Appointments at Mayo Clinic
- Pregnancy week by week
- Fetal presentation before birth
The way a baby is positioned in the uterus just before birth can have a big effect on labor and delivery. This positioning is called fetal presentation.
Babies twist, stretch and tumble quite a bit during pregnancy. Before labor starts, however, they usually come to rest in a way that allows them to be delivered through the birth canal headfirst. This position is called cephalic presentation. But there are other ways a baby may settle just before labor begins.
Following are some of the possible ways a baby may be positioned at the end of pregnancy.

Head down, face down
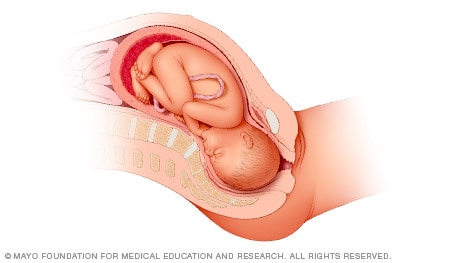
When a baby is head down, face down, the medical term for it is the cephalic occiput anterior position. This the most common position for a baby to be born in. With the face down and turned slightly to the side, the smallest part of the baby's head leads the way through the birth canal. It is the easiest way for a baby to be born.
Head down, face up
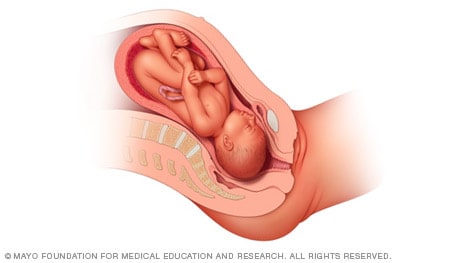
When a baby is head down, face up, the medical term for it is the cephalic occiput posterior position. In this position, it might be harder for a baby's head to go under the pubic bone during delivery. That can make labor take longer.
Most babies who begin labor in this position eventually turn to be face down. If that doesn't happen, and the second stage of labor is taking a long time, a member of the health care team may reach through the vagina to help the baby turn. This is called manual rotation.
In some cases, a baby can be born in the head-down, face-up position. Use of forceps or a vacuum device to help with delivery is more common when a baby is in this position than in the head-down, face-down position. In some cases, a C-section delivery may be needed.
Frank breech
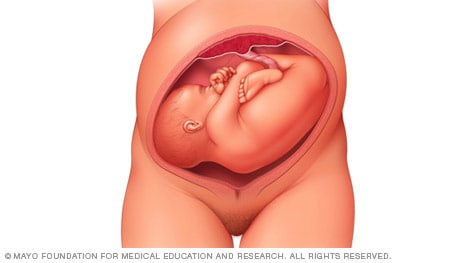
When a baby's feet or buttocks are in place to come out first during birth, it's called a breech presentation. This happens in about 3% to 4% of babies close to the time of birth. The baby shown below is in a frank breech presentation. That's when the knees aren't bent, and the feet are close to the baby's head. This is the most common type of breech presentation.
If you are more than 36 weeks into your pregnancy and your baby is in a frank breech presentation, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. It involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a breech position, talk with a member of your health care team about the choices you have for delivery. Most babies in a frank breech position are born by planned C-section.

Complete and incomplete breech
A complete breech presentation, as shown below, is when the baby has both knees bent and both legs pulled close to the body. In an incomplete breech, one or both of the legs are not pulled close to the body, and one or both of the feet or knees are below the baby's buttocks. If a baby is in either of these positions, you might feel kicking in the lower part of your belly.
If you are more than 36 weeks into your pregnancy and your baby is in a complete or incomplete breech presentation, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. It involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a breech position, talk with a member of your health care team about the choices you have for delivery. Many babies in a complete or incomplete breech position are born by planned C-section.

When a baby is sideways — lying horizontal across the uterus, rather than vertical — it's called a transverse lie. In this position, the baby's back might be:
- Down, with the back facing the birth canal.
- Sideways, with one shoulder pointing toward the birth canal.
- Up, with the hands and feet facing the birth canal.
Although many babies are sideways early in pregnancy, few stay this way when labor begins.
