Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 November 2020

Translational research is all-encompassing and lets everyone be a researcher
- Eleanor J. Molloy 1 , 2 , 3 , 4 , 5 &
- Cynthia F. Bearer 6 , 7
Pediatric Research volume 90 , pages 2–3 ( 2021 ) Cite this article
2899 Accesses
4 Citations
3 Altmetric
Metrics details
Translational research
Biomedical research can be categorized based on its aim: (1) to determine the underlying nature of things (basic research) and (2) to understand the underlying nature of things in order to be able to improve human health (translational research). There are a diverse number of definitions of translational research, which usually includes an all-encompassing overview of all biomedical research from basic translational science to clinical implementation. 1 The National Center for Advancing Translational Sciences (NCATS’) definition of translation is broad and inclusive: “translation is the process of turning observations in the laboratory, clinic and community into interventions that improve the health of individuals and the public—from diagnostics and therapeutics to medical procedures and behavioural changes.” 1 This definition technically applies to much of the research in biomedical sciences ranging from preliminary laboratory-based research to epidemiology and implementation science. The core message is that research with the aim of improving health can ultimately be translated from the bench to patient care. 2 We therefore realized in Pediatric Research that defining a subset of articles as a category termed translational research was incorrect and removed this term. Instead, all articles published in Pediatric Research represent translational research and categorized based on methodology: basic translational research, clinical translational research, or population translational research, with translational research as the overriding theme of the journal (Fig. 1 ).
Adapted from image by Macmillan Publishers Ltd. Nature Medicine (Blumberg et al., copyright 2012).
Everyone is a researcher
There is a huge value for all healthcare personnel and families to participate in research. Although career development is another important motivation, the advantages are the ability to cope with rejection and there is increasing evidence that being research-active avoids burnout and builds resilience. 3 , 4 It is postulated that research develops a deep sense of purpose of oneself and allows one the chance to change and improve outcomes. Everyone can be involved in research, either in their daily clinical practice including quality improvement or to laboratory-based or epidemiologic research. Translational research from basic to epidemiologic has been the basis of the seven great advances in pediatrics, including: immunizations to prevent disease, reducing sudden infant death, cure for acute lymphoblastic leukemia, surfactant therapy for premature infants, preventing vertical HIV transmission, improving life expectancy for children with sickle cell anemia and cystic fibrosis, and saving lives with car seats and seat belts. 5
Cheng et al. 6 have also suggested that the future of pediatric research advancements, including new immunizations, cancer immunotherapy, genomics discoveries, identification of early antecedents of adult health, impact of specific social–environmental influences on biology and health, quality improvement science, and implementation and dissemination research to reduce global poverty, would be based on translational research. Further research and programmatic investment early in the life course is essential.
Children and family involvement in research
There are many motivations for families to participate in research. Healthcare workers are predominantly altruistic to get evidence-based answers for clinical applications that can improve outcomes for children and families. 7 Patients’ and public involvement (PPI) is a way of involving anyone not professionally interested or experienced in health care into research. The engagement of patients and the public into research is termed participatory research.
Upholding the rights of the child enshrined in the United Nation’s Convention on the Rights of the Child (UN CRC), which states that all children have the right to an opinion and to express their views. They all should have the right to be informed and give their opinion about the world around them. 8
Developing PPI using resources, such as medical conferences, publishing, editorial, and research boards, are vital. The James Lind alliance is an organization with the idea of bringing together clinicians, patients, and carers to discuss research priorities and to develop the top 10 research priorities ( http://www.jla.nihr.ac.uk/ ). Initiatives for children to lead research projects have been developed. This research projects also increase awareness about the benefits of research and clinical trials. Child-led research has several prominent examples including RCTS by children ( https://www.hrb-tmrn.ie/public-engagement/start-competition/ ) to develop better quality evidence, and more applicable and impactful research. In these PPI, children are involved in all aspects of the project from its inception to publication and implementation and are a source of empowerment.
Developing research capacity
In Pediatric Research , we have started a series of editorials on career development at all stages from early career investigator to senior roles. Training for research is vital. National and international resources are available. For example, in the UK the academic toolkit ( https://academictoolkit.org/ ) has been developed in conjunction with the Royal College of Pediatrics and Child Health (RCPCH) for pediatricians in training. There are multiple resources for families and patients who want to be involved in PPI, including training courses ( http://www.icphr.org/ppiphr-courses.html ) and the RCPCH patient research charter. 9 Improvement in research infrastructure and training were noted by the RCPCH in their document “Turning the Tide 5 years on,” but they noted more development was needed (Turing the Tide 5 years on… https://www.rcpch.ac.uk/sites/default/files/2018-03/turning_the_tide_-_five_years_on_2018-03.pdf ).
Database development, artificial intelligence, and big data will be major resources when combined with new discovery and human capacity in research. The concept that all healthcare professionals are researchers helps to ensure the integration of research in health care. Healthcare professionals have a privileged position to improve family outcomes through translational research and the engagement of all stakeholders.
Austin, C. P. Translation translation. Nat. Rev. Drug Discov. 17 , 455–456 (2018).
Article CAS Google Scholar
Rubio, D. M. et al. Defining translational research: implications for training. Acad. Med. 85 , 470–475 (2010).
Article Google Scholar
Halliday, L., Walker, A., Vig, S., Hines, J. & Brecknell, J. Grit and burnout in UK doctors: a cross-sectional study across specialties and stages of training. Postgrad. Med. J. 93 , 389–394 (2017).
Galaiya, R., Kinross, J. & Arulampalam, T. Factors associated with burnout syndrome in surgeons: a systematic review. Ann. R. Coll. Surg. Engl. 102 , 401–407 (2020).
Cheng, T. L., Bogue, C. W. & Dover, G. J. The next 7 great achievements in Pediatric Research. Pediatrics 139 , e20163803 (2017).
Cheng, T. L. et al. Seven great achievements in pediatric research in the past 40 y. Pediatr. Res. 80 , 330–337 (2016). pmid:27556199.
Molloy, E. J., Mader, S., Modi, N. & Gale, C. Parent, child and public involvement in child health research: core value not just an optional extra. Pediatr. Res. 85 , 2–3 (2019).
Molloy, E. J. Dr Janusz Korczak: paediatrician, children’s advocate and hero. Pediatr. Res. 86 , 783–784 (2019).
Hunter, L. et al. Advancing child health research in the UK: the Royal College of Paediatrics and Child Health Infants’ Children’s and Young People’s Research Charter. Arch. Dis. Child. 102 , 299–300 (2017).
Download references
Acknowledgements
This work was supported by National Children’s Research Centre, Crumlin, Dublin, Ireland and Health Research Board Ireland (to E.J.M.) and NIH/NICHD P01HD085928 (to C.F.B.).
Author information
Authors and affiliations.
Discipline of Paediatrics, Trinity College, The University of Dublin, Dublin, Ireland
- Eleanor J. Molloy
Children’s Health Hospital (CHI) at Tallaght, Tallaght University Hospital, Dublin, Ireland
Trinity Translational Medicine Institute (TTMI), St. James Hospital and Trinity Research in Childhood Centre (TriCC), Dublin, Ireland
Department of Neonatology, CHI at Crumlin, Dublin, Ireland
Department of Paediatrics, Coombe Women’s and Infant’s University Hospital, Dublin, Ireland
Department of Pediatrics, Division of Neonatology, Rainbow Babies and Children’s Hospital, Cleveland, OH, USA
Cynthia F. Bearer
Case Western Reserve University School of Medicine, Cleveland, OH, USA
You can also search for this author in PubMed Google Scholar
Contributions
E.J.M. and C.F.B.: substantial contributions to conception and design, revising the article critically for important intellectual content; final approval of the version to be published.
Corresponding author
Correspondence to Eleanor J. Molloy .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Molloy, E.J., Bearer, C.F. Translational research is all-encompassing and lets everyone be a researcher. Pediatr Res 90 , 2–3 (2021). https://doi.org/10.1038/s41390-020-01225-4
Download citation
Received : 15 September 2020
Accepted : 15 September 2020
Published : 02 November 2020
Issue Date : July 2021
DOI : https://doi.org/10.1038/s41390-020-01225-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Multisystem inflammatory syndrome in children (mis-c) and neonates (mis-n) associated with covid-19: optimizing definition and management.
- Natasha Nakra
- Satyan Lakshminrusimha
Pediatric Research (2023)
Doing a PhD: ten golden rules
- E. J. Molloy
- C. F. Bearer
Pediatric Research and COVID-19: the changed landscape
- C. B. Bearer
Pediatric Research (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

What Is the Translational Gap and How Do You Close It?
Drug discovery has a translatability problem.
The National Institutes of Health (NIH) once famously reported that for every drug that receives FDA approval, another 1,000 fail. In drug development, translatability refers to the basic principle of taking scientific findings from a laboratory setting and successfully translating them as therapeutic patient treatments in a clinical setting. This is sometimes also referred to as the bench-to-bedside process.
But the drug discovery landscape is notoriously difficult to navigate and translatability can be elusive; pre-clinical failure rates for novel therapies are at around ninety percent, with an average time-to-market of 10-15 years and costs ticking upwards of US $2.5 billion .
As a result, the entire biopharmaceutical industry is under increasing pressure to transform drug discovery, particularly its slow and costly R&D processes, in order to find novel ways to close the translational gap – what’s been termed the “valley of death” in drug development.
What is the translational gap?
The translational gap in drug development refers to the routine failure of the bench-to-bedside process – literally the lack of successful drug candidates that make it all the way through discovery and research to clinical implementation in patients.
What is translational medicine?
