Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Systematic Review | Definition, Examples & Guide

Systematic Review | Definition, Examples & Guide
Published on 15 June 2022 by Shaun Turney . Revised on 17 October 2022.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesise all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
They answered the question ‘What is the effectiveness of probiotics in reducing eczema symptoms and improving quality of life in patients with eczema?’
In this context, a probiotic is a health product that contains live microorganisms and is taken by mouth. Eczema is a common skin condition that causes red, itchy skin.
Table of contents
What is a systematic review, systematic review vs meta-analysis, systematic review vs literature review, systematic review vs scoping review, when to conduct a systematic review, pros and cons of systematic reviews, step-by-step example of a systematic review, frequently asked questions about systematic reviews.
A review is an overview of the research that’s already been completed on a topic.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce research bias . The methods are repeatable , and the approach is formal and systematic:
- Formulate a research question
- Develop a protocol
- Search for all relevant studies
- Apply the selection criteria
- Extract the data
- Synthesise the data
- Write and publish a report
Although multiple sets of guidelines exist, the Cochrane Handbook for Systematic Reviews is among the most widely used. It provides detailed guidelines on how to complete each step of the systematic review process.
Systematic reviews are most commonly used in medical and public health research, but they can also be found in other disciplines.
Systematic reviews typically answer their research question by synthesising all available evidence and evaluating the quality of the evidence. Synthesising means bringing together different information to tell a single, cohesive story. The synthesis can be narrative ( qualitative ), quantitative , or both.
Prevent plagiarism, run a free check.
Systematic reviews often quantitatively synthesise the evidence using a meta-analysis . A meta-analysis is a statistical analysis, not a type of review.
A meta-analysis is a technique to synthesise results from multiple studies. It’s a statistical analysis that combines the results of two or more studies, usually to estimate an effect size .
A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarise and evaluate previous work, without using a formal, explicit method.
Although literature reviews are often less time-consuming and can be insightful or helpful, they have a higher risk of bias and are less transparent than systematic reviews.
Similar to a systematic review, a scoping review is a type of review that tries to minimise bias by using transparent and repeatable methods.
However, a scoping review isn’t a type of systematic review. The most important difference is the goal: rather than answering a specific question, a scoping review explores a topic. The researcher tries to identify the main concepts, theories, and evidence, as well as gaps in the current research.
Sometimes scoping reviews are an exploratory preparation step for a systematic review, and sometimes they are a standalone project.
A systematic review is a good choice of review if you want to answer a question about the effectiveness of an intervention , such as a medical treatment.
To conduct a systematic review, you’ll need the following:
- A precise question , usually about the effectiveness of an intervention. The question needs to be about a topic that’s previously been studied by multiple researchers. If there’s no previous research, there’s nothing to review.
- If you’re doing a systematic review on your own (e.g., for a research paper or thesis), you should take appropriate measures to ensure the validity and reliability of your research.
- Access to databases and journal archives. Often, your educational institution provides you with access.
- Time. A professional systematic review is a time-consuming process: it will take the lead author about six months of full-time work. If you’re a student, you should narrow the scope of your systematic review and stick to a tight schedule.
- Bibliographic, word-processing, spreadsheet, and statistical software . For example, you could use EndNote, Microsoft Word, Excel, and SPSS.
A systematic review has many pros .
- They minimise research b ias by considering all available evidence and evaluating each study for bias.
- Their methods are transparent , so they can be scrutinised by others.
- They’re thorough : they summarise all available evidence.
- They can be replicated and updated by others.
Systematic reviews also have a few cons .
- They’re time-consuming .
- They’re narrow in scope : they only answer the precise research question.
The 7 steps for conducting a systematic review are explained with an example.
Step 1: Formulate a research question
Formulating the research question is probably the most important step of a systematic review. A clear research question will:
- Allow you to more effectively communicate your research to other researchers and practitioners
- Guide your decisions as you plan and conduct your systematic review
A good research question for a systematic review has four components, which you can remember with the acronym PICO :
- Population(s) or problem(s)
- Intervention(s)
- Comparison(s)
You can rearrange these four components to write your research question:
- What is the effectiveness of I versus C for O in P ?
Sometimes, you may want to include a fourth component, the type of study design . In this case, the acronym is PICOT .
- Type of study design(s)
- The population of patients with eczema
- The intervention of probiotics
- In comparison to no treatment, placebo , or non-probiotic treatment
- The outcome of changes in participant-, parent-, and doctor-rated symptoms of eczema and quality of life
- Randomised control trials, a type of study design
Their research question was:
- What is the effectiveness of probiotics versus no treatment, a placebo, or a non-probiotic treatment for reducing eczema symptoms and improving quality of life in patients with eczema?
Step 2: Develop a protocol
A protocol is a document that contains your research plan for the systematic review. This is an important step because having a plan allows you to work more efficiently and reduces bias.
Your protocol should include the following components:
- Background information : Provide the context of the research question, including why it’s important.
- Research objective(s) : Rephrase your research question as an objective.
- Selection criteria: State how you’ll decide which studies to include or exclude from your review.
- Search strategy: Discuss your plan for finding studies.
- Analysis: Explain what information you’ll collect from the studies and how you’ll synthesise the data.
If you’re a professional seeking to publish your review, it’s a good idea to bring together an advisory committee . This is a group of about six people who have experience in the topic you’re researching. They can help you make decisions about your protocol.
It’s highly recommended to register your protocol. Registering your protocol means submitting it to a database such as PROSPERO or ClinicalTrials.gov .
Step 3: Search for all relevant studies
Searching for relevant studies is the most time-consuming step of a systematic review.
To reduce bias, it’s important to search for relevant studies very thoroughly. Your strategy will depend on your field and your research question, but sources generally fall into these four categories:
- Databases: Search multiple databases of peer-reviewed literature, such as PubMed or Scopus . Think carefully about how to phrase your search terms and include multiple synonyms of each word. Use Boolean operators if relevant.
- Handsearching: In addition to searching the primary sources using databases, you’ll also need to search manually. One strategy is to scan relevant journals or conference proceedings. Another strategy is to scan the reference lists of relevant studies.
- Grey literature: Grey literature includes documents produced by governments, universities, and other institutions that aren’t published by traditional publishers. Graduate student theses are an important type of grey literature, which you can search using the Networked Digital Library of Theses and Dissertations (NDLTD) . In medicine, clinical trial registries are another important type of grey literature.
- Experts: Contact experts in the field to ask if they have unpublished studies that should be included in your review.
At this stage of your review, you won’t read the articles yet. Simply save any potentially relevant citations using bibliographic software, such as Scribbr’s APA or MLA Generator .
- Databases: EMBASE, PsycINFO, AMED, LILACS, and ISI Web of Science
- Handsearch: Conference proceedings and reference lists of articles
- Grey literature: The Cochrane Library, the metaRegister of Controlled Trials, and the Ongoing Skin Trials Register
- Experts: Authors of unpublished registered trials, pharmaceutical companies, and manufacturers of probiotics
Step 4: Apply the selection criteria
Applying the selection criteria is a three-person job. Two of you will independently read the studies and decide which to include in your review based on the selection criteria you established in your protocol . The third person’s job is to break any ties.
To increase inter-rater reliability , ensure that everyone thoroughly understands the selection criteria before you begin.
If you’re writing a systematic review as a student for an assignment, you might not have a team. In this case, you’ll have to apply the selection criteria on your own; you can mention this as a limitation in your paper’s discussion.
You should apply the selection criteria in two phases:
- Based on the titles and abstracts : Decide whether each article potentially meets the selection criteria based on the information provided in the abstracts.
- Based on the full texts: Download the articles that weren’t excluded during the first phase. If an article isn’t available online or through your library, you may need to contact the authors to ask for a copy. Read the articles and decide which articles meet the selection criteria.
It’s very important to keep a meticulous record of why you included or excluded each article. When the selection process is complete, you can summarise what you did using a PRISMA flow diagram .
Next, Boyle and colleagues found the full texts for each of the remaining studies. Boyle and Tang read through the articles to decide if any more studies needed to be excluded based on the selection criteria.
When Boyle and Tang disagreed about whether a study should be excluded, they discussed it with Varigos until the three researchers came to an agreement.
Step 5: Extract the data
Extracting the data means collecting information from the selected studies in a systematic way. There are two types of information you need to collect from each study:
- Information about the study’s methods and results . The exact information will depend on your research question, but it might include the year, study design , sample size, context, research findings , and conclusions. If any data are missing, you’ll need to contact the study’s authors.
- Your judgement of the quality of the evidence, including risk of bias .
You should collect this information using forms. You can find sample forms in The Registry of Methods and Tools for Evidence-Informed Decision Making and the Grading of Recommendations, Assessment, Development and Evaluations Working Group .
Extracting the data is also a three-person job. Two people should do this step independently, and the third person will resolve any disagreements.
They also collected data about possible sources of bias, such as how the study participants were randomised into the control and treatment groups.
Step 6: Synthesise the data
Synthesising the data means bringing together the information you collected into a single, cohesive story. There are two main approaches to synthesising the data:
- Narrative ( qualitative ): Summarise the information in words. You’ll need to discuss the studies and assess their overall quality.
- Quantitative : Use statistical methods to summarise and compare data from different studies. The most common quantitative approach is a meta-analysis , which allows you to combine results from multiple studies into a summary result.
Generally, you should use both approaches together whenever possible. If you don’t have enough data, or the data from different studies aren’t comparable, then you can take just a narrative approach. However, you should justify why a quantitative approach wasn’t possible.
Boyle and colleagues also divided the studies into subgroups, such as studies about babies, children, and adults, and analysed the effect sizes within each group.
Step 7: Write and publish a report
The purpose of writing a systematic review article is to share the answer to your research question and explain how you arrived at this answer.
Your article should include the following sections:
- Abstract : A summary of the review
- Introduction : Including the rationale and objectives
- Methods : Including the selection criteria, search method, data extraction method, and synthesis method
- Results : Including results of the search and selection process, study characteristics, risk of bias in the studies, and synthesis results
- Discussion : Including interpretation of the results and limitations of the review
- Conclusion : The answer to your research question and implications for practice, policy, or research
To verify that your report includes everything it needs, you can use the PRISMA checklist .
Once your report is written, you can publish it in a systematic review database, such as the Cochrane Database of Systematic Reviews , and/or in a peer-reviewed journal.
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a dissertation , thesis, research paper , or proposal .
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarise yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Turney, S. (2022, October 17). Systematic Review | Definition, Examples & Guide. Scribbr. Retrieved 22 April 2024, from https://www.scribbr.co.uk/research-methods/systematic-reviews/
Is this article helpful?

Shaun Turney
Other students also liked, what is a literature review | guide, template, & examples, exploratory research | definition, guide, & examples, what is peer review | types & examples.
Systematic Literature Review
- First Online: 31 August 2021
Cite this chapter

- Ana Paula Cardoso Ermel ORCID: orcid.org/0000-0002-3874-9792 5 ,
- D. P. Lacerda ORCID: orcid.org/0000-0002-8011-3376 6 ,
- Maria Isabel W. M. Morandi ORCID: orcid.org/0000-0003-1337-1487 7 &
- Leandro Gauss ORCID: orcid.org/0000-0001-5708-5912 8
1238 Accesses
3 Citations
This chapter presents the concept of Systematic Literature Review (SLR) and how it differs from the traditional ways of describing and portraying the literature. Moreover, it critically analyzes the common underlying structure among the SLR methods developed over the past years as well as highlights the improvements required.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Bardin, L.: L’analyse de contenu [Content Analysis], p. 223. Presses Universitaires de France Le Psychologue, Paris (1993)
Google Scholar
Baumeister, R.F., Leary, M.R.: Writing narrative literature reviews. Rev. Gen. Psychol. 1 (3), 311–320 (1997)
Article Google Scholar
Bhattacherjee, A.: Social Science Research: Principles, Methods, and Practices, 3rd edn. Textbooks Collection, [S.l.] (2012). 9781475146127
Borrego, M., Foster, M.J., Froyd, J.E.: Systematic literature reviews in engineering education and other developing interdisciplinary fields. J. Eng. Educ. 103 (1), 45–76 (2014). 1069-4730
Colicchia, C., Strozzi F.: Supply chain risk management: A new methodology for a systematic literature review. Supply Chain Manag. 17 (4), 403–418 (2012). 1359-8546
Cooper, H., Hedges, L.V., Valentine, J.C.: Handbook of Research Synthesis and Meta-Analysis, 2nd edn., p. 610. Russel Sage Foundation, New York (2009). 9780871541635
Cornell University Library Evidence Synthesis Service—A Guide to Evidence Synthesis—LibGuides at Cornell University. Disponível em: https://guides.library.cornell.edu/evidence-synthesis/service . Acesso em: 15 abr. 2021
Denyer, D., Tranfield, D.: Producing a Systematic Review. The Sage Handbook of Organizational Research Methods, 1st edn., pp. 671–689. SAGE Publications, London (2009)
Denyer, D., Tranfield, D., Vanaken, J.E.: Developing design propositions through research synthesis. Organ. Stud. 29 (3), 393–413 (2008). 0170-8406
Dresch, A., Lacerda, D.P., Antunes, J.A.V.: Design Science Research: A Method for Scientific and Technology Advancement, p. 161. Springer, [S.l.] (2015). 978-3-319-07373-6
Finfgeld, D.L.: Metasynthesis: The state of the art—so far. Qual. Health Res. 13 (7), 893–904 (2003). 1049-7323 (Print)r1049-7323 (Linking)
Gauss, L., Lacerda, D.P., Cauchick, M.P.A.: Module-based product family design: systematic literature review and meta-synthesis. J. Intell. Manuf. 32 (1), 265–312 (2021)
Glass, G.V.: Primary, secondary, and meta-analysis of research. Educ. Res. 5 (10), 3–8 (1976)
Gough, D., Oliver, S., Thomas, J.: An Introduction to Systematic Reviews, 1st edn., p. 288. SAGE Publications, Los Angeles (2012). 9781849201803
Hart, C.: Doing a Literature Review: Realising the Social Science Research Imagination, 1st edn., p. 230. SAGE Publications, London (1988)
Higgins, J., Green, S.: Cochrane Handbook for Systematic Reviews of Interventions
Khan, K.S., et al.: Five steps for a sistematic review. J. r. Soc. Med. 96 (1), 118–121 (2003)
Kitchenham, B., Charters, S.: Guidelines for performing systematic literature reviews in software engineering. Engineering 45 (4ve), 1051 (2007). 1595933751
Krippendorff, K.: Content Analysis: An Introduction to its Methodology, 4th edn., p. 356. Sage Publications Inc, Thousand Oaks (2019)
Littell, J.H. Corcoran, J., Pillai, V.: Systematic Review and Meta-Analysis, pp. 1–211. s.n., [S.l] (2008). 978-0-19-532654-3
Morandi, M.I.W.M., Camargo, L.F.R.: Systematic Literature Review. Design Science Research, P. 161. Springer, [S.l.] (2015)
Palomino, M., Abraham, D., Melendez, K.: Methodologies, methods, techniques and tools used on SLR elaboration: A mapping Studs. Trends and Applications in Software Engineering, pp.14–30. Springer, Cham (2019). 978-3-319-69340-8
Petticrew, M., Roberts, H.: Systematic Reviews in the Social Sciences: A Practical Guide. Malden, 1st edn., p. 336. Blackwell Publishing, MA (2006). 1473314060098
Robinson, P., Lowe, J.: Literature reviews vs systematic reviews. Aust. n. z. J. Public Health 39 (2), 103–103 (2015)
Siluo, Y., Qingli, Y.: Are Scientometrics, Informetrics, and Bibliometrics Different? 2017, pp. 1–12. [s.n.], Wuhan (2017)
Smith, V., et al.: Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 11 (15), 1–6 (2011)
Snyder, H.: Literature review as a research methodology: an overview and guidelines. J. Bus. Res. 104, 333–339 (2019). Disponível em: < https://doi.org/10.1016/j.jbusres.2019.07.039 >
Systematic vs Literature reviews—Systematic and Literature Reviews—LibGuides at Brown University. Disponível em: https://libguides.brown.edu/Reviews/types . Acesso em: 22 July 2020
Thomé, A.M.T., Scavarda, L.F., Scavarda, A.J.: Conducting systematic literature review in operations management. Prod. Plan. Control 27 (5), 408–420 (2016)
Tranfield, D., Denyer, D., Smart, P.: Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 14 , 207–222 (2003)
Webster, J., Watson, R.T.: Analyzing the past to prepare for the future: writing a literature review. MIS Quarterly 26 (2), 133–151 (2002) 0959-5309
Whiting, P., et al.: ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 69 , 225–234 (2016)
Zupic, I., Čater, T.: Bibliometric methods in management and organization. Organ. Res. Methods 18 (3), 429–472 (2015)
Download references
Author information
Authors and affiliations.
Production and Systems Engineering, Universidade do Vale do Rio dos Sinos, São Leopoldo, Rio Grande do Sul, Brazil
Ana Paula Cardoso Ermel
D. P. Lacerda
Maria Isabel W. M. Morandi
Leandro Gauss
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ana Paula Cardoso Ermel .
Rights and permissions
Reprints and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cardoso Ermel, A.P., Lacerda, D.P., Morandi, M.I.W.M., Gauss, L. (2021). Systematic Literature Review. In: Literature Reviews. Springer, Cham. https://doi.org/10.1007/978-3-030-75722-9_3
Download citation
DOI : https://doi.org/10.1007/978-3-030-75722-9_3
Published : 31 August 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-75721-2
Online ISBN : 978-3-030-75722-9
eBook Packages : Education Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
1.2.2 What is a systematic review?
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman 1992, Oxman 1993) . The key characteristics of a systematic review are:
a clearly stated set of objectives with pre-defined eligibility criteria for studies;
an explicit, reproducible methodology;
a systematic search that attempts to identify all studies that would meet the eligibility criteria;
an assessment of the validity of the findings of the included studies, for example through the assessment of risk of bias; and
a systematic presentation, and synthesis, of the characteristics and findings of the included studies.
Many systematic reviews contain meta-analyses. Meta-analysis is the use of statistical methods to summarize the results of independent studies (Glass 1976). By combining information from all relevant studies, meta-analyses can provide more precise estimates of the effects of health care than those derived from the individual studies included within a review (see Chapter 9, Section 9.1.3 ). They also facilitate investigations of the consistency of evidence across studies, and the exploration of differences across studies.

Systematic Review
- Library Help
- What is a Systematic Review (SR)?
- Steps of a Systematic Review
- Framing a Research Question
- Developing a Search Strategy
- Searching the Literature
- Managing the Process
- Meta-analysis
- Publishing your Systematic Review
Introduction to Systematic Review
- Introduction
- Types of literature reviews
- Other Libguides
- Systematic review as part of a dissertation
- Tutorials & Guidelines & Examples from non-Medical Disciplines
Depending on your learning style, please explore the resources in various formats on the tabs above.
For additional tutorials, visit the SR Workshop Videos from UNC at Chapel Hill outlining each stage of the systematic review process.
Know the difference! Systematic review vs. literature review

Types of literature reviews along with associated methodologies
JBI Manual for Evidence Synthesis . Find definitions and methodological guidance.
- Systematic Reviews - Chapters 1-7
- Mixed Methods Systematic Reviews - Chapter 8
- Diagnostic Test Accuracy Systematic Reviews - Chapter 9
- Umbrella Reviews - Chapter 10
- Scoping Reviews - Chapter 11
- Systematic Reviews of Measurement Properties - Chapter 12
Systematic reviews vs scoping reviews -
Grant, M. J., & Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information and Libraries Journal , 26 (2), 91–108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
Gough, D., Thomas, J., & Oliver, S. (2012). Clarifying differences between review designs and methods. Systematic Reviews, 1 (28). htt p s://doi.org/ 10.1186/2046-4053-1-28
Munn, Z., Peters, M., Stern, C., Tufanaru, C., McArthur, A., & Aromataris, E. (2018). Systematic review or scoping review ? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology, 18 (1), 143. https://doi.org/10.1186/s12874-018-0611-x. Also, check out the Libguide from Weill Cornell Medicine for the differences between a systematic review and a scoping review and when to embark on either one of them.
Sutton, A., Clowes, M., Preston, L., & Booth, A. (2019). Meeting the review family: Exploring review types and associated information retrieval requirements . Health Information & Libraries Journal , 36 (3), 202–222. https://doi.org/10.1111/hir.12276
Temple University. Review Types . - This guide provides useful descriptions of some of the types of reviews listed in the above article.
UMD Health Sciences and Human Services Library. Review Types . - Guide describing Literature Reviews, Scoping Reviews, and Rapid Reviews.
Whittemore, R., Chao, A., Jang, M., Minges, K. E., & Park, C. (2014). Methods for knowledge synthesis: An overview. Heart & Lung: The Journal of Acute and Critical Care, 43 (5), 453–461. https://doi.org/10.1016/j.hrtlng.2014.05.014
Differences between a systematic review and other types of reviews
Armstrong, R., Hall, B. J., Doyle, J., & Waters, E. (2011). ‘ Scoping the scope ’ of a cochrane review. Journal of Public Health , 33 (1), 147–150. https://doi.org/10.1093/pubmed/fdr015
Kowalczyk, N., & Truluck, C. (2013). Literature reviews and systematic reviews: What is the difference? Radiologic Technology , 85 (2), 219–222.
White, H., Albers, B., Gaarder, M., Kornør, H., Littell, J., Marshall, Z., Matthew, C., Pigott, T., Snilstveit, B., Waddington, H., & Welch, V. (2020). Guidance for producing a Campbell evidence and gap map . Campbell Systematic Reviews, 16 (4), e1125. https://doi.org/10.1002/cl2.1125. Check also this comparison between evidence and gaps maps and systematic reviews.
Rapid Reviews Tutorials
Rapid Review Guidebook by the National Collaborating Centre of Methods and Tools (NCCMT)
Hamel, C., Michaud, A., Thuku, M., Skidmore, B., Stevens, A., Nussbaumer-Streit, B., & Garritty, C. (2021). Defining Rapid Reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. Journal of clinical epidemiology , 129 , 74–85. https://doi.org/10.1016/j.jclinepi.2020.09.041
- Müller, C., Lautenschläger, S., Meyer, G., & Stephan, A. (2017). Interventions to support people with dementia and their caregivers during the transition from home care to nursing home care: A systematic review . International Journal of Nursing Studies, 71 , 139–152. https://doi.org/10.1016/j.ijnurstu.2017.03.013
- Bhui, K. S., Aslam, R. W., Palinski, A., McCabe, R., Johnson, M. R. D., Weich, S., … Szczepura, A. (2015). Interventions to improve therapeutic communications between Black and minority ethnic patients and professionals in psychiatric services: Systematic review . The British Journal of Psychiatry, 207 (2), 95–103. https://doi.org/10.1192/bjp.bp.114.158899
- Rosen, L. J., Noach, M. B., Winickoff, J. P., & Hovell, M. F. (2012). Parental smoking cessation to protect young children: A systematic review and meta-analysis . Pediatrics, 129 (1), 141–152. https://doi.org/10.1542/peds.2010-3209
Scoping Review
- Hyshka, E., Karekezi, K., Tan, B., Slater, L. G., Jahrig, J., & Wild, T. C. (2017). The role of consumer perspectives in estimating population need for substance use services: A scoping review . BMC Health Services Research, 171-14. https://doi.org/10.1186/s12913-017-2153-z
- Olson, K., Hewit, J., Slater, L.G., Chambers, T., Hicks, D., Farmer, A., & ... Kolb, B. (2016). Assessing cognitive function in adults during or following chemotherapy: A scoping review . Supportive Care In Cancer, 24 (7), 3223-3234. https://doi.org/10.1007/s00520-016-3215-1
- Pham, M. T., Rajić, A., Greig, J. D., Sargeant, J. M., Papadopoulos, A., & McEwen, S. A. (2014). A scoping review of scoping reviews: Advancing the approach and enhancing the consistency . Research Synthesis Methods, 5 (4), 371–385. https://doi.org/10.1002/jrsm.1123
- Scoping Review Tutorial from UNC at Chapel Hill
Qualitative Systematic Review/Meta-Synthesis
- Lee, H., Tamminen, K. A., Clark, A. M., Slater, L., Spence, J. C., & Holt, N. L. (2015). A meta-study of qualitative research examining determinants of children's independent active free play . International Journal Of Behavioral Nutrition & Physical Activity, 12 (5), 121-12. https://doi.org/10.1186/s12966-015-0165-9
Videos on systematic reviews
Systematic Reviews: What are they? Are they right for my research? - 47 min. video recording with a closed caption option.
More training videos on systematic reviews:
Books on Systematic Reviews
Books on Meta-analysis
- University of Toronto Libraries - very detailed with good tips on the sensitivity and specificity of searches.
- Monash University - includes an interactive case study tutorial.
- Dalhousie University Libraries - a comprehensive How-To Guide on conducting a systematic review.
Guidelines for a systematic review as part of the dissertation
- Guidelines for Systematic Reviews in the Context of Doctoral Education Background by University of Victoria (PDF)
- Can I conduct a Systematic Review as my Master’s dissertation or PhD thesis? Yes, It Depends! by Farhad (blog)
- What is a Systematic Review Dissertation Like? by the University of Edinburgh (50 min video)
Further readings on experiences of PhD students and doctoral programs with systematic reviews
Puljak, L., & Sapunar, D. (2017). Acceptance of a systematic review as a thesis: Survey of biomedical doctoral programs in Europe . Systematic Reviews , 6 (1), 253. https://doi.org/10.1186/s13643-017-0653-x
Perry, A., & Hammond, N. (2002). Systematic reviews: The experiences of a PhD Student . Psychology Learning & Teaching , 2 (1), 32–35. https://doi.org/10.2304/plat.2002.2.1.32
Daigneault, P.-M., Jacob, S., & Ouimet, M. (2014). Using systematic review methods within a Ph.D. dissertation in political science: Challenges and lessons learned from practice . International Journal of Social Research Methodology , 17 (3), 267–283. https://doi.org/10.1080/13645579.2012.730704
UMD Doctor of Philosophy Degree Policies
Before you embark on a systematic review research project, check the UMD PhD Policies to make sure you are on the right path. Systematic reviews require a team of at least two reviewers and an information specialist or a librarian. Discuss with your advisor the authorship roles of the involved team members. Keep in mind that the UMD Doctor of Philosophy Degree Policies (scroll down to the section, Inclusion of one's own previously published materials in a dissertation ) outline such cases, specifically the following:
" It is recognized that a graduate student may co-author work with faculty members and colleagues that should be included in a dissertation . In such an event, a letter should be sent to the Dean of the Graduate School certifying that the student's examining committee has determined that the student made a substantial contribution to that work. This letter should also note that the inclusion of the work has the approval of the dissertation advisor and the program chair or Graduate Director. The letter should be included with the dissertation at the time of submission. The format of such inclusions must conform to the standard dissertation format. A foreword to the dissertation, as approved by the Dissertation Committee, must state that the student made substantial contributions to the relevant aspects of the jointly authored work included in the dissertation."
- Cochrane Handbook for Systematic Reviews of Interventions - See Part 2: General methods for Cochrane reviews
- Systematic Searches - Yale library video tutorial series
- Using PubMed's Clinical Queries to Find Systematic Reviews - From the U.S. National Library of Medicine
- Systematic reviews and meta-analyses: A step-by-step guide - From the University of Edinsburgh, Centre for Cognitive Ageing and Cognitive Epidemiology
Bioinformatics
- Mariano, D. C., Leite, C., Santos, L. H., Rocha, R. E., & de Melo-Minardi, R. C. (2017). A guide to performing systematic literature reviews in bioinformatics . arXiv preprint arXiv:1707.05813.
Environmental Sciences
Collaboration for Environmental Evidence. 2018. Guidelines and Standards for Evidence synthesis in Environmental Management. Version 5.0 (AS Pullin, GK Frampton, B Livoreil & G Petrokofsky, Eds) www.environmentalevidence.org/information-for-authors .
Pullin, A. S., & Stewart, G. B. (2006). Guidelines for systematic review in conservation and environmental management. Conservation Biology, 20 (6), 1647–1656. https://doi.org/10.1111/j.1523-1739.2006.00485.x
Engineering Education
- Borrego, M., Foster, M. J., & Froyd, J. E. (2014). Systematic literature reviews in engineering education and other developing interdisciplinary fields. Journal of Engineering Education, 103 (1), 45–76. https://doi.org/10.1002/jee.20038
Public Health
- Hannes, K., & Claes, L. (2007). Learn to read and write systematic reviews: The Belgian Campbell Group . Research on Social Work Practice, 17 (6), 748–753. https://doi.org/10.1177/1049731507303106
- McLeroy, K. R., Northridge, M. E., Balcazar, H., Greenberg, M. R., & Landers, S. J. (2012). Reporting guidelines and the American Journal of Public Health’s adoption of preferred reporting items for systematic reviews and meta-analyses . American Journal of Public Health, 102 (5), 780–784. https://doi.org/10.2105/AJPH.2011.300630
- Pollock, A., & Berge, E. (2018). How to do a systematic review. International Journal of Stroke, 13 (2), 138–156. https://doi.org/10.1177/1747493017743796
- Institute of Medicine. (2011). Finding what works in health care: Standards for systematic reviews . https://doi.org/10.17226/13059
- Wanden-Berghe, C., & Sanz-Valero, J. (2012). Systematic reviews in nutrition: Standardized methodology . The British Journal of Nutrition, 107 Suppl 2, S3-7. https://doi.org/10.1017/S0007114512001432
Social Sciences
- Bronson, D., & Davis, T. (2012). Finding and evaluating evidence: Systematic reviews and evidence-based practice (Pocket guides to social work research methods). Oxford: Oxford University Press.
- Petticrew, M., & Roberts, H. (2006). Systematic reviews in the social sciences: A practical guide . Malden, MA: Blackwell Pub.
- Cornell University Library Guide - Systematic literature reviews in engineering: Example: Software Engineering
- Biolchini, J., Mian, P. G., Natali, A. C. C., & Travassos, G. H. (2005). Systematic review in software engineering . System Engineering and Computer Science Department COPPE/UFRJ, Technical Report ES, 679 (05), 45.
- Biolchini, J. C., Mian, P. G., Natali, A. C. C., Conte, T. U., & Travassos, G. H. (2007). Scientific research ontology to support systematic review in software engineering . Advanced Engineering Informatics, 21 (2), 133–151.
- Kitchenham, B. (2007). Guidelines for performing systematic literature reviews in software engineering . [Technical Report]. Keele, UK, Keele University, 33(2004), 1-26.
- Weidt, F., & Silva, R. (2016). Systematic literature review in computer science: A practical guide . Relatórios Técnicos do DCC/UFJF , 1 .
- Academic Phrasebank - Get some inspiration and find some terms and phrases for writing your research paper
- Oxford English Dictionary - Use to locate word variants and proper spelling
- << Previous: Library Help
- Next: Steps of a Systematic Review >>
- Last Updated: Apr 19, 2024 12:47 PM
- URL: https://lib.guides.umd.edu/SR

- Duke NetID Login
- 919.660.1100
- Duke Health Badge: 24-hour access
- Accounts & Access
- Databases, Journals & Books
- Request & Reserve
- Training & Consulting
- Request Articles & Books
- Renew Online
- Reserve Spaces
- Reserve a Locker
- Study & Meeting Rooms
- Course Reserves
- Digital Health Device Collection
- Pay Fines/Fees
- Recommend a Purchase
- Access From Off Campus
- Building Access
- Computers & Equipment
- Wifi Access
- My Accounts
- Mobile Apps
- Known Access Issues
- Report an Access Issue
- All Databases
- Article Databases
- Basic Sciences
- Clinical Sciences
- Dissertations & Theses
- Drugs, Chemicals & Toxicology
- Grants & Funding
- Interprofessional Education
- Non-Medical Databases
- Search for E-Journals
- Search for Print & E-Journals
- Search for E-Books
- Search for Print & E-Books
- E-Book Collections
- Biostatistics
- Global Health
- MBS Program
- Medical Students
- MMCi Program
- Occupational Therapy
- Path Asst Program
- Physical Therapy
- Researchers
- Community Partners
Conducting Research
- Archival & Historical Research
- Black History at Duke Health
- Data Analytics & Viz Software
- Data: Find and Share
- Evidence-Based Practice
- NIH Public Access Policy Compliance
- Publication Metrics
- Qualitative Research
- Searching Animal Alternatives
Systematic Reviews
- Test Instruments
Using Databases
- JCR Impact Factors
- Web of Science
Finding & Accessing
- COVID-19: Core Clinical Resources
- Health Literacy
- Health Statistics & Data
- Library Orientation
Writing & Citing
- Creating Links
- Getting Published
- Reference Mgmt
- Scientific Writing
Meet a Librarian
- Request a Consultation
- Find Your Liaisons
- Register for a Class
- Request a Class
- Self-Paced Learning
Search Services
- Literature Search
- Systematic Review
- Animal Alternatives (IACUC)
- Research Impact
Citation Mgmt
- Other Software
Scholarly Communications
- About Scholarly Communications
- Publish Your Work
- Measure Your Research Impact
- Engage in Open Science
- Libraries and Publishers
- Directions & Maps
- Floor Plans
Library Updates
- Annual Snapshot
- Conference Presentations
- Contact Information
- Gifts & Donations
What is a Systematic Review?
- Types of Reviews
- Manuals and Reporting Guidelines
- Our Service
- 1. Assemble Your Team
- 2. Develop a Research Question
- 3. Write and Register a Protocol
- 4. Search the Evidence
- 5. Screen Results
- 6. Assess for Quality and Bias
- 7. Extract the Data
- 8. Write the Review
- Additional Resources
- Finding Full-Text Articles
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. The key characteristics of a systematic review are:
- a clearly defined question with inclusion and exclusion criteria;
- a rigorous and systematic search of the literature;
- two phases of screening (blinded, at least two independent screeners);
- data extraction and management;
- analysis and interpretation of results;
- risk of bias assessment of included studies;
- and report for publication.
Medical Center Library & Archives Presentations
The following presentation is a recording of the Getting Started with Systematic Reviews workshop (4/2022), offered by the Duke Medical Center Library & Archives. A NetID/pw is required to access the tutorial via Warpwire.
- << Previous: Overview
- Next: Types of Reviews >>
- Last Updated: Mar 20, 2024 2:21 PM
- URL: https://guides.mclibrary.duke.edu/sysreview
- Duke Health
- Duke University
- Duke Libraries
- Medical Center Archives
- Duke Directory
- Seeley G. Mudd Building
- 10 Searle Drive
- [email protected]

- Research Process
Systematic Literature Review or Literature Review?
- 3 minute read
- 43.5K views
Table of Contents
As a researcher, you may be required to conduct a literature review. But what kind of review do you need to complete? Is it a systematic literature review or a standard literature review? In this article, we’ll outline the purpose of a systematic literature review, the difference between literature review and systematic review, and other important aspects of systematic literature reviews.
What is a Systematic Literature Review?
The purpose of systematic literature reviews is simple. Essentially, it is to provide a high-level of a particular research question. This question, in and of itself, is highly focused to match the review of the literature related to the topic at hand. For example, a focused question related to medical or clinical outcomes.
