with ischemic heart disease are best done with an interprofessional team. In most hospitals, there are cardiology teams that are dedicated to the management of these patients.
For patients who present with chest pain, the key to the management of MI is time to treatment. Thus, healthcare professionals, including nurses who work in the emergency department, must be familiar with the symptoms of MI and the importance of rapid triage. A cardiology consult should be made immediately to ensure that the patient gets treated within the time frame recommendations. Because MI can be associated with several serious complications, these patients are best managed in an ICU setting.
There is no cure for ischemic heart disease, and all treatments are symptom-oriented. The key to improving outcomes is to prevent coronary artery disease. The primary care provider and nurse practitioner should educate the patient on the benefits of a healthy diet, the importance of controlling blood pressure and diabetes, exercising regularly, discontinuing smoking, maintaining healthy body weight, and remaining compliant with medications. The pharmacist should educate the patient on types of medication used to treat ischemic heart disease, their benefits, and potential adverse effects.
Only through such a team approach can the morbidity and mortality of myocardial infarction be lowered. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Myocardial Infarction (Heart Attack) Warning Signs in Women. U.S. Department of Health and Human Services Office on Women's Health
ECG With Pardee Waves Indicating AMI. Pardee waves indicate acute myocardial infarction in the inferior leads II, III, and aVF with reciprocal changes in the anterolateral leads. Wikimedia Commons, Glenlarson
Transesophageal echocardiography, Thrombo embolism, Pulmonary artery, Pulmonary Embolism, Thromboembolic , Right Pulmonary artery, TE, RPA, Acute ECG segment elevation mimicking myocardial infarction in a patient with pulmonary embolism Contribute by (more...)
Ischemic ventricular tachycardia in a patient with an old inferior myocardial infarction Contributed by Alina Negru, MD
Disclosure: Niranjan Ojha declares no relevant financial relationships with ineligible companies.
Disclosure: Amit Dhamoon declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Ojha N, Dhamoon AS. Myocardial Infarction. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Myocardial Infarction (Nursing). [StatPearls. 2024] Myocardial Infarction (Nursing). Ojha N, Dhamoon AS, Chapagain R. StatPearls. 2024 Jan
- Review Context-independent identification of myocardial ischemia in the prehospital ECG of chest pain patients. [J Electrocardiol. 2024] Review Context-independent identification of myocardial ischemia in the prehospital ECG of chest pain patients. Swenne CA, Ter Haar CC. J Electrocardiol. 2024 Jan-Feb; 82:34-41. Epub 2023 Nov 7.
- Enhanced External Counterpulsation (EECP): An Evidence-Based Analysis. [Ont Health Technol Assess Ser....] Enhanced External Counterpulsation (EECP): An Evidence-Based Analysis. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2006; 6(5):1-70. Epub 2006 Mar 1.
- Association of Silent Myocardial Infarction and Sudden Cardiac Death. [JAMA Cardiol. 2019] Association of Silent Myocardial Infarction and Sudden Cardiac Death. Vähätalo JH, Huikuri HV, Holmström LTA, Kenttä TV, Haukilahti MAE, Pakanen L, Kaikkonen KS, Tikkanen J, Perkiömäki JS, Myerburg RJ, et al. JAMA Cardiol. 2019 Aug 1; 4(8):796-802.
- Review Prevention of ventricular fibrillation, acute myocardial infarction (myocardial necrosis), heart failure, and mortality by bretylium: is ischemic heart disease primarily adrenergic cardiovascular disease? [Am J Ther. 2004] Review Prevention of ventricular fibrillation, acute myocardial infarction (myocardial necrosis), heart failure, and mortality by bretylium: is ischemic heart disease primarily adrenergic cardiovascular disease? Bacaner M, Brietenbucher J, LaBree J. Am J Ther. 2004 Sep-Oct; 11(5):366-411.
Recent Activity
- Myocardial Infarction - StatPearls Myocardial Infarction - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Search Menu
- Advance Articles
- Editor's Choice
- Supplements
- Image Library
- ESC Journals App
- ESC Content Collections
- Author Guidelines
- Submission Site
- Open Access Options
- Read & Publish
- Author Resources
- Self-Archiving Policy
- About European Journal of Preventive Cardiology
- Editorial Board
- ESC Publications
- About European Society of Cardiology
- Advertising & Corporate Services
- Developing Countries Initiative
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
- < Previous
Cardiology off the map: the impact of remoteness on secondary prevention after myocardial infarction
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Preventive Cardiology or of the European Society of Cardiology.