Translational medicine, sometimes also called translational science, is a multidisciplinary field of investigation that seeks to improve outcomes where they matter most: for patients. The goal of translational medicine is to find novel and effective ways to move basic scientific discoveries into real-world applications and practices with greater speed and efficiency – ultimately improving prevention, diagnosis and therapies.
Why do we need translational medicine?
Translational medicine is helping to bring more efficiency and predictability to diagnostics, therapeutics and medical procedures. The last decade has seen immense progress across a range of translational areas, particularly inside drug development where
AI-powered applications like deep learning, machine learning and natural language processing are being used to help close the translational gap, advancing to trial the drug candidates that have the best chance of success. These AI technologies can detect patterns and find meaningful relationships faster than conventional methods, giving them the ability to sort millions of potential molecular compounds, looking for candidates with the right properties to move forward in early lab experiments.
AI-enabled translational medicine can also help streamline trial design, improve drug efficacy and safety, accelerate speed-to-market, and significantly cut costs and reduce waste. This translational approach is helping biopharmaceutical companies mitigate their risk and scale their operations, using AI techniques that go far beyond the powers of standard modeling to prioritize the experiments that show the most promise. In this way, translational medicine is helping produce better, more targeted therapies that can reach more patients in need.
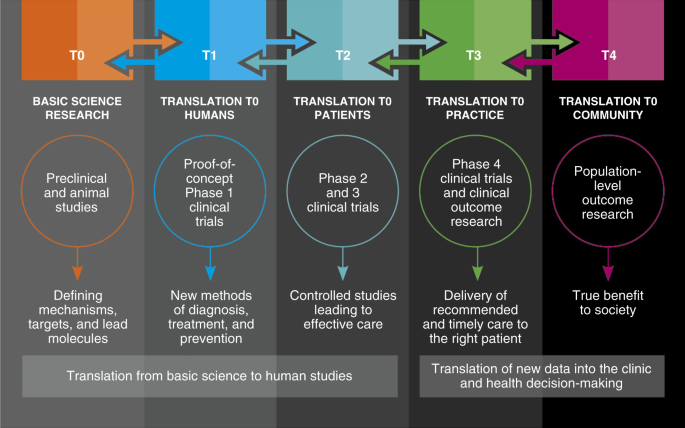
What are the 3 types of translational research?
There are 3 primary types of translational research, which fall into the following broad categories: benchside, bedside, and community. These translational research types are further classified according to their phase of translation .
Benchside
- T0: Basic science research (pre-clinical or animal studies) This translational research tests findings from basic research for clinical significance or impact, and yields knowledge about potential human interventions.
- T1: Translation to humans (phase 1 clinical trials) This translational research tests new biomedical interventions in a small group of people to determine efficacy and safety (i.e. safe dosage; side effects).
- T2: Translation to patients (phases 2 & 3 of clinical trials) This translational research tests new interventions in broader groups of people in controlled environments in order to provide the foundation for clinical application and evidence-based guidelines.
- T3: Translation to practice (phase 4 & clinical outcomes research) This translational research finds different ways of applying recommendations or guidelines in general practice, yielding insights about how interventions are working in real-world settings.
- T4: Translation to community (phase 5 & population-level outcomes research) This translational research looks at factors and interventions that influence the health of populations.
7 Principles for Closing the Translational Gap
According to the National Center for Advancing Translational Sciences (NCATS) , translational medicine relies on these 7 scientific and operational principles, which can be used as tips to help guide researchers and drug developers who are working to improve translatability.
- Prioritize initiatives the address unmet needs Look to pursue initiatives that will contribute new research or solutions in under-investigated areas, like currently untreatable diseases.
- Produce crosscutting solutions for common and persistent challenges Address challenges through generalizable solutions, particularly those that can reach across multiple, disparate research areas, diseases and conditions. For example, problem solving for common R&D roadblocks.
- Emphasize creativity and innovation Increase the impact of your research by investing in innovations that can amplify and transform entrenched research methods and processes. AI-enabled technologies, for example, have demonstrated their ability to accelerate discovery through optimization of traditional screening systems, also serving to reduce late-stage drug attrition.
- Leverage cross-disciplinary team science Pool expertise across disciplines to produce more comprehensive, holistic research that not only aids translatability, but which also produces applications with more real-world relevance and potential.
- Enhance the efficiency and speed of translational research Accelerate the pace of research by improving and streamlining essential work and operational functions. For example, enabling rapid redirection of resources after a failure is detected.
- Utilize boundary-crossing partnerships Use cross-disciplinary research teams and cross-agency partnerships to advance translation. For example, incentivizing collaboration or designing science policy and/or funding opportunities that support cross-disciplinary relationships.
- Use bold and rigorous research approaches Embrace risk-taking and challenge the traditional research ecosystem by developing ambitious approaches that address even the most complex problems in translational research.
Closing the translational gap in drug R&D will mean developing solutions that directly address its most long standing roadblocks. While very real progress has been made toward closing the translational gap using AI-based tools, barriers remain, particularly when it comes to clinical implementation. However, as innovations in AI tech continues to transform translational medicine into a more predictive science, we can expect to see more novel therapies making it from bench to bedside.
VeriSIM Life’s BIOiSIM platform and unique Translational Index™️ technology are helping close the translational gap in drug R&D. Contact us to find out how, and check out our publications for peer-reviewed research, white papers and a range of resources on topics related to AI-powered drug development.
Learn more about VeriSIM Life’s BIOiSIM platform and unique Translational Index™️ technology.
Introduction: What Is Translational Research
- First Online: 01 January 2014
Cite this chapter

- Dennis V. Cokkinos MD, FESC 2
1257 Accesses
1 Citations
There are many definitions of Research, Basic, Clinical and Translational Research. A very practical and short definition of Translational Research could be, the application of findings from Basic Research to patient, community and population care and to the advancement of the delivery of health services.
Usually three steps can be defined:
T1 (T for translation): is the transfer of new understanding of disease mechanisms gained in the laboratory into the development of new methods for diagnosis and therapy.
T2 is the translation of results from clinical studies into everyday clinical practice and health decision making.
T3 is the dissemination and implementation of research translation into practice/community/large populations.
Corresponding blocks or impediments are delineated to the successful employment of these steps. A newly introduced concept is the Valley of Death, separating research results from successful innovation-application.
To overcome these problems, the foundation and collaboration of centers able to conduct Translational Research, such as the National Institutes of Health and the National Clinical and Translational Science Award Consortium is important.
The teaching, training, and formation of translational researchers is difficult, varied and a matter of constant effort.
To overcome increasing costs the combination of “wet” –i.e. biological labs with “dry” or computational data is being increasingly employed.
- Basic research
- Valley of death
Translational Research
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Abbreviations
Association for Clinical Research Training
Academic Health Centers
Biomedical Research Foundation Academy of Athens
Clinical and Translational Science Awards
General Clinical Research Centers
National Center for Advancing Translating Services
Translational Research Institute
National Institutes of Health
NSF. Definition of research. National Science Foundation 2007. http://www.Nsf.gov/statistics/randdef/business. cfm .
Oxford English Dictionary. www.oed.com .
Popper KR. Objective knowledge. 2nd ed. Oxford: Clarendon press; 1972. p. 191–205.
Google Scholar
Illing J. Thinking about research: frameworkers, ethics and scholarships. Edinburgh: Association for the Study of Medical Education; 2007. p. 2–37.
Bush V. Science: the endless frontier; a report to the President by Vannevar Bush, Director of the Office of Scientific research and Development. Washington, DC: United states Government Printing Office; 1945. Accessed 13 Nov 2009. Section 3 (the importance of basic research), chapter 3 (science and the public welfare) http://nsf.gov/about/history/nsf50/vbush1945_content.jsp .
Hecker L, Birla RV. Intangible factors leading to success in research: strategy, innovation and leadership. J Cardiovasc Transl Res. 2008;1:85–92.
Article PubMed Google Scholar
Glossary of themes for human subjects protection and inclusion issues based on the 1997 report of the NIH Director’s Panel on clinical research, entry: “clinical research”. http://grants.nih.gov/grants/peer/tree_glossary.pdf . Accessed 13 Nov 2009.
Schteingart DE. Introduction to principles of clinical and translational research. Athens: ESCI Core Course on Clinical Research; 2010.
Seely WE, Grinspoon S. Patient-oriented research: clinical pathophysiology and clinical therapeutics. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. 3–12.
Chapter Google Scholar
Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–47.
Article CAS PubMed Google Scholar
Karp JE, McCaffrey RP. New avenues of translational research in leukemia and lymphoma: outgrowth of a Leukemia Society of America-National Cancer Institute workshop. J Natl Cancer Inst. 1994;86:1196–201.
Feldman AM, Koch WJ, Force TL. Developing strategies to link basic cardiovascular sciences with clinical drug development: another opportunity for translational sciences. Clin Pharmacol Ther. 2007;81:887–92.
Littman BH, Di Mario L, Plebani M, Marincola FM. What’s next in translational medicine? Clin Sci (Lond). 2007;112:217–27.
Article Google Scholar
Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;290:211–3.
Dische S, Saunders M. Translational research–a new entity? Acta Oncol. 2001;40:995–9.
Coller BS. Translational research: forging a new cultural identity. Mt Sinai J Med. 2008;75:478–87.
Article PubMed Central PubMed Google Scholar
Coller BS. Translating from the rivers of Babylon to the coronary bloodstream. J Clin Invest. 2012;122:4293–9.
Article CAS PubMed Central PubMed Google Scholar
Williams DR. Introduction to clinical research. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. xvii–xx.
Williams GH, Robertson D. Clinical and translational science in infrastructure. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. 171–81.