If your baby is in a transverse lie during week 37 of your pregnancy, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. External cephalic version involves one or two members of your health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a transverse lie, talk with a member of your health care team about the choices you have for delivery. Many babies who are in a transverse lie are born by C-section.
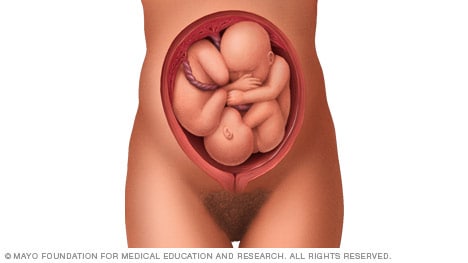
If you're pregnant with twins and only the twin that's lower in the uterus is head down, as shown below, your health care provider may first deliver that baby vaginally.
Then, in some cases, your health care team may suggest delivering the second twin in the breech position. Or they may try to move the second twin into a head-down position. This is done using a procedure called external cephalic version. External cephalic version involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
Your health care team may suggest delivery by C-section for the second twin if:
- An attempt to deliver the baby in the breech position is not successful.
- You do not want to try to have the baby delivered vaginally in the breech position.
- An attempt to move the baby into a head-down position is not successful.
- You do not want to try to move the baby to a head-down position.
In some cases, your health care team may advise that you have both twins delivered by C-section. That might happen if the lower twin is not head down, the second twin has low or high birth weight as compared to the first twin, or if preterm labor starts.
- Landon MB, et al., eds. Normal labor and delivery. In: Gabbe's Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed May 19, 2023.
- Holcroft Argani C, et al. Occiput posterior position. https://www.updtodate.com/contents/search. Accessed May 19, 2023.
- Frequently asked questions: If your baby is breech. American College of Obstetricians and Gynecologists https://www.acog.org/womens-health/faqs/if-your-baby-is-breech. Accessed May 22, 2023.
- Hofmeyr GJ. Overview of breech presentation. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Strauss RA, et al. Transverse fetal lie. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Chasen ST, et al. Twin pregnancy: Labor and delivery. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Cohen R, et al. Is vaginal delivery of a breech second twin safe? A comparison between delivery of vertex and non-vertex second twins. The Journal of Maternal-Fetal & Neonatal Medicine. 2021; doi:10.1080/14767058.2021.2005569.
- Marnach ML (expert opinion). Mayo Clinic. May 31, 2023.
Products and Services
- A Book: Obstetricks
- A Book: Mayo Clinic Guide to a Healthy Pregnancy
- 3rd trimester pregnancy
- Fetal development: The 3rd trimester
- Overdue pregnancy
- Pregnancy due date calculator
- Prenatal care: 3rd trimester
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.
- Getting Pregnant
- Registry Builder
- Baby Products
- Birth Clubs
- See all in Community
- Ovulation Calculator
- How To Get Pregnant
- How To Get Pregnant Fast
- Ovulation Discharge
- Implantation Bleeding
- Ovulation Symptoms
- Pregnancy Symptoms
- Am I Pregnant?
- Pregnancy Tests
- See all in Getting Pregnant
- Due Date Calculator
- Pregnancy Week by Week
- Pregnant Sex
- Weight Gain Tracker
- Signs of Labor
- Morning Sickness
- COVID Vaccine and Pregnancy
- Fetal Weight Chart
- Fetal Development
- Pregnancy Discharge
- Find Out Baby Gender
- Chinese Gender Predictor
- See all in Pregnancy
- Baby Name Generator
- Top Baby Names 2023
- Top Baby Names 2024
- How to Pick a Baby Name
- Most Popular Baby Names
- Baby Names by Letter
- Gender Neutral Names
- Unique Boy Names
- Unique Girl Names
- Top baby names by year
- See all in Baby Names
- Baby Development
- Baby Feeding Guide
- Newborn Sleep
- When Babies Roll Over
- First-Year Baby Costs Calculator
- Postpartum Health
- Baby Poop Chart
- See all in Baby
- Average Weight & Height
- Autism Signs
- Child Growth Chart
- Night Terrors
- Moving from Crib to Bed
- Toddler Feeding Guide
- Potty Training
- Bathing and Grooming
- See all in Toddler
- Height Predictor
- Potty Training: Boys
- Potty training: Girls
- How Much Sleep? (Ages 3+)
- Ready for Preschool?