The components of a systematic literature review are quite different from the standard literature review research theses that most of us are used to (more on this below). And because of the specificity of the research question, typically a systematic literature review involves more than one primary author. There’s more work related to a systematic literature review, so it makes sense to divide the work among two or three (or even more) researchers.
Your systematic literature review will follow very clear and defined protocols that are decided on prior to any review. This involves extensive planning, and a deliberately designed search strategy that is in tune with the specific research question. Every aspect of a systematic literature review, including the research protocols, which databases are used, and dates of each search, must be transparent so that other researchers can be assured that the systematic literature review is comprehensive and focused.
Most systematic literature reviews originated in the world of medicine science. Now, they also include any evidence-based research questions. In addition to the focus and transparency of these types of reviews, additional aspects of a quality systematic literature review includes:
- Clear and concise review and summary
- Comprehensive coverage of the topic
- Accessibility and equality of the research reviewed
Systematic Review vs Literature Review
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper. That is, it includes an introduction, description of the methods used, a discussion and conclusion, as well as a reference list or bibliography.
A systematic review, however, includes entirely different components that reflect the specificity of its research question, and the requirement for transparency and inclusion. For instance, the systematic review will include:
- Eligibility criteria for included research
- A description of the systematic research search strategy
- An assessment of the validity of reviewed research
- Interpretations of the results of research included in the review
As you can see, contrary to the general overview or summary of a topic, the systematic literature review includes much more detail and work to compile than a standard literature review. Indeed, it can take years to conduct and write a systematic literature review. But the information that practitioners and other researchers can glean from a systematic literature review is, by its very nature, exceptionally valuable.
This is not to diminish the value of the standard literature review. The importance of literature reviews in research writing is discussed in this article . It’s just that the two types of research reviews answer different questions, and, therefore, have different purposes and roles in the world of research and evidence-based writing.
Systematic Literature Review vs Meta Analysis
It would be understandable to think that a systematic literature review is similar to a meta analysis. But, whereas a systematic review can include several research studies to answer a specific question, typically a meta analysis includes a comparison of different studies to suss out any inconsistencies or discrepancies. For more about this topic, check out Systematic Review VS Meta-Analysis article.
Language Editing Plus
With Elsevier’s Language Editing Plus services , you can relax with our complete language review of your systematic literature review or literature review, or any other type of manuscript or scientific presentation. Our editors are PhD or PhD candidates, who are native-English speakers. Language Editing Plus includes checking the logic and flow of your manuscript, reference checks, formatting in accordance to your chosen journal and even a custom cover letter. Our most comprehensive editing package, Language Editing Plus also includes any English-editing needs for up to 180 days.

- Publication Recognition
How to Make a PowerPoint Presentation of Your Research Paper

- Manuscript Preparation
What is and How to Write a Good Hypothesis in Research?
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
How-to conduct a systematic literature review: A quick guide for computer science research
Affiliations.
- 1 Faculty of Engineering, Mondragon University.
- 2 Design Innovation Center(DBZ), Mondragon University.
- PMID: 36405369
- PMCID: PMC9672331
- DOI: 10.1016/j.mex.2022.101895
Performing a literature review is a critical first step in research to understanding the state-of-the-art and identifying gaps and challenges in the field. A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in particular early-stage researchers in the computer-science field. The contribution of the article is the following:•Clearly defined strategies to follow for a systematic literature review in computer science research, and•Algorithmic method to tackle a systematic literature review.
Keywords: Systematic literature reviews; computer science; doctoral studies; literature reviews; research methodology.
© 2022 The Author(s).
Systematic Reviews & Literature Reviews
Evidence synthesis: part 1.
This blog post is the first in a series exploring Evidence Synthesis . We’re going to start by looking at two types of evidence synthesis: literature reviews and systemic reviews . To help me with this topic I looked at a number of research guides from other institutions, e.g., Cornell University Libraries.
The Key Differences Between a Literature Review and a Systematic Review
Overall, while both literature reviews and systematic reviews involve reviewing existing research literature, systematic reviews adhere to more rigorous and transparent methods to minimize bias and provide robust evidence to inform decision-making in education and other fields. If you are interested in learning about other evidence synthesis this decision tree created by Cornell Libraries (Robinson, n.d.) is a nice visual introduction.
Along with exploring evidence synthesis I am also interested in generative A.I. I want to be transparent about how I used A.I. to create the table above. I fed this prompt into ChatGPT:
“ List the differences between a literature review and a systemic review for a graduate student of education “
I wanted to see what it would produce. I reformatted the list into a table so that it would be easier to compare and contrast these two reviews much like the one created by Cornell University Libraries (Kibbee, 2024). I think ChatGPT did a pretty good job. I did have to do quite a bit of editing, and make sure that what was created matched what I already knew. There are things ChatGPT left out, for example time frames, and how many people are needed for a systemic review, but we can revisit that in a later post.
Kibbee, M. (2024, April 10). Libguides: A guide to evidence synthesis: Cornell University Library Evidence Synthesis Service. Cornell University Library. https://guides.library.cornell.edu/evidence-synthesis/intro
- Blog Archive 2009-2018
- Library Hours
- Library Salons
- Library Spaces
- Library Workshops
- Reference Desk Questions
Subscribe to the Bank Street Library Blog
- Open access
- Published: 19 April 2024
Person-centered care assessment tool with a focus on quality healthcare: a systematic review of psychometric properties
- Lluna Maria Bru-Luna 1 ,
- Manuel Martí-Vilar 2 ,
- César Merino-Soto 3 ,
- José Livia-Segovia 4 ,
- Juan Garduño-Espinosa 5 &
- Filiberto Toledano-Toledano 5 , 6 , 7
BMC Psychology volume 12 , Article number: 217 ( 2024 ) Cite this article
338 Accesses
Metrics details
The person-centered care (PCC) approach plays a fundamental role in ensuring quality healthcare. The Person-Centered Care Assessment Tool (P-CAT) is one of the shortest and simplest tools currently available for measuring PCC. The objective of this study was to conduct a systematic review of the evidence in validation studies of the P-CAT, taking the “Standards” as a frame of reference.
First, a systematic literature review was conducted following the PRISMA method. Second, a systematic descriptive literature review of validity tests was conducted following the “Standards” framework. The search strategy and information sources were obtained from the Cochrane, Web of Science (WoS), Scopus and PubMed databases. With regard to the eligibility criteria and selection process, a protocol was registered in PROSPERO (CRD42022335866), and articles had to meet criteria for inclusion in the systematic review.
A total of seven articles were included. Empirical evidence indicates that these validations offer a high number of sources related to test content, internal structure for dimensionality and internal consistency. A moderate number of sources pertain to internal structure in terms of test-retest reliability and the relationship with other variables. There is little evidence of response processes, internal structure in measurement invariance terms, and test consequences.
The various validations of the P-CAT are not framed in a structured, valid, theory-based procedural framework like the “Standards” are. This can affect clinical practice because people’s health may depend on it. The findings of this study show that validation studies continue to focus on the types of validity traditionally studied and overlook interpretation of the scores in terms of their intended use.
Peer Review reports
Person-centered care (PCC)
Quality care for people with chronic diseases, functional limitations, or both has become one of the main objectives of medical and care services. The person-centered care (PCC) approach is an essential element not only in achieving this goal but also in providing high-quality health maintenance and medical care [ 1 , 2 , 3 ]. In addition to guaranteeing human rights, PCC provides numerous benefits to both the recipient and the provider [ 4 , 5 ]. Additionally, PCC includes a set of necessary competencies for healthcare professionals to address ongoing challenges in this area [ 6 ]. PCC includes the following elements [ 7 ]: an individualized, goal-oriented care plan based on individuals’ preferences; an ongoing review of the plan and the individual’s goals; support from an interprofessional team; active coordination among all medical and care providers and support services; ongoing information exchange, education and training for providers; and quality improvement through feedback from the individual and caregivers.
There is currently a growing body of literature on the application of PCC. A good example of this is McCormack’s widely known mid-range theory [ 8 ], an internationally recognized theoretical framework for PCC and how it is operationalized in practice. This framework forms a guide for care practitioners and researchers in hospital settings. This framework is elaborated in PCC and conceived of as “an approach to practice that is established through the formation and fostering of therapeutic relationships between all care providers, service users, and others significant to them, underpinned by values of respect for persons, [the] individual right to self-determination, mutual respect, and understanding” [ 9 ].
Thus, as established by PCC, it is important to emphasize that reference to the person who is the focus of care refers not only to the recipient but also to everyone involved in a care interaction [ 10 , 11 ]. PCC ensures that professionals are trained in relevant skills and methodology since, as discussed above, carers are among the agents who have the greatest impact on the quality of life of the person in need of care [ 12 , 13 , 14 ]. Furthermore, due to the high burden of caregiving, it is essential to account for caregivers’ well-being. In this regard, studies on professional caregivers are beginning to suggest that the provision of PCC can produce multiple benefits for both the care recipient and the caregiver [ 15 ].
Despite a considerable body of literature and the frequent inclusion of the term in health policy and research [ 16 ], PCC involves several complications. There is no standard consensus on the definition of this concept [ 17 ], which includes problematic areas such as efficacy assessment [ 18 , 19 ]. In addition, the difficulty of measuring the subjectivity involved in identifying the dimensions of the CPC and the infrequent use of standardized measures are acute issues [ 20 ]. These limitations and purposes motivated the creation of the Person-Centered Care Assessment Tool (P-CAT; [ 21 ]), which emerged from the need for a brief, economical, easily applied, versatile and comprehensive assessment instrument to provide valid and reliable measures of PCC for research purposes [ 21 ].
Person-centered care assessment tool (P-CAT)
There are several instruments that can measure PCC from different perspectives (i.e., the caregiver or the care recipient) and in different contexts (e.g., hospitals and nursing homes). However, from a practical point of view, the P-CAT is one of the shortest and simplest tools and contains all the essential elements of PCC described in the literature. It was developed in Australia to measure the approach of long-term residential settings to older people with dementia, although it is increasingly used in other healthcare settings, such as oncology units [ 22 ] and psychiatric hospitals [ 23 ].
Due to the brevity and simplicity of its application, the versatility of its use in different medical and care contexts, and its potential emic characteristics (i.e., constructs that can be cross-culturally applicable with reasonable and similar structure and interpretation; [ 24 ]), the P-CAT is one of the most widely used tests by professionals to measure PCC [ 25 , 26 ]. It has expanded to several countries with cultural and linguistic differences. Since its creation, it has been adapted in countries separated by wide cultural and linguistic differences, such as Norway [ 27 ], Sweden [ 28 ], China [ 29 ], South Korea [ 30 ], Spain [ 25 ], and Italy [ 31 ].
The P-CAT comprises 13 items rated on a 5-point ordinal scale (from “strongly disagree” to “strongly agree”), with high scores indicating a high degree of person-centeredness. The scale consists of three dimensions: person-centered care (7 items), organizational support (4 items) and environmental accessibility (2 items). In the original study ( n = 220; [ 21 ]), the internal consistency of the instrument yielded satisfactory values for the total scale ( α = 0.84) and good test-retest reliability ( r =.66) at one-week intervals. A reliability generalization study conducted in 2021 [ 32 ] that estimated the internal consistency of the P-CAT and analyzed possible factors that could affect the it revealed that the mean α value for the 25 meta-analysis samples (some of which were part of the validations included in this study) was 0.81, and the only variable that had a statistically significant relationship with the reliability coefficient was the mean age of the sample. With respect to internal structure validity, three factors (56% of the total variance) were obtained, and content validity was assessed by experts, literature reviews and stakeholders [ 33 ].
Although not explicitly stated, the apparent commonality between validation studies of different versions of the P-CAT may be influenced by an influential decades-old validity framework that differentiates three categories: content validity, construct validity, and criterion validity [ 34 , 35 ]. However, a reformulation of the validity of the P-CAT within a modern framework, which would provide a different definition of validity, has not been performed.
Scale validity
Traditionally, validation is a process focused on the psychometric properties of a measurement instrument [ 36 ]. In the early 20th century, with the frequent use of standardized measurement tests in education and psychology, two definitions emerged: the first defined validity as the degree to which a test measures what it intends to measure, while the second described the validity of an instrument in terms of the correlation it presents with a variable [ 35 ].
However, in the past century, validity theory has evolved, leading to the understanding that validity should be based on specific interpretations for an intended purpose. It should not be limited to empirically obtained psychometric properties but should also be supported by the theory underlying the construct measured. Thus, to speak of classical or modern validity theory suggests an evolution in the classical or modern understanding of the concept of validity. Therefore, a classical approach (called classical test theory, CTT) is specifically differentiated from a modern approach. In general, recent concepts associated with a modern view of validity are based on (a) a unitary conception of validity and (b) validity judgments based on inferences and interpretations of the scores of a measure [ 37 , 38 ]. This conceptual advance in the concept of validity led to the creation of a guiding framework to for obtaining evidence to support the use and interpretation of the scores obtained by a measure [ 39 ].
This purpose is addressed by the Standards for Educational and Psychological Testing (“Standards”), a guide created by the American Educational Research Association (AERA), the American Psychological Association (APA) and the National Council on Measurement in Education (NCME) in 2014 with the aim of providing guidelines to assess the validity of the interpretations of scores of an instrument based on their intended use. Two conceptual aspects stand out in this modern view of validity: first, validity is a unitary concept centered on the construct; second, validity is defined as “the degree to which evidence and theory support the interpretations of test scores for proposed uses of tests” [ 37 ]. Thus, the “Standards” propose several sources that serve as a reference for assessing different aspects of validity. The five sources of valid evidence are as follows [ 37 ]: test content, response processes, internal structure, relations to other variables and consequences of testing. According to AERA et al. [ 37 ], test content validity refers to the relationship of the administration process, subject matter, wording and format of test items to the construct they are intended to measure. It is measured predominantly with qualitative methods but without excluding quantitative approaches. The validity of the responses is based on analysis of the cognitive processes and interpretation of the items by respondents and is measured with qualitative methods. Internal structure validity is based on the interrelationship between the items and the construct and is measured by quantitative methods. Validity in terms of the relationship with other variables is based on comparison between the variable that the instrument intends to measure and other theoretically relevant external variables and is measured by quantitative methods. Finally, validity based on the results of the test analyses consequences, both intended and unintended, that may be due to a source of invalidity. It is measured mainly by qualitative methods.
Thus, although validity plays a fundamental role in providing a strong scientific basis for interpretations of test scores, validation studies in the health field have traditionally focused on content validity, criterion validity and construct validity and have overlooked the interpretation and use of scores [ 34 ].
“Standards” are considered a suitable validity theory-based procedural framework for reviewing the validity of questionnaires due to its ability to analyze sources of validity from both qualitative and quantitative approaches and its evidence-based method [ 35 ]. Nevertheless, due to a lack of knowledge or the lack of a systematic description protocol, very few instruments to date have been reviewed within the framework of the “Standards” [ 39 ].
Current study
Although the P-CAT is one of the most widely used instruments by professionals and has seven validations [ 25 , 27 , 28 , 29 , 30 , 31 , 40 ], no analysis has been conducted of its validity within the framework of the “Standards”. That is, empirical evidence of the validity of the P-CAT has not been obtained in a way that helps to develop a judgment based on a synthesis of the available information.
A review of this type is critical given that some methodological issues seem to have not been resolved in the P-CAT. For example, although the multidimensionality of the P-CAT was identified in the study that introduced it, Bru-Luna et al. [ 32 ] recently stated that in adaptations of the P-CAT [ 25 , 27 , 28 , 29 , 30 , 40 ], the total score is used for interpretation and multidimensionality is disregarded. Thus, the multidimensionality of the original study was apparently not replicated. Bru-Luna et al. [ 32 ] also indicated that the internal structure validity of the P-CAT is usually underreported due to a lack of sufficiently rigorous approaches to establish with certainty how its scores are calculated.
The validity of the P-CAT, specifically its internal structure, appears to be unresolved. Nevertheless, substantive research and professional practice point to this measure as relevant to assessing PCC. This perception is contestable and judgment-based and may not be sufficient to assess the validity of the P-CAT from a cumulative and synthetic angle based on preceding validation studies. An adequate assessment of validity requires a model to conceptualize validity followed by a review of previous studies of the validity of the P-CAT using this model.
Therefore, the main purpose of this study was to conduct a systematic review of the evidence provided by P-CAT validation studies while taking the “Standards” as a framework.
The present study comprises two distinct but interconnected procedures. First, a systematic literature review was conducted following the PRISMA method ( [ 41 ]; Additional file 1; Additional file 2) with the aim of collecting all validations of the P-CAT that have been developed. Second, a systematic description of the validity evidence for each of the P-CAT validations found in the systematic review was developed following the “Standards” framework [ 37 ]. The work of Hawkins et al. [ 39 ], the first study to review validity sources according to the guidelines proposed by the “Standards”, was also used as a reference. Both provided conceptual and pragmatic guidance for organizing and classifying validity evidence for the P-CAT.
The procedure conducted in the systematic review is described below, followed by the procedure for examining the validity studies.
Systematic review
Search strategy and information sources.
Initially, the Cochrane database was searched with the aim of identifying systematic reviews of the P-CAT. When no such reviews were found, subsequent preliminary searches were performed in the Web of Science (WoS), Scopus and PubMed databases. These databases play a fundamental role in recent scientific literature since they are the main sources of published articles that undergo high-quality content and editorial review processes [ 42 ]. The search formula was as follows. The original P-CAT article [ 21 ] was located, after which all articles that cited it through 2021 were identified and analyzed. This approach ensured the inclusion of all validations. No articles were excluded on the basis of language to avoid language bias [ 43 ]. Moreover, to reduce the effects of publication bias, a complementary search in Google Scholar was also performed to allow the inclusion of “gray” literature [ 44 ]. Finally, a manual search was performed through a review of the references of the included articles to identify other articles that met the search criteria but were not present in any of the aforementioned databases.
This process was conducted by one of the authors and corroborated by another using the Covidence tool [ 45 ]. A third author was consulted in case of doubt.
Eligibility criteria and selection process
The protocol was registered in PROSPERO, and the search was conducted according to these criteria. The identification code is CRD42022335866.
The articles had to meet the following criteria for inclusion in the systematic review: (a) a methodological approach to P-CAT validations, (b) an experimental or quasiexperimental studies, (c) studies with any type of sample, and (d) studies in any language. We discarded studies that met at least one of the following exclusion criteria: (a) systematic reviews or bibliometric reviews of the instrument or meta-analyses or (b) studies published after 2021.
Data collection process
After the articles were selected, the most relevant information was extracted from each article. Fundamental data were recorded in an Excel spreadsheet for each of the sections: introduction, methodology, results and discussion. Information was also recorded about the limitations mentioned in each article as well as the practical implications and suggestions for future research.
Given the aim of the study, information was collected about the sources of validity of each study, including test content (judges’ evaluation, literature review and translation), response processes, internal structure (factor analysis, design, estimator, factor extraction method, factors and items, interfactor R, internal replication, effect of the method, and factor loadings), and relationships with other variables (convergent, divergent, concurrent and predictive validity) and consequences of measurement.
Description of the validity study
To assess the validity of the studies, an Excel table was used. Information was recorded for the seven articles included in the systematic review. The data were extracted directly from the texts of the articles and included information about the authors, the year of publication, the country where each P-CAT validation was produced and each of the five standards proposed in the “Standards” [ 37 ].
The validity source related to internal structure was divided into three sections to record information about dimensionality (e.g., factor analysis, design, estimator, factor extraction method, factors and items, interfactor R, internal replication, effect of the method, and factor loadings), reliability expression (i.e., internal consistency and test-retest) and the study of factorial invariance according to the groups into which it was divided (e.g., sex, age, profession) and the level of study (i.e., metric, intercepts). This approach allowed much more information to be obtained than relying solely on source validity based on internal structure. This division was performed by the same researcher who performed the previous processes.
Study selection and study characteristics
The systematic review process was developed according to the PRISMA methodology [ 41 ].
The WoS, Scopus, PubMed and Google Scholar databases were searched on February 12, 2022 and yielded a total of 485 articles. Of these, 111 were found in WoS, 114 in Scopus, 43 in PubMed and 217 in Google Scholar. In the first phase, the title and abstracts of all the articles were read. In this first screening, 457 articles were eliminated because they did not include studies with a methodological approach to P-CAT validation and one article was excluded because it was the original P-CAT article. This resulted in a total of 27 articles, 19 of which were duplicated in different databases and, in the case of Google Scholar, within the same database. This process yielded a total of eight articles that were evaluated for eligibility by a complete reading of the text. In this step, one of the articles was excluded due to a lack of access to the full text of the study [ 31 ] (although the original manuscript was found, it was impossible to access the complete content; in addition, the authors of the manuscript were contacted, but no reply was received). Finally, a manual search was performed by reviewing the references of the seven studies, but none were considered suitable for inclusion. Thus, the review was conducted with a total of seven articles.
Of the seven studies, six were original validations in other languages. These included Norwegian [ 27 ], Swedish [ 28 ], Chinese (which has two validations [ 29 , 40 ]), Spanish [ 25 ], and Korean [ 30 ]. The study by Selan et al. [ 46 ] included a modification of the Swedish version of the P-CAT and explored the psychometric properties of both versions (i.e., the original Swedish version and the modified version).
The item selection and screening process are illustrated in detail in Fig. 1 .
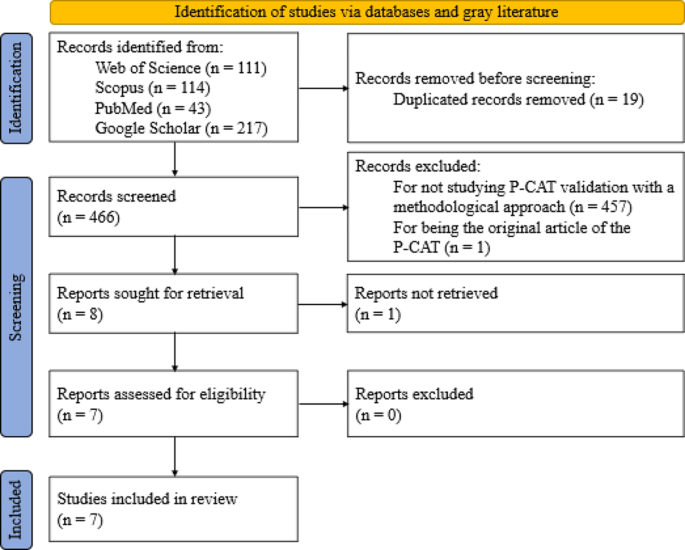
PRISMA 2020 flow diagram for new systematic reviews including database searches
Validity analysis
To provide a clear overview of the validity analyses, Table 1 descriptively shows the percentages of items that provide information about the five standards proposed by the “Standards” guide [ 37 ].
The table shows a high number of validity sources related to test content and internal structure in relation to dimensionality and internal consistency, followed by a moderate number of sources for test-retest and relationship with other variables. A rate of 0% is observed for validity sources related to response processes, invariance and test consequences. Below, different sections related to each of the standards are shown, and the information is presented in more detail.
Evidence based on test content
The first standard, which focused on test content, was met for all items (100%). Translation, which refers to the equivalence of content between the original language and the target language, was met in the six articles that conducted validation in another language and/or culture. These studies reported that the validations were translated by bilingual experts and/or experts in the area of care. In addition, three studies [ 25 , 29 , 40 ] reported that the translation process followed International Test Commission guidelines, such as those of Beaton et al. [ 47 ], Guillemin [ 48 ], Hambleton et al. [ 49 ], and Muñiz et al. [ 50 ]. Evaluation by judges, who referred to the relevance, clarity and importance of the content, was divided into two categories: expert evaluation (a panel of expert judges for each of the areas to consider in the evaluation instrument) and experiential evaluation (potential participants testing the test). The first type of evaluation occurred in three of the articles [ 28 , 29 , 46 ], while the other occurred in two [ 25 , 40 ]. Only one of the items [ 29 ] reported that the scale contained items that reflected the dimension described in the literature. The validity evidence related to the test content presented in each article can be found in Table 2 .
Evidence based on response processes
The second standard, related to the validity of the response process, was obtained according to the “Standards” from the analysis of individual responses: “questioning test takers about their performance strategies or response to particular items (…), maintaining records that monitor the development of a response to a writing task (…), documentation of other aspects of performance, like eye movement or response times…” [ 37 ] (p. 15). According to the analysis of the validity of the response processes, none of the articles complied with this evidence.
Evidence based on internal structure
The third standard, validity related to internal structure, was divided into three sections. First, the dimensionality of each study was examined in terms of factor analysis, design, estimator, factor extraction method, factors and items, interfactor R, internal replication, effect of the method, and factor loadings. Le et al. [ 40 ] conducted an exploratory-confirmatory design while Sjögren et al. [ 28 ] conducted a confirmatory-exploratory design to assess construct validity using confirmatory factor analysis (CFA) and investigated it further using exploratory factor analysis (EFA). The remaining articles employed only a single form of factor analysis: three employed EFA, and two employed CFA. Regarding the next point, only three of the articles reported the factor extraction method used, including Kaiser’s eigenvalue, criterion, scree plot test, parallel analysis and Velicer’s MAP test. Instrument validations yielded a total of two factors in five of the seven articles, while one yielded a single dimension [ 25 ] and the other yielded three dimensions [ 29 ], as in the original instrument. The interfactor R was reported only in the study by Zhong and Lou [ 29 ], whereas in the study by Martínez et al. [ 25 ], it could be easily obtained since it consisted of only one dimension. Internal replication was also calculated in the Spanish validation by randomly splitting the sample into two to test the correlations between factors. The effectiveness of the method was not reported in any of the articles. This information is presented in Table 3 in addition to a summary of the factor loadings.
The second section examined reliability. All the studies presented measures of internal consistency conducted in their entirety with Cronbach’s α coefficient for both the total scale and the subscales. The ω coefficient of McDonald was not used in any case. Four of the seven articles performed a test-retest test. Martínez et al. [ 25 ] conducted a test-retest after a period of seven days, while Le et al. [ 40 ] and Rokstad et al. [ 27 ] performed it between one and two weeks later and Sjögren et al. [ 28 ] allowed approximately two weeks to pass after the initial test.
The third section analyzes the calculation of invariance, which was not reported in any of the studies.
Evidence based on relationships with other variables
In the fourth standard, based on validity according to the relationship with other variables, the articles that reported it used only convergent validity (i.e., it was hypothesized that the variables related to the construct measured by the test—in this case, person-centeredness—were positively or negatively related to another construct). Discriminant validity hypothesizes that the variables related to the PCC construct are not correlated in any way with any other variable studied. No article (0%) measured discriminant evidence, while four (57%) measured convergent evidence [ 25 , 29 , 30 , 46 ]. Convergent validity was obtained through comparisons with instruments such as the Person-Centered Climate Questionnaire–Staff Version (PCQ-S), the Staff-Based Measures of Individualized Care for Institutionalized Persons with Dementia (IC), the Caregiver Psychological Elder Abuse Behavior Scale (CPEAB), the Organizational Climate (CLIOR) and the Maslach Burnout Inventory (MBI). In the case of Selan et al. [ 46 ], convergent validity was assessed on two items considered by the authors as “crude measures of person-centered care (i.e., external constructs) giving an indication of the instruments’ ability to measure PCC” (p. 4). Concurrent validity, which measures the degree to which the results of one test are or are not similar to those of another test conducted at more or less the same time with the same participants, and predictive validity, which allows predictions to be established regarding behavior based on comparison between the values of the instrument and the criterion, were not reported in any of the studies.
Evidence based on the consequences of testing
The fifth and final standard was related to the consequences of the test. It analyzed the consequences, both intended and unintended, of applying the test to a given sample. None of the articles presented explicit or implicit evidence of this.
The last two sources of validity can be seen in Table 4 .
Table 5 shows the results of the set of validity tests for each study according to the described standards.
The main purpose of this article is to analyze the evidence of validity in different validation studies of the P-CAT. To gather all existing validations, a systematic review of all literature citing this instrument was conducted.
The publication of validation studies of the P-CAT has been constant over the years. Since the publication of the original instrument in 2010, seven validations have been published in other languages (taking into account the Italian version by Brugnolli et al. [ 31 ], which could not be included in this study) as well as a modification of one of these versions. The very unequal distribution of validations between languages and countries is striking. A recent systematic review [ 51 ] revealed that in Europe, the countries where the PCC approach is most widely used are the United Kingdom, Sweden, the Netherlands, Northern Ireland, and Norway. It has also been shown that the neighboring countries seem to exert an influence on each other due to proximity [ 52 ] such that they tend to organize healthcare in a similar way, as is the case for Scandinavian countries. This favors the expansion of PCC and explains the numerous validations we found in this geographical area.
Although this approach is conceived as an essential element of healthcare for most governments [ 53 ], PCC varies according to the different definitions and interpretations attributed to it, which can cause confusion in its application (e.g., between Norway and the United Kingdom [ 54 ]). Moreover, facilitators of or barriers to implementation depend on the context and level of development of each country, and financial support remains one of the main factors in this regard [ 53 ]. This fact explains why PCC is not globally widespread among all territories. In countries where access to healthcare for all remains out of reach for economic reasons, the application of this approach takes a back seat, as does the validation of its assessment tools. In contrast, in a large part of Europe or in countries such as China or South Korea that have experienced decades of rapid economic development, patients are willing to be involved in their medical treatment and enjoy more satisfying and efficient medical experiences and environments [ 55 ], which facilitates the expansion of validations of instruments such as the P-CAT.
Regarding validity testing, the guidelines proposed by the “Standards” [ 37 ] were followed. According to the analysis of the different validations of the P-CAT instrument, none of the studies used a structured validity theory-based procedural framework for conducting validation. The most frequently reported validity tests were on the content of the test and two of the sections into which the internal structure was divided (i.e., dimensionality and internal consistency).
In the present article, the most cited source of validity in the studies was the content of the test because most of the articles were validations of the P-CAT in other languages, and the authors reported that the translation procedure was conducted by experts in all cases. In addition, several of the studies employed International Test Commission guidelines, such as those by Beaton et al. [ 47 ], Guillemin [ 48 ], Hambleton et al. [ 49 ], and Muñiz et al. [ 50 ]. Several studies also assessed the relevance, clarity and importance of the content.
The third source of validity, internal structure, was the next most often reported, although it appeared unevenly among the three sections into which this evidence was divided. Dimensionality and internal consistency were reported in all studies, followed by test-retest consistency. In relation to the first section, factor analysis, a total of five EFAs and four CFAs were presented in the validations. Traditionally, EFA has been used in research to assess dimensionality and identify key psychological constructs, although this approach involves a number of inconveniences, such as difficulty testing measurement invariance and incorporating latent factors into subsequent analyses [ 56 ] or the major problem of factor loading matrix rotation [ 57 ]. Studies eventually began to employ CFA, a technique that overcame some of these obstacles [ 56 ] but had other drawbacks; for example, the strict requirement of zero cross-loadings often does not fit the data well, and misspecification of zero loadings tends to produce distorted factors [ 57 ]. Recently, exploratory structural equation modeling (ESEM) has been proposed. This technique is widely recommended both conceptually and empirically to assess the internal structure of psychological tools [ 58 ] since it overcomes the limitations of EFA and CFA in estimating their parameters [ 56 , 57 ].
The next section, reliability, reports the total number of items according to Cronbach’s α reliability coefficient. Reliability is defined as a combination of systematic and random influences that determine the observed scores on a psychological test. Reporting the reliability measure ensures that item-based scores are consistent, that the tool’s responses are replicable and that they are not modified solely by random noise [ 59 , 60 ]. Currently, the most commonly employed reliability coefficient in studies with a multi-item measurement scale (MIMS) is Cronbach’s α [ 60 , 61 ].
Cronbach’s α [ 62 ] is based on numerous strict assumptions (e.g., the test must be unidimensional, factor loadings must be equal for all items and item errors should not covary) to estimate internal consistency. These assumptions are difficult to meet, and their violation may produce small reliability estimates [ 60 ]. One of the alternative measures to α that is increasingly recommended by the scientific literature is McDonald’s ω [ 63 ], a composite reliability measure. This coefficient is recommended for congeneric scales in which tau equivalence is not assumed. It has several advantages. For example, estimates of ω are usually robust when the estimated model contains more factors than the true model, even with small samples, or when skewness in univariate item distributions produces lower biases than those found when using α [ 59 ].
The test-retest method was the next most commonly reported internal structure section in these studies. This type of reliability considers the consistency of the scores of a test between two measurements separated by a period [ 64 ]. It is striking that test-retest consistency does not have a prevalence similar to that of internal consistency since, unlike internal consistency, test-retest consistency can be assessed for practically all types of patient-reported outcomes. It is even considered by some measurement experts to report reliability with greater relevance than internal consistency since it plays a fundamental role in the calculation of parameters for health measures [ 64 ]. However, the literature provides little guidance regarding the assessment of this type of reliability.
The internal structure section that was least frequently reported in the studies in this review was invariance. A lack of invariance refers to a difference between scores on a test that is not explained by group differences in the structure it is intended to measure [ 65 ]. The invariance of the measure should be emphasized as a prerequisite in comparisons between groups since “if scale invariance is not examined, item bias may not be fully recognized and this may lead to a distorted interpretation of the bias in a particular psychological measure” [ 65 ].
Evidence related to other variables was the next most reported source of validity in the studies included in this review. Specifically, the four studies that reported this evidence did so according to convergent validity and cited several instruments. None of the studies included evidence of discriminant validity, although this may be because there are currently several obstacles related to the measurement of this type of validity [ 66 ]. On the one hand, different definitions are used in the applied literature, which makes its evaluation difficult; on the other hand, the literature on discriminant validity focuses on techniques that require the use of multiple measurement methods, which often seem to have been introduced without sufficient evidence or are applied randomly.
Validity related to response processes was not reported by any of the studies. There are several methods to analyze this validity. These methods can be divided into two groups: “those that directly access the psychological processes or cognitive operations (think aloud, focus group, and interviews), compared to those which provide indirect indicators which in turn require additional inference (eye tracking and response times)” [ 38 ]. However, this validity evidence has traditionally been reported less frequently than others in most studies, perhaps because there are fewer clear and accepted practices on how to design or report these studies [ 67 ].
Finally, the consequences of testing were not reported in any of the studies. There is debate regarding this source of validity, with two main opposing streams of thought. On the one hand [ 68 , 69 ]) suggests that consequences that appear after the application of a test should not derive from any source of test invalidity and that “adverse consequences only undermine the validity of an assessment if they can be attributed to a problem of fit between the test and the construct” (p. 6). In contrast, Cronbach [ 69 , 70 ] notes that adverse social consequences that may result from the application of a test may call into question the validity of the test. However, the potential risks that may arise from the application of a test should be minimized in any case, especially in regard to health assessments. To this end, it is essential that this aspect be assessed by instrument developers and that the experiences of respondents be protected through the development of comprehensive and informed practices [ 39 ].