Davis D, Evans M, Jadad A, Perrier L, Rath D, Ryan D, et al. The case for knowledge translation: shortening the journey from evidence to effect. BMJ. 2003;327(7405):33–5.
National Cancer Institute. Translational research working group definition of translational research. http://www.cancer.gov/trwg/TRWG-definition–ND-tr-continuum .
Sung NS, Crowley Jr WF, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–87.
Lauer M, Scarlatos S. Translational research for cardiovascular diseases at the national heart lung and blood institute. Moving from bench to bedside and from bedside to community. Circulation. 2010;121:929–33.
Westfall JM, Mold J, Fagnan L. Practice-based research–“Blue Highways” on the NIH roadmap. JAMA. 2007;297:403–6.
Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532–41.
Waldman SA, Terzic A. Clinical and translational science: from bench-bedside to global village. Clin Transl Sci. 2010;5:254–7.
Mc Garland Rubio D, Schoenbaum E, Lee S, Schteinggard DE, Marantz PR, Anderson KE, et al. Defining translational research: implications for training. Acad Med. 2010;85:470–5.
Koch WJ. Targeting the beta-adrenergic receptor kinase in heart failure. Lecture presented at the 1st international scientific conference on cardiovascular biotechnology: from cell to man. Biomedical Research Foundation of the Academy of Athens, 31 May 2013.
Kieburtz K, Olanow CW. Translational experimental therapeutics: the translation of laboratory-based discovery into disease-related therapy. Mt Sinai J Med. 2007;74:7–14.
Antoniades CH. Bridging the gap between basic science and clinical practice: the role of translational medicine. Lecture presented at the Biomedical Research Foundation Academy of Athens, 31 Oct 2012.
Murry CE, Jennings KA, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Chautard E, Thierry-Mieg N, Ricard-Blum S. Interaction networks: from protein functions to drug discovery. A review. Pathol Biol (Paris). 2009;57:324–33.
Article CAS Google Scholar
Kerner JF, Hall KL. Research dissemination and diffusion. Res Soc Work Practice 2009;19:519–30.
Zucker DR. What is needed to promote translational research and how do we get it? J Investig Med. 2009;57:468–70.
PubMed Google Scholar
Bernard GR. Preparedness of the CTSA’s structural and scientific assets to support the mission of the National Center for Advancing Translational Sciences (NCATS). Clin Transl Sci. 2012;5:121–9.
Hartmann KE, Heitman E, Brown NJ. Training basic, clinical and translational investigators. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. 191–9.
Williams GH, Robertson D. The future of clinical research. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. 565–70.
Nadler LM, Roberts WC. Lee Marshall Nadler, MD: a conversation with the editor. Proc Baylor Univ Med Cent. 2007;20:381–9.
Verkoeijen PPJL, Tabbers HK. Good research requires productive theories and guidelines. Med Educ. 2013;47:858–65.
Pang T, Terry RF, The PLoS Medicine Editors WHO/PLoS Collection. No health without research: a call for papers. PLoS Med. 2011;8:4 e1001008.
White KL, Williams TF, Greenberg BG. The ecology of medical care. N Engl J Med. 1961;265:885–92.
Green LA, Fryer Jr GE, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344:2021–5.
Martin SS, Ou FS, Newby LK, Sutton V, Adams P, Felker GM, Wang TY. Patient- and trial-specific barriers to participation in cardiovascular randomized clinical trials. J Am Coll Cardiol. 2013;61:762–9.
Brutsaert DL. Heart failure: Quo Vadis. Lecture presented at cardiovascular biotechnology: from cell to man. Biomedical Research Foundation Academy of Athens, 31 May–1 June 2013.
Califf RM. Clinical trials. In: Robertson D, Williams GH, editors. Clinical and translational science. 1st ed. London: Elsevier; 2009. p. 13–37.
Welke KF, Ferguson Jr TB, Cooms LP, Dokholygan RS, Murray CJ, Sehrader MI, et al. Validity of the society of thoracic surgeons national adult cardiac surgery database. Ann Thorac Surg. 2004;77:1137–9.
Moss AJ, Francis CW, Rayan D. Collaborative clinical trials. N Engl J Med. 2011;364:789791.
Sipido KR, Casadei B, Holvoet P, Janssens S, Luttun A, Sampaolesi M. Bedside to bench: a look at experimental research with a clinical trial checklist. Cardiovasc Res. 2014;101:1–3.
Kaul S, Diamond GA. Trial and error. How to avoid commonly encountered limitations of published clinical trials. J Am Coll Cardiol. 2010;55:415–27.
Balas EA, Boren SA. In: Balas EA, editor. Yearbook of medical informatics: managing clinical knowledge for health care improvement. Stuttgart: Shattauer Verlagsgesellschaft mbH; 2000, p. 65–70.
Contopoulos-Ioannidis DG, Alexiou GA, Grouvias TC, Ioannidis JPA. Life circle of translational research for medical interventions. Science. 2008;321:1298–9.
Hudson J, Khazhragui HF. Into the valley of death; research to innovation. Drug Discov Today. 2012;18:610–3.
Butler D. Translational research; crossing the valley of death. Nature. 2008;453:840–2.
Roberts SF, Fischhoff MA, Sakowski SA, Feldman EL. Perspective: transforming science into medicine: how clinician-scientists can build bridges across research’s “valley of death”. Acad Med. 2012;87:266–70.
Coller BS, Califf RM. Traversing the valley of death: a guide to assessing prospects for translational success. Sci Transl Med. 2009;1:10cm9. doi: 10.1126/scitranslmed.3000265 .
Bodi V, Marrachelli VG, Husser O, Chorro FJ, Viña JR, Monleon D. Metabolomics in the diagnosis of acute myocardial ischemia. J Cardiovasc Trans Res. 2013;6:808–15.
Moses 3rd H, Dorsey ER, Matheson DH, Their SO. Financial anatomy of biomedical research. JAMA. 2005;294:1333–42.
Quinn T, Kohl P. Combining wet and dry research: experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc Res. 2013;97:601–11.
The Royal Swedish Academy of Science. press release: pressmeddecande, 9 october 2013. Nobel prize in Chemistry. 2013.
Curry SH. Translational science: past, present, and future. Bio Tech. 2008;44:Pii–vii.
Braunwald E. Cardiovascular science: opportunities for translating research into improved care. J Clin Invest. 2013;123:6–10.
Stamler JS, Taber RL, Califf RM. Translation of academic discovery into societal benefit: proposal for a balanced approach–part 1. Am J Med. 2003;115:596–9.
Download references
Author information
Authors and affiliations.
Heart and Vessel Department, Biomedical Research Foundation, Academy of Athens, Athens, Greece
Dennis V. Cokkinos MD, FESC
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Dennis V. Cokkinos MD, FESC .
Editor information
Editors and affiliations.
Academy of Athens, Biomedical Research Foundation, Athens, Greece
Dennis V. Cokkinos
Rights and permissions
Reprints and permissions

Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cokkinos, D.V. (2015). Introduction: What Is Translational Research. In: Cokkinos, D. (eds) Introduction to Translational Cardiovascular Research. Springer, Cham. https://doi.org/10.1007/978-3-319-08798-6_1
Download citation
DOI : https://doi.org/10.1007/978-3-319-08798-6_1
Published : 22 September 2014
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-08797-9
Online ISBN : 978-3-319-08798-6
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: translational approaches to combat emerging viral infections: diagnosis, immunopathogenesis, and therapeutics.

- 1 Department of Applied Biology, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad, Telangana, India
- 2 Allergy Immunology and Cell Biology Unit, Department of Immunology and Molecular Medicine, University of Sri Jayewardenepura, Nugegoda, Sri Lanka
- 3 MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom
Editorial on the Research Topic Translational approaches to combat emerging viral infections: diagnosis, immunopathogenesis, and therapeutics
Several novel viral infections have caused global outbreaks in the past. However, the recent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) developed as a pandemic, disrupting global human health and affected the economy. The SARS-CoV-2 has been reported to show continuous changes in the genetic code, due to rapid mutations, giving rise to many variants of concern (VOCs). Due to mutations within primer binding site, diagnosing these variants from the clinical samples of SARS-CoV-2 infected patients has also been challenging. Understanding the immunopathogenesis of the emerging SARS-CoV-2 variants is crucial development of preventive or therapeutic strategies. Many novel vaccine candidates were established to combat SARS-CoV-2 infection. However, most of them subsequently demonstrated reduced efficacy to in preventing infection due to the emerging SARS-CoV-2 VOCs, although they have remained effective against severe disease and hospitalizations. A large number of compounds were shown to interfere with the SARS-CoV-2 life cycle or initiate host responses to thereby reducing viral load in the preclinical studies. However, as many such compounds failed to show efficacy in clinical trials, it would be important to carefully validate all novel and repurposed compounds in randomized controlled trials. Evaluating the efficacy of these candidate drugs in the contect of natural infection of SARS-CoV-2 is currently impossible, and not required as the global population have either been naturally infected, vaccinated and in most instances, both. Several neutralizing antibodies to SARS-CoV-2 have been identified, and most of them target the receptor binding domain (RBD), N-terminal domain (NTD), and S2 domain of the SARS-CoV-2 spike protein. Hence, translational approaches targeting those critical gaps might pave the way for the rapid diagnosis and development of therapeutic strategies to combat emerging SARS-CoV- variants.