- Thumb-Sucking
- Gross Motor Skills
- Napping (Ages 2 to 3)
- See all in Child
- Photos: Rashes & Skin Conditions
- Symptom Checker
- Vaccine Scheduler
- Reducing a Fever
- Acetaminophen Dosage Chart
- Constipation in Babies
- Ear Infection Symptoms
- Head Lice 101
- See all in Health
- Second Pregnancy
- Daycare Costs
- Family Finance
- Stay-At-Home Parents
- Breastfeeding Positions
- See all in Family
- Baby Sleep Training
- Preparing For Baby
- My Custom Checklist
- My Registries
- Take the Quiz
- Best Baby Products
- Best Breast Pump
- Best Convertible Car Seat
- Best Infant Car Seat
- Best Baby Bottle
- Best Baby Monitor
- Best Stroller
- Best Diapers
- Best Baby Carrier
- Best Diaper Bag
- Best Highchair
- See all in Baby Products
- Why Pregnant Belly Feels Tight
- Early Signs of Twins
- Teas During Pregnancy
- Baby Head Circumference Chart
- How Many Months Pregnant Am I
- What is a Rainbow Baby
- Braxton Hicks Contractions
- HCG Levels By Week
- When to Take a Pregnancy Test
- Am I Pregnant
- Why is Poop Green
- Can Pregnant Women Eat Shrimp
- Insemination
- UTI During Pregnancy
- Vitamin D Drops
- Best Baby Forumla
- Postpartum Depression
- Low Progesterone During Pregnancy
- Baby Shower
- Baby Shower Games
Breech, posterior, transverse lie: What position is my baby in?

Fetal presentation, or how your baby is situated in your womb at birth, is determined by the body part that's positioned to come out first, and it can affect the way you deliver. At the time of delivery, 97 percent of babies are head-down (cephalic presentation). But there are several other possibilities, including feet or bottom first (breech) as well as sideways (transverse lie) and diagonal (oblique lie).
Fetal presentation and position
During the last trimester of your pregnancy, your provider will check your baby's presentation by feeling your belly to locate the head, bottom, and back. If it's unclear, your provider may do an ultrasound or an internal exam to feel what part of the baby is in your pelvis.
Fetal position refers to whether the baby is facing your spine (anterior position) or facing your belly (posterior position). Fetal position can change often: Your baby may be face up at the beginning of labor and face down at delivery.
Here are the many possibilities for fetal presentation and position in the womb.
Medical illustrations by Jonathan Dimes
Head down, facing down (anterior position)
A baby who is head down and facing your spine is in the anterior position. This is the most common fetal presentation and the easiest position for a vaginal delivery.
This position is also known as "occiput anterior" because the back of your baby's skull (occipital bone) is in the front (anterior) of your pelvis.
Head down, facing up (posterior position)
In the posterior position , your baby is head down and facing your belly. You may also hear it called "sunny-side up" because babies who stay in this position are born facing up. But many babies who are facing up during labor rotate to the easier face down (anterior) position before birth.
Posterior position is formally known as "occiput posterior" because the back of your baby's skull (occipital bone) is in the back (posterior) of your pelvis.
Frank breech
In the frank breech presentation, both the baby's legs are extended so that the feet are up near the face. This is the most common type of breech presentation. Breech babies are difficult to deliver vaginally, so most arrive by c-section .
Some providers will attempt to turn your baby manually to the head down position by applying pressure to your belly. This is called an external cephalic version , and it has a 58 percent success rate for turning breech babies. For more information, see our article on breech birth .
Complete breech
A complete breech is when your baby is bottom down with hips and knees bent in a tuck or cross-legged position. If your baby is in a complete breech, you may feel kicking in your lower abdomen.
Incomplete breech
In an incomplete breech, one of the baby's knees is bent so that the foot is tucked next to the bottom with the other leg extended, positioning that foot closer to the face.