This work is not without limitations. First, not all published validation studies of the P-CAT, such as the Italian version by Brugnolli et al. [ 31 ], were available. These studies could have provided relevant information. Second, many sources of validity could not be analyzed because the studies provided scant or no data, such as response processes [ 25 , 27 , 28 , 29 , 30 , 40 , 46 ], relationships with other variables [ 27 , 28 , 40 ], consequences of testing [ 25 , 27 , 28 , 29 , 30 , 40 , 46 ], or invariance [ 25 , 27 , 28 , 29 , 30 , 40 , 46 ] in the case of internal structure and interfactor R [ 27 , 28 , 30 , 40 , 46 ], internal replication [ 27 , 28 , 29 , 30 , 40 , 46 ] or the effect of the method [ 25 , 27 , 28 , 29 , 30 , 40 , 46 ] in the case of dimensionality. In the future, it is hoped that authors will become aware of the importance of validity, as shown in this article and many others, and provide data on unreported sources so that comprehensive validity studies can be performed.
The present work also has several strengths. The search was extensive, and many studies were obtained using three different databases, including WoS, one of the most widely used and authoritative databases in the world. This database includes a large number and variety of articles and is not fully automated due to its human team [ 71 , 72 , 73 ]. In addition, to prevent publication bias, gray literature search engines such as Google Scholar were used to avoid the exclusion of unpublished research [ 44 ]. Finally, linguistic bias was prevented by not limiting the search to articles published in only one or two languages, thus avoiding the overrepresentation of studies in one language and underrepresentation in others [ 43 ].
Conclusions
Validity is understood as the degree to which tests and theory support the interpretations of instrument scores for their intended use [ 37 ]. From this perspective, the various validations of the P-CAT are not presented in a structured, valid, theory-based procedural framework like the “Standards” are. After integration and analysis of the results, it was observed that these validation reports offer a high number of sources of validity related to test content, internal structure in dimensionality and internal consistency, a moderate number of sources for internal structure in terms of test-retest reliability and the relationship with other variables, and a very low number of sources for response processes, internal structure in terms of invariance, and test consequences.
Validity plays a fundamental role in ensuring a sound scientific basis for test interpretations because it provides evidence of the extent to which the data provided by the test are valid for the intended purpose. This can affect clinical practice as people’s health may depend on it. In this sense, the “Standards” are considered a suitable and valid theory-based procedural framework for studying this modern conception of questionnaire validity, which should be taken into account in future research in this area.
Although the P-CAT is one of the most widely used instruments for assessing PCC, as shown in this study, PCC has rarely been studied. The developers of measurement tests applied to the health care setting, on which the health and quality of life of many people may depend, should use this validity framework to reflect the clear purpose of the measurement. This approach is important because the equity of decision making by healthcare professionals in daily clinical practice may depend on the source of validity. Through a more extensive study of validity that includes the interpretation of scores in terms of their intended use, the applicability of the P-CAT, an instrument that was initially developed for long-term care homes for elderly people, could be expanded to other care settings. However, the findings of this study show that validation studies continue to focus on traditionally studied types of validity and overlook the interpretation of scores in terms of their intended use.
Data availability
All data relevant to the study were included in the article or uploaded as additional files. Additional template data extraction forms are available from the corresponding author upon reasonable request.
Abbreviations
American Educational Research Association
American Psychological Association
Confirmatory factor analysis
Organizational Climate
Caregiver Psychological Elder Abuse Behavior Scale
Exploratory factor analysis
Exploratory structural equation modeling
Staff-based Measures of Individualized Care for Institutionalized Persons with Dementia
Maslach Burnout Inventory
Multi-item measurement scale
Maximum likelihood
National Council on Measurement in Education
Person-Centered Care Assessment Tool
- Person-centered care
Person-Centered Climate Questionnaire–Staff Version
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
International Register of Systematic Review Protocols
Standards for Educational and Psychological Testing
weighted least square mean and variance adjusted
Web of Science
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy; 2001.
Google Scholar
International Alliance of Patients’ Organizations. What is patient-centred healthcare? A review of definitions and principles. 2nd ed. London, UK: International Alliance of Patients’ Organizations; 2007.
World Health Organization. WHO global strategy on people-centred and integrated health services: interim report. Geneva, Switzerland: World Health Organization; 2015.
Britten N, Ekman I, Naldemirci Ö, Javinger M, Hedman H, Wolf A. Learning from Gothenburg model of person centred healthcare. BMJ. 2020;370:m2738.
Article PubMed Google Scholar
Van Diepen C, Fors A, Ekman I, Hensing G. Association between person-centred care and healthcare providers’ job satisfaction and work-related health: a scoping review. BMJ Open. 2020;10:e042658.
Article PubMed PubMed Central Google Scholar
Ekman N, Taft C, Moons P, Mäkitalo Å, Boström E, Fors A. A state-of-the-art review of direct observation tools for assessing competency in person-centred care. Int J Nurs Stud. 2020;109:103634.
American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64:15–8.
Article Google Scholar
McCormack B, McCance TV. Development of a framework for person-centred nursing. J Adv Nurs. 2006;56:472–9.
McCormack B, McCance T. Person-centred practice in nursing and health care: theory and practice. Chichester, England: Wiley; 2016.
Nolan MR, Davies S, Brown J, Keady J, Nolan J. Beyond person-centred care: a new vision for gerontological nursing. J Clin Nurs. 2004;13:45–53.
McCormack B, McCance T. Person-centred nursing: theory, models and methods. Oxford, UK: Wiley-Blackwell; 2010.
Book Google Scholar
Abraha I, Rimland JM, Trotta FM, Dell’Aquila G, Cruz-Jentoft A, Petrovic M, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open. 2017;7:e012759.
Anderson K, Blair A. Why we need to care about the care: a longitudinal study linking the quality of residential dementia care to residents’ quality of life. Arch Gerontol Geriatr. 2020;91:104226.
Bauer M, Fetherstonhaugh D, Haesler E, Beattie E, Hill KD, Poulos CJ. The impact of nurse and care staff education on the functional ability and quality of life of people living with dementia in aged care: a systematic review. Nurse Educ Today. 2018;67:27–45.
Smythe A, Jenkins C, Galant-Miecznikowska M, Dyer J, Downs M, Bentham P, et al. A qualitative study exploring nursing home nurses’ experiences of training in person centred dementia care on burnout. Nurse Educ Pract. 2020;44:102745.
McCormack B, Borg M, Cardiff S, Dewing J, Jacobs G, Janes N, et al. Person-centredness– the ‘state’ of the art. Int Pract Dev J. 2015;5:1–15.
Wilberforce M, Challis D, Davies L, Kelly MP, Roberts C, Loynes N. Person-centredness in the care of older adults: a systematic review of questionnaire-based scales and their measurement properties. BMC Geriatr. 2016;16:63.
Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351–79.
Sharma T, Bamford M, Dodman D. Person-centred care: an overview of reviews. Contemp Nurse. 2016;51:107–20.
Ahmed S, Djurkovic A, Manalili K, Sahota B, Santana MJ. A qualitative study on measuring patient-centered care: perspectives from clinician-scientists and quality improvement experts. Health Sci Rep. 2019;2:e140.
Edvardsson D, Fetherstonhaugh D, Nay R, Gibson S. Development and initial testing of the person-centered Care Assessment Tool (P-CAT). Int Psychogeriatr. 2010;22:101–8.
Tamagawa R, Groff S, Anderson J, Champ S, Deiure A, Looyis J, et al. Effects of a provincial-wide implementation of screening for distress on healthcare professionals’ confidence and understanding of person-centered care in oncology. J Natl Compr Canc Netw. 2016;14:1259–66.
Degl’ Innocenti A, Wijk H, Kullgren A, Alexiou E. The influence of evidence-based design on staff perceptions of a supportive environment for person-centered care in forensic psychiatry. J Forensic Nurs. 2020;16:E23–30.
Hulin CL. A psychometric theory of evaluations of item and scale translations: fidelity across languages. J Cross Cult Psychol. 1987;18:115–42.
Martínez T, Suárez-Álvarez J, Yanguas J, Muñiz J. Spanish validation of the person-centered Care Assessment Tool (P-CAT). Aging Ment Health. 2016;20:550–8.
Martínez T, Martínez-Loredo V, Cuesta M, Muñiz J. Assessment of person-centered care in gerontology services: a new tool for healthcare professionals. Int J Clin Health Psychol. 2020;20:62–70.
Rokstad AM, Engedal K, Edvardsson D, Selbaek G. Psychometric evaluation of the Norwegian version of the person-centred Care Assessment Tool. Int J Nurs Pract. 2012;18:99–105.
Sjögren K, Lindkvist M, Sandman PO, Zingmark K, Edvardsson D. Psychometric evaluation of the Swedish version of the person-centered Care Assessment Tool (P-CAT). Int Psychogeriatr. 2012;24:406–15.
Zhong XB, Lou VW. Person-centered care in Chinese residential care facilities: a preliminary measure. Aging Ment Health. 2013;17:952–8.
Tak YR, Woo HY, You SY, Kim JH. Validity and reliability of the person-centered Care Assessment Tool in long-term care facilities in Korea. J Korean Acad Nurs. 2015;45:412–9.
Brugnolli A, Debiasi M, Zenere A, Zanolin ME, Baggia M. The person-centered Care Assessment Tool in nursing homes: psychometric evaluation of the Italian version. J Nurs Meas. 2020;28:555–63.
Bru-Luna LM, Martí-Vilar M, Merino-Soto C, Livia J. Reliability generalization study of the person-centered Care Assessment Tool. Front Psychol. 2021;12:712582.
Edvardsson D, Innes A. Measuring person-centered care: a critical comparative review of published tools. Gerontologist. 2010;50:834–46.
Hawkins M, Elsworth GR, Nolte S, Osborne RH. Validity arguments for patient-reported outcomes: justifying the intended interpretation and use of data. J Patient Rep Outcomes. 2021;5:64.
Sireci SG. On the validity of useless tests. Assess Educ Princ Policy Pract. 2016;23:226–35.
Hawkins M, Elsworth GR, Osborne RH. Questionnaire validation practice: a protocol for a systematic descriptive literature review of health literacy assessments. BMJ Open. 2019;9:e030753.
American Educational Research Association, American Psychological Association. National Council on Measurement in Education. Standards for educational and psychological testing. Washington, DC: American Educational Research Association; 2014.
Padilla JL, Benítez I. Validity evidence based on response processes. Psicothema. 2014;26:136–44.
PubMed Google Scholar
Hawkins M, Elsworth GR, Hoban E, Osborne RH. Questionnaire validation practice within a theoretical framework: a systematic descriptive literature review of health literacy assessments. BMJ Open. 2020;10:e035974.
Le C, Ma K, Tang P, Edvardsson D, Behm L, Zhang J, et al. Psychometric evaluation of the Chinese version of the person-centred Care Assessment Tool. BMJ Open. 2020;10:e031580.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2008;22:338–42.
Grégoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a tower of Babel bias? J Clin Epidemiol. 1995;48:159–63.
Arias MM. Aspectos metodológicos Del metaanálisis (1). Pediatr Aten Primaria. 2018;20:297–302.
Covidence. Covidence systematic review software. Veritas Health Innovation, Australia. 2014. https://www.covidence.org/ . Accessed 28 Feb 2022.
Selan D, Jakobsson U, Condelius A. The Swedish P-CAT: modification and exploration of psychometric properties of two different versions. Scand J Caring Sci. 2017;31:527–35.
Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25:3186–91.
Guillemin F. Cross-cultural adaptation and validation of health status measures. Scand J Rheumatol. 1995;24:61–3.
Hambleton R, Merenda P, Spielberger C. Adapting educational and psychological tests for cross-cultural assessment. Mahwah, NJ: Lawrence Erlbaum Associates; 2005.
Muñiz J, Elosua P, Hambleton RK. International test commission guidelines for test translation and adaptation: second edition. Psicothema. 2013;25:151–7.
Rosengren K, Brannefors P, Carlstrom E. Adoption of the concept of person-centred care into discourse in Europe: a systematic literature review. J Health Organ Manag. 2021;35:265–80.
Alharbi T, Olsson LE, Ekman I, Carlström E. The impact of organizational culture on the outcome of hospital care: after the implementation of person-centred care. Scand J Public Health. 2014;42:104–10.
Bensbih S, Souadka A, Diez AG, Bouksour O. Patient centered care: focus on low and middle income countries and proposition of new conceptual model. J Med Surg Res. 2020;7:755–63.
Stranz A, Sörensdotter R. Interpretations of person-centered dementia care: same rhetoric, different practices? A comparative study of nursing homes in England and Sweden. J Aging Stud. 2016;38:70–80.
Zhou LM, Xu RH, Xu YH, Chang JH, Wang D. Inpatients’ perception of patient-centered care in Guangdong province, China: a cross-sectional study. Inquiry. 2021. https://doi.org/10.1177/00469580211059482 .
Marsh HW, Morin AJ, Parker PD, Kaur G. Exploratory structural equation modeling: an integration of the best features of exploratory and confirmatory factor analysis. Annu Rev Clin Psychol. 2014;10:85–110.
Asparouhov T, Muthén B. Exploratory structural equation modeling. Struct Equ Model Multidiscip J. 2009;16:397–438.
Cabedo-Peris J, Martí-Vilar M, Merino-Soto C, Ortiz-Morán M. Basic empathy scale: a systematic review and reliability generalization meta-analysis. Healthc (Basel). 2022;10:29–62.
Flora DB. Your coefficient alpha is probably wrong, but which coefficient omega is right? A tutorial on using R to obtain better reliability estimates. Adv Methods Pract Psychol Sci. 2020;3:484–501.
McNeish D. Thanks coefficient alpha, we’ll take it from here. Psychol Methods. 2018;23:412–33.
Hayes AF, Coutts JJ. Use omega rather than Cronbach’s alpha for estimating reliability. But… Commun Methods Meas. 2020;14:1–24.
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334.
McDonald R. Test theory: a unified approach. Mahwah, NJ: Erlbaum; 1999.
Polit DF. Getting serious about test-retest reliability: a critique of retest research and some recommendations. Qual Life Res. 2014;23:1713–20.
Ceylan D, Çizel B, Karakaş H. Testing destination image scale invariance for intergroup comparison. Tour Anal. 2020;25:239–51.
Rönkkö M, Cho E. An updated guideline for assessing discriminant validity. Organ Res Methods. 2022;25:6–14.
Hubley A, Zumbo B. Response processes in the context of validity: setting the stage. In: Zumbo B, Hubley A, editors. Understanding and investigating response processes in validation research. Cham, Switzerland: Springer; 2017. pp. 1–12.
Messick S. Validity of performance assessments. In: Philips G, editor. Technical issues in large-scale performance assessment. Washington, DC: Department of Education, National Center for Education Statistics; 1996. pp. 1–18.
Moss PA. The role of consequences in validity theory. Educ Meas Issues Pract. 1998;17:6–12.
Cronbach L. Five perspectives on validity argument. In: Wainer H, editor. Test validity. Hillsdale, MI: Erlbaum; 1988. pp. 3–17.
Birkle C, Pendlebury DA, Schnell J, Adams J. Web of Science as a data source for research on scientific and scholarly activity. Quant Sci Stud. 2020;1:363–76.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6:245.
Web of Science Group. Editorial selection process. Clarivate. 2024. https://clarivate.com/webofsciencegroup/solutions/%20editorial-selection-process/ . Accessed 12 Sept 2022.
Download references
Acknowledgements
The authors thank the casual helpers for their aid in information processing and searching.
This work is one of the results of research project HIM/2015/017/SSA.1207, “Effects of mindfulness training on psychological distress and quality of life of the family caregiver”. Main researcher: Filiberto Toledano-Toledano Ph.D. The present research was funded by federal funds for health research and was approved by the Commissions of Research, Ethics and Biosafety (Comisiones de Investigación, Ética y Bioseguridad), Hospital Infantil de México Federico Gómez, National Institute of Health. The source of federal funds did not control the study design, data collection, analysis, or interpretation, or decisions regarding publication.
Author information
Authors and affiliations.
Departamento de Educación, Facultad de Ciencias Sociales, Universidad Europea de Valencia, 46010, Valencia, Spain
Lluna Maria Bru-Luna
Departamento de Psicología Básica, Universitat de València, Blasco Ibáñez Avenue, 21, 46010, Valencia, Spain
Manuel Martí-Vilar
Departamento de Psicología, Instituto de Investigación de Psicología, Universidad de San Martín de Porres, Tomás Marsano Avenue 242, Lima 34, Perú
César Merino-Soto
Instituto Central de Gestión de la Investigación, Universidad Nacional Federico Villarreal, Carlos Gonzalez Avenue 285, 15088, San Miguel, Perú
José Livia-Segovia
Unidad de Investigación en Medicina Basada en Evidencias, Hospital Infantil de México Federico Gómez Instituto Nacional de Salud, Dr. Márquez 162, 06720, Doctores, Cuauhtémoc, Mexico
Juan Garduño-Espinosa & Filiberto Toledano-Toledano
Unidad de Investigación Multidisciplinaria en Salud, Instituto Nacional de Rehabilitación Luis Guillermo Ibarra Ibarra, México-Xochimilco 289, Arenal de Guadalupe, 14389, Tlalpan, Mexico City, Mexico
Filiberto Toledano-Toledano
Dirección de Investigación y Diseminación del Conocimiento, Instituto Nacional de Ciencias e Innovación para la Formación de Comunidad Científica, INDEHUS, Periférico Sur 4860, Arenal de Guadalupe, 14389, Tlalpan, Mexico City, Mexico
You can also search for this author in PubMed Google Scholar
Contributions
L.M.B.L. conceptualized the study, collected the data, performed the formal anal- ysis, wrote the original draft, and reviewed and edited the subsequent drafts. M.M.V. collected the data and reviewed and edited the subsequent drafts. C.M.S. collected the data, performed the formal analysis, wrote the original draft, and reviewed and edited the subsequent drafts. J.L.S. collected the data, wrote the original draft, and reviewed and edited the subsequent drafts. J.G.E. collected the data and reviewed and edited the subsequent drafts. F.T.T. conceptualized the study and reviewed and edited the subsequent drafts. L.M.B.L. conceptualized the study and reviewed and edited the subsequent drafts. M.M.V. conceptualized the study and reviewed and edited the subsequent drafts. C.M.S. reviewed and edited the subsequent drafts. J.G.E. reviewed and edited the subsequent drafts. F.T.T. conceptualized the study; provided resources, software, and supervision; wrote the original draft; and reviewed and edited the subsequent drafts.
Corresponding author
Correspondence to Filiberto Toledano-Toledano .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Commissions of Research, Ethics and Biosafety (Comisiones de Investigación, Ética y Bioseguridad), Hospital Infantil de México Federico Gómez, National Institute of Health. HIM/2015/017/SSA.1207, “Effects of mindfulness training on psychological distress and quality of life of the family caregiver”.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bru-Luna, L.M., Martí-Vilar, M., Merino-Soto, C. et al. Person-centered care assessment tool with a focus on quality healthcare: a systematic review of psychometric properties. BMC Psychol 12 , 217 (2024). https://doi.org/10.1186/s40359-024-01716-7
Download citation
Received : 17 May 2023
Accepted : 07 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s40359-024-01716-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Person-centered care assessment tool
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.2(1); Jan-Mar 2013
Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare
S. gopalakrishnan.
Department of Community Medicine, SRM Medical College, Hospital and Research Centre, Kattankulathur, Tamil Nadu, India
P. Ganeshkumar
Healthcare decisions for individual patients and for public health policies should be informed by the best available research evidence. The practice of evidence-based medicine is the integration of individual clinical expertise with the best available external clinical evidence from systematic research and patient's values and expectations. Primary care physicians need evidence for both clinical practice and for public health decision making. The evidence comes from good reviews which is a state-of-the-art synthesis of current evidence on a given research question. Given the explosion of medical literature, and the fact that time is always scarce, review articles play a vital role in decision making in evidence-based medical practice. Given that most clinicians and public health professionals do not have the time to track down all the original articles, critically read them, and obtain the evidence they need for their questions, systematic reviews and clinical practice guidelines may be their best source of evidence. Systematic reviews aim to identify, evaluate, and summarize the findings of all relevant individual studies over a health-related issue, thereby making the available evidence more accessible to decision makers. The objective of this article is to introduce the primary care physicians about the concept of systematic reviews and meta-analysis, outlining why they are important, describing their methods and terminologies used, and thereby helping them with the skills to recognize and understand a reliable review which will be helpful for their day-to-day clinical practice and research activities.
Introduction
Evidence-based healthcare is the integration of best research evidence with clinical expertise and patient values. Green denotes, “Using evidence from reliable research, to inform healthcare decisions, has the potential to ensure best practice and reduce variations in healthcare delivery.” However, incorporating research into practice is time consuming, and so we need methods of facilitating easy access to evidence for busy clinicians.[ 1 ] Ganeshkumar et al . mentioned that nearly half of the private practitioners in India were consulting more than 4 h per day in a locality,[ 2 ] which explains the difficulty of them in spending time in searching evidence during consultation. Ideally, clinical decision making ought to be based on the latest evidence available. However, to keep abreast with the continuously increasing number of publications in health research, a primary healthcare professional would need to read an insurmountable number of articles every day, covered in more than 13 million references and over 4800 biomedical and health journals in Medline alone. With the view to address this challenge, the systematic review method was developed. Systematic reviews aim to inform and facilitate this process through research synthesis of multiple studies, enabling increased and efficient access to evidence.[ 1 , 3 , 4 ]
Systematic reviews and meta-analyses have become increasingly important in healthcare settings. Clinicians read them to keep up-to-date with their field and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research and some healthcare journals are moving in this direction.[ 5 ]
This article is intended to provide an easy guide to understand the concept of systematic reviews and meta-analysis, which has been prepared with the aim of capacity building for general practitioners and other primary healthcare professionals in research methodology and day-to-day clinical practice.
The purpose of this article is to introduce readers to:
- The two approaches of evaluating all the available evidence on an issue i.e., systematic reviews and meta-analysis,
- Discuss the steps in doing a systematic review,
- Introduce the terms used in systematic reviews and meta-analysis,
- Interpret results of a meta-analysis, and
- The advantages and disadvantages of systematic review and meta-analysis.

Application
What is the effect of antiviral treatment in dengue fever? Most often a primary care physician needs to know convincing answers to questions like this in a primary care setting.
To find out the solutions or answers to a clinical question like this, one has to refer textbooks, ask a colleague, or search electronic database for reports of clinical trials. Doctors need reliable information on such problems and on the effectiveness of large number of therapeutic interventions, but the information sources are too many, i.e., nearly 20,000 journals publishing 2 million articles per year with unclear or confusing results. Because no study, regardless of its type, should be interpreted in isolation, a systematic review is generally the best form of evidence.[ 6 ] So, the preferred method is a good summary of research reports, i.e., systematic reviews and meta-analysis, which will give evidence-based answers to clinical situations.
There are two fundamental categories of research: Primary research and secondary research. Primary research is collecting data directly from patients or population, while secondary research is the analysis of data already collected through primary research. A review is an article that summarizes a number of primary studies and may draw conclusions on the topic of interest which can be traditional (unsystematic) or systematic.
Terminologies
Systematic review.
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ] To this end, systematic reviews may or may not include a statistical synthesis called meta-analysis, depending on whether the studies are similar enough so that combining their results is meaningful.[ 8 ] Systematic reviews are often called overviews.
The evidence-based practitioner, David Sackett, defines the following terminologies.[ 3 ]
- Review: The general term for all attempts to synthesize the results and conclusions of two or more publications on a given topic.
- Overview: When a review strives to comprehensively identify and track down all the literature on a given topic (also called “systematic literature review”).
- Meta-analysis: A specific statistical strategy for assembling the results of several studies into a single estimate.
Systematic reviews adhere to a strict scientific design based on explicit, pre-specified, and reproducible methods. Because of this, when carried out well, they provide reliable estimates about the effects of interventions so that conclusions are defensible. Systematic reviews can also demonstrate where knowledge is lacking. This can then be used to guide future research. Systematic reviews are usually carried out in the areas of clinical tests (diagnostic, screening, and prognostic), public health interventions, adverse (harm) effects, economic (cost) evaluations, and how and why interventions work.[ 9 ]
Cochrane reviews
Cochrane reviews are systematic reviews undertaken by members of the Cochrane Collaboration which is an international not-for-profit organization that aims to help people to make well-informed decisions about healthcare by preparing, maintaining, and promoting the accessibility of systematic reviews of the effects of healthcare interventions.
Cochrane Primary Health Care Field is a systematic review of primary healthcare research on prevention, treatment, rehabilitation, and diagnostic test accuracy. The overall aim and mission of the Primary Health Care Field is to promote the quality, quantity, dissemination, accessibility, applicability, and impact of Cochrane systematic reviews relevant to people who work in primary care and to ensure proper representation in the interests of primary care clinicians and consumers in Cochrane reviews and review groups, and in other entities. This field would serve to coordinate and promote the mission of the Cochrane Collaboration within the primary healthcare disciplines, as well as ensuring that primary care perspectives are adequately represented within the Collaboration.[ 10 ]
Meta-analysis
A meta-analysis is the combination of data from several independent primary studies that address the same question to produce a single estimate like the effect of treatment or risk factor. It is the statistical analysis of a large collection of analysis and results from individual studies for the purpose of integrating the findings.[ 11 ] The term meta-analysis has been used to denote the full range of quantitative methods for research reviews.[ 12 ] Meta-analyses are studies of studies.[ 13 ] Meta-analysis provides a logical framework to a research review where similar measures from comparable studies are listed systematically and the available effect measures are combined wherever possible.[ 14 ]
The fundamental rationale of meta-analysis is that it reduces the quantity of data by summarizing data from multiple resources and helps to plan research as well as to frame guidelines. It also helps to make efficient use of existing data, ensuring generalizability, helping to check consistency of relationships, explaining data inconsistency, and quantifies the data. It helps to improve the precision in estimating the risk by using explicit methods.
Therefore, “systematic review” will refer to the entire process of collecting, reviewing, and presenting all available evidence, while the term “meta-analysis” will refer to the statistical technique involved in extracting and combining data to produce a summary result.[ 15 ]
Steps in doing systematic reviews/meta-analysis
Following are the six fundamental essential steps while doing systematic review and meta-analysis.[ 16 ]
Define the question
This is the most important part of systematic reviews/meta-analysis. The research question for the systematic reviews may be related to a major public health problem or a controversial clinical situation which requires acceptable intervention as a possible solution to the present healthcare need of the community. This step is most important since the remaining steps will be based on this.
Reviewing the literature
This can be done by going through scientific resources such as electronic database, controlled clinical trials registers, other biomedical databases, non-English literatures, “gray literatures” (thesis, internal reports, non–peer-reviewed journals, pharmaceutical industry files), references listed in primary sources, raw data from published trials and other unpublished sources known to experts in the field. Among the available electronic scientific database, the popular ones are PUBMED, MEDLINE, and EMBASE.
Sift the studies to select relevant ones
To select the relevant studies from the searches, we need to sift through the studies thus identified. The first sift is pre-screening, i.e., to decide which studies to retrieve in full, and the second sift is selection which is to look again at these studies and decide which are to be included in the review. The next step is selecting the eligible studies based on similar study designs, year of publication, language, choice among multiple articles, sample size or follow-up issues, similarity of exposure, and or treatment and completeness of information.
It is necessary to ensure that the sifting includes all relevant studies like the unpublished studies (desk drawer problem), studies which came with negative conclusions or were published in non-English journals, and studies with small sample size.
Assess the quality of studies
The steps undertaken in evaluating the study quality are early definition of study quality and criteria, setting up a good scoring system, developing a standard form for assessment, calculating quality for each study, and finally using this for sensitivity analysis.
For example, the quality of a randomized controlled trial can be assessed by finding out the answers to the following questions:
- Was the assignment to the treatment groups really random?
- Was the treatment allocation concealed?
- Were the groups similar at baseline in terms of prognostic factors?
- Were the eligibility criteria specified?
- Were the assessors, the care provider, and the patient blinded?
- Were the point estimates and measure of variability presented for the primary outcome measure?
- Did the analyses include intention-to-treat analysis?
Calculate the outcome measures of each study and combine them
We need a standard measure of outcome which can be applied to each study on the basis of its effect size. Based on their type of outcome, following are the measures of outcome: Studies with binary outcomes (cured/not cured) have odds ratio, risk ratio; studies with continuous outcomes (blood pressure) have means, difference in means, standardized difference in means (effect sizes); and survival or time-to-event data have hazard ratios.
Combining studies
Homogeneity of different studies can be estimated at a glance from a forest plot (explained below). For example, if the lower confidence interval of every trial is below the upper of all the others, i.e., the lines all overlap to some extent, then the trials are homogeneous. If some lines do not overlap at all, these trials may be said to be heterogeneous.
The definitive test for assessing the heterogeneity of studies is a variant of Chi-square test (Mantel–Haenszel test). The final step is calculating the common estimate and its confidence interval with the original data or with the summary statistics from all the studies. The best estimate of treatment effect can be derived from the weighted summary statistics of all studies which will be based on weighting to sample size, standard errors, and other summary statistics. Log scale is used to combine the data to estimate the weighting.
Interpret results: Graph
The results of a meta-analysis are usually presented as a graph called forest plot because the typical forest plots appear as forest of lines. It provides a simple visual presentation of individual studies that went into the meta-analysis at a glance. It shows the variation between the studies and an estimate of the overall result of all the studies together.
Forest plot
Meta-analysis graphs can principally be divided into six columns [ Figure 1 ]. Individual study results are displayed in rows. The first column (“study”) lists the individual study IDs included in the meta-analysis; usually the first author and year are displayed. The second column relates to the intervention groups and the third column to the control groups. The fourth column visually displays the study results. The line in the middle is called “the line of no effect.” The weight (in %) in the fifth column indicates the weighting or influence of the study on the overall results of the meta-analysis of all included studies. The higher the percentage weight, the bigger the box, the more influence the study has on the overall results. The sixth column gives the numerical results for each study (e.g., odds ratio or relative risk and 95% confidence interval), which are identical to the graphical display in the fourth column. The diamond in the last row of the graph illustrates the overall result of the meta-analysis.[ 4 ]

Interpretation of meta-analysis[ 4 ]
Thus, the horizontal lines represent individual studies. Length of line is the confidence interval (usually 95%), squares on the line represent effect size (risk ratio) for the study, with area of the square being the study size (proportional to weight given) and position as point estimate (relative risk) of the study.[ 7 ]
For example, the forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults is shown in Figure 2 .[ 17 ]

Forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults[ 17 ]
The overall effect is shown as diamond where the position toward the center represents pooled point estimate, the width represents estimated 95% confidence interval for all studies, and the black plain line vertically in the middle of plot is the “line of no effect” (e.g., relative risk = 1).
Therefore, when examining the results of a systematic reviews/meta-analysis, the following questions should be kept in mind:
- Heterogeneity among studies may make any pooled estimate meaningless.
- The quality of a meta-analysis cannot be any better than the quality of the studies it is summarizing.
- An incomplete search of the literature can bias the findings of a meta-analysis.
- Make sure that the meta-analysis quantifies the size of the effect in units that you can understand.
Subgroup analysis and sensitivity analysis
Subgroup analysis looks at the results of different subgroups of trials, e.g., by considering trials on adults and children separately. This should be planned at the protocol stage itself which is based on good scientific reasoning and is to be kept to a minimum.
Sensitivity analysis is used to determine how results of a systematic review/meta-analysis change by fiddling with data, for example, what is the implication if the exclusion criteria or excluded unpublished studies or weightings are assigned differently. Thus, after the analysis, if changing makes little or no difference to the overall results, the reviewer's conclusions are robust. If the key findings disappear, then the conclusions need to be expressed more cautiously.
Advantages of Systematic Reviews
Systematic reviews have specific advantages because of using explicit methods which limit bias, draw reliable and accurate conclusions, easily deliver required information to healthcare providers, researchers, and policymakers, help to reduce the time delay in the research discoveries to implementation, improve the generalizability and consistency of results, generation of new hypotheses about subgroups of the study population, and overall they increase precision of the results.[ 18 ]
Limitations in Systematic Reviews/Meta-analysis
As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers’ ability to assess the strengths and weaknesses of those reviews.[ 5 ]
Even though systematic review and meta-analysis are considered the best evidence for getting a definitive answer to a research question, there are certain inherent flaws associated with it, such as the location and selection of studies, heterogeneity, loss of information on important outcomes, inappropriate subgroup analyses, conflict with new experimental data, and duplication of publication.
Publication Bias
Publication bias results in it being easier to find studies with a “positive” result.[ 19 ] This occurs particularly due to inappropriate sifting of the studies where there is always a tendency towards the studies with positive (significant) outcomes. This effect occurs more commonly in systematic reviews/meta-analysis which need to be eliminated.
The quality of reporting of systematic reviews is still not optimal. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias even though there is overwhelming evidence both for its existence and its impact on the results of systematic reviews. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately.[ 20 ]
To overcome certain limitations mentioned above, the Cochrane reviews are currently reported in a format where at the end of every review, findings are summarized in the author's point of view and also give an overall picture of the outcome by means of plain language summary. This is found to be much helpful to understand the existing evidence about the topic more easily by the reader.
A systematic review is an overview of primary studies which contains an explicit statement of objectives, materials, and methods, and has been conducted according to explicit and reproducible methodology. A meta-analysis is a mathematical synthesis of the results of two or more primary studies that addressed the same hypothesis in the same way. Although meta-analysis can increase the precision of a result, it is important to ensure that the methods used for the reviews were valid and reliable.
High-quality systematic reviews and meta-analyses take great care to find all relevant studies, critically assess each study, synthesize the findings from individual studies in an unbiased manner, and present balanced important summary of findings with due consideration of any flaws in the evidence. Systematic review and meta-analysis is a way of summarizing research evidence, which is generally the best form of evidence, and hence positioned at the top of the hierarchy of evidence.
Systematic reviews can be very useful decision-making tools for primary care/family physicians. They objectively summarize large amounts of information, identifying gaps in medical research, and identifying beneficial or harmful interventions which will be useful for clinicians, researchers, and even for public and policymakers.
Source of Support: Nil
Conflict of Interest: None declared.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 March 2024
A systematic review of the methodology of trade-off analysis in agriculture
- Timo S. Breure ORCID: orcid.org/0000-0001-5695-8064 1 ,
- Natalia Estrada-Carmona ORCID: orcid.org/0000-0003-4329-5470 2 ,
- Athanasios Petsakos ORCID: orcid.org/0000-0003-0224-4087 3 ,
- Elisabetta Gotor ORCID: orcid.org/0000-0003-0533-3077 3 ,
- Boris Jansen ORCID: orcid.org/0000-0002-4493-1734 4 &
- Jeroen C. J. Groot ORCID: orcid.org/0000-0001-6516-5170 1
Nature Food volume 5 , pages 211–220 ( 2024 ) Cite this article
4652 Accesses
33 Altmetric
Metrics details
- Agroecology
- Ecological modelling
- Ecosystem services
- Environmental impact
- Sustainability
Trade-off analysis (TOA) is central to policy and decision-making aimed at promoting sustainable agricultural landscapes. Yet, a generic methodological framework to assess trade-offs in agriculture is absent, largely due to the wide range of research disciplines and objectives for which TOA is used. In this study, we systematically reviewed 119 studies that have implemented TOAs in landscapes and regions dominated by agricultural systems around the world. Our results highlight that TOAs tend to be unbalanced, with a strong emphasis on productivity rather than environmental and socio-cultural services. TOAs have mostly been performed at farm or regional scales, rarely considering multiple spatial scales simultaneously. Mostly, TOAs fail to include stakeholders at study development stage, disregard recommendation uncertainty due to outcome variability and overlook risks associated with the TOA outcomes. Increased attention to these aspects is critical for TOAs to guide agricultural landscapes towards sustainability.