The US Centers for Disease Control (CDC) and World Health Organization (WHO) release opportune warnings through notices/bulletins on emerging and reemerging infections worldwide. However, the diagnosis, immunopathogenesis, and designing therapeutic strategies for these viral infections were challenging. The themes covered in this Research Topic focused on novel translational research aspects in the diagnosis, immunopathogenesis, and therapeutic designs to combat recent emerging viral diseases. The nomogram model was widely used to represent disease prediction, which explicitly determines the association of a specific disease with its risk factors through a graphical scoring tool. Chen et al. compared the calibration curves obtained through the SARS-CoV-2 infection prediction tool with the actual observations from a SARS-CoV-2 retrospective study. The data from the 6189 vaccinated individuals in the Fujian province of China during the delta variant outbreak in September 2021 were analyzed. The authors developed and validated the nomogram data using univariate and multivariate regression analysis, representing the SARS-CoV-2 breakthrough probability using a calibration plot; however, this study was conducted with a small sample size for the number of SARS-CoV-2 breakthrough cases. This method may be further evaluated in a higher sample size, which would benefit the management of SARS-CoV-2 breakthrough infection. In another study by Zhang et al. , a lung mask-weighted global average pooling-based (GAP-based) deep learning model was developed to differentiate normal and pneumonia cases with the SARS-CoV-2 infection. This machine-learning algorithm for the chest computed tomography (CT) images could diagnose SARS-CoV-2 infection triage with a sensitivity of 96.5% and specificity of 87.88%. This method would benefit a more sensible diagnosis of SARS-CoV-2 triage using chest CT scan results; however, this method is not an applicable quantitative method. The authors also pointed out that respiration and heart motion may affect the accuracy of this learning method.
Metagenomic Next Generation Sequencing (mNGS) was used to diagnose pathogens in patients with fevers of unknown origin (FUO). Song et al. correlated this method with diagnosing positive cases of Epstein-Barr virus (EBV) associated malignancies. In this preliminary study conducted on 29 EBV-positive participants, mNGS displayed 90% sensitivity and 89.5% specificity in identifying the EBV-associated tumors; however, a more detailed evaluation on a larger sample size is necessary before its clinical practice.
The severity of the SARS-CoV-2 pandemic and the higher death toll in developed nations have made a significant impact on the development of prevention and treatment strategies for SARS-CoV-2 infection. Several small molecules targeting the viral proteins (mostly interfering with the viral enzymes) or host proteins (mainly the cell receptors contributing to viral attachment and internalization) were evaluated in preclinical and clinical studies. Azvudine was the first double-target nucleoside drug developed as an anti-SARS CoV-2 agent by China, and the National Medical Products Administration (NMPA) has approved its emergency use to treat SARS-CoV-2 infection based on its efficacy in randomized controlled trials. Mao et al. identified the level of lactate dehydrogenase (LDH) as a predictive marker to evaluate the effectiveness of Azvudine in the treatment of SARS-CoV-2 infection. The authors identified in their retrospective study conducted in the SARS-CoV-2 infected clinical samples treated with or without Azvudine that the higher level of LDH was found to worsen the SARS-CoV-2 progression with other outcome measures. For the first time, this study identified LDH as a disease progression predictor in SARS-CoV-2 infected patients treated with Azvudine; however, more detailed studies with the isoforms of LDH warrant more insights, mainly identifying the source of higher LDH levels. Neutralizing antibodies are another promising treatment strategy that has been evaluated for its efficacy in combating SARS-CoV-2 infection. Bajpai et al. reviewed the recent progress in the novel neutralizing antibodies to combat human coronaviruses, including the recently emerged SARS-CoV-2. The authors concluded that targeting the conserved epitopes on the spike protein may be the best approach for identifying effective neutralizing antibodies against pan-SARS-CoV-2 VOCs.
Overall, this Research Topic discussed the recent translational developments in viral infections, mainly the diagnostic and therapeutic strategies for SARS-CoV-2 infection. More detailed studies on rapid kits and clinical markers may ease the diagnosis. Artificial intelligence-based prediction tools may illustrate the disease progression and severity. The lessons learned from the recent SARS-CoV-2 pandemic may aid in developing translational approaches to combat emerging and reemerging viral infections.
Author contributions
GS: Writing – original draft, Writing – review & editing. GM: Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: viral infection, translational research, diagnosis, immunopathogenesis, therapeutics
Citation: Sreekanth GP and Malavige GN (2024) Editorial: Translational approaches to combat emerging viral infections: diagnosis, immunopathogenesis, and therapeutics. Front. Cell. Infect. Microbiol. 14:1406240. doi: 10.3389/fcimb.2024.1406240
Received: 24 March 2024; Accepted: 26 March 2024; Published: 05 April 2024.
Edited and Reviewed by:
Copyright © 2024 Sreekanth and Malavige. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gopinathan Pillai Sreekanth, [email protected] ; Gathsaurie Neelika Malavige, [email protected]
This article is part of the Research Topic
Translational Approaches to Combat Emerging Viral Infections: Diagnosis, Immunopathogenesis, and Therapeutics
Projects with Promise
Vol. 28 No. 1
Translational Research Approaches in Land-Grant Institutions: A Case Study of the REDI Movement
- David. A. Julian
- Melissa C. Ross
- Kenyona N. Walker
- Gabrielle C. Johnson
- Ana-Paula Correia
In this case study we explore the concept of translational research: specifically, how common tools were employed in the context of the translational research process to design and implement a formal intervention to address racism at the individual and structural level. This approach to translational research focuses on the implementation of evidence-based interventions to address issues in communities, schools, and other organizations and is ideally suited to support researchers and practitioners in the nation’s land-grant institutions. We discuss the suitability of translational research as an approach to identifying and resolving issues and implications for training and day-to-day operations of translational research organizations. Finally, we point to the necessity of incorporating principles of equity and engagement in the translational research process.
Login Register
Forgot your password?
Filling the Gap: Training the Workforce in Evidence-Based Public Health
For more than two decades, the Prevention Research Center (PRC) at Washington University has been training public-health practitioners from around the world in Evidence-Based Public Health, showing them how to develop programs and policies for communities based on local preferences and proven solutions to public health problems.
In its most recent training at the university’s Knight Center in March, the PRC team welcomed 28 participants from six state health departments and the Federated States of Micronesia.

“Less than 20 percent of public health employees have a degree in public health,” said Ross Brownson , Director of the PRC and the Steven H. and Susan U. Lipstein Distinguished Professor at the Brown School. “Our course, a partnership with the National Association of Chronic Disease Directors, plays an ongoing role building the public health workforce.”
The March training was a three and a half day course attended by people working in day-to-day public health practice in Arkansas, Texas, Rhode Island, Kansas, Missouri, Iowa and Micronesia. Among the leaders attending the training were Nancy Sutton, Chief for the Center for Chronic Care & Disease Management at the Rhode Island Department of Health; and X-ner Luther, Non-Communicable Diseases Section Chief, Federated States of Micronesia Department of Health and Social Affairs.

Participants gave the session high marks.
“This training is not only a place where you improve your skills in addressing the vast challenges of chronic diseases, it’s where you learn to appreciate perspectives from a diverse team of experts in public health,” Luther said. “It enabled me to better understand the importance of having a framework that supports evidence-based decision making. It has equipped me to improve my work to better serve my people in the big ocean state/country of Micronesia.”
Sutton agreed.
“This training was exceptional,” she said. “Both those of us who have been in our careers for over 25 years and those who are newer in their careers benefited from the guidance and tools provided. Staff have already shared how they have applied what was learned to improve their approach in framing policy position papers, completing grant applications, and building relationships internally and external to the department of health.”
Evidence based public health (EBPH) is the development, implementation, and evaluation of effective programs and policies in public health through application of principles of scientific reasoning. The process involves integrating research-based interventions with community preferences to improve the health of populations and health equity. The course has focused primarily on skills for practitioners in chronic disease prevention and control. Through presentations, practice exercises, and case studies, the course takes an applied approach and emphasizes information that is readily available to busy practitioners. A 10-week online format is also available.
Since 1997, the EBPH course has reached nearly 4,000 public health practitioners representing all 50 states, 2 territories, 34 countries, and 4 continents. Findings from evaluations of the course have been published in multiple peer-reviewed journals, and a book based on the course is now in its third edition.
In a 3–5-day in person training, the course focuses on 10 specific skill sets to improve public health practice. In evaluations participants provided after the March training, participants applauded the program and said it provided them with practical guidance to improve their work in a variety of ways, among them:
- Involving people who are impacted or at high risk for specific health issues in decision-making and program planning.
- Ensuring equity is central to evidence-based decision making.
- Developing data briefs about cancer to present to legislators.
- Using data to effectively describe who the disproportionately affected populations are in a given chronic disease/condition in a geographic area and why they are affected.
One participant said the training changed their views about their job dramatically.
“I went in feeling like my work wasn’t all that important,” they said. “Now, I have a different perspective on what I do and how it affects the health and well-being of those in my state.”
For more information about Evidence-based Public Health, see https://prcstl.wustl.edu/items/evidence-based-public-health-training/ . For more information about the National Association of Chronic Disease Directors, go to https://chronicdisease.org .