Single footling breech
In the single footling breech presentation, one of the baby's feet is pointed toward your cervix.
Double footling breech
In the double footling breech presentation, both of the baby's feet are pointed toward your cervix.
Transverse lie
In a transverse lie, the baby is lying horizontally in your uterus and may be facing up toward your head or down toward your feet. Babies settle this way less than 1 percent of the time, but it happens more commonly if you're carrying multiples or deliver before your due date.
If your baby stays in a transverse lie until the end of your pregnancy, it can be dangerous for delivery. Your provider will likely schedule a c-section or attempt an external cephalic version , which is highly successful for turning babies in this position.
Oblique lie
In rare cases, your baby may lie diagonally in your uterus, with his rump facing the side of your body at an angle.
Like the transverse lie, this position is more common earlier in pregnancy, and it's likely your provider will intervene if your baby is still in the oblique lie at the end of your third trimester.
Was this article helpful?
What to know if your baby is breech

What's a sunny-side up baby?

What happens to your baby right after birth

Perineal massage

BabyCenter's editorial team is committed to providing the most helpful and trustworthy pregnancy and parenting information in the world. When creating and updating content, we rely on credible sources: respected health organizations, professional groups of doctors and other experts, and published studies in peer-reviewed journals. We believe you should always know the source of the information you're seeing. Learn more about our editorial and medical review policies .
Ahmad A et al. 2014. Association of fetal position at onset of labor and mode of delivery: A prospective cohort study. Ultrasound in obstetrics & gynecology 43(2):176-182. https://www.ncbi.nlm.nih.gov/pubmed/23929533 Opens a new window [Accessed September 2021]
Gray CJ and Shanahan MM. 2019. Breech presentation. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448063/ Opens a new window [Accessed September 2021]
Hankins GD. 1990. Transverse lie. American Journal of Perinatology 7(1):66-70. https://www.ncbi.nlm.nih.gov/pubmed/2131781 Opens a new window [Accessed September 2021]
Medline Plus. 2020. Your baby in the birth canal. U.S. National Library of Medicine. https://medlineplus.gov/ency/article/002060.htm Opens a new window [Accessed September 2021]

Where to go next

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Breech presentation.
Caron J. Gray ; Meaghan M. Shanahan .
Affiliations
Last Update: November 6, 2022 .
- Continuing Education Activity
Breech presentation refers to the fetus in the longitudinal lie with the buttocks or lower extremity entering the pelvis first. The three types of breech presentation include frank breech, complete breech, and incomplete breech. In a frank breech, the fetus has flexion of both hips, and the legs are straight with the feet near the fetal face, in a pike position. This activity reviews the cause and pathophysiology of breech presentation and highlights the role of the interprofessional team in its management.
- Describe the pathophysiology of breech presentation.
- Review the physical exam of a patient with a breech presentation.
- Summarize the treatment options for breech presentation.
- Explain the importance of improving care coordination among interprofessional team members to improve outcomes for patients affected by breech presentation.
- Introduction
Breech presentation refers to the fetus in the longitudinal lie with the buttocks or lower extremity entering the pelvis first. The three types of breech presentation include frank breech, complete breech, and incomplete breech. In a frank breech, the fetus has flexion of both hips, and the legs are straight with the feet near the fetal face, in a pike position. The complete breech has the fetus sitting with flexion of both hips and both legs in a tuck position. Finally, the incomplete breech can have any combination of one or both hips extended, also known as footling (one leg extended) breech, or double footling breech (both legs extended). [1] [2] [3]
Clinical conditions associated with breech presentation include those that may increase or decrease fetal motility, or affect the vertical polarity of the uterine cavity. Prematurity, multiple gestations, aneuploidies, congenital anomalies, Mullerian anomalies, uterine leiomyoma, and placental polarity as in placenta previa are most commonly associated with a breech presentation. Also, a previous history of breech presentation at term increases the risk of repeat breech presentation at term in subsequent pregnancies. [4] [5] These are discussed in more detail in the pathophysiology section.