Conflict of interest: VA: AstraZeneca, Boehringer-Ingelheim, Bayer, NovoNordisk, Novartis. AMB: none.
- Article contents
- Figures & tables
- Supplementary Data
Victor Aboyans, Amine Mamoun Boutaleb, Cardiology off the map: the impact of remoteness on secondary prevention after myocardial infarction, European Journal of Preventive Cardiology , Volume 31, Issue 5, March 2024, Pages 578–579, https://doi.org/10.1093/eurjpc/zwae091
- Permissions Icon Permissions
This editorial refers to ‘Use of secondary prevention medications in metropolitan and non-metropolitan areas: an analysis of 41925 myocardial infarctions in Australia’, by A.C. Livori et al ., https://doi:10.1093/eurjpc/zwad360 .
The burden of cardiovascular diseases (CVDs) is considerable, and the prognosis of patients after a first cardiovascular event depends highly on the quality of management during the hospitalization phase, as well as the long-term implementation of treatments and measures to reduce the risk of recurrent events and mortality. 1 The so-called secondary prevention of CVD has emerged as a cornerstone of risk management. It encompasses for multiple levels of actions including educational programmes for healthy diet, physical activity, and smoking cessation, as well as cardiovascular rehabilitation and pharmacotherapies. Regarding the latter, the European Society of Cardiology recommends the regular use of statins, antiplatelet drugs, angiotensin converting enzyme inhibitors, and beta-blockers to improve the long-term prognosis in patients who experienced myocardial infarction. 1
One of the major challenges in preventive cardiology is in the quality of guidelines implementation. In Europe, the series of EUROASPIRE surveys highlighted a significant residual gap for optimal preventive management of patients with coronary artery disease. 2 , 3 More globally, risk factors and cardiovascular care management are not similar among countries and ethnic groups, representing a barrier to the generalization of studies results in diverse populations. Among different hurdles, one of the major barriers for an adequate secondary prevention are the socio-demographic factors, including low education level and poor socio-economic status (SES). 4 Communities with limited healthcare access, especially those at distance from medical centres, may face a triple challenge: the first can be related to a less favourable cardiovascular risk factor profile and a higher cardiovascular risk, in combination with looser medical attention. 5–7 In a nationwide study in Denmark, almost 10% of the 120 000 victims of a first myocardial infarction did not visit any general physician within the year preceding the cardiac event, and the distance to the first general practitioner was a factor associated with the lack of medical visit. 8 The second is related to the delay for management of acute coronary syndromes at its initial phase. In the STEMI Accelerator-2 project conducted in 12 metropolitan areas in the USA, cath lab activation within 20 min across a geographically diverse group of hospitals was associated with a doubling of primary percutaneous coronary intervention within the recommended guidelines timelines of 120 min. 9 The third challenge is the implementation of cardiac rehabilitation 10 and all the secondary cardiovascular prevention measures in the long term. Geographical remoteness may pose a multifaceted hurdle, not only because of physical distance but also limited healthcare facilities, scarcity of specialized medical practitioners, and logistical difficulties for reaching adequate infrastructures with medical expertise.
From this perspective, the study published by Livori et al . in this issue of the Journal is of big interest: the authors analyzed the effect of remoteness on the implementation of pharmacotherapy for secondary prevention, by analyzing the data of 37 320 patients who experienced a myocardial infarction in the state of Victoria, Australia. 11 As the 6th largest country worldwide, the pertinence of such study in Australia is undisputable. The authors assessed the medication use over 12 months according to the level of remoteness of the patients, based on a country-specific indicator, the Accessibility/Remoteness Index of Australia (ARIA). 11 According to the authors, remoteness had no clinically significant impact for the 3-month post-discharge dispensing, although, similar to elsewhere, 12 the rates of recommended therapies were suboptimal, with only 36% of the study population receiving the four recommended drug classes. Similar findings were reported for the 12-month medication use. The authors have emphasized that subsidizing the drugs for secondary prevention plays a crucial role in medications access despite geographic remoteness. It is however noteworthy that only 10% of the studied population was classified as ‘accessible’ to ‘moderately accessible’ areas according to the ARIA classification, and no area within the state of Victoria is classified as ‘remote’.