Bridging the translational research gap: a successful partnership involving a physician and a basic scientist
- PMID: 20463666
- PMCID: PMC3519100
- DOI: 10.1038/jid.2010.65
Publication types
- Biomedical Research / organization & administration
- Biomedical Research / trends
- Clinical Medicine / organization & administration
- Clinical Medicine / trends
- Interdisciplinary Communication
- Medical Laboratory Personnel*
- Physicians*
- Translational Research, Biomedical / organization & administration*
- Translational Research, Biomedical / trends
Grants and funding
- ZIA HG000180-10/ImNIH/Intramural NIH HHS/United States
Advertisement
Translational and Therapeutic Evaluation of RAS-GTP Inhibition by RMC-6236 in RAS-Driven Cancers
L. Jiang and B.J. Maldonato contributed equally to this article.
Cancer Discov 2024;14:1–24

- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Proof April 9 2024
Jingjing Jiang , Lingyan Jiang , Benjamin J. Maldonato , Yingyun Wang , Matthew Holderfield , Ida Aronchik , Ian P. Winters , Zeena Salman , Cristina Blaj , Marie Menard , Jens Brodbeck , Zhe Chen , Xing Wei , Michael J. Rosen , Yevgeniy Gindin , Bianca J. Lee , James W. Evans , Stephanie Chang , Zhican Wang , Kyle J. Seamon , Dylan Parsons , James Cregg , Abby Marquez , Aidan C.A. Tomlinson , Jason K. Yano , John E. Knox , Elsa Quintana , Andrew J. Aguirre , Kathryn C. Arbour , Abby Reed , W. Clay Gustafson , Adrian L. Gill , Elena S. Koltun , David Wildes , Jacqueline A.M. Smith , Zhengping Wang , Mallika Singh; Translational and Therapeutic Evaluation of RAS-GTP Inhibition by RMC-6236 in RAS-Driven Cancers. Cancer Discov 2024; https://doi.org/10.1158/2159-8290.CD-24-0027
Download citation file:
- Ris (Zotero)
- Reference Manager
RAS-driven cancers comprise up to 30% of human cancers. RMC-6236 is a RAS(ON) multi-selective noncovalent inhibitor of the active, GTP-bound state of both mutant and wild-type variants of canonical RAS isoforms with broad therapeutic potential for the aforementioned unmet medical need. RMC-6236 exhibited potent anticancer activity across RAS-addicted cell lines, particularly those harboring mutations at codon 12 of KRAS . Notably, oral administration of RMC-6236 was tolerated in vivo and drove profound tumor regressions across multiple tumor types in a mouse clinical trial with KRAS G12X xenograft models. Translational PK/efficacy and PK/PD modeling predicted that daily doses of 100 mg and 300 mg would achieve tumor control and objective responses, respectively, in patients with RAS-driven tumors. Consistent with this, we describe here objective responses in two patients (at 300 mg daily) with advanced KRAS G12X lung and pancreatic adenocarcinoma, respectively, demonstrating the initial activity of RMC-6236 in an ongoing phase I/Ib clinical trial (NCT05379985).
The discovery of RMC-6236 enables the first-ever therapeutic evaluation of targeted and concurrent inhibition of canonical mutant and wild-type RAS-GTP in RAS-driven cancers. We demonstrate that broad-spectrum RAS-GTP inhibition is tolerable at exposures that induce profound tumor regressions in preclinical models of, and in patients with, such tumors.
Article PDF first page preview
Supplementary data.
RMC-6236 synthetic route and detailed PK/PD/Efficacy Modeling method
Supplementary Table S1 shows information on the PRISM cell set comprised of 845 cell lines representing more than 45 lineages.
Supplementary Table S2 shows cell panel screening data with a panel of 78 cancer cell lines harboring mutant and wild-type RAS selected for screening at Crown Bioscience.
Supplementary Table S3 shows PK and/or PD data of RMC-6236 in subcutaneous xenograft tumors, intracranially implanted xenograft tumors, normal brain from naïve mice, and ear skin and colon from xenograft tumor bearing mice.
Supplementary Table S4 shows % mean tumor volume change, % mean body weight change of RMC-6236 treatment group on mRECIST response calling date; and PPIA mRNA expression levels of each xenograft model in RMC-6236 mouse clinical trial.
Supplementary Table S5 shows time to tumor doubling of individual animals in RMC-6236 mouse clinical trial.
Supplementary Table S6 shows PK/PD data used to derive PK/PD relationship curves in Fig. 5 and Fig. S5.
Supplementary Table S7 showes final processing and refinement statistics used for RMC-6236 Crystallography data collection and refinement.
Supplementary Table S8 shows all parameter estimates for PK/Efficacy and PK/PD modeling.
Supplementary Table S9 shows datasets used for PK/Efficacy and PK/PD modeling.
Supplementary Figure 1 shows RMC-6236 crystal structure in tri-complex, as well as biophysical and cellular potencies of RMC-6236 by genotype. Supplementary Figure 2 shows RMC-6236 demonstrates dose-dependent anti-tumor activities at tolerable doses; and pharmacodynamic effects on RAS signaling in NCI-H441 xenograft tumors as assessed by IHC, and in relatively refractory KP-4 and NCI-H2122 xenograft tumors as assessed by human DUSP6 mRNA expression in vivo. Supplementary Figure 3 shows genotype dependent response of RMC-6236 across NSCLC, PDAC and CRC; and potential modifiers to the durability of response of KRASG12C NSCLC models upon RMC-6236 treatment assessed by Kaplan-Meier analyses. Supplementary Figure 4 shows Efficacy of RMC-6236 on KrasG12C–driven autochthonous lung tumors harboring cis second-site mutations within KrasG12C (KrasG12C,H95D or KrasG12C,Y96C) and eCT26 (KrasG12D/G12D) syngeneic model in immunocompetent mice; anti-tumor immunity of RMC-6236; and in intracranially implanted NCI-H1373-Luc xenograft model on nude mice. Supplementary Figure 5 shows effects of RMC-6236 mediated pharmacological modulation in KP-4 xenograft tumors and normal colon tissues isolated from xenograft tumor bearing mice. Supplementary Figure 6 shows a graphical representation of the combined mouse PK-Efficacy and PK/PD model.
Citing articles via
Email alerts.
- Online First
- Online ISSN 2159-8290
- Print ISSN 2159-8274
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Transl Vis Sci Technol
- v.9(8); 2020 Jul
What Constitutes Translational Research? Implications for the Scope of Translational Vision Science and Technology
“I have been impressed with the urgency of doing. Knowing is not enough; we must apply.” Leonardo da Vinci
- • The conceptualization of translational research has expanded since the inception of Translational Vision Science and Technology ( TVST ).
- • The expanded scope of translational research necessitates that we likewise expand the scope of the journal.
- ○ T1: Development and validation of animal models, preclinical drug studies, development of clinically relevant technologies, and phase 1 and 2 clinical studies (“bench to bedside” research).
- ○ T2: Phase 3 clinical trials (including comparative effectiveness trials), phase 4 clinical research, and development of clinical guidelines (“bedside to practice” research).
- ○ T3: Research focused on implementation and dissemination of phase 3 and 4 clinical research results (dissemination and implementation research).
- ○ T4: Research focused on outcomes and effectiveness in populations, including assessment of benefit to communities through public health policies and programs, as well as adoption of proven interventions’ best practices in communities (diffusion research), and cost-benefit analyses.
- • This classification scheme is best conceived as a continuum, a natural progression of investigative activity, rather than as a series of clearly defined categories.
- • As a result of the change in scope, the number and diversity of publications accepted by the journal is likely to increase compared with past years.
- • ARVO's commitment to this expanded scope will enable TVST to better represent the diversity of research that is already represented in platform and poster presentations at the annual ARVO meeting.
- • This scope change will enable ARVO to represent the interests of its members and to advance the development and assessment of treatments for blinding diseases worldwide.
Defining Translational Research
TVST was established to provide a venue for multidisciplinary research that bridges the gap between basic research and clinical care. Although the journal's focus may have been unique in the vision science community at the time of its inception, the concept of translational research was not.
In 1945, the Director of the National Science Foundation described the nexus between basic and applied research:
Basic research is performed without thought of practical ends. It results in general knowledge and an understanding of nature and its laws. This general knowledge provides the means of answering a large number of important practical problems, though it may not give a complete specific answer to any one of them. The function of applied research is to provide such complete answers…
One of the peculiarities of basic science is the variety of paths, which lead to productive advance. Many of the most important discoveries have come as a result of experiments undertaken with very different purposes in mind. Statistically it is certain that important and highly useful discoveries will result from some fraction of the undertakings in basic science; but the results of any one particular investigation cannot be predicted with accuracy.
Basic research leads to new knowledge. It provides scientific capital. It creates the fund from which the practical applications of knowledge must be drawn. New products and new processes do not appear full-grown. They are founded on new principles and new conceptions, which in turn are painstakingly developed by research in the purest realms of science.
Today, it is truer than ever that basic research is the pacemaker of technological progress. ( https://www.nsf.gov/about/history/nsf50/vbush1945_content.jsp )
Nonetheless, the distinction between basic and early stage translational research is not always clear, nor is the distinction between late stage translational research and research focused on clinical practice. Some investigators envision translational research as development of laboratory discoveries for clinical application. Public health agencies, however, envision a different role in which translational research establishes the evidence that not only validates the incorporation of these applications into clinical practice but also demonstrates benefit at a population level (vs. the artificial environment of a clinical trial). This ambiguity may underlie the numerous attempts to define translational research. 1 – 6 An emerging consensus of what defines translational research exists, 7 and I believe the ARVO journals should reflect this consensus.
Importance of Translational Research
In one study of 101 very promising claims of scientific discoveries with unambiguous clinical potential published in major science journals between 1979 and 1983, only five resulted in interventions with licensed clinical use by 2003. 8 Estimates of the median time required for new scientific discoveries to result in successfully completed clinical trials or entrance into clinical practice range from 17 to 24 years. 9 , 10 These facts are a matter of great concern to policy makers and have led to significant efforts to identify and resolve the obstacles impeding the translation of basic science discoveries into clinical studies and clinical practice. 1 The purpose of TVST is to highlight translational research in vision science, and in so doing to accelerate our progress toward developing and assessing treatments for blinding diseases. During the past decade, the scope of translational research has been expanded. 1 – 7 TVST should reflect this broader definition of translational research in its published manuscripts.