Similar content being viewed by others
Long-term evidence for ecological intensification as a pathway to sustainable agriculture
An interactive model to assess pathways for agriculture and food sector contributions to country-level net-zero targets

Ecological sensitivity within human realities concept for improved functional biodiversity outcomes in agricultural systems and landscapes
Contemporary agriculture should not only provide food, fibre, feed and fuel but also environmental and socio-economic benefits for rural communities and beyond 1 . To ensure that agriculture delivers multiple services while minimizing its negative impacts, society must be aware of the trade-offs and synergies that may arise. The nature of these trade-offs depends on location-specific natural, social and cultural conditions that place constraints on the inputs and outputs of an agricultural system. For example, market-based farmers are concerned with enhancing commodity production, whereas the priority of subsistence farmers lies with improving food security 2 . The global imperative to achieve the United Nations Sustainable Development Goals (SDGs) underscores the need to reduce the environmental impact of land use practices and strengthen equitable social outcomes at both landscape and community levels. However, achieving the SDGs might require sacrifices to primary productivity and economic revenues. Thus, to reconcile the demands of agriculture and inform decision-making, an analysis is required of potential trade-offs measured against agronomic, environmental, economic and social indicators 3 .
Trade-off analysis (TOA) was established as a concept to generate quantitative information on competing (trade-offs) or complementary (synergies) indicators that can be used to guide policy and decision-making 4 . A typical TOA project starts with three preparatory steps: formulation of the research question, identification of which indicators to assess, and formulation of hypotheses about the relationships between the indicators and the associated trade-offs and synergies. Subsequently, the management, policy or technological changes that affect the TOA indicators can be identified and included in the analysis framework. Then, the trade-offs and synergies under changing conditions or scenarios can be quantified and, finally, the results are communicated to relevant stakeholders to inform decision-making and policy 4 . Since its first implementation in the context of agriculture, a wide range of methods have been used to conduct TOAs, including optimization, simulations, qualitative, econometric and narrative-based approaches. In some cases, these approaches are deployed in a spatially explicit manner with the support of geographic information systems (GIS) 5 .
Although important advances have been made regarding TOA in agricultural contexts, researchers have expressed concerns about the scope and methodological limitations of published studies. These concerns relate to the limited transfer of the academic knowledge generated by TOA into decision- and policy-making due to the inability to take into account social and cultural factors 6 , the sparsity of multi- and cross-scale assessments 3 , 5 , 6 , 7 , and the limited representation of uncertainty 8 , 9 and risk analysis 5 .
The concerns reported in the literature on the limitations of TOA analysis can indeed have important implications. First, failure to recognize the importance of scale (spatial, temporal, jurisdictional and legislative) in TOA may lead to erroneous inferences on how the relationships between trade-offs and indicators develop across scales. Multiple scales can be analysed without interactions between them or a cross-scale analysis can be performed that accounts for interactions between scales 10 . Furthermore, adverse effects appearing outside the TOA case study area (off-site effects) may offset any gains stemming from a TOA-informed policy 11 . Second, recognition of social interactions and cultural values is needed to assure representation of beneficiaries and non-beneficiaries relevant to the topic at hand, that is, distributional justice 9 , 10 . Representation among stakeholders and their involvement in the design and implementation of a TOA can increase the legitimacy of its findings, assure that the data used are relevant to the context and thus enhance adoption of a study’s findings 12 . Third, validation and acknowledgement of uncertainty in both data and model estimates increase the robustness of a TOA and can facilitate risk-based decision-making 13 , 14 , 15 .
Previous literature reviews on TOA in agriculture adopted a ‘storytelling’ approach, where key studies were selected from the literature to discuss research trends. However, given the wide scope of TOAs applied in the context of agriculture, a systematic review could reveal the variety of approaches used and potential knowledge gaps, as well as the indicators that were studied and by which methods, ultimately facilitating the comparability of results.
Here we report on the TOA indicators, methodology and analysis used in 119 peer-reviewed articles. Descriptive statistics are used to characterize articles based on the extent to which they considered (1) indicators relevant to environmental and socio-economic services, (2) multiple spatial scales and their interactions, (3) the comprehensive involvement of stakeholders, and (4) the validity of trade-offs and recommendations in the context of associated uncertainties and risks (see Table 1 for further details). Finally, a cluster analysis shows which indicators were frequently studied together and which TOA methods were associated with each cluster.
The aim of this study was thus to provide an overview of the peer-reviewed literature on TOA in the context of agriculture using a systematic approach. For this purpose, we sought to define how trade-offs in agriculture are conceptualized, characterized and analysed in the TOA literature. Based on these findings, we have identified common gaps in the implementation of TOA.
The distribution of publication dates for the articles in the sample was mainly centred in the years 2015–2021 (Extended Data Fig. 1a ). Specifically, 73% of the articles were published after 2014, which indicates an increasing research effort directed towards TOAs in an agricultural context (Extended Data Fig. 1b ).
Common interrelationships and co-occurrences among TOA indicators
The articles examined included a median of 3.8 ± 1.9 (s.d.) TOA indicators, ranging from 1 to 10. Based on the cumulative distribution, 52% of the articles included three or fewer TOA indicators, while 90% included six or fewer TOA indicators (Extended Data Fig. 2a ). The most prevalent indicators across all articles were ‘profitability’ (57%, economic), ‘yield’ (44%, agronomic) and ‘water quantity’ (34%, sustainable resource management). The second most common set of indicators encompassed a selection of biophysical (for example, ‘water quality’ and ‘greenhouse gases’), agronomic (for example, ‘input efficiency’ and ‘land use efficiency’) and economic indicators (for example, ‘assets’), ranging between 13% and 21% (Fig. 1 ). The remaining TOA indicators were used less frequently and related to economic (that is, ‘labour productivity’ and ‘poverty’), human health (for example, ‘nutrition’, ‘health’ or ‘food security’) and agronomic (that is, ‘self-sufficiency’) aspects, representing a share of 5–6% (Fig. 1 ). Rarely considered TOA indicators (less than 5%) included ‘market supply or demand’ (economic), ‘yield stability’ (agronomic), ‘empowerment’ and ‘gender equity’ (both human health; Fig. 1 ).
Percentage of articles that include a TOA indicator (black dotted line and circles) and the share of each TOA method M1–M9 used to study that indicator (coloured bars). The prefixes of the TOA indicators refer to their class association (A, E, H, S) and number of occurrence within that class as provided in Table 1 . Table 1 also describes the TOA methods M1–M9.
The articles were grouped into 11 clusters, depending on which TOA indicators were assessed (left y -axis dendrogram in Fig. 2 ). These clusters show a dominant theme based on the co-occurrence of TOA indicators (right y- axis in Fig. 2 ). For example, in cluster 7, ‘poverty’ was studied in conjunction with ‘soil nutrients’, whereas in cluster 5, ‘poverty’ was studied in conjunction with ‘profitability’, ‘food security’ and ‘nutrition’. The clustering of articles by TOA indicator reveals which TOA indicators are often studied together. Indicators of ‘profitability’ and ‘yield’ were the most commonly used (Figs. 1 and 2 ) and were generally combined with case-specific environmental and social indicators (Fig. 2 ). This suggests that agronomic and economic viability are conditional for the exploration of improvements in agricultural system sustainability. The cluster with the largest number of articles (cluster 6, Fig. 2 ) concerned agricultural production and water quality. This highlights the strong focus on solving pressing issues related to pollution by surplus nutrients from fertilizers and manure.
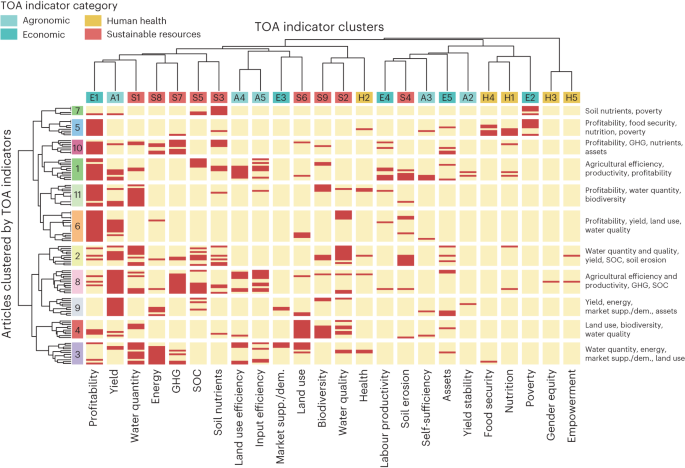
The articles were clustered by TOA indicator (row-wise) and TOA indicator clusters (column-wise). The associations of articles with clusters are indicated by the colours and labels on the left of the figure; the colours are arbitrary. TOA indicator clusters (top x axis) are specified by colour, corresponding to the main indicator categories (legend in top left of the figure), and their name (bottom x axis). The matrix indicates whether a TOA indicator has been included in an article (red) or not (beige). The labels on the right list the main TOA indicators included in each cluster. GHG, greenhouse gases; SOC, soil organic carbon; supp./dem., supply or demand.
The clustering of TOA indicators (top x -axis dendrogram in Fig. 2 ) shows that for 50% of the indicators, the indicator closest in the dendrogram belongs to the same category (sustainable resource management, agronomic, economic or human health). In particular, four out of five human health indicators were studied in isolation from other indicators, forming closely paired branches (top x -axis dendrogram, orange colour, in Fig. 2 ).
The application of TOA methods varied across different TOA indicators and clusters. For example, the TOA indicators ‘labour productivity’, ‘empowerment’, ‘gender equity’ and ‘yield stability’ lacked cases involving spatially explicit methods (M1 or M8; Fig. 1 ). This same observation applies to the clusters in which these TOA indicators belong (Fig. 3 ). While the absence of spatially explicit methods for social indicators such as ‘empowerment’ and ‘gender equity’ is expected, given that their spatial dimension is often disregarded, it is worth noting that gender and empowerment may relate to the spatial distribution of fields and resources in the landscape. For instance, their distance from the location of the homestead or decision-making processes regarding the (distribution of) use and ownership of these resources. Clusters of articles associated with ‘yield’, ‘energy’, ‘biodiversity’ and ‘land use’ exhibited a high use of GIS (M8), qualitative (M6) and other (M9) methods, with fewer articles applying optimization methods (M3; Fig. 3 ). Lastly, an interesting anomaly is the ‘health’ indicator, where methods M1–M3, encompassing (spatially explicit) simulations and optimization methods, were conspicuously absent (Fig. 1 ).
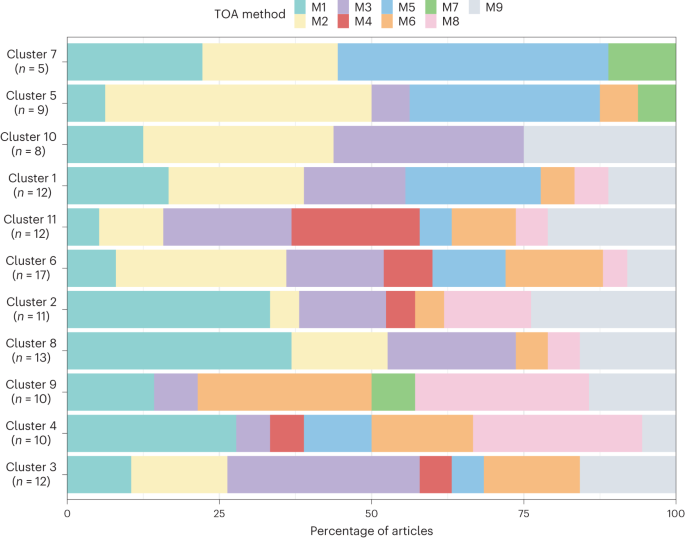
Cluster associations are as per Fig. 2 and the number of articles within each cluster is given by n .
Frequency of criteria levels
The majority of TOAs were conducted at regional (65%) and farm (17%) scales, followed by field (7%) and national (6%) scales. The TOAs conducted at multi-country (4%) and global scales, along with ‘other’, accounted for only a small proportion of the analyses (Fig. 4a ). The spatial scales for TOAs differed from the scales at which modelling was performed or data were collected, with the farm and field scale contributing to a combined share of 48%. Of the articles considered, 12% implemented cross-scale analyses and 17% considered off-site effects (Fig. 4a ). Case study areas were predominantly delineated using administrative borders (54%), followed by biophysical delineation (24%), with 18% of the articles using both methods (Fig. 4b ).
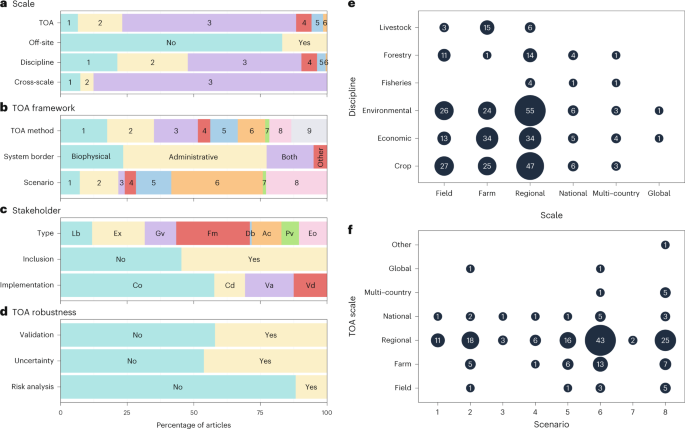
a , Criteria related to the scale of the analysis. TOA: the spatial scale at which the TOA was conducted. The numbers refer to the spatial scales of field (1), farm (2), regional (3), national (4), multi-country (5) and global (6). Off-site: whether off-site effects have been considered in the TOA. Discipline: the spatial scale at which modelling or data collection was performed for a discipline. The numbers refer to the spatial scales detailed above for TOA. Cross-scale: whether aggregative (1), interactive (2) or no cross-scale modelling was performed (3). b , Criteria related to the TOA framework. TOA method: the methods used to perform the TOAs. The numbers refer to the TOA methods M1–M9 defined in Table 1 . System border: which boundaries were used to define the TOA case study area. Scenario: whether the article considered a scenario and, if so, which type of scenario. The numbers refer to the scenarios 1–8 defined in Table 1 . c , Criteria related to stakeholders. Type: whether local beneficiaries and non-beneficiaries, experts, government, farmers, distant beneficiaries and non-beneficiaries, academics, private organizations or environmental organizations were involved. Inclusion: whether the study included stakeholders. Implementation: whether stakeholders were involved in consultation, co-development, valuation or validation. d , Criteria related to TOA robustness: whether the article performed a validation, risk analysis or acknowledged uncertainty. e , The frequency (shown in the circles) for each spatial scale at which the modelling or data collection was performed for a given discipline. f , The frequency (shown in the circles) at which an article considered a given scenario in TOA for each spatial scale. The scenario numbers 1–8 are defined in Table 1 .
Including a scenario in the TOA allows investigation of the effect of a postulated event or driver on the TOA indicators. In our analysis, scenarios focusing on climate, behavioural or demographic change accounted for 14% of the articles, while scenarios involving alternative intensities of resource use constituted 37% of the articles. Scenarios were absent in 25% of the articles (Fig. 4b ). Over half of the articles included stakeholders in their analysis, with a relatively equal spread across stakeholder types, except for ‘distant beneficiaries and non-beneficiaries’, which were under-represented. Farmers and experts constituted a larger share (48%) compared to other categories (Fig. 4c ). Stakeholders were mainly involved in consultation and valuation, with co-development and validation implemented in less than 25% of the articles considered (Fig. 4c ). Overall, the robustness of the TOA results was not widely considered, as the criteria ‘uncertainty’ and ‘validation’ were logged for less than 50% of the articles. Articles incorporating risk analysis constituted 12% of the sample (Fig. 4d ).
Links between spatial scales and criteria
Of the articles considered, ‘livestock’, ‘fisheries’ and ‘forestry’ accounted for a relatively small share (16%) compared with ‘crop’, ‘economic’ and ‘environmental’ disciplines. For the livestock discipline, modelling and data collection were predominantly carried out at the farm scale, while for forestry, they were primarily conducted at the field or regional scale (Fig. 4e ). For the economic discipline, modelling and data collection were evenly distributed between the farm ( n = 34) and regional ( n = 34) scales (Fig. 4e ), in contrast to the overall share of these scales across all of the articles, where ‘regional’ constituted 65% and ‘farm’ constituted 17% of the articles (Fig. 4a ). In general, for a large share of the reviewed articles, data were collected and modelling was performed at the field and farm scales, but the TOA was conducted at the regional scale. These findings show that, before the TOA, some form of aggregation occurs in the majority of the reviewed articles. Regarding the spatial scale at which the TOA was conducted for articles including a scenario, two observations can be made. First, all of the scenarios (except the resource use scenario) were rarely studied at scales larger than the national scale. Second, the climate, behavioural and demographic change scenarios were almost exclusively studied at the regional scale (Fig. 4f ). These results show that few studies investigated how scenarios unfolding at smaller or larger scales affect the indicators at the TOA scale.
Multi-scale, cross-scale and robustness criteria
Figure 5 shows the percentage of articles that include a TOA indicator (black line, the same as shown in Fig. 1 ). The articles were then divided into subsets according to whether they included a cross-scale, multi-scale or robustness criterion. The coloured lines represent the percentage of articles in the subset that include a specific TOA indicator. With the exception of indicators rarely included in all articles (for example, those related to nutrition or health), most TOA indicators were present in articles adopting a cross-scale modelling framework (Fig. 5a ). These findings occur despite the overall low number of articles (<20%) reporting cross-scale analyses (Fig. 5a ). Notably, articles applying an interactive modelling framework did not include ‘water quality’, ‘soil erosion’, ‘soil organic carbon’ and ‘biodiversity’, despite these indicators having a relatively high frequency across all articles (Fig. 5a ).
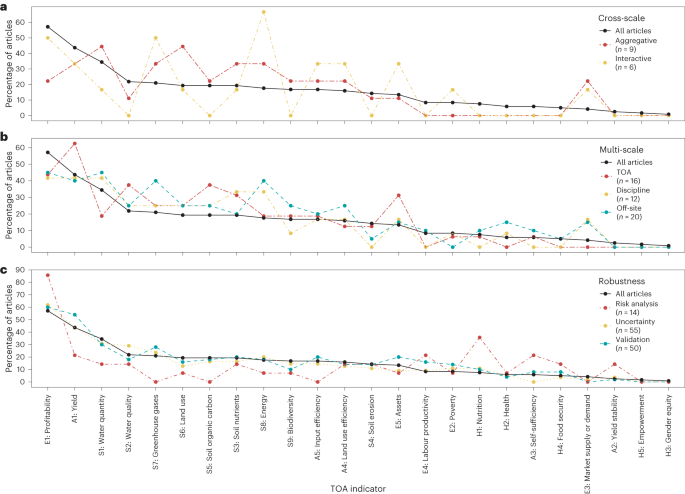
The percentages of all reviewed articles and subsets of articles that include specific TOA indicators. a – c , The subsets comprise articles that included cross-scale ( a ), multi-scale ( b ) and robustness ( c ) criteria. In b , TOA refers to articles in which the TOA was conducted on multiple spatial scales, ‘Discipline’ refers to articles that considered multiple spatial scales for modelling or data collection, and ‘Off-site’ refers to articles in which effects outside the TOA case study area were considered.
Across all articles, 17% considered off-site effects (Fig. 4a ). Notably, the ‘poverty’ and ‘soil erosion’ indicators were under-represented in articles considering off-site effects (Fig. 5b ). Eight indicators were excluded in articles considering multiple spatial scales in modelling or data collection (‘discipline’ in Fig. 5b ). This finding is particularly striking for ‘biodiversity’, given that it constitutes a large share of spatially explicit TOA methods (Fig. 1 ).
Thirteen per cent of articles reported TOA on multiple spatial scales, with seven indicators excluded in these cases (‘TOA’ in Fig. 5b ). Among the excluded indicators, those related to human health dominated (except for ‘nutrition’). For certain indicators, these findings are to be expected. For instance, market supply or demand (economic) is irrelevant at low geographical scales (field and farm) as prices are determined at the regional (local), national or international scale. The articles that included a risk analysis showed stark contrasts between TOA indicators with respect to their representation relative to all articles. Economic and human health indicators were particularly over-represented, while ‘yield’, ‘input efficiency’ and a set of biophysical indicators were under-represented (Fig. 5c ). For articles in which uncertainty was acknowledged or validation was performed, no indicators were over- or under-represented relative to their inclusion across all articles (Fig. 5c ).
Limitations on the inclusion of TOA indicators
Recent reviews on TOA have stated that there is little to no representation of indicators related to social interactions, justice and gender issues in TOAs for agricultural systems 5 , 6 . These studies referred in particular to intra-household equity, asset ownership, health, education and nutrition. Our results also demonstrate that social and cultural TOA indicators are largely absent, mostly considered in isolation and studied by statistical approaches. These findings are probably a result of the limited data availability and the inability of TOA methods to include socio-cultural indicators for features and processes that are difficult to capture quantitatively 16 , 17 . We further note a similarly low frequency for the following indicators: food security, self-sufficiency and yield stability. These findings raise questions about the rationale behind the selection of TOA indicators. That is, the prevalent use of profitability and crop yield as primary indicators reflects the focus on profit and crop yield maximization in the literature 5 . The outcomes and priorities of a TOA depend on the chosen objectives and indicators. Alternative indicators might therefore facilitate a more comprehensive analysis of the delivery of environmental, economic and socio-cultural services from agriculture. One illustrative example is the metric ‘nutritional yield’, defined as “the number of hectares required to provide sufficient quantity to fulfil 100% of dietary reference intake for a nutrient for one adult” 2 . Nutritional yield thus allows the assessment of land use efficiency in both agronomic and social terms. Integrating nutritional yield into TOA in the context of subsistence agriculture could unveil the need for changes in farmers’ crop plans to balance food security and economic profitability objectives.
TOA methodologies
The formulation of research objectives, questions and methodology determines the information base that a TOA can provide 16 , 18 . Decisions regarding TOA objectives and methodology determine the degree to which scales, disciplines and indicators are compartmentalized. In addition, these decisions influence the range of interventions and scenarios explored for alternative agro-environmental management of land, resources and technologies 7 , 18 . The results of our analysis reveal associations between TOA methods and indicators, indicating common gaps, such as the absence of articles reporting the use of spatially explicit methods to study the indicators ‘human health’ and ‘yield stability’. Studying these indicators in a spatially explicit manner could allow for targeted land use planning at the local scale. For instance, Prestele and Verburg demonstrated that spatially explicit analysis of climate-smart agriculture adoptions unveils local-scale trade-offs affecting yield and soil carbon sequestration at an aggregated scale 19 . Our results also underscore expected patterns, with socio-economic indicators predominantly studied through statistical approaches and qualitative methods. These methods, static and based on existing datasets, differ from mechanistic models, which allow extrapolation and ex ante assessment under alternative future scenarios. Simulations based on mechanistic models hold the potential to explore scenarios that minimize trade-offs between indicators 3 , 7 . However, the validity of this kind of optimization depends on having sufficient understanding of relevant processes and feedbacks in the socio-environmental system 3 . For example, while crop models vary in their capacity to assess climate change impacts, they share common limitations, such as inadequate representation of low-intensity agricultural systems 20 . We found that a description of study limitations in the context of the TOA framework, for example, excluded aspects, was often absent. Ideally, models and associated uncertainties would be assessed in the design phase of the TOA. This could ensure the availability of adequate information for quantifying all desired parameters at the desired resolution, allowing the study to comprehensively represent the agricultural system. Such an approach is crucial to guide planning in future management decisions aligned with research objectives 17 .
Involvement of stakeholders and practical application of TOA results
One recurring concern in the literature is the frequent omission of stakeholders at the onset of the TOA, potentially limiting the practical application of TOA results 6 , 8 . Our findings partially support these concerns, given that co-development with stakeholders was observed in only 10% of the articles. However, making a definitive statement on equal representation among stakeholders proved challenging as there was generally an absence of a systematic inventory outlining the relevance of different stakeholders to the decision-making process based on their interests and influence 21 . Our analysis shows that farmers and experts were the primary stakeholders included in the articles. Nonetheless, the omission of distant beneficiaries and non-beneficiaries is noteworthy as they are likely to be relevant to the decision-making process in numerous cases, especially when off-site effects are considered in TOAs conducted on multiple scales.
Multi- and cross-scale analysis
Depending on the research objectives, the TOA literature underscores the importance of acknowledging processes across scales and including them in research 3 , 6 , 7 , 8 , 9 , 22 . In many of the articles, data were collected or modelling was performed at field and farm scales, yet the TOA was conducted at the regional scale. This highlights an opportunity for multi-scale TOA analysis, potentially enhancing the relevance of TOA studies to policy. For example, bilevel optimization is a promising approach to facilitating nested decision-making processes at different scales. In this approach, the solution at the higher level (for example, larger spatial scale) depends on the solution at the lower level (for example, smaller spatial scale). Bostian et al. demonstrated the application of this methodology in recognizing multiple spatial scales inherent to non-point pollution regulation 23 . However, the restricted application of cross-scale analysis in our sample (12%) shows the limited extent to which TOA in agriculture captures the hierarchical nature of social, cultural, environmental, economic and agronomic processes.
Furthermore, 17% of the articles considered effects outside the TOA case study area, considering off-site effects in a diverse array of subjects, including transnational emission permits, water trading and increased demand for scarce resources, anticipated to influence their shadow prices 24 , 25 , 26 . However, off-site effects might have feedbacks, such as dependencies between alternative production systems within a supply chain 27 . In such cases, the delineation of the system boundary must be considered in the context of these feedbacks to ensure their inclusion within the system. In cases where off-site effects do not have feedbacks, these can be classified as ‘teleconnections’, denoting processes whose cause and effect are widely separated 28 . A case in point is a study of the water quality of the Danube River, in which distant beneficiaries and non-beneficiaries, represented by an international committee, were considered in the TOA 29 . The results also show that climate, behavioural and demographic scenarios were rarely assessed at lower or higher scales (compared to the regional scale). This underscores that the extent to which these scales are relevant to TOA is understudied and merits further research. For example, generic methods, such as the carbon 30 or water 31 footprint, can provide a broad assessment of which off-site effects at larger scales are relevant to TOA outcomes. These approaches may facilitate the inclusion of underlying causes, the involvement of more inclusive stakeholders and account for leakage effects, such as the expansion of agricultural lands beyond the TOA case study area 32 .
Ideally, a TOA methodological framework is conceptualized such that (1) it recognizes multi- and cross-scale interactions where applicable, (2) the system boundary aligns with substantiated biophysical and relevant socio-institutional boundaries, and (3) it recognizes the heterogeneity in which scales and associated consequences are perceived as well as valued by different stakeholders 10 .
Robustness of TOA results
The risk associated with TOA extends across spatial, temporal and jurisdictional scales, carrying implications for the dissemination of TOA results 13 . The under-representation of ‘yield’ in articles considering risk analysis highlights the dichotomy between yield and profitability as the most prominent indicators. That is, risk analysis appears to be mainly associated with the economic domain 5 . However, it is important to recognize that the evaluation of risk and the formulation of relevant strategies (risk aversion, mitigation or offsetting) are critical for farmers adopting system transformations, such as alternative forms of land use to mitigate inputs and associated greenhouse gas emissions. Integrating risk into TOA enables the study of the policies and incentives necessary for achieving whole-system transformations towards sustainable agricultural practices 13 , 14 . Decision-making under uncertainty becomes interpretable when recommendations are accompanied by an assessment of associated risks. Ideally, these risks are context-specific. For example, Hochman et al. provided TOA results on crop rotations alongside a minimum risk threshold quantified as the highest gross margin for the poorest 20% of years 33 .
While a moderate number of the articles considered uncertainty, only a few articles quantified changes in trade-offs as a function of uncertainty. The inclusion of stochastic components and the associated uncertainty inherent in biological systems could facilitate a more realistic description of outcomes, proving valuable for decision-making 13 , 15 . Varying input data or model parameterization within an expected range could reveal the sensitivity of results. For instance, when climate scenarios are used, realizations of these scenarios can be used to assess the stochasticity of the objectives for which the TOA is implemented 34 . This approach enables the acknowledgement of both the frequency and pattern of stochastic events, including extreme weather events, and their impact on TOA outcomes. Consequently, an analysis of the adaptability of a farming system would not solely rely on optimal solutions given the mean output but would also account for associated variability and unexpected events 15 . However, it is crucial to contextualize the effect of stochasticity. For example, the relative impact of model or parameter uncertainty on optimization outcomes has been shown to vary depending on the prioritization of objectives and site conditions 35 .
Limitations of this study
An important limitation of our review lies in the use of ‘trade-off analysis’ as a single term in our Web of Science search string. There are research areas that address trade-offs and synergies across various disciplines, scales and methods without explicitly using the term ‘trade-off analysis’ to describe their research objectives. Examples include the ‘food–energy–water nexus’ literature 36 , as well as research under the auspices of the Agricultural Model Intercomparison and Improvement Project (AgMIP) ( https://agmip.org/ ) and the Food, Agriculture, Biodiversity, Land-Use and Energy (FABLE) Consortium 37 . Both AgMIP and FABLE are particularly concerned with the relevance of TOA to policy. AgMIP explicitly states the use of “multiple scenarios and models to assess and probabilistically manage risk” 38 . Given the focus of these studies on global and regional assessments, we anticipate that our findings for those spatial scales could be affected. Indeed, the identified gaps in TOA implementation need to be viewed in the context of our sample, which mostly comprises studies in which modelling or data analysis was performed up to the regional level and TOA at the regional scale.
The method used to log the occurrence of pre-set criteria not only affects the variance within a criterion but also influences its abundance. For example, Sanon et al. included a large number of TOA indicators that were all classified under ‘biodiversity’ 29 . Thus, binary criteria logging does not capture the intensity with which a criterion is considered, a well-known phenomenon in the field of ecology 39 . This limitation may have resulted in the underestimation of both the intensity with which certain TOA indicators and their classes have been studied (Fig. 1 ) and the total number of TOA indicators considered per article (Extended Data Fig. 2 ).
Conclusions
Based on our analysis, it is possible to identify some actions that would increase the contribution of TOAs to SDG-aligned agricultural landscapes.
For instance, future studies should include multi- and cross-scale effects when relevant to the research objectives. We have identified an opportunity for multi-scale analysis, given that many studies aggregated farm- or field-scale data before performing TOA at a regional scale. As the inclusion of multiple scales, indicators and methods may in some cases reduce the generalizability of results and make them more context-specific, an alternative would be to discuss the anticipated implications of multi- and cross-scale effects on the study findings.
Furthermore, the relevance of TOA to society and policy can be improved by formulating research objectives such that TOA indicators lie within the scope of frameworks such as the SDGs. The most frequent indicators were biophysical or informed by profit maximization theory (for example, profitability and yield). However, indicators relevant to human well-being, security and farm resilience (for example, empowerment, nutrition and yield stability) occurred less frequently. To aid the interpretation of TOA results, the rationale behind the TOA methodology that is used to assess indicators should be listed together with a critical review of how the agricultural system under study is represented and what is excluded as a consequence.
In the reviewed articles, the most consulted stakeholders were farmers and experts, stakeholder co-development and validation were rare, and scenarios were predominantly based on resource use with little consideration of off-site effects. These findings suggest that TOAs mostly explore alternative management across a set of farms rather than policies and incentives that would facilitate whole landscape and food system transformations.
Agricultural policy- and decision-making carry an inherent risk. TOAs will become more operational when they evaluate associated risks and list strategies to manage these risks. This process could promote the robustness of quantified trade-offs with respect to the associated uncertainty of data and variability in outcomes. Finally, an inventory of stakeholders that are relevant to the decision-making process and their respective roles in the study would provide legitimacy of results. While this element has already been recognized in the literature 12 , 29 , some of the shortcomings that we have identified here would probably occur less frequently, particularly the lack of stakeholder inclusion and the over-representation of specific stakeholder types and methods of stakeholder engagement.
Closer adherence to these guidelines could enhance the relevance of TOA to the scientific community, policy-makers and farmers.
We followed the approach of Lautenbach et al. and Seppelt et al. in their systematic review of the literature on ecosystem services 9 , 22 . The generic structure involved (1) the identification, screening and selection of relevant peer-reviewed literature from a global repository, (2) formulation of the criteria against which to evaluate each article (Table 1 and Supplementary Table 1 ), and (3) descriptive statistics and cluster analysis to assess common interrelationships between criteria and identify knowledge gaps.
We used the following search string “ALL=agricultur* AND (“trade off* analysis” OR “trade-off* analysis” OR “tradeoff* analysis”)” in the Web of Science (on 14 September 2021) to identify peer-reviewed articles in English reporting TOA. We found 153 articles with publication dates spanning from 1993 to 2021. We excluded studies that mentioned the existence of trade-offs but did not assess relationships between indicators. For this reason, review and opinion papers were considered off-topic and were excluded from the search results. Furthermore, methodological papers that did not involve a case study were also excluded, leading to a total sample of 119 articles.
We selected criteria based on current TOA research 5 , 6 , 7 , 8 , 9 , 16 , 22 and recorded information on these criteria that were relevant to the conceptualization, characterization and analysis of trade-offs in agriculture (research objective 1). Briefly, the criteria included the type of TOA methods used, the spatial scales at which the analyses were performed and/or data collected, the indicators assessed in the TOA, which stakeholder types were included as well as how the stakeholders were engaged in the case study, whether the case study included alternative scenarios and of what type, how the case study area was delineated, whether effects outside the case study area were considered, and whether the case study acknowledged and accounted for uncertainty, validated results or performed a risk analysis. To assess whether cross-scale analyses were performed in case studies, we adopted the definition of Kanter et al., who distinguished between model frameworks that aggregate outputs at lower scales to use as inputs at higher scales (aggregative) and model frameworks that have submodels operating at different spatial and temporal resolutions (interactive) 6 . Thus, whereas an aggregative model framework follows a sequential approach, an interactive model framework performs analysis across scales simultaneously, allowing for interactions between scales and emergent indicators at higher levels. Furthermore, descriptive information was collected for three criteria: the agricultural system(s) studied, agricultural activities and knowledge gaps reported in the discussion section of the article. All of the criteria are listed in Table 1 with a generic description. We refer the reader to Supplementary Table 1 for more detailed information on the criteria. Based on these criteria, knowledge gaps were then assessed through descriptive statistics and cluster analysis (research objective 2).