This is not the first study on remoteness and cardiovascular prevention in Australia: in the Central Australian Heart Protection Study, the number of visits for secondary prevention appeared lower with greater distance, but multivariate analysis suggested that distance did not influence successful completion of visits. 13 In another study, SES explained a substantial proportion of the association between the residence area and CVD mortality rates, but the remoteness effect remained significant beyond SES for a number of sub-populations. 14 In another large sample of more than 1 million individuals with diabetes classified according to the ARIA remoteness indicator, the highest cardiovascular mortality was found in major urban and remote areas. 15 Importantly, remote areas are often less confronted to air and noise pollution, which can somehow temper the poorer prognosis found in those areas.
These results however can hardly be extrapolated to other world regions. Currently, even in the most remoted areas of high-income countries, access to the post-MI recommended therapeutic drug is eased by the presence of local healthcare facilities and services, medication subsidies and assistance programmes, in addition to education and awareness campaigns. Conversely, in secluded regions, the absence of these factors can affect the treatment availability and consequently the patient’s prognosis. Globally, the PURE study 16 showed higher rates of patients without any secondary prevention therapy in low-income countries (80.2%) as compared to those living with a history of stroke or coronary artery disease in lower-middle- (69.3%), upper-middle- (45.1%), and high-income countries (11.2%). While the prescription rates were similar in urban and rural zones in the high-income countries, a gap in disfavour of rural habitants was progressively increasing with the level of country’s wealth. Looking more granularly, a study in the China Kadoorie Biobank Study, the rate of use of any therapy for secondary prevention was 33% lower in rural areas. 17 In South-East Asia 18 as well as in South America, 19 the rates of use of evidence-based preventive drugs were lower in rural vs. urban patients with a history of coronary artery disease or stroke. Similarly, the rate of medication use for secondary prevention was significantly higher among urban patients in Turkey, as compared to rural counterparts. 20
Addressing these challenges demands a multi-pronged approach. Telemedicine emerges as a solution to improve access to healthcare for remote communities. Distant patients monitoring, virtual consultations, and telehealth services present a promising method to ensure continuous access to medical guidance and support. In a retrospective study in Australia, telehealth cardiology was shown to have a favourable impact on adherence to secondary prevention medications and outcomes. 21 In a multi-centre study in the USA, a multifaceted intervention with close collaboration of the pharmacist, the general physician, and the cardiologist, along with automatic refill reminders, improved substantially the medication adherence, blood pressure control, and attainment of LDL targets after myocardial infarction. 22 A combination of these approached seem definitely useful to empower individuals in remote areas to adhere to their prescribed therapies, thereby mitigating the risk of further cardiac events.
Visseren FLJ , Mach F , Smulders YM , Carballo D , Koskinas KC , Bäck M , et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice . Eur J Prev Cardiol 2022 ; 29 : 5 – 115 .
Google Scholar
Vynckier P , Ferrannini G , Rydén L , Jankowski P , De Backer T , Gevaert S , et al. Gender gap in risk factor control of coronary patients far from closing: results from the European Society of Cardiology EUROASPIRE V registry . Eur J Prev Cardiol 2022 ; 29 : 344 – 351 .
Moerschel KS , De Bacquer D , De Backer G , Wood D , Kotseva K , Wellmann J , et al. Assessing the probability of risk factor control in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey . Eur J Prev Cardiol 2022 ; 29 : 1465 – 1475 .
Bahit MC , Korjian S , Daaboul Y , Baron S , Bhatt DL , Kalayci A , et al. Patient adherence to secondary prevention therapies after an acute coronary syndrome: a scoping review . Clin Ther 2023 ; 45 : 1119 – 1126 .
Noor Hassim I , Norazman MR , Diana M , Khairul Hazdi Y , Rosnah I . Cardiovascular risk assessment between urban and rural population in Malaysia . Med J Malaysia 2016 ; 71 : 331 – 337 .
Nowicki GJ , Ślusarska B , Piasecka H , Bartoszek A , Kocka K , Deluga A . The status of cardiovascular health in rural and urban areas of Janów Lubelski district in eastern Poland: a population-based study . Int J Environ Res Public Health 2018 ; 15 : 2388 .