Current Classification of Translational Research: Phases T1, T2, T3, and T4
Phase T1 translational research involves work that develops concepts and discoveries from basic research through early phase clinical trials in humans. 7 Drug development, some studies of disease mechanisms, including proteomics, genomics, genetics, metabolomics, and animal models, are examples of T1 phase translational research. Other examples include development of diagnostic devices and modalities, application of artificial intelligence to identify ocular or systemic disease using ocular imaging technology, development of treatment technologies (e.g., sustained drug delivery systems), and phase 1 and 2 clinical trials. Phase T1 translational research typically is described as “bench to bedside.”
Phase T2 translational research refers to work that establishes efficacy in humans, as well as clinical guidelines. 7 Phase 3 clinical trials, development of clinical guidelines, and assessment of whether treatments that have proved effective in the highly controlled environment of registration trials are effective in less controlled conditions (external validity) are examples of phase T2 research. Phase 4 clinical studies are an example of T2-phase translational research, as they enable assessment of a drug or treatment's effectiveness in diverse populations, as well as the incidence of infrequent but important off-target effects. 5 Comparative effectiveness trials (e.g., CATT, 11 Protocol T 12 ) are classified as T2 activity by some authors and as T3 phase translational research by others. Phase T2 translational research has been described as “bedside to practice.” 5
Phase T3 translational research refers to work that is focused on implementation and dissemination of phase T2 research results. 7 Phase T3 research involves studies that aim to spread knowledge regarding evidence-based interventions (dissemination research) and integrate interventions into existing programs (implementation research). 6 , 10 Some authorities classify phase 4 clinical trials as phase T3 translational research. 6
Phase T4 translational research is focused on outcomes and effectiveness in populations 7 and involves studies that assess the benefit to communities through public health policies and programs, as well as adoption of proven interventions’ best practices in communities (diffusion research). 13 Cost-benefit analyses, surveillance studies, and program evaluations are examples of T4 phase translational research. 5 Thus epidemiology plays an important role in translational research. 14
Areas of overlap in this classification scheme demonstrate that the different phases of translational research are best conceived as a continuum, a natural progression of investigative activity, rather than as a series of clearly defined categories.
Although translational research as defined in phases T1 through T4 includes a broad spectrum of work, its boundaries are finite. Observational studies, such as case reports or case series, for example, generally would not qualify as translational research. Quality of life studies or comparative treatment studies involving patient cohorts in which there is no adequate control group would have limited translational value due to limitations in study design.
The Virtuous Cycle
The transition from basic to translational research is not unidirectional. The results of phase T3 and T4 translational research can be hypothesis-generating and stimulate additional basic research. Limitations in the clinical effectiveness of anti-vascular endothelial growth factor therapy in patients with the neovascular complications of age-related macular degeneration, for example, have stimulated additional research into the pathophysiology of this condition and resulted in the development of drugs modulating different pathways, and in which preclinical experiments and early phase trials suggest efficacy. 15 , 16
Implications for the Scope of TVST
Currently, TVST emphasizes multidisciplinary research that bridges the gap between basic research and clinical care. The scope includes a broad spectrum of work, for example, refinement of data analysis algorithms to improve in vivo imaging technology, nanoengineering to improve virus-based gene delivery, nanoengineering of artificial extracellular matrices, development of new animal models of human disease, applications of stem cell technology for regenerative medicine, development of surgical technology, results of phase 1 clinical trials, and reverse translational (“bedside to bench”) research. Short updates on new developments and controversies and summaries of symposia are considered on an individual basis.
The conceptualization of translational research has expanded since the inception of TVST . The expanded scope of translational research necessitates that we likewise expand the scope of the journal, while we maintain high standards regarding study design, method of data analysis, and impact in evaluating submitted research. TVST will publish work that fits into phase T1 to T4 translational research, as defined earlier. As a result, the number and diversity of clinical publications accepted by the journal is likely to increase compared with past years. This commitment will enable the journal to better represent the diversity of research that is accepted for platform and poster presentations at the annual ARVO meeting. It will thus better enable ARVO to represent the interests of its members, and to advance the development and assessment of treatments for blinding diseases worldwide.
- Get 7 Days Free
Revolution Medicines Announces Publication on the Discovery of and Translational Research for RMC-6236, an Investigational RAS(ON) Multi-Selective Tri-Complex Inhibitor Designed to Block Full Spectrum of Oncogenic RAS(ON) Proteins
REDWOOD CITY, Calif., April 09, 2024 (GLOBE NEWSWIRE) -- Revolution Medicines, Inc. (Nasdaq: RVMD), a clinical-stage oncology company developing targeted therapies for patients with RAS-addicted cancers, today announced the publication of a peer-reviewed research paper in Cancer Discovery. The scientific paper details the discovery and preclinical to clinical translation for RMC-6236, an investigational RAS(ON) multi-selective inhibitor, and includes exemplary case studies from the current Phase 1/1b clinical trial demonstrating the initial anti-tumor activity of RMC-6236. This original research was led by scientists at Revolution Medicines and conducted in collaboration with researchers from across the U.S. and Europe.
Oncogenic RAS proteins drive up to 30 percent of all human cancers, most notably non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC). RAS G12 mutations, such as G12D, G12V and G12C, predominate in human cancers. Currently approved KRAS-targeted cancer therapies target one particular KRAS mutation, KRAS G12C, in the GDP-bound (OFF) state. The paper describes the discovery of RMC-6236, an oral, multi-selective inhibitor of the active GTP-bound (ON) state of both mutant and wild-type RAS. In preclinical studies, RMC-6236 was effective in inhibiting the growth of RAS-dependent tumor cells, while sparing normal tissues. RMC-6236 was found to be well-tolerated and drove deep and durable tumor regressions across multiple cancer types including NSCLC, PDAC, CRC, gastric and gynecologic cancers, with tumor models dependent on KRAS G12 mutations being particularly sensitive. This benefit was found to extend to models with K/N/HRAS hotspot mutations at G13 and Q61 as well.
The paper also highlights translational pharmacokinetics (PK)/efficacy and PK/pharmacodynamics modeling, which predicted that daily doses of 100 mg and 300 mg would achieve tumor control and objective responses, respectively, in patients with RAS-driven tumors. Consistent with this, case studies from the Phase 1/1b RMC-6236 monotherapy clinical trial are featured, describing two patients with advanced KRAS-G12V NSCLC and KRAS-G12D PDAC, respectively, who were treated with 300 mg of RMC-6236 daily. Each of these two patients achieved a complete response as best response demonstrating the potential anti-tumor activity of RMC-6236.
“The discovery of RMC-6236 allowed for the first-ever therapeutic evaluation of targeted concurrent inhibition of both canonical mutant and wild-type RAS-GTP (RAS(ON)) in RAS-driven cancers. The RMC-6236 clinical data that we have shared not only provide platform validation of our tri-complex inhibitor approach, but also refute the dogma that one could not induce anti-tumor activity by broad inhibition of multiple RAS variants, including wild-type RAS, at doses that would be well tolerated,” said Steve Kelsey, M.D., president, research and development of Revolution Medicines. “The research summarized in our Cancer Discovery paper, combined with the preliminary RMC-6236 clinical data presented in late 2023, provide us and our investigators with the confidence to advance and expand our RMC-6236 clinical development program.”
Revolution Medicines is currently evaluating RMC-6236 as monotherapy in a Phase 1/1b trial in patients with advanced solid tumors harboring G12X, G13X and Q61X mutations ( NCT05379985 ). Following promising preliminary data in this Phase 1/1b study, planning is underway to initiate pivotal studies of RMC-6236 as monotherapy in NSCLC and PDAC. RMC-6236 is also being evaluated in combination with pembrolizumab with or without chemotherapy in patients with advanced RAS-mutated solid tumors ( NCT06162221 ) and in combination with RMC-6291, the company’s investigational RAS(ON) G12C-selective inhibitor, for patients with advanced KRAS G12C-mutated solid tumors ( NCT06128551 ).
Today’s publication coincides with the company’s presentation of RMC-6236 preclinical data and additional clinical case studies during the “KRAS: Broadening the Attack Beyond G12C with Small Molecules and Immuno-Oncology” session today at the American Association for Cancer Research (AACR) Annual Meeting 2024 in San Diego.
The scientific paper can be accessed at the following link: https://doi.org/10.1158/2159-8290.CD-24-0027 .
About Revolution Medicines, Inc.
Revolution Medicines is a clinical-stage oncology company developing novel targeted therapies for RAS-addicted cancers. The company’s R&D pipeline comprises RAS(ON) inhibitors designed to suppress diverse oncogenic variants of RAS proteins, and RAS companion inhibitors for use in combination treatment strategies. The company’s RAS(ON) inhibitors RMC-6236, a RAS(ON) multi-selective inhibitor, RMC-6291, a RAS(ON) G12C-selective inhibitor, and RMC-9805, a RAS(ON) G12D-selective inhibitor, are currently in clinical development. Additional RAS(ON) mutant-selective inhibitors in the company’s development pipeline include RMC-5127 (G12V), RMC-0708 (Q61H) and RMC-8839 (G13C).