The decision of which TOA indicators to include is a major methodological decision in TOA as it determines which interrelations are considered and analysed, and therefore which trade-offs and synergies can be identified. We anticipated thematic clusters of TOA indicators based on the discipline, scale, geography and method considered. To identify co-occurrences between TOA indicators, we performed hierarchical Ward clustering to group articles by TOA indicators as well as the TOA indicators themselves based on the Jaccard similarity coefficient 40 . Through the use of the Jaccard similarity metric, we accounted for the double-zero problem. Namely, the absence of a TOA indicator in two articles does not indicate a similarity, whereas its presence does 9 . For the clustering of articles by the TOA indicators used, the number of clusters to be retained was decided by the ‘elbow’ method based on the Mantel correlation between the data for each cluster and the raw distance matrix 40 . For the clustering of TOA indicators, the dendrogram was not cut to visualize common co-occurrences for all of the TOA indicators.
Criteria were logged in a Microsoft Office Excel (2021) spreadsheet (Supplementary Data 1 ). The data collected during this systematic review were further analysed and visualized in R (ref. 41 ). Data handling, visualizations and analysis were performed using the following R packages: tidyverse 42 , dendextend 43 , cluster 44 , vegan 45 and pheatmap 46 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The dataset created has been made available as extended data.
Code availability
The code created for data handling, analysis and visualizations is available on request.
Renting, H. et al. Exploring multifunctional agriculture. A review of conceptual approaches and prospects for an integrative transitional framework. J. Environ. Manag. 90 , 112–123 (2009).
Article Google Scholar
DeFries, R. et al. Synergies and trade-offs for sustainable agriculture: nutritional yields and climate-resilience for cereal crops in Central India. Glob. Food Sec. 11 , 44–53 (2016).
Seppelt, R., Lautenbach, S. & Volk, M. Identifying trade-offs between ecosystem services, land use, and biodiversity: a plea for combining scenario analysis and optimization on different spatial scales. Curr. Opin. Environ. Sustain. 5 , 458–463 (2013).
Stoorvogel, J. J., Antle, J. M., Crissman, C. C. & Bowen, W. The tradeoff analysis model: integrated bio-physical and economic modeling of agricultural production systems. Agric. Syst. 80 , 43–66 (2004).
Antle, J. M. & Valdivia, R. O. Trade-off analysis of agri-food systems for sustainable research and development. Q Open https://doi.org/10.1093/qopen/qoaa005 (2021).
Kanter, D. R. et al. Evaluating agricultural trade-offs in the age of sustainable development. Agric. Syst. 163 , 73–88 (2018).
Groot, J. C. J. et al. On the contribution of modelling to multifunctional agriculture: learning from comparisons. J. Environ. Manag. 90 , 147–160 (2009).
Klapwijk, C. J. et al. Analysis of trade-offs in agricultural systems: current status and way forward. Curr. Opin. Environ. Sustain. 6 , 110–115 (2014).
Lautenbach, S. et al. Blind spots in ecosystem services research and challenges for implementation. Reg. Environ. Change 19 , 2151–2172 (2019).
Cash, D. W. et al. Scale and cross-scale dynamics: governance and information in a multilevel world. Ecol. Soc . https://www.jstor.org/stable/26265993 (2006).
Gibson, R. B. Sustainability assessment: basic components of a practical approach. Impact Assess. Proj. Apprais. 24 , 170–182 (2006).
Cash, D. W. et al. Knowledge systems for sustainable development. Proc. Natl Acad. Sci. USA 100 , 8086–8091 (2003).
Article ADS CAS PubMed PubMed Central Google Scholar
Anderson, J. R. Risk in rural development: challenges for managers and policy makers. Agric. Syst. 75 , 161–197 (2003).
Hardaker, J. B., Lien, G., Anderson, J. R. & Huirne, R. B. Coping with Risk in Agriculture: Applied Decision Analysis (Cabi, 2015).
Uusitalo, L., Lehikoinen, A., Helle, I. & Myrberg, K. An overview of methods to evaluate uncertainty of deterministic models in decision support. Environ. Model. Softw. 63 , 24–31 (2015).
Thornton, P. K. et al. A framework for priority-setting in climate smart agriculture research. Agric. Syst. 167 , 161–175 (2018).
Jones, J. W. et al. Toward a new generation of agricultural system data, models, and knowledge products: state of agricultural systems science. Agric. Syst. 155 , 269–288 (2017).
Article PubMed PubMed Central Google Scholar
Morrison-Saunders, A. & Pope, J. Conceptualising and managing trade-offs in sustainability assessment. Environ. Impact Assess. Rev. 38 , 54–63 (2013).
Prestele, R. & Verburg, P. H. The overlooked spatial dimension of climate‐smart agriculture. Glob. Change Biol. 26 , 1045–1054 (2020).
Article ADS Google Scholar
Ewert, F. et al. Crop modelling for integrated assessment of risk to food production from climate change. Environ. Model. Softw. 72 , 287–303 (2015).
Norström, A. V. et al. Principles for knowledge co-production in sustainability research. Nat. Sustain. 3 , 182–190 (2020).
Seppelt, R., Dormann, C. F., Eppink, F. V., Lautenbach, S. & Schmidt, S. A quantitative review of ecosystem service studies: approaches, shortcomings and the road ahead. J. Appl. Ecol. 48 , 630–636 (2011).
Bostian, M., Whittaker, G., Barnhart, B., Fare, R. & Grosskopf, S. Valuing water quality tradeoffs at different spatial scales: an integrated approach using bilevel optimization. Water Res. Econ. 11 , 1–12 (2015).
Google Scholar
Popp, A. et al. The economic potential of bioenergy for climate change mitigation with special attention given to implications for the land system. Environ. Res. Lett . https://doi.org/10.1088/1748-9326/6/3/034017 (2011).
Hayha, T., Franzese, P. P., Paletto, A. & Fath, B. D. Assessing, valuing, and mapping ecosystem services in Alpine forests. Ecosyst. Serv. 14 , 12–23 (2015).
Maraseni, T., An-Vo, D. A., Mushtaq, S. & Reardon-Smith, K. Carbon smart agriculture: an integrated regional approach offers significant potential to increase profit and resource use efficiency, and reduce emissions. J. Clean. Prod . https://doi.org/10.1016/j.jclepro.2020.124555 (2021).
Modongo, O. & Kulshreshtha, S. N. Economics of mitigating greenhouse gas emissions from beef production in western Canada. Agric. Syst. 162 , 229–238 (2018).
Kinzig, A. P. in The Princeton Guide to Ecology (eds Levin, S. A. et al.) 573–670 (Princeton Univ. Press, 2012).
Sanon, S., Hein, T., Douven, W. & Winkler, P. Quantifying ecosystem service trade-offs: the case of an urban floodplain in Vienna, Austria. J. Environ. Manag. 111 , 159–172 (2012).
Wright, L. A., Kemp, S. & Williams, I. ‘Carbon footprinting’: towards a universally accepted definition. Carbon Manag. 2 , 61–72 (2011).
Chapagain, A. K. & Hoekstra, A. Y. The water footprint of Morocco and the Netherlands: global water use as a result of domestic consumption of agricultural commodities. Ecol. Econ. 64 , 109–118 (2007).
Lambin, E. F. & Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc. Natl Acad. Sci. USA 108 , 3465–3472 (2011).
Hochman, Z. et al. Cropping system yield gaps can be narrowed with more optimal rotations in dryland subtropical Australia. Agric. Syst. 184 , 102896 (2020).
Karner, K., Schmid, E., Schneider, U. A. & Mitter, H. Computing stochastic Pareto frontiers between economic and environmental goals for a semi-arid agricultural production region in Austria. Ecol. Econ . https://doi.org/10.1016/j.ecolecon.2021.107044 (2021).
Holzkämper, A., Klein, T., Seppelt, R. & Fuhrer, J. Assessing the propagation of uncertainties in multi-objective optimization for agro-ecosystem adaptation to climate change. Environ. Model. Softw. 66 , 27–35 (2015).
D’Odorico, P. et al. The global food–energy–water nexus. Rev. Geophys. 56 , 456–531 (2018).
FABLE Consortium. Pathways to Sustainable Land-Use and Food Systems. 2020 Report of the FABLE Consortium (International Institute of Applied Systems Analysis and Sustainable Development Solutions Network, 2020); https://doi.org/10.22022/ESM/12-2020.16896
Agricultural Model Intercomparison and Improvement Project. Approach. Track 2: Climate change multi-model assessment AgMIP https://agmip.org/approach-4/ (2023).
Royle, J. A. & Nichols, J. D. Estimating abundance from repeated presence–absence data or point counts. Ecology 84 , 777–790 (2003).
Legendre, P. & Legendre, L. Numerical Ecology (Elsevier, 2012).
R Core Team. R: A language and environment for statistical computing. R package version 4.1.2. https://www.R-project.org/ (2021).
Wickham, H. et al. Welcome to the tidyverse. J. Open Source Softw . https://doi.org/10.21105/joss.01686 (2019).
Galili, T. dendextend: an R package for visualizing, adjusting, and comparing trees of hierarchical clustering. Bioinformatics 31 , 3718–3720 (2015).
Article CAS PubMed PubMed Central Google Scholar
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M. & Hornik, K. cluster: Cluster analysis basics and extensions. R package version 2.1.2 (2021).
Oksanen, J. et al. vegan: Community ecology package. R package version 2.5-7 https://CRAN.R-project.org/package=vegan (2020).
Kolde, R. pheatmap: Pretty heatmaps. R package version 1.0.12 https://CRAN.R-project.org/package=pheatmap (2019).
Download references
Acknowledgements
We acknowledge R. Seppelt for his comments on the initial methodology. This work was made possible by the CGIAR Research Program on Roots, Tubers and Bananas (RTB) and the One CGIAR Initiatives ‘Nexus Gains—Realizing Multiple Benefits Across Water, Energy, Food and Ecosystems’ and ‘Nature Positive Solutions’, together with all of the donors who supported this research through their contributions to the CGIAR and One CGIAR Fund. For a list of One CGIAR Fund donors, please see http://www.cgiar.org/our-funders . This research was partly funded by the United States Agency for International Development (USAID; AID-BFS-G-11-00002) as part of the US government’s Feed the Future Initiative. The contents of this Article are the responsibility of the producing organizations and do not necessarily reflect the opinion of USAID or the US government.
Author information
Authors and affiliations.
Farming Systems Ecology, Wageningen University and Research, Wageningen, The Netherlands
Timo S. Breure & Jeroen C. J. Groot
Bioversity International, Montpellier, France
Natalia Estrada-Carmona
Bioversity International, Rome, Italy
Athanasios Petsakos & Elisabetta Gotor
Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, The Netherlands
Boris Jansen
You can also search for this author in PubMed Google Scholar
Contributions
T.S.B. conceived and designed the study, led and performed the review and data analyses, interpretations and writing. N.E.-C. contributed to the study’s design, interpretations and writing. A.P., E.G. and B.J. contributed to interpretations and writing. J.C.J.G. contributed to the study’s design, analysis, interpretations and writing.
Corresponding author
Correspondence to Jeroen C. J. Groot .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Food thanks John Antle and Emma Stephens for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 articles per year of publication..
Number of articles by publication year ( a ) and its cumulative distribution ( b ).
Extended Data Fig. 2 Figures on the number of trade-off analysis (TOA) indicators considered.
Cumulative distribution of articles per number of TOA indicators included within an article ( a ). Frequency (%) of the number of TOA indicators included within an article, color-coded by cluster as specified in Fig. 2 in the main text ( b ).
Supplementary information
Supplementary information.
Supplementary Table 1, Figs. 1–9 and a list of articles included in the systematic review.
Reporting Summary
Supplementary data 1.
Criteria assessed in the systematic review. This file was used to perform the analysis and create the figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Breure, T.S., Estrada-Carmona, N., Petsakos, A. et al. A systematic review of the methodology of trade-off analysis in agriculture. Nat Food 5 , 211–220 (2024). https://doi.org/10.1038/s43016-024-00926-x
Download citation
Received : 25 August 2022
Accepted : 15 January 2024
Published : 05 March 2024
Issue Date : March 2024
DOI : https://doi.org/10.1038/s43016-024-00926-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
SYSTEMATIC REVIEW article
The histological and molecular characteristics of early-onset colorectal cancer: a systematic review and meta-analysis.

- 1 School of Medicine and Public Health, Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States
- 2 School of Medicine and Public Health, Department of Population Health Sciences, University of Wisconsin-Madison, Madison, WI, United States
Background: Early-onset colorectal cancer (CRC), defined as diagnosis before age 50, has increased in recent decades. Although more often diagnosed at advanced stage, associations with other histological and molecular markers that impact prognosis and treatment remain to be clarified. We conducted a systematic review and meta-analysis concerning the prevalence of prognostic and predictive tumor markers for early- vs. late-onset CRC, including oncogene mutations, microsatellite instability (MSI), and emerging markers including immune cells and the consensus molecular subtypes.
Methods: We systematically searched PubMed for original research articles published between April 2013–January 2024. Included studies compared the prevalence of tumor markers in early- vs. late-onset CRC. A meta-analysis was completed and summary odds ratios (ORs) with 95% confidence intervals (CIs) were obtained from a random effects model via inverse variance weighting. A sensitivity analysis was completed to restrict the meta-analysis to studies that excluded individuals with Lynch syndrome, a hereditary condition that influences the distribution of tumor markers for early-onset CRC.
Results: In total, 149 articles were identified. Tumors from early-onset CRC are less likely to include mutations in KRAS (OR, 95% CI: 0.91, 0.85-0.98), BRAF (0.63, 0.51-0.78), APC (0.70, 0.58-0.84), and NRAS (0.88, 0.78-1.00) but more likely to include mutations in PTEN (1.68, 1.04-2.73) and TP53 (1.34, 1.24-1.45). After limiting to studies that excluded Lynch syndrome, the associations between early-onset CRC and BRAF (0.77, 0.64-0.92) and APC mutation (0.81, 0.67-0.97) were attenuated, while an inverse association with PIK3CA mutation was also observed (0.88, 0.78-0.99). Early-onset tumors are less likely to develop along the CpG Island Methylator Phenotype pathway (0.24, 0.10-0.57), but more likely to possess adverse histological features including high tumor grade (1.20, 1.15-1.25), and mucinous (1.22, 1.16-1.27) or signet ring histology (2.32, 2.08-2.57). A positive association with MSI status (1.31, 1.11-1.56) was also identified. Associations with immune markers and the consensus molecular subtypes are inconsistent.
Discussion: A lower prevalence of mutations in KRAS and BRAF is consistent with extended survival and superior response to targeted therapies for metastatic disease. Conversely, early-onset CRC is associated with aggressive histological subtypes and TP53 and PTEN mutations, which may serve as therapeutic targets.
1 Introduction
Colorectal cancer (CRC) is the second leading cause of cancer mortality in the United States ( 1 ). The incidence of CRC has steadily declined since the 1980s, largely attributed to greater uptake of colonoscopy screening by adults aged 50 years and older ( 2 ). Concurrently, the incidence of sporadic early-onset CRC, generally defined as CRC diagnosis before age 50 without an underlying hereditary cause, has significantly increased since the mid-1990s ( 2 ). Data from the Surveillance, Epidemiology, and End Results (SEER) program reflect a 2-3% annual increase in the incidence of early-onset CRC ( 3 ). The elevated incidence of early-onset CRC may be explained by birth cohort effects where more recent birth cohorts have increased prevalence of obesity and type 2 diabetes, lower levels of physical activity, and more often consume western-style diets characterized by lower consumption of fruits and vegetables ( 4 ), as well as changes in the composition of the gut microbiome ( 2 ). While early-onset CRC may be caused by hereditary conditions defined by germline mutations in DNA mismatch-repair genes (i.e. Lynch syndrome) or in the tumor suppressor APC (i.e. familial adenomatous polyposis) ( 5 ), these inherited conditions account for a relatively small percentage of early-onset CRC and do not explain the increased prevalence observed in recent decades ( 2 ).
CRC is a heterogeneous disease and the clinicopathological and molecular characteristics of tumors may influence prognosis and response to treatment ( 6 ). Beyond tumor stage, multiple potential prognostic and predictive markers have been identified, including mutations in oncogenes such as KRAS , BRAF , PIK3CA , and TP53 , histological subtypes including mucinous and signet ring carcinomas, and the microsatellite instability (MSI) phenotype ( 7 ). Further, several novel prognostic markers have recently been identified, including immune markers in the tumor microenvironment ( 8 ) and the CRC consensus molecular subtypes ( 9 ). It is anticipated that the continued characterization of molecular phenotypes in CRC will augment traditional clinical markers for therapeutic decision making and support the development of targeted approaches to treatment ( 10 ).
Given the increasing rate of early-onset CRC, recent publications have highlighted potential differences in the clinicopathological and molecular characteristics of tumors based on age of onset ( 11 – 14 ). However, it is currently unclear whether early-onset CRC is distinct from late-onset disease in terms of molecular characteristics and tumor developmental pathways ( 15 ). Understanding the molecular characteristics of early-onset CRC is necessary to guide the development of therapeutic approaches for this condition and to address underlying causes. Therefore, we have completed a systematic review and meta-analysis to comprehensively summarize the evidence linking early-onset CRC to differences in prognostic and predictive tumor markers, including oncogene mutations, histological subtypes, MSI status, as well as anti-tumor immunity and the consensus molecular subtypes.
2.1 Literature review
Articles for this systematic review were identified utilizing a Pubmed search incorporating PRISMA guidelines ( 16 ). Given the wide breadth of the topic and the limited number of relevant articles published prior to 2013, the search was limited to peer-reviewed, original research articles published in English from the last 10 years (April 2013 – April 2023), with relevant keywords and medical subject headings included in the title and/or abstract. The literature review was repeated in January 2024 to identify recently published articles. Specific biomarker terms to include in the literature search were identified from prior reviews, and the search terms “biomark*”, “mark*”, and “character*” were included to capture potentially novel prognostic markers. All search terms included for the literature review are displayed in Supplementary Table S1 . Manuscripts were included that reported the prevalence of prognostic biomarkers in CRC tumors separately for early- vs. late-onset disease. Articles were excluded if the prevalence of tumor clinicopathological or molecular biomarkers were not provided for participants with CRC (see Figure 1 flowchart), or if there was no comparison between early- vs. late-onset CRC (or if the comparison was limited to tumor stage or location only). Articles were also excluded that described hereditary CRC only (e.g. Lynch syndrome), site-specific metastases, or included non-CRC cancers in the analysis samples. For the purposes of this analysis, early-onset disease was defined as CRC diagnosed prior to age 50. To avoid misclassification of early- and late-onset CRC, we excluded papers where late-onset CRC was defined as ≥ 40 years at diagnosis or younger, or where early-onset CRC was defined as ≤ 60 years at diagnosis or older. Lastly, to limit sample overlap where possible, we excluded studies if there was evidence of complete overlap in sample and markers reported with a previously published study, or if a study reported the same outcome in a subsample of a previous study.
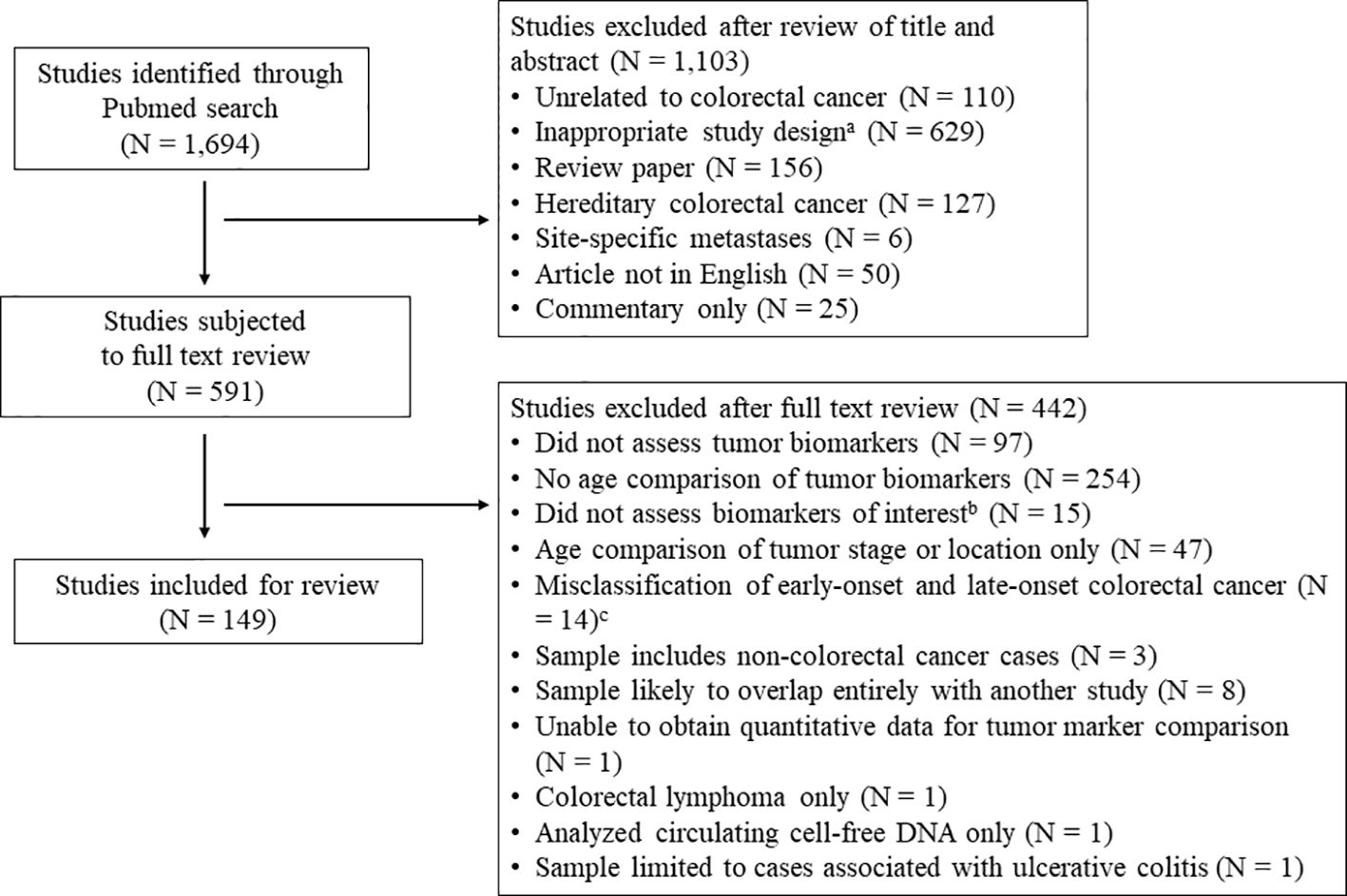
Figure 1 Literature review flowchart. a Inappropriate study design includes studies concerning colorectal cancer incidence, colonoscopy or other colorectal cancer screening, population level summary statistics for colorectal cancer, and studies of colorectal cancer in model organisms or in vitro studies. b Markers of interest include oncogene mutations in KRAS , NRAS , BRAF , PIK3CA , PTEN , TP53 , APC , and HER2 ; histological phenotypes including high-grade tumors and mucinous or signet ring histology; molecular carcinogenesis pathways including microsatellite instability and the CpG island methylator phenotype (CIMP); and novel tumor prognostic phenotypes including immune markers in the tumor microenvironment and the consensus molecular subtypes. c Studies where late-onset colorectal cancer was defined as ≥ 40 years at diagnosis (or younger), or early-onset CRC was defined as ≤ 60 years at diagnosis (or older).
The systematic review and meta-analysis was limited to the following markers that have been shown associations with CRC survival and/or therapeutic response in CRC: oncogene mutations in KRAS ( 17 – 20 ), NRAS ( 17 , 21 , 22 ), BRAF ( 17 , 19 , 23 , 24 ), PIK3CA ( 17 , 25 , 26 ), PTEN ( 27 , 28 ), TP53 ( 29 ), APC ( 30 , 31 ), and HER2 amplifications ( 32 – 34 ); histological phenotypes including high-grade tumors ( 35 , 36 ) and mucinous ( 37 , 38 ) or signet ring histology ( 38 , 39 ); molecular carcinogenesis pathways including MSI ( 40 ) and the CpG island methylator phenotype (CIMP) ( 41 ); and novel tumor prognostic phenotypes including immune markers ( 42 , 43 ) in the tumor microenvironment and the consensus molecular subtypes ( 9 , 44 ). Because it is well-established that early-onset CRC is associated with advanced tumor summary stage at diagnosis and rectal tumor location, these markers are not summarized in this review. The literature review was completed by two authors (T.L. and L.P) independently. Disagreements between reviewers were resolved by further review of the manuscript to determine whether the study included a comparison of tumor markers of interest between early- and late-onset CRC. The final decision to include a manuscript was made by the lead author. In total, 1,694 articles were identified from the literature search and 149 were eligible for review ( Figure 1 ). For each study, the potential for bias was evaluated by the lead author using the Newcastle-Ottawa Scale adapted for cross-sectional studies ( 45 ). Pre-registration of the systematic review protocol was not performed.
2.2 Meta-analysis
From each eligible study, the number of mutant and wild-type tumors for each marker in early- and late-onset CRC was extracted by the lead author. Data extraction was completed in duplicate, and the results from the two extractions were compared to identify any errors or inconsistencies in the sample sizes, which were subsequently revised after further review of the original article. If these data were not available from the manuscript, sample sizes were requested from the corresponding author. One study was excluded for which we were unable to obtain the necessary sample sizes from each group ( 46 ). When necessary, sample sizes for separate age groups were combined to create a single category for early-onset and late-onset CRC. For most studies, age 45 or 50 at diagnosis was utilized as the threshold to distinguish early- vs. late-onset CRC, although occasionally other classifications were employed (see Supplementary S2 ). For each study, sample characteristics including overall sample size, country, tumor stage, sex, or other distinguishing features were also extracted. For each marker, an odds ratio (OR) and 95% confidence interval (CI) were calculated using a standard equation ( 47 ). For mutations in oncogenes KRAS, NRAS, BRAF, PIK3CA, PTEN, TP53 , and APC , as well as MSI status and histological subtypes, meta-analyses were completed to compare the prevalence in tumors from early- vs. late-onset CRC. Due to the wide variety of immune markers that have been reported, a meta-analysis was not attempted for the comparison of immune phenotypes in the tumor microenvironment. For each marker that was meta-analyzed, a pooled OR with 95% CI was obtained from a random effects model via inverse variance weighting. The random effects model was selected a priori , as between-study heterogeneity is plausible given variability in the definition of early-onset CRC, as well as differences in tumor location, race, nationality and stage between studies. The random effects meta-analysis is capable of providing unbiased estimates in the presence of heterogeneity and will generally provide more conservative estimates than the fixed-effects model (which assumes no between-study heterogeneity) ( 48 ). Heterogeneity was determined via the Cochrane’s Q statistic and the I 2 statistic. Significant heterogeneity was defined as P <.05 for Cochrane’s Q or I 2 ≥ 50%. To determine whether the meta-analysis estimates were influenced by a single study, a ‘leave-one-out’ sensitivity analysis was conducted for each marker. Because Lynch syndrome may influence the prevalence of tumor markers for individuals with early-onset CRC, a second sensitivity analysis was completed to limit the analysis to studies that specifically excluded individuals with Lynch syndrome or family history of CRC, or that restricted the sample to microsatellite stable tumors. All statistical tests were two-sided, with statistical significance defined using a threshold of P <.05. All meta-analyses were completed using Review Manager 5.4.1 from Cochrane.
In total, 149 articles were reviewed that compared the prevalence of clinicopathological tumor markers in early- vs. late-onset CRC. All meta-analysis results are summarized in Table 1 . Sample characteristics and references for all included studies are presented in Supplementary Table S2 . Results of the bias assessment utilizing the Newcastle-Ottawa Scale are presented in Supplementary Table S4 .
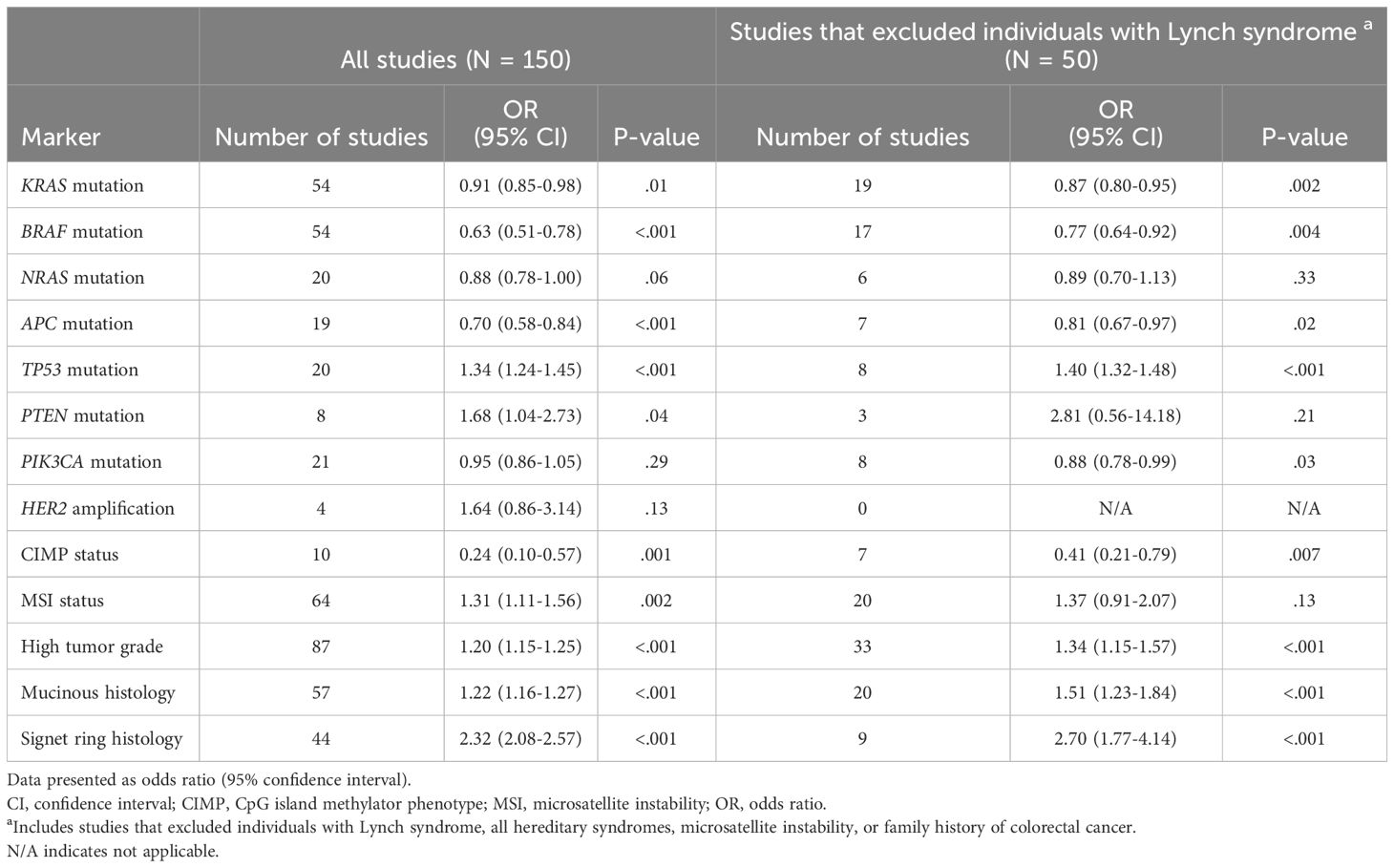
Table 1 Summary of meta-analysis results showing associations between early-onset colorectal cancer and the prevalence of tumor markers, compared to late-onset colorectal cancer.
3.1 Oncogene mutations
The number of studies identified for the following markers is as follows: KRAS mutation ( 49 ); BRAF mutation ( 49 ); NRAS mutation ( 20 ); PIK3CA mutation ( 21 ); PTEN mutation ( 8 ); HER2 amplifications ( 5 ); APC mutation ( 19 ); TP53 mutation ( 20 ). For early-onset CRC, there is evidence for a significantly lower prevalence of mutations in KRAS ( Figure 2 , OR 0.91, 95% CI 0.85-0.98), BRAF ( Figure 3 , OR 0.63, 95% CI 0.51-0.78) and APC ( Figure 4 , OR 0.70, 95% CI 0.58-0.84) compared to late-onset CRC. Early-onset CRC was associated with non-significantly lower prevalence of mutations in NRAS ( Figure 5 , OR 0.88, 95% CI 0.78-1.00, p = .06). Conversely, early-onset CRC is associated with a higher prevalence of mutations in TP53 ( Figure 6 , OR 1.34, 95% CI 1.24-1.45) and PTEN ( Figure 7 , OR 1.68, 95% CI 1.04-2.73). There was no significant difference in the prevalence of PIK3CA mutations ( Supplementary Figure S1 , OR 0.95, 95% CI 0.86-1.05), or HER2 amplifications ( Supplementary Figure S2 , OR 1.64, 95% CI 0.86-3.14). Significant inter-study heterogeneity was observed for mutations in KRAS , BRAF , PTEN , and APC . Hazard ratios for oncogene mutations were stable in the leave-one-out sensitivity analysis ( Supplementary Table S3 ), although the association for NRAS and PTEN mutations did not always reach statistical significance.
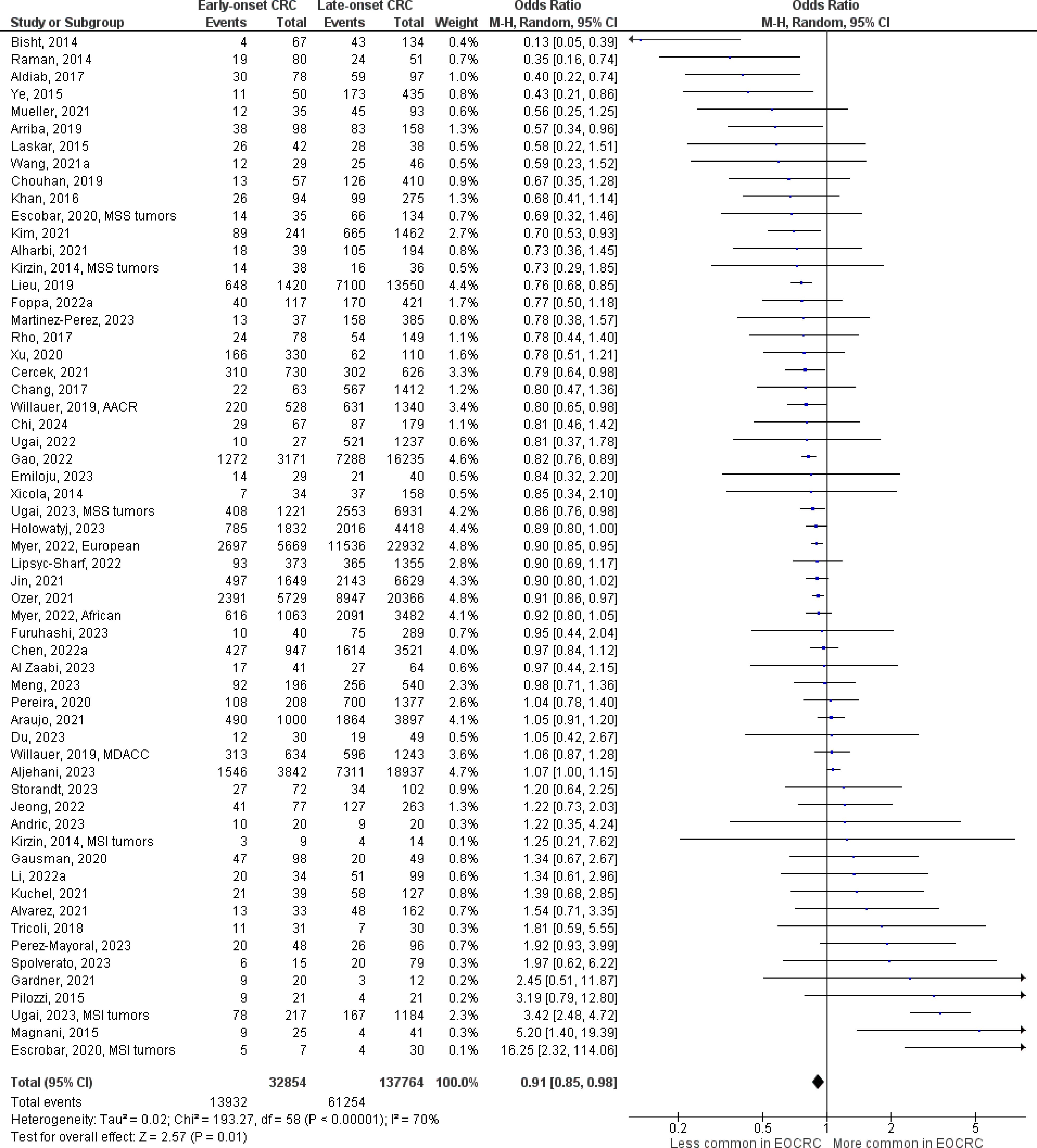
Figure 2 Odds ratios for KRAS mutation in early-onset CRC. Data presented as odds ratios (95% confidence interval) for KRAS mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; MDACC, MD Anderson Cancer Center; MSI, microsatellite instability; MSS, microsatellite stable.