- Epidemiology
Breech presentation occurs in 3% to 4% of all term pregnancies. A higher percentage of breech presentations occurs with less advanced gestational age. At 32 weeks, 7% of fetuses are breech, and 28 weeks or less, 25% are breech.
Specifically, following one breech delivery, the recurrence rate for the second pregnancy was nearly 10%, and for a subsequent third pregnancy, it was 27%. Prior cesarean delivery has also been described by some to increase the incidence of breech presentation two-fold.
- Pathophysiology
As mentioned previously, the most common clinical conditions or disease processes that result in the breech presentation are those that affect fetal motility or the vertical polarity of the uterine cavity. [6] [7]
Conditions that change the vertical polarity or the uterine cavity, or affect the ease or ability of the fetus to turn into the vertex presentation in the third trimester include:
- Mullerian anomalies: Septate uterus, bicornuate uterus, and didelphys uterus
- Placentation: Placenta previa as the placenta is occupying the inferior portion of the uterine cavity. Therefore, the presenting part cannot engage
- Uterine leiomyoma: Mainly larger myomas located in the lower uterine segment, often intramural or submucosal, that prevent engagement of the presenting part.
- Prematurity
- Aneuploidies and fetal neuromuscular disorders commonly cause hypotonia of the fetus, inability to move effectively
- Congenital anomalies: Fetal sacrococcygeal teratoma, fetal thyroid goiter
- Polyhydramnios: Fetus is often in unstable lie, unable to engage
- Oligohydramnios: Fetus is unable to turn to vertex due to lack of fluid
- Laxity of the maternal abdominal wall: Uterus falls forward, the fetus is unable to engage in the pelvis.
The risk of cord prolapse varies depending on the type of breech. Incomplete or footling breech carries the highest risk of cord prolapse at 15% to 18%, while complete breech is lower at 4% to 6%, and frank breech is uncommon at 0.5%.
- History and Physical
During the physical exam, using the Leopold maneuvers, palpation of a hard, round, mobile structure at the fundus and the inability to palpate a presenting part in the lower abdomen superior to the pubic bone or the engaged breech in the same area, should raise suspicion of a breech presentation.
During a cervical exam, findings may include the lack of a palpable presenting part, palpation of a lower extremity, usually a foot, or for the engaged breech, palpation of the soft tissue of the fetal buttocks may be noted. If the patient has been laboring, caution is warranted as the soft tissue of the fetal buttocks may be interpreted as caput of the fetal vertex.
Any of these findings should raise suspicion and ultrasound should be performed.
Diagnosis of a breech presentation can be accomplished through abdominal exam using the Leopold maneuvers in combination with the cervical exam. Ultrasound should confirm the diagnosis.
On ultrasound, the fetal lie and presenting part should be visualized and documented. If breech presentation is diagnosed, specific information including the specific type of breech, the degree of flexion of the fetal head, estimated fetal weight, amniotic fluid volume, placental location, and fetal anatomy review (if not already done previously) should be documented.
- Treatment / Management
Expertise in the delivery of the vaginal breech baby is becoming less common due to fewer vaginal breech deliveries being offered throughout the United States and in most industrialized countries. The Term Breech Trial (TBT), a well-designed, multicenter, international, randomized controlled trial published in 2000 compared planned vaginal delivery to planned cesarean delivery for the term breech infant. The investigators reported that delivery by planned cesarean resulted in significantly lower perinatal mortality, neonatal mortality, and serious neonatal morbidity. Also, there was no significant difference in maternal morbidity or mortality between the two groups. Since that time, the rate of term breech infants delivered by planned cesarean has increased dramatically. Follow-up studies to the TBT have been published looking at maternal morbidity and outcomes of the children at two years. Although these reports did not show any significant difference in the risk of death and neurodevelopmental, these studies were felt to be underpowered. [8] [9] [10] [11]
Since the TBT, many authors since have argued that there are still some specific situations that vaginal breech delivery is a potential, safe alternative to planned cesarean. Many smaller retrospective studies have reported no difference in neonatal morbidity or mortality using these specific criteria.