Forward Looking Statements This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this press release that are not historical facts may be considered "forward-looking statements," including without limitation statements regarding the potential advantages of Revolution Medicines’ preclinical and preclinical candidates, including the potential efficacy, durability, tolerability and combination potential of RMC-6236; validation of the company’s tri-complex platform; and the company’s RMC-6236 and RMC-6291 development plans. Forward-looking statements are typically, but not always, identified by the use of words such as "may," "will," "would," "believe," "intend," "plan," "anticipate," "estimate," "expect," and other similar terminology indicating future results. Such forward-looking statements are subject to substantial risks and uncertainties that could cause the company’s development programs, future results, performance or achievements to differ materially from those anticipated in the forward-looking statements. Such risks and uncertainties include without limitation risks and uncertainties inherent in the drug development process, including the company’s programs’ early stage of development, the process of designing and conducting preclinical studies and clinical trials, risks that the results of prior preclinical models or studies may not be predictive of future clinical trials, clinical efficacy or other future results, the regulatory approval processes, the timing of regulatory filings, the challenges associated with manufacturing drug products, the company’s ability to successfully establish, protect and defend its intellectual property, other matters that could affect the sufficiency of the company’s capital resources to fund operations, reliance on third parties for manufacturing and development efforts, changes in the competitive landscape, the risk that the wind-down of EQRx, Inc. could take longer than anticipated or result in unexpected costs, and the effects on the company’s business of global events, such as international conflicts or pandemics. For a further description of the risks and uncertainties that could cause actual results to differ from those anticipated in the forward-looking statements, as well as risks relating to the business of Revolution Medicines in general, see Revolution Medicines’ Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2024, and its future periodic reports to be filed with the Securities and Exchange Commission. Except as required by law, Revolution Medicines undertakes no obligation to update any forward-looking statements to reflect new information, events, or circumstances, or to reflect the occurrence of unanticipated events.
Market Updates
Forecasts for march cpi report show more mixed signals on inflation, 5 dirt-cheap stocks to buy in april, markets brief: what fewer rate cuts could mean for stock valuations, will small-cap stocks ever catch up, what’s happening in the markets this week, these stocks led a stealth q1 value rally, top- and bottom-performing stock etfs for the quarter, march jobs report forecasts show still-strong but slowing hiring gains, stock picks, 5 top dividend stocks to buy from the best managers, tesla stock is down 30% in 2024. is it a buy, the best reits to buy, 3 dividend stocks for april 2024, investment opportunities in the biopharma industry, basic material stocks: as sector underperforms, we see strong opportunities, industrial stocks: sector continues to benefit from resilient us economy, but is now overvalued, consumer defensive stocks: brand investments remain key to weathering competitive pressures, sponsor center.
- UB Directory
- Office of the Provost >
- Academic & Administrative Units >
- U.S. health care providers don’t know much about female genital cutting; UB medical students want to change that
U.S. health care providers don’t know much about female genital cutting; UB medical students want to change that

As immigration increases, a student-run group at the Jacobs School aims to fill a concerning gap
By Ellen Goldbaum
Release Date: April 9, 2024

Kim Griswold, MD, professor emerita of family medicine and psychiatry in the Jacobs School, is the founder and faculty advisor of UB's Human Rights Initiative.

BUFFALO, N.Y. – For more than a decade, a medical student group at the University at Buffalo has played a critical role documenting medical evidence of torture in immigrants seeking asylum. Knowledge of the work done by UB’s Human Rights Initiative (HRI) contributes to Buffalo’s reputation as a city where individuals persecuted in their home countries can access the medical documentation they need to pursue asylum in the U.S.
Now these students at the Jacobs School of Medicine and Biomedical Sciences at UB are working to educate providers — and their fellow classmates — about the medical and psychological consequences of female genital cutting (FGC) among those seeking asylum in Buffalo.
The effort comes at a time when an increase in immigration means that more physicians in the U.S. will encounter FGC, but none of the health care professions cover it in their training.
“Everyone lacks knowledge in this topic,” says Alyssa D. Reese of HRI, who will graduate with her MD from the Jacobs School later this month. “But as we see more refugees, we are going to see a lot more of it, especially people in primary care or those delivering babies.”
And if a provider hasn’t been educated about FGC, encountering their first patient with it could be overwhelming. “It could lead to a really poor therapeutic relationship,” she says.
Since her first year in medical school, Reese has been a member of HRI, working to improve knowledge of FGC. HRI has held conferences and grand rounds on FGC.
On April 12, at the Physicians for Human Rights National Student Program conference in Phoenix, Reese will present her FGC projects, including a systematic review of health care provider knowledge of FGC and HRI’s experience with gynecological exams for these clients. She is also developing a handbook about FGC for providers and medical students.
Taboo topic
“We talk about domestic violence and physical abuse, but this is a taboo topic,” says Reese, who conducted the research for her honors thesis.
“As medical students, we are responsible for learning about taboo topics so that we can talk about them in a trauma-informed way and provide trauma-informed care to our patients.”
FGC has been illegal in the U.S. since 1996 and is internationally condemned by the World Health Organization and the United Nations as a human rights violation. Reese knew about it from global advocacy classes she took as an undergraduate at UB.
“But it wasn’t until I heard these stories from the women who experienced it, where it becomes an emotional, raw connection with them,” she says. “From a medical point of view, it’s obviously traumatic and can interfere with intercourse and childbirth, but then you hear about the psychological impacts. Every part of their life can be impacted.”
More than 200 million women
FGC involves the forced partial or total removal of the external genitals of girls and women for religious, cultural or other nonmedical reasons.
The procedure is done throughout the world, but primarily in sub-Saharan Africa, the Middle East and Asia. It has been performed on more than 200 million women living today; it is a leading cause of death among girls and women in those regions.
Performed on females as young as infants and up through the teen years or later by non-medical individuals — often close female relatives — without anesthesia, FGC is seen in the cultures that practice it as a kind of “women’s rite.” It is based on false beliefs that the procedure will increase the male partner’s pleasure, make the girl more desirable for marriage, increase hygiene, preserve virginity or promote fertility.
There is anecdotal evidence that some American girls whose parents are from cultures that practice FGC may be forced to undergo the procedure when they travel to those regions on family vacations.
Acute medical consequences include severe pain, bleeding and loss of consciousness; chronic consequences include urinary tract infections, pain with sex, inability to have intercourse, infertility, pain during menses, clitoral neuroma and abnormal growth of neural and other tissue, in addition to obstetric complications. And because FGC is typically done in non-sterile conditions, there is the risk of serious, even life-threatening, infection.
In some instances, the physical condition can be surgically repaired but the procedure can also cause lifelong psychological consequences, from depression to anxiety and post-traumatic stress disorder.
Affidavits for asylum seekers
HRI collaborates with local groups like Journey’s End Refugee Services, Jericho Road Community Health Center, VIVE and the Erie County Bar Association Volunteer Lawyers Project.
Since 2014, HRI has completed over 250 affidavits based on forensic evaluations for asylum seekers. It began performing gynecological forensic evaluations for survivors of FGC in 2017 and since then has evaluated 15 women who have undergone FGC.
Before meeting with clients, students must attend a scribe training session. They learn about practicing cultural humility when talking to these individuals and how to use trauma-informed language, care and education with them. They learn how to work effectively with an interpreter and that the client may be shackled or come with a guard if they are being held in a detention center.
During client evaluations, under the supervision of UB and Western New York physician volunteers, students act as medical scribes and help write the affidavits that are presented in court.
“These affidavits really do make a difference to our clients,” says Adela Smehlik, a Buffalo-based immigration attorney who addressed the students during a recent HRI conference. “I want you to know how amazing HRI is and how invaluable the group is to the work that we do.”
Once the lawyer requests a forensic evaluation, the client is scheduled for a psychological, gynecologic and/or physical exam performed by one or two clinicians and documented by the medical students. The students write the affidavit for the court, including the chronology of the client’s story, clinical presentation and diagnoses. Clinicians may then be called as an expert witness to testify.
The affidavits that HRI prepares for women who have undergone FGC greatly increase their prospects for asylum.
Witnessing resilience
The HRI students and physicians say it can be hard to communicate how rewarding it is to engage in this work, which can improve the life of someone who has gone through unimaginable pain and despair.
“The clients make an imprint on you, not because of the violence they endured but because of their resilience,” says Kim Griswold, MD, HRI founder, faculty adviser and professor emerita of family medicine and psychiatry in the Jacobs School.
Because they must remain impartial, neither the physicians nor the students can stay in touch with clients once their case has gone to court. But the students say the power of bearing witness to people who have lived through such extraordinary difficulties and can now begin to heal is, in many ways, a life-changing experience. Some say it was among the most meaningful experiences they’ve had in medical school.
“I have grown so much as a future physician talking to these people,” Reese says. “They’re survivors of torture. I am so grateful to hear their stories of resilience. Witnessing their resilience gives me resilience.”
Media Contact Information
Ellen Goldbaum News Content Manager Medicine Tel: 716-645-4605 [email protected]
Featured Centers and Institutes

PhD Excellence Initiative
A campus-wide, student-centric effort to ensure that UB’s PhD programs remain among the strongest in the world.
Recent University News
- 4/9/24 UB Pharmacy School continues sustained leadership with Top 20 U.S. News & World Report ranking
- 4/9/24 UB schools among the best in U.S. News graduate rankings
- 4/9/24 UB School of Social Work is again top 25 in U.S. News & World Report
- 4/9/24 Total eclipse steals UB’s heart
- 4/9/24 Fast track to law school
April 5, 2024 - Notice of Intent to Publish a Notice of Funding Opportunity for ML/AI Tools to Advance Genomic Translational Research (MAGen) - Coordinating Center NOT-HG-24-030
The National Human Genome Research Institute intends to issue a Notice of Funding Opportunity (NOFO) to solicit applications to explore the feasibility of developing Machine Learning (ML) and Artificial Intelligence (AI) tools that can enhance the accuracy and precision of predicting how individuals with pathogenic genetic variants manifest disease. The objective of the NOFO is to collaboratively identify both genomic and non-genomic factors influencing disease development in individuals carrying pathogenic genetic variants within a research consortium, ML/AI Tools to Advance Genomic Translational Research (MAGen). This consortium will include the MAGen Sites funded by the NOFO and MAGen Coordinating Center funded by the companion NOFO (see NOT-HG-24-030). The ML/AI tools will leverage existing multimodal genomic and non-genomic data and will be cross validated in genomic translational research settings to ensure the robustness and generalizability of the tools. In addition, the Consortium will explore the ethical, legal, and social implications (ELSI) of integrating ML/AI tools into genomic medicine through the establishment of an ELSI framework for their development and through implementation of ELSI research projects.