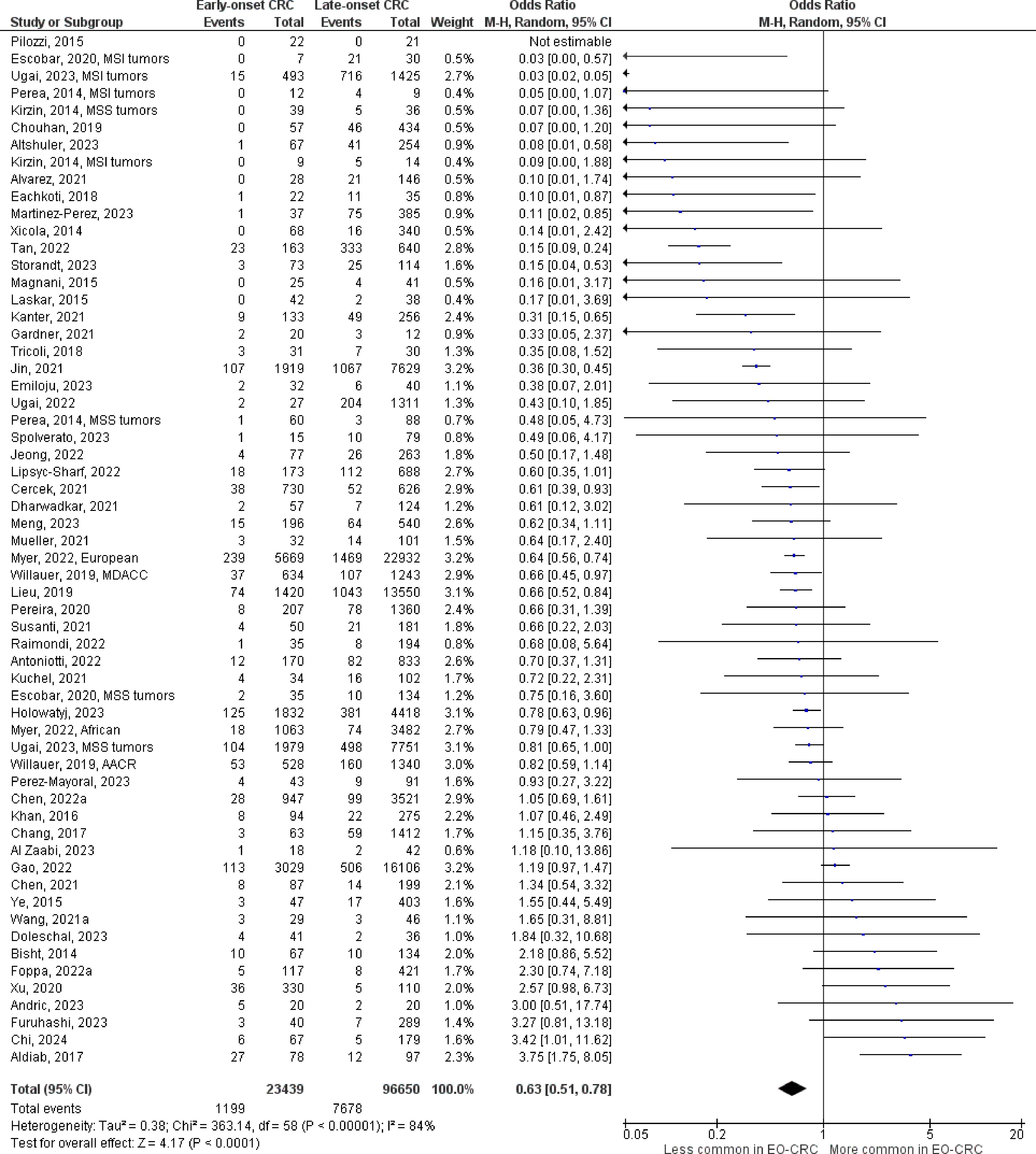
Figure 3 Odds ratios for BRAF mutation in early-onset CRC. Data presented as odds ratios (95% confidence interval) for BRAF mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; MDACC, MD Anderson Cancer Center; MSI, microsatellite instability; MSS, microsatellite stable.
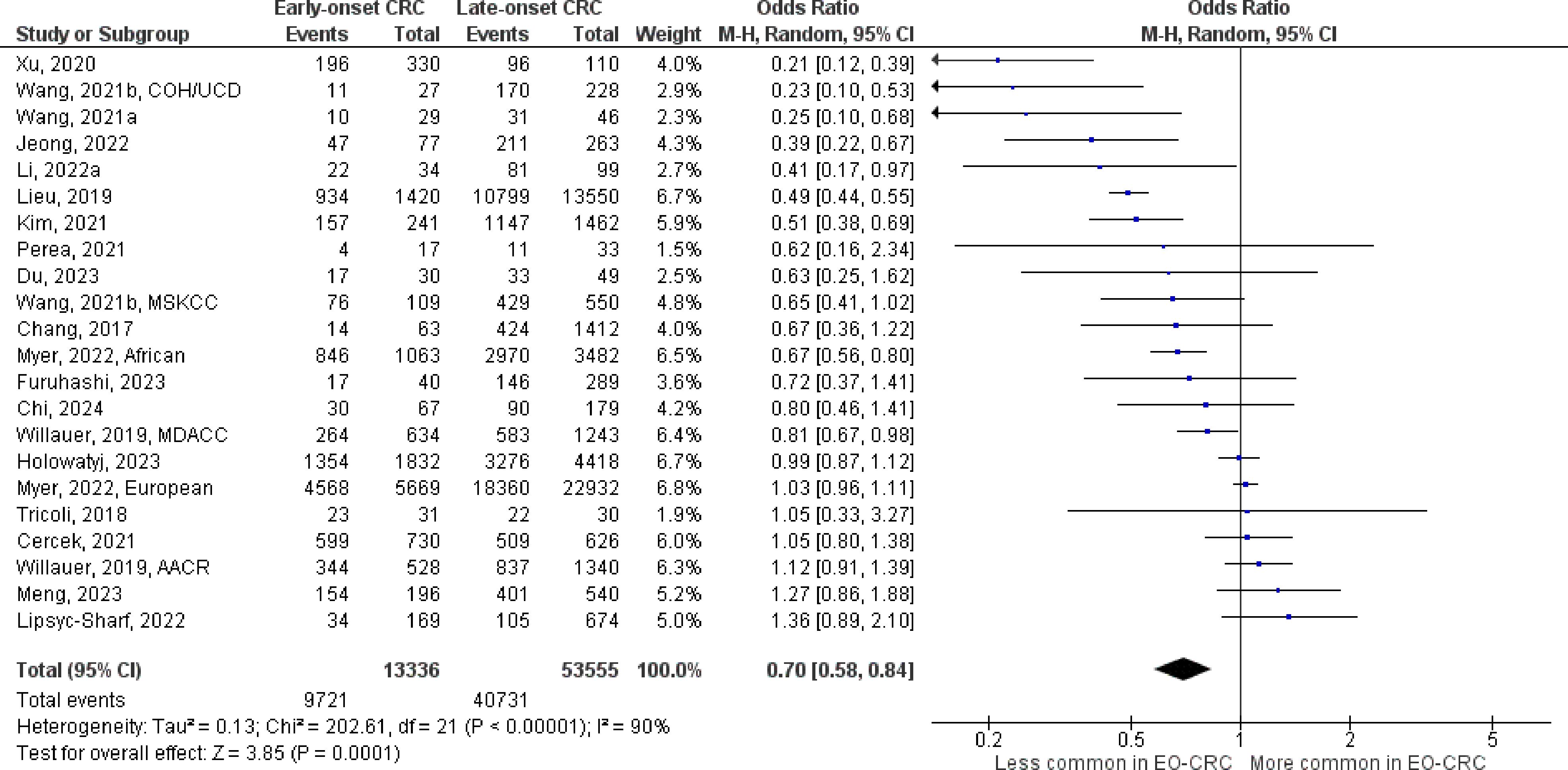
Figure 4 Odds ratios for APC mutation in early-onset colorectal cancer. Data presented as odds ratios (95% confidence interval) for APC mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; COH, City of Hope National Medical Center; CI, confidence interval; EO-CRC, early-onset colorectal cancer; MDACC, MD Anderson Cancer Center; MSKCC, Memorial Sloan Kettering Cancer Center; UCD, University of California, Davis.
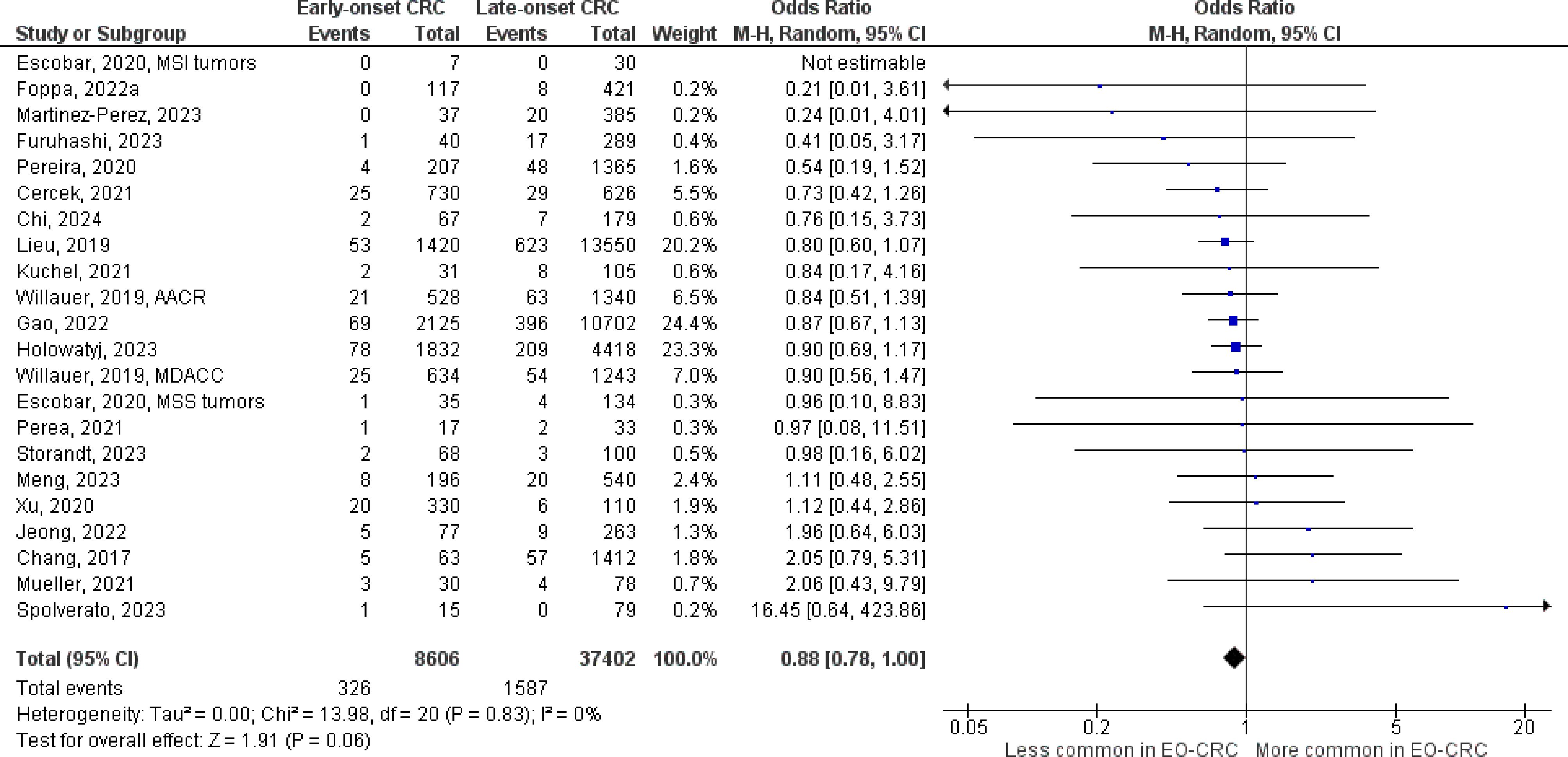
Figure 5 Odds ratios for NRAS mutation in early-onset colorectal cancer. Data presented as odds ratios (95% confidence interval) for NRAS mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; CI, confidence interval; EO-CRC, early-onset colorectal cancer; MDACC, MD Anderson Cancer Center.
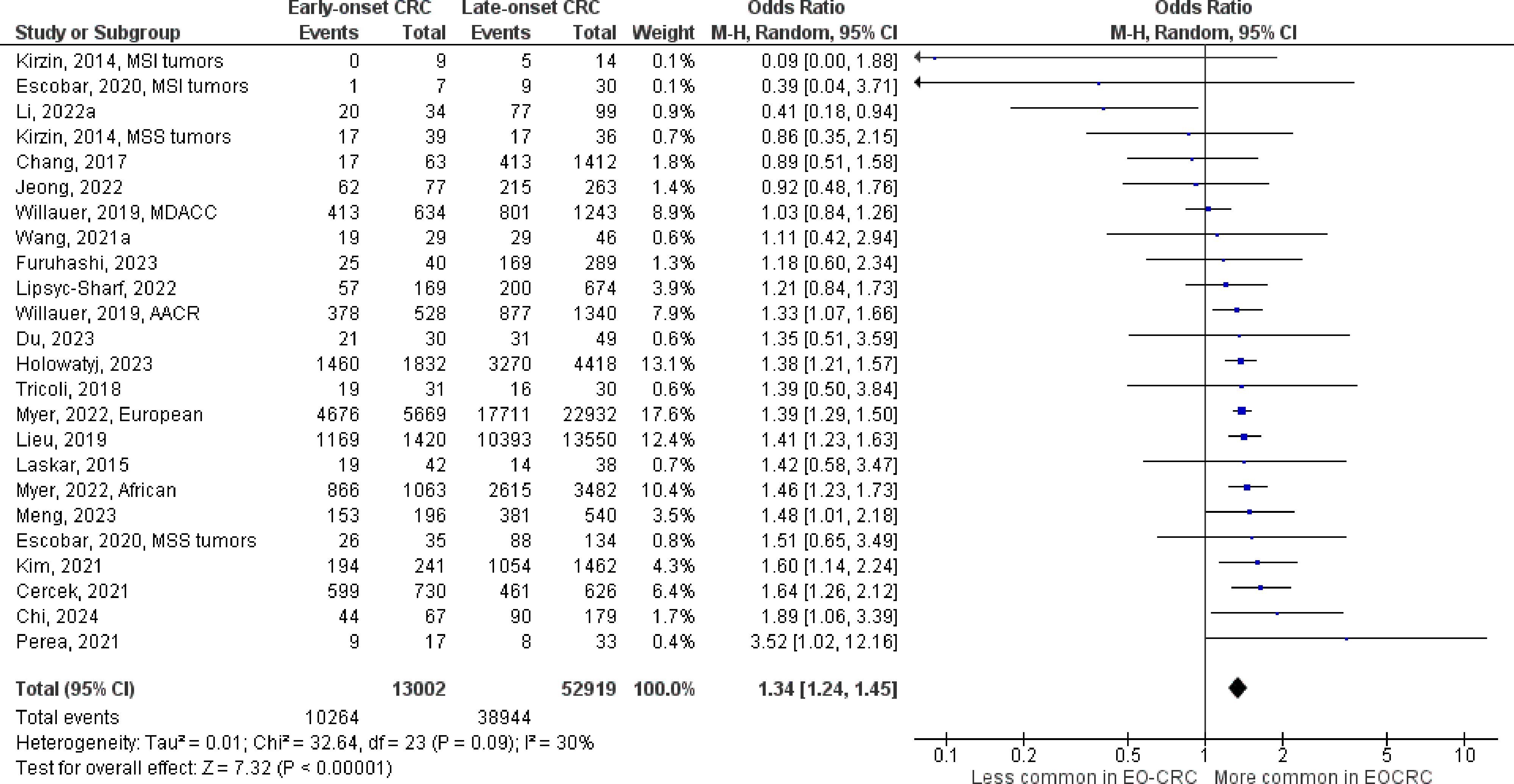
Figure 6 Odds ratios for TP53 mutation in early-onset colorectal cancer. Data presented as odds ratios (95% confidence interval) for TP53 mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; CI, confidence interval; EO-CRC, early-onset colorectal cancer; MDACC, MD Anderson Cancer Center; MSI, microsatellite instability; MSS, microsatellite stability.
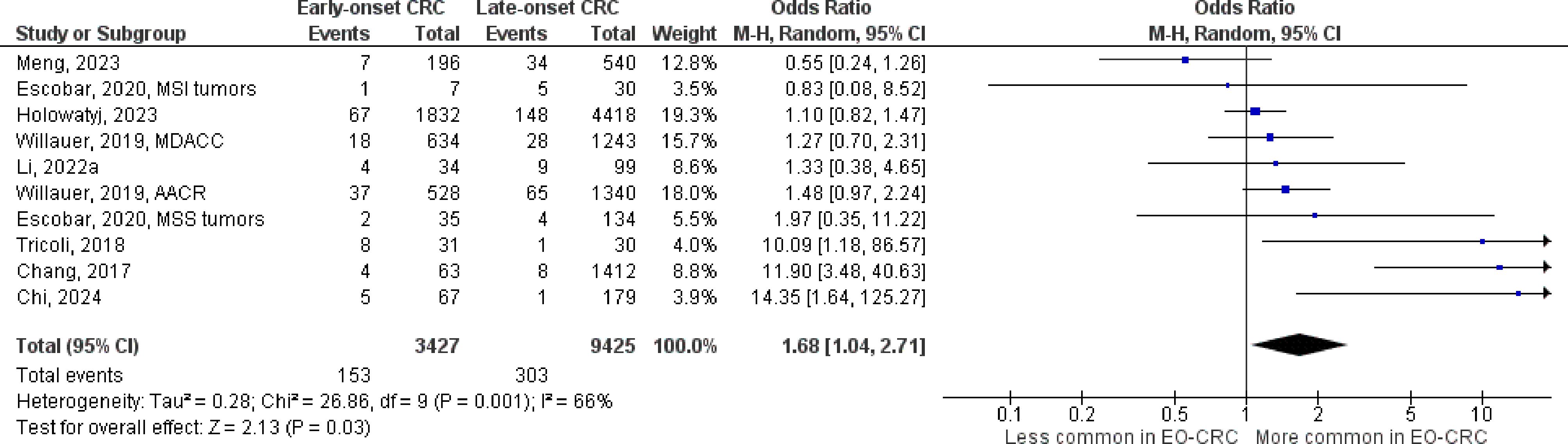
Figure 7 Odds ratios for PTEN mutation in early-onset colorectal cancer. Data presented as odds ratios (95% confidence interval) for PTEN mutation in early-onset relative to late-onset colorectal cancer. The pooled odds ratio is obtained via a random effects model using inverse variance weighting. AACR, American Association for Cancer Research; CI, confidence interval; EO-CRC, early-onset colorectal cancer; MDACC, MD Anderson Cancer Center.
Fifty studies were identified that specifically excluded individuals with Lynch syndrome or family history of CRC, or that restricted the analysis to individuals with microsatellite stable tumors ( Table 1 ; Supplementary Table S2 ). Compared to the full analysis, the association between early-onset CRC and BRAF (OR 0.77, 95% CI 0.64-0.92) and APC mutations (OR 0.81, 95% CI 0.67-0.97) were attenuated but remained statistically significant, while the associations with KRAS , NRAS , and TP53 mutations were similar. Further, an inverse association between early-onset CRC and PIK3CA mutation was also observed (OR 0.88, 95% CI 0.78-0.99).
3.2 Molecular carcinogenesis pathways
There were 10 studies that compared the prevalence of CIMP-high status in early- vs. late-onset CRC, and 64 studies that compared MSI status. Individuals with early-onset CRC had significantly lower odds for CIMP-high tumors compared to individuals with late-onset disease ( Supplementary Figure S3 , OR 0.24, 0.10-0.57), but significantly higher odds for the MSI phenotype ( Supplementary Figure S4 , OR 1.31, 1.11-1.56). Significant heterogeneity was observed for both markers. Associations were stable in the leave-one-out sensitivity analysis ( Supplementary Table S3 ), and after limiting the analysis to studies that excluded individuals with Lynch syndrome or family history of CRC ( Table 1 ).
3.3 Histological characteristics
There were 86 studies that compared the prevalence of high-grade tumors (i.e. poorly differentiated or undifferentiated tumors) in early- vs. late-onset CRC, 57 studies that compared the prevalence of mucinous histology (or mucinous characteristics), and 44 studies that reported on signet ring cell carcinomas. In early-onset CRC, there was evidence for a significantly higher prevalence of high-grade (i.e., poorly differentiated) tumors ( Supplementary Figure S5 , OR 1.20, 95% CI 1.15-1.25), as well as mucinous tumors ( Supplementary Figure S6 , OR 1.22, 95% CI 1.16-1.27), and signet ring cell carcinomas ( Supplementary Figure S7 , OR 2.32, 2.08-2.57). Significant inter-study heterogeneity was observed for all histological markers. All associations were stable in the leave-one-out sensitivity analysis ( Supplementary Table S3 ) and after limiting the analysis to studies that excluded individuals with Lynch syndrome or family history of CRC ( Table 1 ).
3.4 Immune markers
There have been nine studies to investigate age differences in the immune cell populations of CRC tumors, with inconsistent results ( 49 – 57 ). Du et al. reported that Chinese patients with sporadic early-onset CRC showed significantly higher densities of multiple immune cell populations in the tumor microenvironment compared to patients with late-onset disease, including higher levels of B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells ( 50 ). By contrast, Ugai et al. reported no significant differences in the populations of T cells, macrophages, and other myeloid cells in participants with early- vs. late-onset CRC from the Nurses’ Health Study and Health Professionals Follow-up Study ( 51 ). In a small study of 14 tumors utilizing single cell RNA sequencing, Li et al. reported that early-onset CRC was associated with lower levels of effector CD8+ T cells and antigen-presentation in the tumor microenvironment, but higher levels of naïve CD8+ T cells and immunosuppressive regulatory T cells compared to individuals with late-onset disease, suggesting an impaired anti-tumor immune response for early-onset CRC ( 54 ). Because MSI status may influence the anti-tumor immune response, recent studies have examined associations between early-onset CRC and tumor lymphocyte populations in samples limited to microsatellite stable tumors, or after careful exclusion of participants with Lynch syndrome ( 56 , 57 ). In a matched analysis of microsatellite stable tumors, Lu et al. (2023) reported that there was no significant differences between early- and late-onset CRC for the infiltration of 22 different lymphocyte populations in the tumor microenvironment ( 57 ). Likewise, Andric et al. found no significant difference for five lymphocyte populations (total T cells, conventional CD4+ and CD8+ T cells, regulatory T cells, and γδ T cells) in a matched sample limited to cases of sporadic CRC ( 56 ). Other studies have reported no significant differences between early and late-onset CRC for the density of total tumor infiltrating lymphocytes ( 53 , 55 ).
3.5 The consensus molecular subtypes
There have been six studies to determine the distribution of consensus molecular subtypes (CMS) for CRC by age at diagnosis ( 50 , 57 – 61 ). Utilizing tumor tissues samples from 626 individuals diagnosed with CRC from The Cancer Genome Atlas and MD Anderson Cancer Center, Willauer reported that the CMS1 subtype was more common among patients aged 30-39 years at diagnosis (46%) compared to older participants, while the CMS4 subtype was less common (13%) ( 58 ). Conversely, in a smaller study from the Nanjing Colorectal Cancer Cohort, Du et al. reported a higher prevalence of the CMS4 subtype in early- vs. late-onset CRC (36.7% vs. 12.2%, respectively), although the comparison between age groups did not reach statistical significance ( 50 ). Recent results, including from a small sample of South Korean participants ( 59 ) and additional analyses of The Cancer Genome Atlas ( 60 , 61 ) did not show any significant association between early-onset tumors and the distribution of consensus molecular subtypes.
4 Discussion
Sporadic early-onset CRC is a significant public health concern, increasing by 2-3% per year in the U.S. since 1990 ( 3 , 62 ). Early-onset CRC is more often diagnosed at advanced stages compared to late-onset disease ( 63 , 64 ). However, there is inconsistent evidence that survival varies between early- and late-onset CRC ( 65 , 66 ), complicated by reports that younger patients receive more aggressive systemic treatment ( 67 – 69 ). Thus, international guidelines do not endorse separate treatment recommendations for early-onset disease ( 70 ). Investigating the associations between early-onset tumors and molecular and histological characteristics, and novel tumor markers including immune cell populations, may help to guide the development of therapies that benefit early-onset CRC. Further, highlighting associations between early-onset CRC and tumor markers may aid in the design of clinical trials for targeted therapies. To the authors’ knowledge, this is the first comprehensive systematic review and meta-analysis of tumor prognostic and predictive markers in early-onset CRC. We found that early-onset CRC was associated with a lower prevalence of oncogene mutations in KRAS , BRAF , NRAS , and APC , but a higher prevalence of TP53 and PTEN mutations and adverse histologic subtypes, with inconsistent associations for immune cell populations and the consensus molecular subtypes.
KRAS , BRAF , and NRAS encode proteins that act downstream of the epidermal growth factor receptor (EGFR) and activate Mek/Erk signaling ( 21 , 71 ). Mutations in these oncogenes are negative predictive markers for EGFR inhibition in metastatic CRC ( 17 , 18 ) and are associated with inferior survival outcomes across tumor stage ( 19 , 20 , 23 , 72 ), including for early-onset CRC ( 73 – 75 ). Early-onset CRC is associated with a lower prevalence of mutations in these genes compared to late-onset disease, indicating that individuals with metastatic early-onset CRC may be more likely to benefit from EGFR inhibition. Notably, the association with NRAS mutations was not statistically significant, which may be due to the scarcity of this marker ( 76 ). Further, the association with BRAF mutation was attenuated but still statistically significant in studies that excluded individuals with Lynch syndrome, who are less likely to have BRAF mutations compared to sporadic disease ( 77 ). Further, this sensitivity analysis revealed an inverse association with PIK3CA mutation, which has also been linked to higher risk for mortality and resistance to EGFR inhibition ( 17 , 78 ). Conversely, early-onset CRC was associated with a higher proportion of mutations in tumor suppressor PTEN , which encodes a lipid-phosphatase that suppresses the activity of PI3k/Akt/mTOR signaling and interacts with the EGFR pathway ( 27 ). Loss of PTEN activity has been linked to resistance to EGFR inhibition in metastatic CRC ( 79 ) but is not currently used in clinical decision making. Pharmaceutical therapies to restore normal PTEN activity are under development but have not been evaluated in CRC. Early-onset CRC was associated with a significantly higher prevalence of TP53 mutations, which cause loss of p53 tumor suppressor activity and pro-tumorigenic gain of function effects that accelerate cell proliferation, angiogenesis, and metastasis ( 80 ). TP53 mutations are found in approximately 60% of tumors and may promote resistance to EGFR inhibitors and chemotherapies that rely on wild type p53 to induce cellular apoptosis (e.g. 5-fluorouracil and Oxaliplatin) ( 29 ). Consequently, targeted therapies to restore wild type p53 activity or degrade mutant p53, or to inhibit downstream effector pathways, are currently being investigated in clinical trials ( 81 ). Potentially, individuals with early-onset CRC may be more likely to benefit from treatments that inhibit pro-tumorigenic p53 activity and should be targeted for enrollment in these trials.
Early-onset CRC was associated with a lower prevalence of APC mutation, a key driver of the canonical adenoma-carcinoma pathway ( 82 ). APC mutations are present in approximately 80% of CRC tumors ( 11 , 12 , 14 ), and recent evidence indicates that APC -mutant tumors are associated with extended overall and progression-free survival compared to wild type ( 30 , 31 ) ( 5 ). Notably, the association with APC mutation was attenuated but still statistically significant when limiting the analysis to studies that excluded individuals with Lynch syndrome, or that included microsatellite stable tumors only. Individuals with early-onset CRC had a higher prevalence of MSI, defined by a high density of somatic mutations in short, non-coding sequences caused by defects in DNA mismatch repair ( 40 ). MSI is associated with lower risk for overall mortality and distant metastases compared to microsatellite stable tumors, including in early-onset CRC ( 75 ). Further, MSI tumors secrete truncated proteins that trigger an anti-tumor immune response ( 83 ), and consequently MSI is a positive predictor for response to immune checkpoint inhibitors ( 83 ). Our findings therefore highlight the importance of MSI testing for individuals younger than 50, in accordance with clinical guidelines ( 70 ). Unexpectedly, the association between early-onset CRC and MSI status was modestly strengthened in studies that excluded individuals with known Lynch syndrome, which causes tumors with MSI ( 84 ). Because a significant proportion of individuals with Lynch syndrome may be unaware of the condition ( 85 ), it is possible that the exclusion of Lynch syndrome was incomplete in some studies. Early-onset CRC was associated with a lower prevalence of the CpG island methylator phenotype (CIMP), characterized by methylation and inactivation of tumor-suppressor genes ( 86 ). Although CIMP has been linked to poor prognosis in multiple studies, it currently has limited value as a prognostic marker due to a lack of standardized assessment and competing effects of MSI and BRAF mutation, which are associated with CIMP ( 41 ).
We also found that early-onset CRC is associated with higher odds for tumors with more aggressive histological features, including poorly differentiated tumors, mucinous carcinomas, and signet ring cell carcinomas ( 38 , 87 ). The association with signet ring features was especially pronounced (OR [95% CI]: 2.32 [2.08-2.57]). Although signet ring carcinomas comprise only 1% of CRC tumors ( 39 ), this feature is present in 2-3% of early-onset tumors. A recent meta-analysis showed that signet ring carcinomas were associated with significantly higher risk for overall mortality and recurrence compared to conventional adenocarcinomas ( 88 ). Results were similar for mucinous tumors, which comprise approximately 10-15% of CRCs ( 89 ). The associations between histological subtypes and colorectal cancer mortality, especially poorly differentiated tumors and signet ring carcinomas, have been validated in early-onset CRC ( 90 – 93 ). Currently, there are no treatments that specifically target mucinous or signet ring cell carcinomas and treatment guidelines do not distinguish between histological subtypes ( 70 ).
The observed associations between early-onset CRC and certain histological and molecular tumor characteristics may be explained in part by differences in tumor location ( 94 ). Approximately 30% of early-onset tumors are located in the rectum, versus 20% of late-onset tumors ( 64 , 95 ). KRAS, BRAF, PIK3CA , and NRAS mutations are enriched in proximal tumors ( 96 , 97 ) while TP53 mutations are enriched in rectal tumors ( 98 ). Notably, studies that were limited to individuals with tumors in the distal colon or rectum have not shown a consistent association between early-onset CRC and the presence of oncogene mutations ( 46 , 55 , 56 , 99 – 102 ). For example, a study with more than 1,000 distal and rectal tumors showed no significant age difference in KRA S, BRAF, NRAS , PIK3CA , TP53 , or APC mutations ( 46 ). Conversely, in a large-scale analysis with detailed stratification by tumor location, Ugai et al. found that early-onset CRC had a lower prevalence of BRAF mutations for all tumor sites except the sigmoid colon and rectum ( 103 ). Notably, aggressive histological subtypes are overrepresented in the proximal colon ( 104 ), and consequently the association with early-onset CRC is not explained by differences in tumor location.
We found inconsistent evidence linking early-onset CRC to differences in ‘novel’ tumor prognostic and predictive markers including populations of immune cells in the tumor microenvironment ( 8 ). A recent meta-analysis demonstrated that a higher density of tumor infiltrating lymphocytes was associated with reduced overall mortality among 20,015 individuals with CRC (HR [95% CI]: 0.65 [0.54-0.77]) ( 42 ), while others have shown that an ‘immunoscore’ encompassing cytotoxic T cells and CD3+ cells was a superior prognostic marker compared to the tumor stage ( 105 , 106 ). Currently, the association between early-onset CRC and the anti-tumor immune response has been inconsistent ( 48 – 50 , 52 , 53 , 55 , 56 , 58 ). Notably, higher rates of MSI in early-onset CRC due to Lynch syndrome may obscure associations with immune markers in sporadic disease, as MSI tumors trigger a robust anti-tumor immune response ( 83 ). Studies limited to microsatellite stable tumors or that carefully excluded participants with hereditary syndromes have tended to show no significant differences in immune cell populations between early- and late-onset CRC ( 51 , 56 , 57 ). Likewise, there is currently no consistent evidence that the distribution of consensus molecular subtypes differs between early- and late-onset CRC, with most studies reporting null findings ( 50 , 57 , 59 – 61 ). The consensus molecular subtypes have shown to be a robust predictor of mortality outcomes independent of tumor stage ( 107 ), but to the authors’ knowledge have not been validated specifically in early-onset CRC. Further, the identification of novel molecular subtypes in early-onset CRC based on tumor gene expression is an area for future research.