Nuotio J , Vähämurto L , Pahkala K , Magnussen CG , Hutri-Kähönen N , Kähönen M , et al. CVD risk factors and surrogate markers—urban–rural differences . Scand J Public Health 2020 ; 48 : 752 – 761 .
Kjærulff TM , Bihrmann K , Søndergaard J , Gislason G , Larsen ML , Ersbøll AK . Association between travel distance and face-to-face consultations with general practitioners before an incident acute myocardial infarction: a nationwide register-based spatial epidemiological study . BMJ Open 2024 ; 14 : e079124 .
Zeitouni M , HR A-K , Roettig ML , Bolles MM , Doerfler SM , Fordyce CB , et al. Catheterization laboratory activation time in patients transferred with ST-segment–elevation myocardial infarction: insights from the mission: lifeline STEMI accelerator-2 project . Circ Cardiovasc Qual Outcomes 2020 ; 13 : e006204 .
Van Iterson EH , Laffin LJ , Bruemmer D , Cho L . Geographical and urban-rural disparities in cardiac rehabilitation eligibility and center-based use in the US . JAMA Cardiol 2023 ; 8 : 98 – 100 .
Livori AC , Ademi Z , Ilomäki J , Pol D , Morton JI , Bell JS . Use of secondary prevention medications in metropolitan and non-metropolitan areas: an analysis of 41 925 myocardial infarctions in Australia . Eur J Prev Cardiol 2024 ; 31 : 580 – 588 .
Aboyans V , Boukhris M . Secondary prevention after an acute cardiovascular event: far from targets and large room for improvement . Eur J Prev Cardiol 2022 ; 29 : 360 – 361 .
Tuttle CS , Carrington MJ , Stewart S , Brown A . Overcoming the tyranny of distance: an analysis of outreach visits to optimise secondary prevention of cardiovascular disease in high-risk individuals living in Central Australia . Aust J Rural Health 2016 ; 24 : 99 – 105 .
Jacobs J , Peterson KL , Allender S , Alston LV , Nichols M . Regional variation in cardiovascular mortality in Australia 2009–2012: the impact of remoteness and socioeconomic status . Aust N Z J Public Health 2018 ; 42 : 467 – 473 .
Magliano DJ , Cohen K , Harding JL , Shaw JE . Residential distance from major urban areas, diabetes and cardiovascular mortality in Australia . Diabetes Res Clin Pract 2015 ; 109 : 271 – 278 .
Yusuf S , Islam S , Chow CK , Rangarajan S , Dagenais G , Diaz R , et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey . Lancet 2011 ; 378 : 1231 – 1243 .
Chen Y , Li L , Zhang Q , Clarke R , Chen J , Guo Y , et al. Use of drug treatment for secondary prevention of cardiovascular disease in urban and rural communities of China: China Kadoorie Biobank Study of 0.5 million people . Int J Cardiol 2014 ; 172 : 88 – 95 .
Gupta R , Islam S , Mony P , Kutty VR , Mohan V , Kumar R , et al. Socioeconomic factors and use of secondary preventive therapies for cardiovascular diseases in South Asia: the PURE study . Eur J Prev Cardiol 2015 ; 22 : 1261 – 1271 .
Avezum A , Oliveira GBF , Lanas F , Lopez-Jaramillo P , Diaz R , Miranda JJ , et al. Secondary CV prevention in South America in a community setting: the PURE study . Glob Heart 2017 ; 12 : 305 – 313 .
Kılıç S , Saraçoğlu E , Çekici Y , Yıldırım A , Kuzu Z , Kılıç DD , et al. Comparison of secondary prevention in coronary heart disease patients living in rural and urban areas . Turk Kardiyol Dern Ars 2019 ; 47 : 128 – 136 .
Livori AC , Pol D , Levkovich B , Oqueli E . Optimising adherence to secondary prevention medications following acute coronary syndrome utilising telehealth cardiology pharmacist clinics: a matched cohort study . Int J Clin Pharm 2023 ; 45 : 722 – 730 .
Ho PM , Lambert-Kerzner A , Carey EP , Fahdi IE , Bryson CL , Melnyk SD , et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial . JAMA Intern Med 2014 ; 174 : 186 – 193 .