The intended NOFO is based on a concept recently approved by the National Advisory Council on Human Genome Research and accompanying discussion .
This Notice is being provided to allow potential applicants sufficient time to develop collaborations and responsive projects.
The NOFO is expected to be published in Spring 2024 with an expected application due date in Summer 2024.
This NOFO will utilize the UG3/UH3 activity code. Details of the planned NOFO are provided below.
MAGen Sites, with support from the MAGen Coordinating Center, will collaboratively develop the following Consortium-wide resources pertinent to ML/AI tool development and cross validation: an ELSI framework for ML/AI tool development and cross validation for translational research purposes; the consortium’s collective set of human genes with pathogenic variants selected from the list proposed by the MAGen Sites; ML/AI tools' key requirements; a common data model; a cross validation plan; research questions and outcomes that are to be assessed by the ELSI project(s); and a plan for dissemination of the Consortium-generated resources. In accordance with the agreed-upon consortium-wide plans and resources identified above, each MAGen Site will: develop and cross validate tools; conduct ELSI research projects; and generate resources for dissemination.
The MAGen consortium is not focused on any specific disease or condition. However, the proposed genes must have pathogenic variants associated with known human diseases and with variable clinical manifestation.
Important considerations include: (1) funding will not be provided for new data generation; (2) groups need to have sufficient multimodal genomic, other-omic, and non-genomic data (clinical including electronic health records, social determinants of health, environmental, wearable etc.) accessible to them to address a range of diseases to be used for development, validation, and cross validation of ML/AI tools in translational research settings. The MAGen Sites will work collaboratively with each other and the MAGen CC (NOT-HG-24-030) within the Consortium to achieve the goals of the NOFO.
This Notice encourages investigators with multi-disciplinary expertise, including biomedical informatics, data science, genomics, computer science, clinical data management, ELSI research etc., to begin considering applying for this NOFO.
The NOFO will use the UG3/UH3 cooperative agreement mechanism which entails a two-Phase, one-application approach to accomplish the goals.
Funding Information
$4.8 million in FY2025
The maximum award for the UG3 phase is $1.6 million total cost per year for up to two years. The maximum award for the UH3 phase is $1.6 million direct cost per year for up to three years.
Applications are not being solicited at this time.
Please direct all inquiries to:
Sandhya Xirasagar Ph.D. National Human Genome Research Institute (NHGRI) Office of Genomic Data Science Telephone: 240-380-0400 Email: [email protected]

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .

IMAGES
VIDEO
COMMENTS
The translational gap: bridging basic research, clinical practices and society ... industry and tech-transfer experts in four research areas that are bridging the gap between basic science and ...
Introduction. Clinical and translational research (CTR) has been experiencing a resurgence since the mid-2000s along with an embracing of team science within the CTR community [1-4].Around this same time, articles questioning or discussing the validity of published research results began to emerge in academic as well as nonacademic publications [5-7].
As National Institutes of Healthʼs (NIH) mechanism to advance translational research, Clinical and Translational Science Award (CTSA) awardees are uniquely positioned to bridge this gap. ... A primary challenge to addressing the research-policy-practice gap is the lack of sustainable infrastructure bringing researchers, policymakers ...
A growing body of empirical research has begun to address this gap. To date, translational research has been positioned as bridging two seemingly disparate worlds: basic science and clinical medicine, with the former assumed to inform and feed into the latter. ... The first theme, concepts of translational research, identifies a disconnect ...
Instead, all articles published in Pediatric Research represent translational research and categorized based on methodology: basic translational research, clinical translational research, or ...
As National Institutes of Health's (NIH) mechanism to advance translational research, Clinical and Translational Science Award (CTSA) awardees are uniquely positioned to bridge this gap. Delivering on this promise requires sustained collaboration and alignment between research institutions and public health and healthcare programs and services.
Participants included clinicians, scientists, and engineers that brought a wide range of perspectives to the discussion. Key issues raised include the need to better interface fundamental research with the clinic, and how artificial intelligence (AI) can best be incorporated into imaging.
Abstract. The complexity of modern biomedical research continues to increase. Research-to-practice gaps are now widely pervasive; these widening fissures between the disciplines of research and clinical medicine can only be abridged if the translational processes are methodically backed up by robust methods, many of which are yet to be developed.
The terms translational research (TR), translational medicine, and translational science are currently seeing widespread usage in a variety of biomedical and health research fields. Yet, they remain slippery concepts. ... of bringing basic biomedical knowledge findings to bear in clinical contexts in a note called On the Translation Gap. With ...
Abstract. Translational research can be conceptualized within several blocks or spheres of knowledge transfer and focused on closing the gap between new discoveries and their endpoint application to clinical practice, health decision-making, and health policy. Although support for type 1 translational research (the classical bench-to-bedside ...
The evolution of medicine and medical technology hinges on the successful translation of basic science research from the bench to clinical implementation at the bedside. Out of the increasing need to facilitate the transfer of scientific knowledge to patients, translational research has emerged. Significant leaps in improving global health, such as antibiotics, vaccinations, and cancer ...
INTRODUCTION. Translational research can be conceptualized within several blocks or spheres of knowledge transfer and focused on closing the gap between new discoveries and their endpoint application to clinical practice, health decision-making, and health policy. Translational research is an evolving concept across many disciplines in various ...
Gap(s): Majority of OA research has investigated the influence of gait parameters in isolation; however, it is likely that multiple biomechanical variables interplay with each other to characterise movement strategies. ... Optimising translational research opportunities: a systematic review and narrative synthesis of basic and clinician ...
Use bold and rigorous research approaches Embrace risk-taking and challenge the traditional research ecosystem by developing ambitious approaches that address even the most complex problems in translational research. Closing the translational gap in drug R&D will mean developing solutions that directly address its most long standing roadblocks.
Although neither of us had prior experience with a translational research team jointly led by a clinical researcher and a basic scientist, our common enthusiasm propelled us into a high-risk research project that proved to be rewarding and fruitful. A critical issue was learning how to foster a collaboration that promoted translational research.
Dische and Saunders [ 15] mention the definition given by Julie Denekamp: Translational Research is the application of scientific method to address a health need. Barry S. Coller has very simply defined [ 16] it as: Successful implementation of a laboratory concept into a clinical protocol.
The themes covered in this Research Topic focused on novel translational research aspects in the diagnosis, immunopathogenesis, and therapeutic designs to combat recent emerging viral diseases. ... (GAP-based) deep learning model was developed to differentiate normal and pneumonia cases with the SARS-CoV-2 infection. This machine-learning ...
In this case study we explore the concept of translational research: specifically, how common tools were employed in the context of the translational research process to design and implement a formal intervention to address racism at the individual and structural level. This approach to translational research focuses on the implementation of evidence-based interventions to address issues in ...
Alzheimer's & Dementia: Translational Research & Clinical Interventions; Alzheimer's & Dementia: Diagnosis, Assessment and Disease Monitoring; Join ISTAART; ... The Sleep Ancillary Study to AU-ARROW will allow this critical knowledge gap to be addressed. As part of the Sleep Ancillary Study, optional objective sleep assessments will be ...
Mario Mancino, PhD, Translational Research Manager at MEDSIR, expressed enthusiasm: "We are thrilled to present our research at the Annual Meeting of the American Association for Cancer Research. Our commitment to advancing personalized medicine through biomarkers underscores our dedication to improving patient outcomes."
For more than two decades, the Prevention Research Center (PRC) at Washington University has been training public-health practitioners from around the world in Evidence-Based Public Health, showing them how to develop programs and policies for communities based on local preferences and proven solutions to public health problems.. In its most recent training at the university's Knight Center ...
Bridging the translational research gap: a successful partnership involving a physician and a basic scientist. Bridging the translational research gap: a successful partnership involving a physician and a basic scientist J Invest Dermatol. 2010 Jun;130(6):1478-80. doi: 10.1038/jid.2010.65. ...
AbstractRAS-driven cancers comprise up to 30% of human cancers. RMC-6236 is a RAS(ON) multi-selective noncovalent inhibitor of the active, GTP-bound state of both mutant and wild-type variants of canonical RAS isoforms with broad therapeutic potential for the aforementioned unmet medical need. RMC-6236 exhibited potent anticancer activity across RAS-addicted cell lines, particularly those ...
Defining Translational Research. TVST was established to provide a venue for multidisciplinary research that bridges the gap between basic research and clinical care. Although the journal's focus may have been unique in the vision science community at the time of its inception, the concept of translational research was not.
"The research summarized in our Cancer Discovery paper, combined with the preliminary RMC-6236 clinical data presented in late 2023, provide us and our investigators with the confidence to ...
As immigration increases, a student-run group at the Jacobs School aims to fill a concerning gap . By Ellen Goldbaum. Release Date: April 9, 2024 . ... Clinical and Translational Research Center. 2/15/22. Headquartered on the growing Buffalo Niagara Medical Campus and one of only a handful of such buildings in the nation, the state-of-the-art ...
MAGen Sites, with support from the MAGen Coordinating Center, will collaboratively develop the following Consortium-wide resources pertinent to ML/AI tool development and cross validation: an ELSI framework for ML/AI tool development and cross validation for translational research purposes; the consortium's collective set of human genes with pathogenic variants selected from the list ...