Strengths of this study include the comprehensive nature of the search strategy, as we were able to summarize the evidence for age-related differences in the prevalence of established tumor prognostic markers as well as emerging markers including immune cell populations in the tumor microenvironment and the consensus molecular subtypes. Further, the large number of studies identified for most markers allowed for relatively precise estimates of the association with early-onset CRC. Lastly, to better understand the associations between early-onset CRC and tumor markers in sporadic disease, we completed a sensitivity analysis limited to studies that excluded individuals with known Lynch syndrome (or family history of CRC). This analysis is also attended by several limitations. Due to the breadth of the review, our literature search was limited to original research studies published within the last ten years in Pubmed. Consequently, it is possible that a relevant study was missed. However, this is unlikely to be a significant limitation given the paucity of large tumor genomic studies published prior to 2013 and the comprehensive nature of our search strategy. Further, there was evidence for significant heterogeneity in the estimates for most tumor markers, but we were unable to investigate underlying sources of inter-study heterogeneity because the prevalence of tumor prognostic markers was rarely presented in subgroups defined by tumor location, tumor stage, or MSI status. Between-study differences in the definitions of early- and late-onset CRC may also have contributed to heterogeneity, although we excluded studies where misclassification of early-onset CRC was apparent. Lastly, although we attempted to control for bias by performing a sensitivity analysis limited to studies that accounted for Lynch syndrome in the study design, it is possible that residual confounding by hereditary conditions or differences in tumor location may have biased the results.
5 Conclusions
In summary, early-onset CRC was associated with a lower prevalence of mutations in several oncogenes linked to mortality and poor therapeutic response, including KRAS , BRAF , and NRAS compared to individuals with late-onset disease. Conversely, early-onset disease was associated with a higher prevalence of potentially harmful mutations in TP53 and PTEN , as well as aggressive histological subtypes including mucinous and signet ring cell carcinomas. In part, these associations may reflect the higher prevalence of rectal tumors in early-onset CRC and the effect of hereditary syndromes on tumor markers. Given these findings and the alarming rise in the incidence of early-onset CRC, it is essential that clinical trials for targeted therapies enroll sufficient numbers of individuals with early-onset disease to evaluate their efficacy in this subgroup. Additional research is required to clarify the relationships with novel tumor characteristics including immune markers and to identify molecular subtypes specific to early-onset CRC that can inform treatment and prognosis.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
TL: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. LP: Writing – review & editing, Writing – original draft, Investigation, Data curation. SW: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Cancer Institute of the National Institutes of Health [NIH/NCI] under grants R00 CA207848 and R01 CA255318.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1349572/full#supplementary-material
Abbreviations
AACR, American Association for Cancer Research; APC, adenomatous polyposis coli; CI, confidence interval; CIMP, CpG island methylator phenotype; CMS, consensus molecular subtypes; COH, City of Hope (National Medical Center); CRC, colorectal cancer; EGFR, epidermal growth factor receptor; MDACC, MD Anderson Cancer Center; MSI, microsatellite instability; MSKCC, Memorial Sloan Kettering Cancer Center; MSS, microsatellite stable; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results; TIL, tumor infiltrating lymphocytes.
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin . (2023). 73(3):233–54. doi: 10.3322/caac.21772
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Weinberg BA, Marshall JL. Colon cancer in young adults: trends and their implications. Curr Oncol Rep . (2019) 21:3. doi: 10.1007/s11912-019-0756-8
3. Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology . (2018) 155:1716–9. doi: 10.1053/j.gastro.2018.07.045
4. Done JZ, Fang SH. Young-onset colorectal cancer: A review. World J Gastrointest Oncol . (2021) 13:856–66. doi: 10.4251/wjgo.v13.i8.856
5. Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol . (2016) 22:1736–44. doi: 10.3748/wjg.v22.i5.1736
6. Sagaert X, Vanstapel A, Verbeek S. Tumor heterogeneity in colorectal cancer: what do we know so far? Pathobiology . (2018) 85:72–84. doi: 10.1159/000486721
7. Gonzalez-Pons M, Cruz-Correa M. Colorectal cancer biomarkers: where are we now? BioMed Res Int . (2015) 2015:149014. doi: 10.1155/2015/149014
8. Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Tumor-infiltrating lymphocytes in colorectal cancer: the fundamental indication and application on immunotherapy. Front Immunol . (2021) 12:808964. doi: 10.3389/fimmu.2021.808964
9. Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med . (2015) 21:1350–6. doi: 10.1038/nm.3967
10. Lech G, Słotwiński R, Słodkowski M, Krasnodębski IW. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol . (2016) 22:1745–55. doi: 10.3748/wjg.v22.i5.1745
11. Myer PA, Lee JK, Madison RW, Pradhan K, Newberg JY, Isasi CR, et al. The genomics of colorectal cancer in populations with african and european ancestry. Cancer Discovery . (2022) 12:1282–93. doi: 10.1158/2159-8290.CD-21-0813
12. Lieu CH, Golemis EA, Serebriiskii IG, Newberg J, Hemmerich A, Connelly C, et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin Cancer Res . (2019) 25:5852–8. doi: 10.1158/1078-0432.CCR-19-0899
13. Gao XH, Li J, Liu LJ, Zheng NX, Zheng K, Mei Z, et al. Trends, clinicopathological features, surgical treatment patterns and prognoses of early-onset versus late-onset colorectal cancer: A retrospective cohort study on 34067 patients managed from 2000 to 2021 in a Chinese tertiary center. Int J Surg . (2022) 104:106780. doi: 10.1016/j.ijsu.2022.106780
14. Holowatyj AN, Wen W, Gibbs T, Seagle HM, Keller SR, Edwards DRV, et al. Racial/ethnic and sex differences in somatic cancer gene mutations among patients with early-onset colorectal cancer. Cancer Discovery . (2023) 13:570–9. doi: 10.1158/2159-8290.CD-22-0764
15. Venugopal A, Carethers JM. Epidemiology and biology of early onset colorectal cancer. EXCLI J . (2022) 21:162–82. doi: 10.17179/excli2021-4456
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . (2021) 372:n71. doi: 10.1136/bmj.n71
17. Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol . (2014) 53:852–64. doi: 10.3109/0284186X.2014.895036
18. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol . (2010) 28:4706–13. doi: 10.1200/JCO.2009.27.6055
19. Formica V, Sera F, Cremolini C, Riondino S, Morelli C, Arkenau HT, et al. KRAS and BRAF mutations in stage II and III colon cancer: A systematic review and meta-analysis. J Natl Cancer Inst . (2022) 114:517–27. doi: 10.1093/jnci/djab190
20. Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol . (2016) 27:1746–53. doi: 10.1093/annonc/mdw261
21. De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol . (2010) 11:753–62. doi: 10.1016/S1470-2045(10)70130-3
22. Schirripa M, Cremolini C, Loupakis F, Morvillo M, Bergamo F, Zoratto F, et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int J Cancer . (2015) 136:83–90. doi: 10.1002/ijc.v136.1
23. Jones JC, Renfro LA, Al-Shamsi HO, Schrock AB, Rankin A, Zhang BY, et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol . (2017) 35:2624–30. doi: 10.1200/JCO.2016.71.4394
24. Yang ZY, Wu XY, Huang YF, Di MY, Zheng DY, Chen JZ, et al. Promising biomarkers for predicting the outcomes of patients with KRAS wild-type metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a systematic review with meta-analysis. Int J Cancer . (2013) 133:1914–25. doi: 10.1002/ijc.v133.8
25. Tan ES, Fan W, Knepper TC, Schell MJ, Sahin IH, Fleming JB, et al. Prognostic and predictive value of PIK3CA mutations in metastatic colorectal cancer. Target Oncol . (2022) 17:483–92. doi: 10.1007/s11523-022-00898-7
26. Wu S, Gan Y, Wang X, Liu J, Li M, Tang Y. PIK3CA mutation is associated with poor survival among patients with metastatic colorectal cancer following anti-EGFR monoclonal antibody therapy: a meta-analysis. J Cancer Res Clin Oncol . (2013) 139:891–900. doi: 10.1007/s00432-013-1400-x
27. Salvatore L, Calegari MA, Loupakis F, Fassan M, Di Stefano B, Bensi M, et al. PTEN in colorectal cancer: shedding light on its role as predictor and target. Cancers (Basel) . (2019) 11:E1765. doi: 10.3390/cancers11111765
CrossRef Full Text | Google Scholar
28. Molinari F, Frattini M. Functions and regulation of the PTEN gene in colorectal cancer. Front Oncol . (2013) 3:326. doi: 10.3389/fonc.2013.00326
29. Liebl MC, Hofmann TG. The role of p53 signaling in colorectal cancer. Cancers (Basel) . (2021) 13:2125. doi: 10.3390/cancers13092125
30. Wang C, Ouyang C, Cho M, Ji J, Sandhu J, Goel A, et al. Wild-type APC is associated with poor survival in metastatic microsatellite stable colorectal cancer. Oncologist . (2021) 26:208–14. doi: 10.1002/onco.13607
31. Jorissen RN, Christie M, Mouradov D, Sakthianandeswaren A, Li S, Love C, et al. Wild-type APC predicts poor prognosis in microsatellite-stable proximal colon cancer. Br J Cancer . (2015) 113:979–88. doi: 10.1038/bjc.2015.296
32. Park DI, Kang MS, Oh SJ, Kim HJ, Cho YK, Sohn CI, et al. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int J Colorectal Dis . (2007) 22:491–7. doi: 10.1007/s00384-006-0192-8
33. Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, et al. Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer . (2018) 17:198–205. doi: 10.1016/j.clcc.2018.05.006
34. Sartore-Bianchi A, Amatu A, Porcu L, Ghezzi S, Lonardi S, Leone F, et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncologist . (2019) 24:1395–402. doi: 10.1634/theoncologist.2018-0785
35. Marks KM, West NP, Morris E, Quirke P. Clinicopathological, genomic and immunological factors in colorectal cancer prognosis. Br J Surg . (2018) 105:e99–109. doi: 10.1002/bjs.10756
36. Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol . (2003) 16:376–88. doi: 10.1097/01.MP.0000062859.46942.93
37. Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, Huang WH, Luo XZ. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta analysis. BMC Cancer . (2019) 19:123. doi: 10.1186/s12885-019-5324-y
38. Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg . (2013) 258:775–82. doi: 10.1097/SLA.0b013e3182a69f7e
39. An Y, Zhou J, Lin G, Wu H, Cong L, Li Y, et al. Clinicopathological and molecular characteristics of colorectal signet ring cell carcinoma: A review. Pathol Oncol Res . (2021) 27:1609859. doi: 10.3389/pore.2021.1609859
40. Diao Z, Han Y, Chen Y, Zhang R, Li J. The clinical utility of microsatellite instability in colorectal cancer. Crit Rev Oncol Hematol . (2021) 157:103171. doi: 10.1016/j.critrevonc.2020.103171
41. Rhee YY, Kim KJ, Kang GH. CpG island methylator phenotype-high colorectal cancers and their prognostic implications and relationships with the serrated neoplasia pathway. Gut Liver . (2017) 11:38–46. doi: 10.5009/gnl15535
42. Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: A systematic review and meta-analysis. Sci Rep . (2020) 10:3360. doi: 10.1038/s41598-020-60255-4
43. Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res . (2009) 15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438
44. Valenzuela G, Canepa J, Simonetti C, Solo de Zaldívar L, Marcelain K, González-Montero J. Consensus molecular subtypes of colorectal cancer in clinical practice: A translational approach. World J Clin Oncol . (2021) 12:1000–8. doi: 10.5306/wjco.v12.i11.1000
45. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health . (2013) 13:154. doi: 10.1186/1471-2458-13-154
46. Puccini A, Lenz HJ, Marshall JL, Arguello D, Raghavan D, Korn WM, et al. Impact of patient age on molecular alterations of left-sided colorectal tumors. Oncologist . (2019) 24:319–26. doi: 10.1634/theoncologist.2018-0117
47. Tenny S, Hoffman MR. Odds ratio. In: StatPearls . Treasure Island (FL): StatPearls Publishing. (2024) Available at: http://www.ncbi.nlm.nih.gov/books/NBK431098/ .
Google Scholar
48. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ . (2011) 342:d549. doi: 10.1136/bmj.d549
49. Wang MJ, Ping J, Li Y, Adell G, Arbman G, Nodin B, et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep . (2015) 5:10645. doi: 10.1038/srep10645
50. Du M, Gu D, Xin J, Peters U, Song M, Cai G, et al. Integrated multi-omics approach to distinct molecular characterization and classification of early-onset colorectal cancer. Cell Rep Med . (2023) 4:100974. doi: 10.1016/j.xcrm.2023.100974
51. Ugai T, Väyrynen JP, Lau MC, Borowsky J, Akimoto N, Väyrynen SA, et al. Immune cell profiles in the tumor microenvironment of early-onset, intermediate-onset, and later-onset colorectal cancer. Cancer Immunol Immunother . (2022) 71:933–42. doi: 10.1007/s00262-021-03056-6
52. Gardner IH, Siddharthan R, Watson K, Dewey E, Ruhl R, Khou S, et al. A distinct innate immune signature of early onset colorectal cancer. Immunohorizons . (2021) 5:489–99. doi: 10.4049/immunohorizons.2000092
53. Irabor DO, Oluwasola OA, Ogunbiyi OJ, Ogun OG, Okolo CA, Melas M, et al. Microsatellite instability is common in colorectal cancer in native Nigerians. Anticancer Res . (2017) 37:2649–54. doi: 10.21873/anticanres
54. Li GM, Xiao GZ, Qin PF, Wan XY, Fu YJ, Zheng YH, et al. Single-cell RNA sequencing reveals heterogeneity in the tumor microenvironment between young-onset and old-onset colorectal cancer. Biomolecules . (2022) 12:1860. doi: 10.3390/biom12121860
55. Pilozzi E, Maresca C, Duranti E, Giustiniani MC, Catalanotto C, Lucarelli M, et al. Left-sided early-onset vs late-onset colorectal carcinoma: histologic, clinical, and molecular differences. Am J Clin Pathol . (2015) 143:374–84. doi: 10.1309/AJCPNOC55IOLXFUD
56. Andric F, Al-Fairouzi A, Wettergren Y, Szeponik L, Bexe-Lindskog E, Cusack JC, et al. Immune microenvironment in sporadic early-onset versus average-onset colorectal cancer. Cancers (Basel) . (2023) 15:1457. doi: 10.3390/cancers15051457
57. Lu C, Zhang X, Schardey J, Wirth U, Heinrich K, Massiminio L, et al. Molecular characteristics of microsatellite stable early-onset colorectal cancer as predictors of prognosis and immunotherapeutic response. NPJ Precis Oncol . (2023) 7:63. doi: 10.1038/s41698-023-00414-8
58. Willauer AN, Liu Y, Pereira AAL, Lam M, Morris JS, Raghav KPS, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer . (2019) 125:2002–10. doi: 10.1002/cncr.31994
59. Ha YJ, Shin YJ, Tak KH, Park JL, Kim JH, Lee JL, et al. Reduced expression of alanyl aminopeptidase is a robust biomarker of non-familial adenomatous polyposis and non-hereditary nonpolyposis colorectal cancer syndrome early-onset colorectal cancer. Cancer Med . (2023) 12:10091–104. doi: 10.1002/cam4.5675
60. Yang J, Zhao Y, Yuan R, Wang Y, Wang S, Chang Z, et al. Identifying individualized prognostic signature and unraveling the molecular mechanism of recurrence in early-onset colorectal cancer. Eur J Med Res . (2023) 28:533. doi: 10.1186/s40001-023-01491-y
61. Furuhashi S, Bustos MA, Mizuno S, Ryu S, Naeini Y, Bilchik AJ, et al. Spatial profiling of cancer-associated fibroblasts of sporadic early onset colon cancer microenvironment. NPJ Precis Oncol . (2023) 7:118. doi: 10.1038/s41698-023-00474-w
62. Chang SH, Patel N, Du M, Liang PS. Trends in early-onset vs late-onset colorectal cancer incidence by race/ethnicity in the United States cancer statistics database. Clin Gastroenterol Hepatol . (2022) 20:e1365–77. doi: 10.1016/j.cgh.2021.07.035
63. Wang R, Wang MJ, Ping J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: A population-based cohort study of SEER 9 registries data (1988-2011). Med (Baltimore) . (2015) 94:e1402. doi: 10.1097/MD.0000000000001402
64. McClelland PHT, Liu T, Ozuner G. Early-onset colorectal cancer in patients under 50 years of age: demographics, disease characteristics, and survival. Clin Colorectal Cancer . (2022) 21:e135–44. doi: 10.1016/j.clcc.2021.11.003
65. Saraiva MR, Rosa I, Claro I. Early-onset colorectal cancer: A review of current knowledge. World J Gastroenterol . (2023) 29:1289–303. doi: 10.3748/wjg.v29.i8.1289
66. Cavestro GM, Mannucci A, Balaguer F, Hampel H, Kupfer SS, Repici A, et al. Delphi initiative for early-onset colorectal cancer (DIRECt) international management guidelines. Clin Gastroenterol Hepatol . (2023) 21:581–603. doi: 10.1016/j.cgh.2022.12.006
67. Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg . (2015) 150:402–9. doi: 10.1001/jamasurg.2014.3572
68. Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer . (2016) 122:929–34. doi: 10.1002/cncr.29716
69. Kanter K, Fish M, Mauri G, Horick NK, Allen JN, Blaszkowsky LS, et al. Care patterns and overall survival in patients with early-onset metastatic colorectal cancer. JCO Oncol Pract . (2021) 17:e1846–55. doi: 10.1200/OP.20.01010
70. NCCN. Guidelines Detail (2023). Available online at: https://www.nccn.org/guidelines/guidelines-detail .
71. Afrăsânie VA, Marinca MV, Alexa-Stratulat T, Gafton B, Păduraru M, Adavidoaiei AM, et al. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer - practical implications for the clinician. Radiol Oncol . (2019) 53:265–74. doi: 10.2478/raon-2019-0033
72. Taieb J, Zaanan A, Le Malicot K, Julié C, Blons H, Mineur L, et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: A post hoc analysis of the PETACC-8 trial. JAMA Oncol . (2016) 2:643–53. doi: 10.1001/jamaoncol.2015.5225
73. Jácome AA, Vreeland TJ, Johnson B, Kawaguchi Y, Wei SH, Nancy You Y, et al. The prognostic impact of RAS on overall survival following liver resection in early versus late-onset colorectal cancer patients. Br J Cancer . (2021) 124:797–804. doi: 10.1038/s41416-020-01169-w
74. Aljehani MA, Bien J, Lee JSH, Fisher GA, Lin AY. KRAS sequence variation as prognostic marker in patients with young- vs late-onset colorectal cancer. JAMA Netw Open . (2023) 6:e2345801. doi: 10.1001/jamanetworkopen.2023.45801
75. Khan SA, Morris M, Idrees K, Gimbel MI, Rosenberg S, Zeng Z, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg . (2016) 51:1812–7. doi: 10.1016/j.jpedsurg.2016.07.015
76. Irahara N, Baba Y, Nosho K, Shima K, Yan L, Dias-Santagata D, et al. NRAS mutations are rare in colorectal cancer. Diagn Mol Pathol . (2010) 19:157–63. doi: 10.1097/PDM.0b013e3181c93fd1
77. Capper D, Voigt A, Bozukova G, Ahadova A, Kickingereder P, von Deimling A, et al. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer . (2013) 133:1624–30. doi: 10.1002/ijc.28183
78. Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta . (2015) 1855:104–21. doi: 10.1016/j.bbcan.2014.09.008
79. Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin R, et al. Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance. Gastroenterol Rep (Oxf) . (2020) 8:179–91. doi: 10.1093/gastro/goaa026
80. Nakayama M, Oshima M. Mutant p53 in colon cancer. J Mol Cell Biol . (2019) 11:267–76. doi: 10.1093/jmcb/mjy075
81. Michel M, Kaps L, Maderer A, Galle PR, Moehler M. The role of p53 dysfunction in colorectal cancer and its implication for therapy. Cancers (Basel) . (2021) 13:2296. doi: 10.3390/cancers13102296
82. Aghabozorgi AS, Bahreyni A, Soleimani A, Bahrami A, Khazaei M, Ferns GA, et al. Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Biochimie . (2019) 157:64–71. doi: 10.1016/j.biochi.2018.11.003
83. Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev . (2021) 35:787–820. doi: 10.1101/gad.348226.120
84. Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med . (2018) 379:764–73. doi: 10.1056/NEJMcp1714533
85. McRonald FE, Pethick J, Santaniello F, Shand B, Tyson A, Tulloch O, et al. Identification of people with Lynch syndrome from those presenting with colorectal cancer in England: baseline analysis of the diagnostic pathway. Eur J Hum Genet . (2024). doi: 10.1038/s41431-024-01550-w
86. Mezzapesa M, Losurdo G, Celiberto F, Rizzi S, d’Amati A, Piscitelli D, et al. Serrated colorectal lesions: an up-to-date review from histological pattern to molecular pathogenesis. Int J Mol Sci . (2022) 23:4461. doi: 10.3390/ijms23084461
87. Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Histological grading in colorectal cancer: new insights and perspectives. Histol Histopathol . (2015) 30:1059–67. doi: 10.14670/HH-11-633
88. Fadel MG, Malietzis G, Constantinides V, Pellino G, Tekkis P, Kontovounisios C. Clinicopathological factors and survival outcomes of signet-ring cell and mucinous carcinoma versus adenocarcinoma of the colon and rectum: a systematic review and meta-analysis. Discovery Oncol . (2021) 12:5. doi: 10.1007/s12672-021-00398-6
89. Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond) . (2019) 39:13. doi: 10.1186/s40880-019-0361-0
90. Gabriel E, Attwood K, Al-Sukhni E, Erwin D, Boland P, Nurkin S. Age-related rates of colorectal cancer and the factors associated with overall survival. J Gastrointest Oncol . (2018) 9:96–110. doi: 10.21037/jgo
91. Ding X, Yang X, Wu D, Huang Y, Dai Y, Li J, et al. Nomogram predicting the cancer-specific survival of early-onset colorectal cancer patients with synchronous liver metastasis: a population-based study. Int J Colorectal Dis . (2022) 37:1309–19. doi: 10.1007/s00384-022-04175-x
92. Chen Y, He L, Lu X, Tang Y, Luo G, Chen Y, et al. Causes of death among early-onset colorectal cancer population in the United States: a large population-based study. Front Oncol . (2023) 13:1094493. doi: 10.3389/fonc.2023.1094493
93. Benesch MGK, Mathieson A, O’Brien SBL. Effects of tumor localization, age, and stage on the outcomes of gastric and colorectal signet ring cell adenocarcinomas. Cancers (Basel) . (2023) 15:714. doi: 10.3390/cancers15030714
94. De Renzi G, Gaballo G, Gazzaniga P, Nicolazzo C. Molecular biomarkers according to primary tumor location in colorectal cancer: current standard and new insights. Oncology . (2021) 99:135–43. doi: 10.1159/000510944
95. Cheng E, Blackburn HN, Ng K, Spiegelman D, Irwin ML, Ma X, et al. Analysis of survival among adults with early-onset colorectal cancer in the national cancer database. JAMA Netw Open . (2021) 4:e2112539. doi: 10.1001/jamanetworkopen.2021.12539
96. Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF, et al. KRAS testing, tumor location, and survival in patients with stage IV colorectal cancer: SEER 2010-2013. J Natl Compr Canc Netw . (2017) 15:1484–93. doi: 10.6004/jnccn.2017.7011
97. Xie MZ, Li JL, Cai ZM, Li KZ, Hu BL. Impact of primary colorectal Cancer location on the KRAS status and its prognostic value. BMC Gastroenterol . (2019) 19:46. doi: 10.1186/s12876-019-0965-5
98. Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw . (2017) 15:411–9. doi: 10.6004/jnccn.2017.0038
99. Foppa C, Tamburello S, Maroli A, Carvello M, Poliani L, Laghi L, et al. Early age of onset is an independent predictor for worse disease-free survival in sporadic rectal cancer patients. A comparative analysis of 980 consecutive patients. Eur J Surg Oncol . (2022) 48:857–63. doi: 10.1016/j.ejso.2021.10.021
100. Laskar RS, Ghosh SK, Talukdar FR. Rectal cancer profiling identifies distinct subtypes in India based on age at onset, genetic, epigenetic and clinicopathological characteristics. Mol Carcinog . (2015) 54:1786–95. doi: 10.1002/mc.22250
101. Perea J, García JL, Corchete L, Tapial S, Olmedillas-López S, Vivas A, et al. A clinico-pathological and molecular analysis reveals differences between solitary (early and late-onset) and synchronous rectal cancer. Sci Rep . (2021) 11:2202. doi: 10.1038/s41598-020-79118-z
102. Spolverato G, Fassan M, Scarpa M, Stepanyan A, De Simoni O, Scognamiglio F, et al. IMMUNOREACT 6: weak immune surveillance characterizes early-onset rectal cancer. Br J Surg . (2023) 110:1490–501. doi: 10.1093/bjs/znad219
103. Ugai T, Haruki K, Harrison TA, Cao Y, Qu C, Chan AT, et al. Molecular characteristics of early-onset colorectal cancer according to detailed anatomical locations: comparison with later-onset cases. Am J Gastroenterol . (2023) 118:712–26. doi: 10.14309/ajg.0000000000002171
104. Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol Res . (2018) 11:264–73. doi: 10.14740/gr1062w
105. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science . (2006) 313:1960–4. doi: 10.1126/science.1129139
106. Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res . (2020) 26:332–9. doi: 10.1158/1078-0432.CCR-18-1851
107. Ten Hoorn S, de Back TR, Sommeijer DW, Vermeulen L. Clinical value of consensus molecular subtypes in colorectal cancer: A systematic review and meta-analysis. J Natl Cancer Inst . (2022) 114:503–16. doi: 10.1093/jnci/djab106
Keywords: colorectal cancer, colon cancer, rectal cancer, early-onset, oncogenes, prognosis, molecular characteristics
Citation: Lawler T, Parlato L and Warren Andersen S (2024) The histological and molecular characteristics of early-onset colorectal cancer: a systematic review and meta-analysis. Front. Oncol. 14:1349572. doi: 10.3389/fonc.2024.1349572
Received: 04 December 2023; Accepted: 16 April 2024; Published: 26 April 2024.
Reviewed by:
Copyright © 2024 Lawler, Parlato and Warren Andersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaneda Warren Andersen, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 27 April 2024
Prevalence and associated factors of anemia among postpartum mothers in public health facilities in Ethiopia, 2024: a systematic review and meta-analysis
- Gebeyehu Lakew 1 ,
- Amlaku Nigussie Yirsaw 1 ,
- Alemshet Yirga Berhie 2 ,
- Asnake Gashaw Belayneh 3 ,
- Solomon Ketema Bogale 4 ,
- Eyob Getachew 1 ,
- Getnet Alemu Andarge 4 ,
- Kedir Seid 5 &
- Eyob Ketema Bogale 6
BMC Pregnancy and Childbirth volume 24 , Article number: 327 ( 2024 ) Cite this article
Metrics details
Postpartum anemia, characterized by hematocrit or hemoglobin levels below the defined cutoff point (< 11gm/dl or hematocrit < 33%), is a prevalent global issue. It serves as an indirect contributor to maternal mortality and morbidity. Mothers in the postpartum period experience diminished quality of life, impaired cognitive function, emotional instability, and an increased risk of postpartum depression due to anemia. Additionally, infants of affected mothers may face challenges such as insufficient breast milk supply and a lack of proper care. Examining the combined prevalence and factors associated with postpartum anemia is crucial for addressing maternal health risks and complications during the postnatal phase attributed to anemia.
The study aimed to synthesize the existing literature on the prevalence and associated factors of postpartum anemia in public health facilities of Ethiopia, in 2024.
The study was conducted by searching through the Google Scholar, PubMed, and Cochrane Library search engines. The search utilized keywords and MeSH terms such as anemia, low hemoglobin, postpartum, postnatal women, and Ethiopia. The collected data underwent analysis and comparison with the WHO criteria to determine if it met the threshold for declaring a public health concern. Heterogeneity was evaluated through the Cochran Q test and I2 statistics. Prevalence and odds ratio estimations were performed using a random-effects model with a 95% confidence interval.
Four studies were included in this systematic review and meta-analysis. The overall pooled prevalence of anemia among postpartum women in Ethiopia was 69% (95% CI: 60- 77%).Lack of formal education(OR = 3.5;CI:2.639,4.408),Low Pre-delivery hemoglobin (OR = 4.2;CI: 1.768–6.668), Postpartum women < 4 ANC visit (OR = 2.72; 95% CI:2.14,3.3 ),history of post partum hemorrhage (OR = 2.49; CI: 1.075–3.978),history of Forceps/vacuum delivery(OR = 3.96; CI:2.986–4.947), Poor iron and folic acid adherence (OR = 2.8;95% CI:2.311,3.297), C/S (OR = 4.04; 95% CI: 3.426,4.671),lower dietary diversity (OR = 4.295% CI:1.768,6.668) were significantly associated postpartum anemia.
Postpartum women in Ethiopia continue to face a considerable public health challenge in the form of anemia. Consequently, there is a pressing need for the government to formulate comprehensive, multi-sectorial policies and strategies. These initiatives should be designed to address the substantial regional disparities influenced by interconnected factors, with the aim of reducing the prevalence of anemia among postpartum women in Ethiopia.
Peer Review reports
Anemia is characterized by a drop in red blood cell mass (RBC mass) or a low hemoglobin (Hb) level in comparison to the normal reference range [ 1 ]. When hemoglobin levels are less than 11 gm/dl at one week postpartum and less than 12 gm/dl at eight weeks postpartum, postpartum anemia—a persistent iron deficiency occurs [ 2 ].
Women who were nursing and had hemoglobin levels ≥ 12 g/dl were regarded as having a normal value. Mild anemia is defined as hemoglobin levels 11–11.9 g/dl, whereas moderate and severe anemia is defined as hemoglobin values 8–10.9 g/dl and < 8 g/dl, respectively [ 3 ]. Within the first 24 h following birth, hemoglobin concentration is reduced due to hemodynamic changes, fluid loss, and blood loss. But increases 48 h later and takes 7 days to reach the non-pregnant level [ 4 ].
Anemia afflicted 613 million (33%) of all women of reproductive age worldwide in 2016, with Asia and Africa having higher rates than the other two continents combined [ 4 ]. Anemia prevalence among new mothers varies from 10 to 30% in wealthy nations and 50–80% in underdeveloped nations; it is lower in Kenya (16.4%) and higher in south Rajasthan (90.68%) [ 5 , 6 ]. Ethiopia’s 2020 objective was reduce anemia in the reproductive age group from 19.3 to 12%, but it resulted in an increase in anemia burden to 24%. In particular, the percentage of lactating mothers increased from 18.6% in 2011 to 28.6% in 2016 [ 7 , 8 ].
The majority of maternal deaths worldwide happen in the postpartum phase; yet, several Sub-Saharan African nations, including Ethiopia, have a disproportionately higher burden of these deaths. Postpartum anemia is linked to postpartum depression, exhaustion, poor cognitive function, and disrupted mother-infant attachment. It affects up to 80% of women in low-income and rural communities and up to 50% of women in Europe and the US [ 9 ]. The postnatal period is the most crucial yet most ignored time in mothers’ and babies’ lives, according to the World Health Organization (WHO) [ 10 ]. The annual report from the Ethiopian Ministry of Health states that over 70% of maternal mortality occurred in the postpartum period in 2019 alone [ 11 ].
Because they are more prone to iron deficiency and anemia during pregnancy due to dietary and physiological factors, breastfeeding moms are viewed as being more vulnerable than non-lactating mothers [ 12 ].
Iron deficiency anemia during the postpartum period can have very serious and long-term effects on mothers and their babies. Anemia is a contributing factor to maternal morbidity, mortality, and complications indirectly [ 7 ]. Anemia damages women’s health and well-being and raises the possibility of unfavorable results for mothers and newborns [ 13 ]. It represents 2% of Ethiopia’s overall maternal death rate [ 14 ]. A 10% g/l increase in maternal hemoglobin has been estimated to reduce maternal mortality by 29% and perinatal mortality by 28%. Anemia and mother death have a linear association [ 15 ]. Due to bleeding, postnatal moms lose a substantial amount of iron during birth; every milliliter of blood lost is equivalent to 0.5 milligrams of iron lost [ 4 , 16 ]. It can cause morbidity throughout the reproductive cycle if it is not detected and treated early [ 16 ].
A major issue in public health is postpartum anemia. As a result of postpartum anemia, some moms experience despair, emotional instability, exhaustion, infection, and decreased quality of life. Additionally, their babies endure poor care and insufficient breast milk production [ 17 ].
Negative effects of postpartum anemia include reduced quality of life, dyspnea, palpitations, infections, exhaustion, altered cognitive function, unstable emotions, and postpartum depression. Therefore it has an impact on breastfeeding, caregiver capacity, and the attachment between a mother and her kid [ 5 , 18 ]. Iron deficiency anemia in babies is increased by early weaning from breastfeeding, as breast milk itself has low iron content. Limited birth weight and preterm newborns have limited iron stores at birth, which is critical for development, immunity, and growth. As a result, babies exposed to infections experience poor growth and development in addition to morbidity and mortality [ 4 , 19 ].
Preventing postpartum anemia is critical to the health of expectant mothers and their babies. Therefore, the WHO advises postpartum women to take oral iron supplements for three months following delivery, either with or without folic acid [ 17 ]. Additionally, weekly supplements of 60 mg iron and 2.8 mg folic acid for all women of reproductive age and 60 mg + 400 µg iron folic acid for expectant mothers were advised in populations where the prevalence of anemia was higher than 20% [ 20 ]. Even in Ethiopia, postpartum moms were not given IFA or evaluated for anemia in the research area. On the other hand, inadequate adherence poses a challenge to the emphasis placed on IFA supplementation during pregnancy. 5% of those who took iron supplements for 90 days or longer remained below the inadequate level, indicating poor iron use [ 7 ].
Dietary content and quantity have a significant impact on the amount of iron absorbed from prescribed iron folate or diet. It is challenging to improve maternal iron status and meet the second global nutrition target, which calls for a 50% reduction in anemia among reproductive-age individuals by 2025 unless iron supplementation is continued for periods ranging from 12 weeks to two years and “nutrition-specific interventions” are implemented for all women in the reproductive cycle [ 4 , 20 ].
To lessen this issue, the WHO advises postpartum women to take iron supplements for six to twelve weeks following birth in areas where anemia during pregnancy is a public health risk [ 17 ]. However, in the study area, there is no postnatal anemia screening and iron supplementation. Poor adherence can lead to postpartum anemia by affecting the iron reserve from the recommended IFA during pregnancy and making it difficult to handle iron loss during childbirth [ 21 ]. The EDHS found that the prevalence of anemia among nursing moms increased by 10.6% between 2011 and 2016, despite a reduction goal of 18–12% [ 7 , 8 ]. This discrepancy may be due to failure to emphasize during preconception and postnatal period. It has many contributions to offer evidence for policymakers and stakeholders, aiding in the development and implementation of evidence-based interventions to address the morbidity and mortality associated with anemia in postpartum women in Ethiopia.