Author notes
Email alerts.
- Use of secondary prevention medications in metropolitan and non-metropolitan areas: an analysis of 41 925 myocardial infarctions in Australia
Citing articles via
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2047-4881
- Print ISSN 2047-4873
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
Part 4. He was taken to the catheterization lab where the left anterior descending coronary artery (LAD) was shown to be completely occluded. Following successful percutaneous intervention and one drug eluding stent implantation in the LAD normal flow is restored (Thrombosis in myocardial infarction, TIMI = 3). 72 hours later, he is ready to be discharged home.
Additional laboratory studies were ... coronary arteries that results in myocardial infarction. However, this young woman did not have any known cardiovascular risk factors or coronary ...
Most deaths from myocardial infarction occur in the first hours of disease onset, with 40-65% occurring within the first hour and approximately 80% in the first 24 hours 5,6. The recently implemented therapies for MI treatment have been proven to modify patient evolution and prognosis.
This case study aims to explain and illustrate how to care for patients with acute myocardial. infarction based on the rationale and nursing practice evidence underlying the holistic approach ...
Inferior Wall Myocardial Infarction An inferior wall myocardial infarction (MI)—the event that occurred in this case study—is usually caused by occlusion of the right coro-nary artery, resulting in damage to part of the inferior wall of the heart (the shaded area). It is sometimes referred to as a diaphrag-
The time course of myocardial rupture is bimodal, with one peak occurring within the first 24 hours and the other peak occurring 3 to 5 days after myocardial infarction. 22 Shared risk factors for ...
Myocardial infarction with non-obstructive CAD (MINOCA) is clinically defined as acute myocardial infarction in the absence of (≥50% stenosis) obstructive CAD in any artery . The Women's Ischemic Syndrome Evaluation (WISE) study at 10-year follow-up demonstrated that women with no obstructive CAD were at increased risk of death or nonfatal ...
Introduction. Myocardial infarction in the absence of obstructive (>50% stenosis) coronary artery disease (MINOCA) is found in approximately 6% of all patients with acute myocardial infarction (MI) who are referred for coronary angiography. 1, 2 The term MINOCA should be reserved for patients in whom there is an ischaemic basis for their clinical presentation and should be considered a ...
Acute myocardial infarction is the most severe manifestation of coronary artery disease, which causes more than 2·4 million deaths in the USA, more than 4 million deaths in Europe and northern Asia,1and more than a third of deaths in developed nations annually.2. Increased use of evidence-based therapies and lifestyle changes have spurred ...
Dr. David F.M. Brown: The case of this patient high-lights two major issues that must be decisively addressed in the emergency department. One is the management of myocardial infarction with ST-segment elevation (STEMI), and the second is ventricular fibrillation that persists after a num-ber of shocks from an external defibrillator
Securing a patient with myocardial infarction requires a rapid pre-hospital procedure and a fast cardiac intervention at an invasive cardiology centre. The paper describes a case of a 55-year-old man diagnosed with acute coronary syndrome with ST-segment elevation myocardial infarction (STEMI), i.e. myocardial infarction of the bottom wall.
Myocardial infarction type 2. sudden death episodes that may be cardiac (nonisch-emic) or noncardiac in origin. When a type 3 MI is diagnosed and a subsequent autopsy reveals recent evidence of an MI, with a fresh or recent thrombus in the infarct-related artery, the type 3 MI should be re-classified to a type 1 MI.
This case study aims to explain and illustrate how to care for patients with acute myocardial infarction based on the rationale and nursing practice evidence underlying the holistic approach. To achieve high-quality nursing care and management of acute myocardial infarction patients must be in accordance with evidence-based nursing practice and nurses' willingness to modify nursing practice ...
7. Mr. Bob Carlson is a 59 year old male who came to Ventura County Medical Center (VCMC) with nausea, upper back pain he rated 7/10, and diaphoretic. His vital signs were BP 156/92, HR 90, RR 22 SpO2 90%, and temperature 99.5. Physical examination revealed clear lung sounds, mild tachypnea, S1 S2 present, and several ulcerations to the right foot.
Myocardial infraction (MI) is defined as the necrosis in the myocardium due to the lack of the. oxygen supply of heart which cannot be supplied by the coronary artery [2]. It is also known as a ...
A Case Report: Acute Myocardial Infarction in a 29-year-old Male. 2/5/2019 Aaron Tiffee, MD, FACEP , Zariad Saran, DO , Tyler Ingersoll, MS. The HEART score is a go-to tool in assessing the risk of an acute coronary syndrome. But in this case, a score of 3 did not mean the 29-year-old patient was safe. Cardiovascular disease (CVD) is currently ...
Owing to recent advances in early reperfusion strategies, pharmacological therapy, standardized care, and the identification of vulnerable patient subsets, the prognosis of acute myocardial infarction has improved. However, there is still considerable room for improvement. This review article summarizes the latest evidence concerning clinical diagnosis and treatment of acute myocardial infarction.
PDF | This is a bedside case discussion of a patient presenting with acute myocardial infarction. The symptoms and signs are discussed. ... Case-control studies in India have identified that the ...
Understanding the risk factors associated with myocardial infarction is vital for prevention and early detection. This case study will examine both modifiable and non-modifiable risk factors, including age, gender, family history, smoking, high blood pressure, diabetes, and high cholesterol levels. Recognizing these risk factors is instrumental ...
ED treatment. Internal STEMI Code paged at 22:55 Patient arrived at 22:57 Interventions Chest X‐Ray Assessment Repeat 12 Lead EKG. 5,000 Units IV Heparin. ED treatment. 22:05 (8 minutes after arrival) Compressions started Defibrillated at 150 J. ED treatment. 18 minutes in ED. Interventions Continued 180 mg ticagrelor Patient undressed ...
A 67-year-old woman sought emergency medical care due to prolonged chest pain. In April 2009 the patient had prolonged chest pain and at that time she sought medical care. She was admitted at the hospital and diagnosed with myocardial infarction. The patient had hypertension, diabetes mellitus, dyslipidemia and was a smoker.
1 Introduction. Myocardial infarction (MI), or other acute coronary syndromes in patients with hemophilia A, are not exceptional events. Even severe clotting factor VIII (FVIII) deficiency does not offer protection against atherothrombotic complications, although MI may occur infrequently in these patients. Conditions predisposing to arterial occlusion, such as obesity, hypertension, smoking ...
Prolonged ischaemia leads to myocardial infarction. The prognosis for each condition may depend on their varying aetiologies.1 Recent studies demonstrate aberrant myo-cardial reinnervation in ventricular arrhyth-mias, cardiomyopathies and after myocardial infarction; in some circum-stances periarteriolar reinnervation takes place.2
coronary-artery-disease-an-example-case-study - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
risk for complications. However, constant in the management of the patient with myocardial infarction is the commitment of the nursing to an evidence-based holistic approach. This article applies a case study method to explore the present evidence-based nursing practice (EBNP) that informs the assessment, clinical decision-
Acute myocardial infarction (AMI) is characterized by inflammation, oxidative stress, and atherosclerosis, contributing to increased mortality risk. High-density lipoprotein (HDL) takes a crucial part in mitigating atherosclerosis and inflammation through its diverse functionalities. Conversely, fibrinogen is implicated in the development of atherosclerotic plaques.
The management of perioperative acute myocardial infarction (AMI) following oncologic neurosurgery requires balancing competing risks of myocardial ischemia and postoperative bleeding. There are limited human data to establish the safest timing of antiplatelet or anticoagulation therapy following neurosurgical procedures. For patients with malignancy experiencing AMI in the acute postoperative ...
Accurately predicting post-discharge mortality risk in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI) remains a complex and critical challenge. The primary objective of this study was to develop and validate a robust risk prediction model to assess the 12-month and 24-month mortality risk in STEMI patients after ...
Myocardial infarction (MI), colloquially known as "heart attack," is caused by decreased or complete cessation of blood flow to a portion of the myocardium. Myocardial infarction may be "silent" and go undetected, or it could be a catastrophic event leading to hemodynamic deterioration and sudden death.[1] Most myocardial infarctions are due to underlying coronary artery disease, the ...
From this perspective, the study published by Livori et al. in this issue of the Journal is of big interest: the authors analyzed the effect of remoteness on the implementation of pharmacotherapy for secondary prevention, by analyzing the data of 37 320 patients who experienced a myocardial infarction in the state of Victoria, Australia. 11 As ...