Study design and search strategy
A systematic review and meta-analysis of published studies were used. Searching was made from beginning of January to end of January 2024.A comprehensive examination of all available research studies was conducted across major databases, including Google Scholar, PubMed, and the Cochrane Library. Additionally, efforts were made to retrieve new articles by reaching out to experts and researchers, and manual searches were performed to identify unpublished studies. The search utilized specific Keywords and MeSH terms such as “Anemia,” “low hemoglobin,” “Postpartum,” “post-natal women,” and “Ethiopia.” The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines will be adhered to meticulously during the course of this review.
Study selection and eligibility criteria
This systematic review and meta-analysis encompassed cross-sectional studies conducted in Ethiopia. Eligible studies included those with primary research designs, while review articles, conference abstracts, and editorials were excluded. The criteria for inclusion also specified studies measuring the prevalence of anemia among postpartum women in Ethiopia between 2015 and 2023. The selection process adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Two authors independently screened the studies for eligibility, and any disparities were resolved through discussion and consensus.
The protocol for this review has been prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024505959.
Data extraction process
A standardized data extraction format was created using Microsoft Excel for retrieving information from the chosen studies. The format included categories such as author details (name and year of publication), study year, study setting, study design, sample size, study population, sampling procedures, data collection procedures, and findings. Two authors independently carried out the data extraction, and the results were cross-checked for consistency. In instances of discrepancies, a thorough review of the articles was conducted, and any disagreements were resolved through verification and subsequent discussion.
Outcome of interest
The systematic meta-analysis focused on the outcome variable of anemia in postpartum mothers. According to the World Health Organization (WHO) criteria, anemia is defined as a hemoglobin concentration below 12.0 g/dl, with severity categorized as mild, moderate, and severe. The cutoff points for these categories are 10.0–11.9 g/dl, 7.0–9.9 g/dl, and < 7.0 g/dl, respectively.
Study quality and risk of bias
Two authors independently conducted a risk of bias assessment for the selected studies using the Hoy 2012 tool, which comprises ten criteria. These criteria encompass the representation of the population, sampling frame, methods of participant selection, non-response bias, data collection directly from subjects, acceptability of case definition, reliability and validity of study tools, mode of data collection, length of prevalence period, and appropriateness of the numerator and denominator. The tool divides into four items assessing selection and non-response bias, five items evaluating measurement bias, and one item addressing bias related to analysis and results reporting. Each criterion was evaluated as either low or high risk of bias, and the overall risk of bias for each study was determined based on the total score of high-risk items: low (≤ 2), moderate ( 3 – 4 ), and high (≥ 5).
We utilized the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) tool to assess the level of certainty of evidence for the outcome. The GRADE quality evaluation tool initiates observational studies with a low quality of evidence, and this quality may further be downgraded to very low based on considerations such as risk of bias, inconsistency, indirectness, imprecision, and publication bias. However, there is an option for upgrading if no other limitations are identified within these factors. Assessments were conducted for the five primary domains (risk of bias, consistency, directness, precision, and publication bias), along with an evaluation of the overall quality of evidence. Following the GRADE recommendations, the study design served as the starting point, and for each domain not met, a one-step downgrade was applied [ 22 ].
The quality of studies was evaluated using the Joanna Briggs Institute (JBI) critical appraisal checklist. Following a protocol, the reviewers (GL, EKB) employed a blinded review approach to assess the quality of the original articles. Studies scoring 5 or more on the JBI criteria [ 23 ] were deemed to have good quality and were consequently included in the review. Any discrepancies in the quality assessment were resolved through consultation with the first author.
Statistical analysis and synthesis
The collected data were inputted and analyzed using STATA version 17 statistical software. To calculate the variance of postpartum anemia prevalence for each article, the binomial distribution formula was applied by extracting the frequency of the outcome and the sample size. The random-effects model was used to calculate the pooled odds ratio (OR) with a 95% confidence interval, determining factors associated with anemia among postpartum women in Ethiopia. Heterogeneity among the studies was evaluated through the Cochran Q test (a P -value < 0.10 was considered significant) and I2 statistics (a significance level of at least 50%).
Estimation was conducted using a random-effects model with a 95% confidence interval (CI) due to notable variations among the study findings. The choice of a random-effects model, known for its conservative approach, was made to accommodate the inherent heterogeneity in meta-analysis. Subgroup analysis was carried out according to the study’s location. To identify publication bias, funnel plot analysis, Egger weighted regression, and Begg rank correlation tests were employed, with a significance level set at P < 0.05. The outcomes of the meta-analysis were visually presented through forest plots and tables.
Characteristics of the studies
A total of 11,123 published studies were retrieved through searching from different databases. Out of 11,123 studies, 6320 studies were excluded due to duplication, and 3959 studies were excluded after reading the title and abstract using inclusion and exclusion criteria since they did not relate to the aim of this study. The remaining 844 full- articles were assessed for eligibility. Finally, 4 studies were included in the systematic review and meta-analysis (Fig. 1 ).All four studies included in a systematic review and meta-analysis were cross-sectional studies.
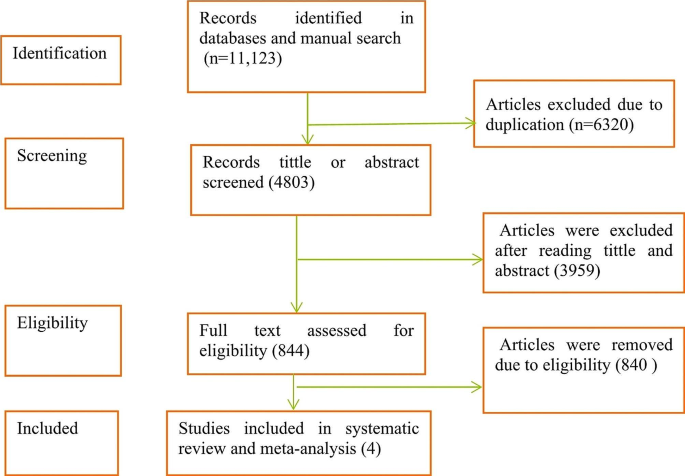
PRISMA flow chart diagram describing selection of studies for systematic review and meta-analysis on the prevalence of Anemia among postpartum women in Ethiopia, 2024
The assessment of bias risk for the four individual articles included in the systematic review and meta-analysis utilized the Hoy 2012 tool with ten specified criteria, as outlined in the methodology section. Among the four studies, two (50%) were identified as having a low risk of bias, while the remaining two studies (50%) were categorized as having a moderate risk of bias. These four studies, which examined the prevalence of anemia in postpartum women, demonstrated significant heterogeneity, as indicated by the Cochrane Q test ( p = 0.00) and I2 test (93.1%), warranting the use of a random-effects model (Fig. 2 ). However, the Egger weighted regression statistics for studies on anemia prevalence ( P = 0.00) and Begg rank correlation statistics ( p = 0.0) revealed evidence suggesting the presence of publication bias. The funnel plot has also an asymmetry by visual inspection also shows there is a sign of publication bias (Fig. 3 ). To decrease the heterogeneity, subgroup analysis was performed based on the region (Tables 1 and Fig. 4 ). More over to treat publication bias we run non parametric trim-and-fill analysis, however no imputed studies observed.
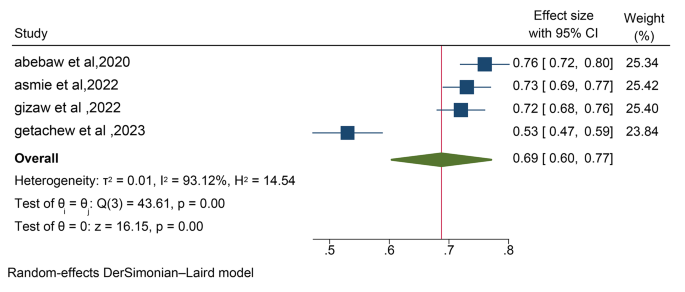
Forest plots of four studies on the prevalence of anemia among postpartum women in Ethiopia, 2024
A Funnel plot of studies conducted on prevalence of anemia among post partum women in Ethiopia, 2024
Sub group analysis based on place of study on prevalence of postpartum anemia in Ethiopia, 2024
Fig A: sub group analysis of western Ethiopia on postpartum anemia in Ethiopia
Fig B: sub group analysis of eastern Ethiopia among postpartum anemia in Ethiopia
Prevalence of anemia among postpartum women in Ethiopia
A total of four studies were included in this systematic review and Meta-analysis to reveal the prevalence of anemia in postpartum women in Ethiopia from 2015 to 2023. A study done in 2020 reported there was a 24.3% prevalence of anemia among postpartum women in the Amhara region in Debre Markos. Whereas in a study conducted in 2022, the prevalence of anemia in postpartum women was 26.9% and 28.1% in Diredawa and Harer respectively. Another study conducted in the Amhara region, Gondar documented a relatively high prevalence (47.1%) of anemia in postpartum women in 2023 (Table 2 ).
The pooled prevalence of anemia among postpartum women in Ethiopia was 69% (95%CI: 60- 77%) (Fig. 2 ).
Furthermore, subgroup analysis based on the place of study showed that the prevalence of anemia in postpartum women was significantly higher (72.5% ) in Eastern Ethiopia (Diredewa and Harer) compared to the lowest prevalence ( 64.6%) in Western Ethiopia (Gondar and debremarkos (Table 1 ).
Meta-regression and sensitivity analysis
Meta-regression.
Meta-regression was performed with the place of study considered as covariates, employing a random-effects model. The outcome indicated the absence of heterogeneity based on the place of study ( p = 0.445) (Table 3 ).
Sensitivity analysis
A sensitivity analysis was carried out using the leave-one-out method to evaluate the impact of each individual study on the overall pooled prevalence of postpartum anemia. The findings indicated that the estimated prevalence obtained when each study was excluded from the analysis fell within the confidence interval of the pooled prevalence. Consequently, none of the included studies had a significant effect on the overall pooled estimate, as demonstrated below (Table 4 and Fig. 5 ).
Leave-one-out sensitivity analysis of prevalence of postpartum women in Ethiopia, 2024
Factors associated with Anemia among postpartum women in Ethiopia
Before doing the pooled associated factors, there were twelve associated factors for post partum anemia : Lack of formal education [ 25 , 26 ], ANC visit < 4 [ 24 , 25 , 26 ] ,history of PPH [ 1 , 24 ] ,history of APH [ 24 ], C/S [ 1 , 26 ] ,vaccum/forceps [ 24 , 25 ], pre delivery Hgb < 11gm/dl [ 25 , 26 ], poor adherence to IFA supplementation [ 1 , 24 , 25 , 26 ] ,low diet [ 1 , 25 ] ,maternal blod loss [ 26 ] ,MUAC < 23 cm [ 24 ], GIT [ 25 ] and unemployment [ 25 ] were the independent variables associated with post partum anemia .
Lack of formal education was one of the socio economic factor that has significantly associated with post partum anemia.Women who lacked formal education were 3.5 times more likely to be anemic than their educated counter parts (OR = 3.5; 95% CI: 2.639, 4.408).
ANC visit less than 4, history of PPH, history of Forceps/vaccum delivery, pre delivery Hgb less than 11gm/dl and history of cesarean delivery are obstetric factors that are significantly associated with post partum anemia .Women who had lower antenatal care visits (below 4) (OR = 2.72; 95% CI:2.14, 3.3) had 2.72 times more likely odds of developing anemia compared to their respective counterparts. Mothers who had history of PPH had 2.49 times higher odds of anemia than those that had not history of PPH (OR = 2.49, CI:1.075,3.978). Women who had forceps/vaccum delivery were 3.96 times more likely to be anemic than their counter parts (OR = 3.96; 95% CI: 2.986, 4.947).Mothers who had also pre delivery Hgb less than 11gm/dl were 4.2 times more likely to be anemic than those who had normal pre delivery Hgb (OR = 4.2CI:1.768,6.668). Women who delivered via C/S are 4.4 times at higher risk of developing anemia compared with their SVD counter parts.
The meta-analysis also showed that poor adherence for iron and folic acid supplementation during pregnancy and having low diet are the nutritional factor that were significantly associated with post-partum anemia. Mothers who had poor adherence for iron and folic acid supplementation are also 2.8 times more likely to be anemic than their counter parts (OR = 2.8 CI:2.311,3.297).Women with a low level of dietary diversity were 2.45 times more likely to suffer from anemia than those with a minimum level of dietary diversity (OR = 2.45 CI :1.214–3.703) (Table 5 ).
This systematic review and meta-analysis compiled evidence on the prevalence and factors associated with anemia among postpartum women in Ethiopia in the year 2024.
The pooled prevalence of anemia among post-partum women in Ethiopia was found to be 69%. This discovery exceeds the results of a study carried out by the Ethiopian Demographic and Health Survey (EDHS), which reported a 28.6% prevalence of anemia among postpartum women in the country in 2016 [ 27 ]. This could be linked to the comparatively elevated prevalence of infectious diseases and the presence of vulnerable health institutions [ 27 ] and this finding is also higher than studies conducted in Germany(22%) [ 28 ], Kenya(16.4%) [ 29 ]. The variation observed may be attributed to differences in the timing of the studies and certain Sociodemographic factors such as age and educational attainment. Additionally, such discrepancies could arise from variations in sample sizes and the utilization of different time frames for postpartum anemia assessment [ 27 ].
Subgroup analysis based on place of study indicated that significantly higher (72.5%) prevalence of anemia in Eastern Ethiopia (Harer and Dire dawa) compared with 64.6% in studies conducted in Western Ethiopia (Gondar and Debremarkos). The disparities in anemia prevalence across the regions may be linked to regional variations in food consumption preferences [ 30 , 31 ], the rate of infectious disease occurrence [ 31 ] and variation in healthcare services accessibility [ 27 ]. The differences across study locations emphasize the significance of disaggregated data for informed policymaking and program development. Context-specific interventions are necessary to address anemia in Ethiopia.
Post-partum women’s educational status was significantly associated with anemia. Women who had formal education had a significantly lower likelihood of developing anemia than educated counter parts. This finding is consistent with other studies from developing countries [ 32 ]. This is because women with higher education are more inclined to utilize healthcare services and have a more diverse diet compared to mothers with lower education levels [ 27 ].
Women who had received the recommend at least four antenatal care visits were found to be less likely to develop anemia during the postpartum period. This could be attributed to the provision of education on nutrition and health, particularly emphasizing the importance of including diverse sources of iron-rich foods to alleviate anemia [ 27 ].
Women who did not receive iron-folate supplementation during their latest pregnancy were susceptible to the onset of postpartum anemia. This finding is in consistent with studies from countries such as Karachi, Pakistan [ 33 ], and India [ 34 ]. One plausible explanation is that iron serves as a vital replenishment for blood loss and tissue growth during pregnancy and childbirth, given the heightened physiological demands and depletion of iron during these phases [ 35 ]. Research suggests that the intake of a minimum of 90 iron-containing tablet supplements during pregnancy has the potential to decrease maternal anemia by as much as 70% [ 36 ]. Another reason could be that women who initially have iron deficiency without anemia early in pregnancy may develop anemia later due to reduced or ineffective erythrocyte production, leading to immediate postpartum anemia [ 37 ].
The odds of developing immediate PPA increased among women who gave birth via cesarean section compared with those who gave birth via SVD. This result aligns with earlier studies conducted in various countries. Such as Jeddah, Saudi Arabia [ 38 ], and Pakistan [ 33 ]and developed countries like Madrid, Spain [ 10 ], and Bursa, Turkey [ 39 ]. One potential explanation is that women who have experienced a cesarean section may be more prone to postpartum hemorrhage (PPH), leading to a reduction in red blood cell (RBC) production and an increase in nutrient losses due to bleeding [ 40 ]. Another proposition might involve uterine atony resulting from prolonged labor, uterine tears, lacerations due to obstructed labor, and retroplacental clot formation following placental abruption. These factors lead to severe bleeding by impeding uterine contraction, and they collectively serve as indications for a cesarean delivery [ 41 ].
Pre delivery hemoglobin levels less than eleven were another independent factor strongly associated with PPA during the immediate postpartum period. This finding is in line with different studies conducted in developed countries such as [ 34 ]Spain [ 10 ], and Tamil Nadu, India [ 34 ]. The potential reasons could include a pre-delivery low hemoglobin (Hgb) level, reduced myometrial contractility, and compromised coagulation due to the impaired transport of Hgb and oxygen to the uterus. This situation leads to tissue enzyme and cellular dysfunction, ultimately resulting in uterine atony, which stands as the most prevalent cause of postpartum hemorrhage (PPH) (53).
The odds of anemia among postpartum mothers who experienced massive postpartum blood loss were 2.49 times higher than the odds of anemia among postnatal mothers who did not develop postpartum haemorrhage. These similar findings were conducted in Saudi Arabia, and Tamil Nadu, India [32, 36].
Mothers who gave birth by instrumental (vacuum or forceps) assisted mode of delivery were almost 3.96 times more likely to be anemic in the postpartum period when compared to those who gave birth through spontaneous vaginal delivery. This finding were consistent with the studies done in Spain (two studies) and Saudi Arabia [ 10 , 37 ]. This could be attributed to the fact that instrumental assisted vaginal delivery heightens the likelihood of episiotomy, spontaneous perineal, and/or cervical tears, which may also extend to the uterus. Clinicians often misdiagnose these tears and perform repairs after significant bleeding occurs in mothers.
Maternal nutritional status was found to be significantly associated with anemia. Women with a low level of dietary diversity were 2.4 times more likely to suffer from anemia than those with a minimum level of dietary diversity. This finding was consistent with a study conducted among lactating women in Jimma District [ 42 ]. This may stem from insufficient dietary intake, resulting in deficiencies of iron, vitamin B12, folate, and vitamin A. Another factor could be a deficiency in protein and foods containing iron [ 43 ].
Implications of the study
This research aimed to ascertain the collective prevalence and factors linked to anemia in postpartum mothers in Ethiopia. The objective was to offer evidence for policymakers and stakeholders, aiding in the development and implementation of evidence-based interventions to address the morbidity and mortality associated with anemia in postpartum women in Ethiopia.
Strength and limitations of the study
Thorough searches were conducted using various strategies, both manual and electronic, to include a range of published and unpublished articles. To mitigate bias, two authors independently extracted data using a predefined tool, and one author performed a quality assessment. Addressing high heterogeneity, subgroup analysis and the random-effects model were employed to calculate the pooled prevalence and odds ratio.
Potential biases, such as inaccurate selection of study participants, small sample sizes in some studies, limitations in data collection and analysis, and selective reporting of results in the included studies, could impact the findings of the meta-analysis. The cross-sectional design of the original studies in this review raises the possibility of confounding variables influencing the estimates. Unexamined confounders may contribute to the heterogeneity observed in the prevalence of anemia among the studies reviewed.
The study indicated that anemia in post-partum women is a major public health problem in Ethiopia. ANC visit less than 4, history of PPH, vaccum/forceps delivery, poor adherence to Iron and folic acid supllemtation during pregnancy, low diet, predelivery hemoglobin less than 11, history of cesearn delivery and lack of eduction were factors associated with higher odds of developing anemia in post-partum women in Ethiopia. Therefore, it is imperative to integrate health education and promote the usage of iron and folate supplements during pregnancy, along with encouraging dietary diversity practices. Additionally, it is crucial to align these interventions with women’s sustained income-generating activities. Preventing anemia in women who undergo cesarean deliveries entails ensuring efficient CS delivery, promoting a positive long-term health outlook after CS, and implementing postoperative monitoring. Hence, focused attention is essential to ensure effective antepartum, intrapartum, and postpartum maternal care.
The government of Ethiopia also needs to monitor and evaluate the implementation and effectiveness of nutrition programs in Ethiopia in order to strengthen, design, and effectively implement comprehensive multi-sectorial community and facility-based interventions like micronutrient supplementation, and nutrition education in order to prevent and reduce anemia morbidity among post-partum women in Ethiopia.
Data availability
All the data included in systematic review and Meta-analysis are available in the main manuscript.
Bambo GM, Kebede SS, Sitotaw C, Shiferaw E, Melku M. Postpartum anemia and its determinant factors among postnatal women in two selected health institutes in Gondar, Northwest Ethiopia: a facility-based, cross-sectional study. Front Med. 2023;10:1105307.
Article Google Scholar
Darmawati D, Syahbandi S, Fitri A, Audina M. Prevalence and risk factors of Iron Deficiency Anemia among Postpartum Women. J Nurs Care. 2020;3(3).
WHO. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. 2011.
WHO. Nutritional anaemias: tools for effective prevention and control. 2017.
N. M. Postpartum anemia I: definition, prevalence, causes, and consequences.. Annals of hematology. 2011;;90(11)::1247.
Rukiya A GO, Machoki J. Determinants of post-partum anaemia–a cross sectional study. Age.. 2015;;14(19)::33–82.
Central Statistical Agency - CSA/Ethiopia I. Ethiopia Demographic and Health Sruvey 2016. Addis Ababa, Ethiopia: CSA and ICF; 2017.
Google Scholar
Ayana GHA, Tessema M, Belay A, Zerfu D, Bekele T et al. Ethiopian National Nutrition Program End-Line Survey. 2015.
Butwick A, McDonnell N. Antepartum and postpartum anemia: a narrative review. Int J Obstet Anesth. 2021;47:102985.
Article CAS PubMed Google Scholar
Medina Garrido C, León J, Romani Vidal A. Maternal anaemia after delivery: prevalence and risk factors. J Obstet Gynaecol. 2018;38(1):55–9.
Article PubMed Google Scholar
Neamin TesfayID1 RT. Alemu Zenebe1, Fitsum WoldeyohannesID2. Critical factors associated with postpartummaternal death in Ethiopia. PLOS ONE; 2022.
Dagmawi C. 2 Fantu Mamo Aragaw, 3Daniel Gashaneh Belay, 4,5Melaku Hunie Asratie, 6Mequanint Melesse Bicha,7Adugnaw Zeleke Alem 8. anemia among lactating and nonlactating women in low-income and middle-income countries: a comparative cross-sectional study. BMJ open. 2022.
Zhao A, Zhang J, Wu W, Wang P, Zhang Y. Postpartum anemia is a neglected public health issue in China: a cross-sectional study. Asia Pac J Clin Nutr. 2019;28(4):793–9.
CAS PubMed Google Scholar
Ethiopia EPHI. Ethiopian emergency Obstetric and Newborn Care (EmONC) Assessment 2016. Federal Ministry of Health Addis Ababa; 2017.
Black REVC, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51.
Rathod S SS, Mahapatra PC, Samal S. Ferric carboxymaltose: A revolution in the treatment of postpartum anemia in Indian women. International Journal of Applied and Basic Medical Research. 2015;;5(1):: 25.
WHO, Guideline. Iron supplementation in postpartum women. 2016.
Haas JD BIT. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship.. The Journal of nutrition 2001;;131(2):676S-90S.
Abu-Ouf NM JM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi medical journal 2015;;36(2)::146.
WHO. Weekly iron–folic acid supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. WHO/NMH/NHD/MNM/092[PubMed]. [21 August 2012)].
Abebaw A, Gudayu TW, Kelkay B. Proportion of Immediate Postpartum Anaemia and Associated factors among postnatal mothers in Northwest Ethiopia: a cross-sectional study. Anemia. 2020;2020:8979740.
Article PubMed PubMed Central Google Scholar
Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–10.
Barker TH, Stone JC, Sears K, Klugar M, Leonardi-Bee J, Tufanaru C, et al. Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process. JBI Evid Synthesis. 2023;21(3):478–93.
Abebaw A, Gudayu TW, Kelkay B. Proportion of immediate postpartum Anaemia and associated factors among postnatal mothers in Northwest Ethiopia: a cross-sectional study. Anemia. 2020;2020.
Bireda A, Mohammed A, Hailu M, Ali K. Magnitude and Factors Associated with Immediate Postpartum Anemia among Singleton postpartum women at Public Hospitals in Dire Dawa Administration, Eastern Ethiopia. 2022.
Abebe GT, Kure MA, Yadeta TA, Roba KT, Amante TD. Immediate postpartum anemia and associated factors among women admitted to maternity ward at public hospitals in Harari Regional State, Eastern Ethiopia: a facility-based cross-sectional study. Front Global Women’s Health. 2022;3:916245.
Csa I. Central statistical agency (CSA)[Ethiopia] and ICF. Ethiopia demographic and health survey, Addis Ababa, Ethiopia and Calverton, Maryland, USA. 2016;1.
Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW. Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reproductive Biology. 2010;150(2):126–31.
Rukiya A, Gachuno O, Machoki J. Determinants of post-partum anaemia–a cross sectional study. J Obstet Gynecol East Cent Afr. 2015;27(1):2–6.
Berhane G, Paulos Z, Tafere K, Tamru S. Foodgrain consumption and calorie intake patterns in Ethiopia. IFPRI Ethiopia Strategy Support Program II (ESSP II) Working Paper. 2011;23:1–17.
Eregata GT, Hailu A, Memirie ST, Norheim OF. Measuring progress towards universal health coverage: national and subnational analysis in Ethiopia. BMJ Global Health. 2019;4(6).
Haidar J. Prevalence of anaemia, deficiencies of iron and folic acid and their determinants in Ethiopian women. J Health Popul Nutr. 2010;28(4):359.
Rabia S, Jalil N, Feroze S, Iqbal M. Frequency and determinants of maternal anaemia in early postpartum period. ANNALS OF ABBASI SHAHEED HOSPITAL AND KARACHI MEDICAL & DENTAL COLLEGE. 2018;23(1):15–20.
Rakesh P, Gopichandran V, Jamkhandi D, Manjunath K, George K, Prasad J. Determinants of postpartum anemia among women from a rural population in southern India. Int J women’s health. 2014:395–400.
Grammatikopoulou MG, Nigdelis MP, Haidich A-B, Kyrezi M, Ntine H, Papaioannou M, et al. Diet Quality and Nutritional Risk based on the FIGO Nutrition Checklist among Greek pregnant women: a cross-sectional routine Antenatal Care Study. Nutrients. 2023;15(9):2019.
Article CAS PubMed PubMed Central Google Scholar
Abraha I, Bonacini MI, Montedori A, Di Renzo GC, Angelozzi P, Micheli M, et al. Oral iron-based interventions for prevention of critical outcomes in pregnancy and postnatal care: an overview and update of systematic reviews. J Evidence‐Based Med. 2019;12(2):155–66.
Auerbach M, Abernathy J, Juul S, Short V, Derman R. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Maternal-Fetal Neonatal Med. 2021;34(6):1002–5.
Mattar G, Alsahafi N, Shami B, Abulkhair S, Alhazmi N, Alsaleh R. Incidence of postpartum anemia among postpartum patients in East Jeddah Hospital. Int J Pharma Bio Sci. 2019;9(2):39–46.
Dündar B, Çakmak BD. The prevalence and analysis of risk factors for postpartum anemia in women without prepartum anemia. Haydarpaşa Numune Train Res Hosp Med J. 2019;59(2):165–70.
Butwick AJ, Walsh EM, Kuzniewicz M, Li SX, Escobar GJ. Patterns and predictors of severe postpartum anemia after C esarean section. Transfusion. 2017;57(1):36–44.
Fawcus S, Moodley J. Postpartum haemorhage associated with caesarean section and caesarean hysterectomy. Best Pract Res Clin Obstet Gynecol. 2013;27(2):233–49.
Alemayehu M, Argaw A, Mariam AG. Factors associated with malnutrition among lactating women in subsistence farming households from Dedo and Seqa-Chekorsa districts, Jimma Zone, 2014. Developing Ctry Stud. 2015;5(21):117–8.
Zhang Y. Anemia among lactating mothers in Kokang, Myanmar. Southeast Asian J Trop Med Public Health. 2016;47:1298–305.
PubMed Google Scholar
Download references
Acknowledgements
We would like to acknowledge the authors who conducted the primary studies.
Have no financial and non-financial support.
Author information
Authors and affiliations.
Health Promotion and Communication Department, School of public health, College of medicine and health sciences, Gondar University, Gondar, Ethiopia
Gebeyehu Lakew, Amlaku Nigussie Yirsaw & Eyob Getachew
Nursing department, college of medicine and health science, Bahir Dar University, Bahir Dar, Ethiopia
Alemshet Yirga Berhie
Department of emergency and critical care nursing, College of Medicine and Health Science, Bahir Dar University, Bahir Dar, Ethiopia
Asnake Gashaw Belayneh
Department of Nutrition, Antsokiya Gemza wereda Health Office, North Shoa, North East, Ethiopia
Solomon Ketema Bogale & Getnet Alemu Andarge
Bati Primary Hospital, Oromia Special Zone, North Shoa, North Central, Ethiopia
Health Promotion and Behavioral science department, school of public health, College of medicine and health science, Bahir Dar University , Bahir Dar, Ethiopia
Eyob Ketema Bogale
You can also search for this author in PubMed Google Scholar
Contributions
GL, EKB, GAA, KS, EG, ANY, AYB, AGB and SKB search and extract the articles, EG, EKB and GAA check the quality of the articles, GL, EKB, GAA, KS, EG, ANY, AYB, AGB and SKB search and extract the articles, GL, EKB and GAA do the analysis part and write the result, GL, EKB, GAA, KS, EG and ANY review the manuscript. GL and EKB revised the manuscript. Finally, all authors gave approval of the version to be published; agreed on the journal to which the article had been submitted; and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Gebeyehu Lakew .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
This section is not applicable because this study is a systematic review and Meta-analysis.
Consent for publication
Not applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lakew, G., Yirsaw, A.N., Berhie, A.Y. et al. Prevalence and associated factors of anemia among postpartum mothers in public health facilities in Ethiopia, 2024: a systematic review and meta-analysis. BMC Pregnancy Childbirth 24 , 327 (2024). https://doi.org/10.1186/s12884-024-06525-9
Download citation
Received : 17 February 2024
Accepted : 16 April 2024
Published : 27 April 2024
DOI : https://doi.org/10.1186/s12884-024-06525-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Postpartum women
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Systematic Review | Definition, Example & Guide. Published on June 15, 2022 by Shaun Turney. Revised on November 20, 2023. ... Systematic review vs. literature review. A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize and ...
Cite this article: Lame, G. (2019) 'Systematic Literature Reviews: An Introduction', in Proceedings of the 22nd International Conference on Engineering Design (ICED19), Delft, The Netherlands, 5-8 August 2019. DOI:10.1017/ ... Define SRs and give an overview of the methodology as used in health sciences, with its processes, its strengths ...
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
A systematic review attempts to gather all available empirical research by using clearly defined, systematic methods to obtain answers to a specific question. A meta-analysis is the statistical process of analyzing and combining results from several similar studies. ... When performing a systematic literature review or meta-analysis, if the ...
Systematic reviews: Structure, form and content. This article aims to provide an overview of the structure, form and content of systematic reviews. It focuses in particular on the literature searching component, and covers systematic database searching techniques, searching for grey literature and the importance of librarian involvement in the ...
Method details Overview. A Systematic Literature Review (SLR) is a research methodology to collect, identify, and critically analyze the available research studies (e.g., articles, conference proceedings, books, dissertations) through a systematic procedure [12].An SLR updates the reader with current literature about a subject [6].The goal is to review critical points of current knowledge on a ...
A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesise all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer. Example: Systematic review. In 2008, Dr Robert Boyle and his colleagues published a systematic review in ...
A comprehensive and systematic assessment of existing literature will avoid unnecessary duplication and provide the most scientific and ethical justification for a new trial. ... Krnic Martinic M, Pieper D, Glatt A, Puljak L (2019) Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and ...
Abstract. This chapter presents the concept of Systematic Literature Review (SLR) and how it differs from the traditional ways of describing and portraying the literature. Moreover, it critically analyzes the common underlying structure among the SLR methods developed over the past years as well as highlights the improvements required.
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
Systematic literature reviews (SRs) are a way of synt hesising scientific evidence to answer a particular. research question in a way that is transparent and reproducible, while seeking to include ...
It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman 1992, Oxman 1993). The key characteristics of a systematic review are: a clearly stated set of objectives with pre-defined eligibility criteria for studies ...
Introduction and background. A literature review provides an important insight into a particular scholarly topic. It compiles published research on a topic, surveys different sources of research, and critically examines these sources [].A literature review may be argumentative, integrative, historical, methodological, systematic, or theoretical, and these approaches may be adopted depending ...
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic (in the scientific literature), then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based ...
Doing a systematic literature review in legal scholarship by Marnix Snel, Janaína de Moraes "This book is indispensable for any beginning legal academic, and a useful guide for even the most experienced researcher. With the rapid proliferation of legal literature, and its increasing globalization, neither memory nor intuition is sufficient to ...
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. The key characteristics of a systematic review are: a clearly defined question with inclusion and exclusion criteria; a rigorous and systematic search of the literature;
2.1.1. Systematic literature review. What is it and when should we use it? Systematic reviews have foremost been developed within medical science as a way to synthesize research findings in a systematic, transparent, and reproducible way and have been referred to as the gold standard among reviews (Davis et al., 2014).Despite all the advantages of this method, its use has not been overly ...
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper.
A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in particular early-stage researchers in the computer-science field. The contribution of the article is the following:•Clearly defined strategies to follow for a ...
Inclusion criteria are clearly defined and applied consistently to all studies. Typically includes factors such as study design, population characteristics, intervention/exposure, outcome measures, and language of publication. ... Overall, while both literature reviews and systematic reviews involve reviewing existing research literature ...
This systematic literature review examined the research on prior knowledge and its activation to ascertain how these terms are defined, what specific techniques have been empirically investigated, and the conditions under which prior knowledge activation facilitated students' comprehension. Fifty-four articles met the inclusion criteria and revealed that the terms prior knowledge and prior ...
The present study comprises two distinct but interconnected procedures. First, a systematic literature review was conducted following the PRISMA method ( []; Additional file 1; Additional file 2) with the aim of collecting all validations of the P-CAT that have been developed.Second, a systematic description of the validity evidence for each of the P-CAT validations found in the systematic ...
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ]
This systematic review examines the existing trade-off analysis literature in terms of methods, spatial scale, farming system, indicators and other analytical features. ... we sought to define how ...
This systematic literature review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method (Moher et al., Citation 2010). A literature search was carried out in June 2023. To cover a wide range of articles that were published recently, the review sought to investigate studies published in the last ten years.
A systematic literature review design was used in this study following the guidelines of Paul and ... and Google Scholar. The relevance was determined by deciding whether articles fit the used keywords. The defined keywords for the search were 'Organizational', 'business', and 'work'. These terms were cross-referenced with the term ...
Our systematic review assembles an inaugural comprehensive corpus of cross-border commuting literature. It reveals three transversal key topics (transport-oriented topic, qualitative approaches versus a lack of quantitative data, and a large majority of European papers) and four sub-topics (patterns, determinants, impacts and policies).
2.1 Literature review. Articles for this systematic review were identified utilizing a Pubmed search incorporating PRISMA guidelines . Given the wide breadth of the topic and the limited number of relevant articles published prior to 2013, the search was limited to peer-reviewed, original research articles published in English from the last 10 ...
Background Postpartum anemia, characterized by hematocrit or hemoglobin levels below the defined cutoff point (< 11gm/dl or hematocrit < 33%), is a prevalent global issue. It serves as an indirect contributor to maternal mortality and morbidity. Mothers in the postpartum period experience diminished quality of life, impaired cognitive function, emotional instability, and an increased risk of ...