The initial criteria used in these reports were similar: gestational age greater than 37 weeks, frank or complete breech presentation, no fetal anomalies on ultrasound examination, adequate maternal pelvis, and estimated fetal weight between 2500 g and 4000 g. In addition, the protocol presented by one report required documentation of fetal head flexion and adequate amniotic fluid volume, defined as a 3-cm vertical pocket. Oxytocin induction or augmentation was not offered, and strict criteria were established for normal labor progress. CT pelvimetry did determine an adequate maternal pelvis.
Despite debate on both sides, the current recommendation for the breech presentation at term includes offering external cephalic version (ECV) to those patients that meet criteria, and for those whom are not candidates or decline external cephalic version, a planned cesarean section for delivery sometime after 39 weeks.
Regarding the premature breech, gestational age will determine the mode of delivery. Before 26 weeks, there is a lack of quality clinical evidence to guide mode of delivery. One large retrospective cohort study recently concluded that from 28 to 31 6/7 weeks, there is a significant decrease in perinatal morbidity and mortality in a planned cesarean delivery versus intended vaginal delivery, while there is no difference in perinatal morbidity and mortality in gestational age 32 to 36 weeks. Of note, due to lack of recruitment, no prospective clinical trials are examining this issue.
- Differential Diagnosis
- Face and brow presentation
- Fetal anomalies
- Fetal death
- Grand multiparity
- Multiple pregnancies
- Oligohydramnios
- Pelvis Anatomy
- Preterm labor
- Primigravida
- Uterine anomalies
- Pearls and Other Issues
In light of the decrease in planned vaginal breech deliveries, thus the decrease in expertise in managing this clinical scenario, it is prudent that policies requiring simulation and instruction in the delivery technique for vaginal breech birth are established to care for the emergency breech vaginal delivery.
- Enhancing Healthcare Team Outcomes
A breech delivery is usually managed by an obstetrician, labor and delivery nurse, anesthesiologist and a neonatologist. The ultimate decison rests on the obstetrician. To prevent complications, today cesarean sections are performed and experienced with vaginal deliveries of breech presentation is limited. For healthcare workers including the midwife who has no experience with a breech delivery, it is vital to communicate with an obstetrician, otherwise one risks litigation if complications arise during delivery. [12] [13] [14]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Caron Gray declares no relevant financial relationships with ineligible companies.
Disclosure: Meaghan Shanahan declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Gray CJ, Shanahan MM. Breech Presentation. [Updated 2022 Nov 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- [What effect does leg position in breech presentation have on mode of delivery and early neonatal morbidity?]. [Z Geburtshilfe Neonatol. 1997] [What effect does leg position in breech presentation have on mode of delivery and early neonatal morbidity?]. Krause M, Fischer T, Feige A. Z Geburtshilfe Neonatol. 1997 Jul-Aug; 201(4):128-35.
- The effect of intra-uterine breech position on postnatal motor functions of the lower limbs. [Early Hum Dev. 1993] The effect of intra-uterine breech position on postnatal motor functions of the lower limbs. Sival DA, Prechtl HF, Sonder GH, Touwen BC. Early Hum Dev. 1993 Mar; 32(2-3):161-76.
- The influence of the fetal leg position on the outcome in vaginally intended deliveries out of breech presentation at term - A FRABAT prospective cohort study. [PLoS One. 2019] The influence of the fetal leg position on the outcome in vaginally intended deliveries out of breech presentation at term - A FRABAT prospective cohort study. Jennewein L, Allert R, Möllmann CJ, Paul B, Kielland-Kaisen U, Raimann FJ, Brüggmann D, Louwen F. PLoS One. 2019; 14(12):e0225546. Epub 2019 Dec 2.
- Review Breech vaginal delivery at or near term. [Semin Perinatol. 2003] Review Breech vaginal delivery at or near term. Tunde-Byass MO, Hannah ME. Semin Perinatol. 2003 Feb; 27(1):34-45.
- Review [Breech Presentation: CNGOF Guidelines for Clinical Practice - Epidemiology, Risk Factors and Complications]. [Gynecol Obstet Fertil Senol. 2...] Review [Breech Presentation: CNGOF Guidelines for Clinical Practice - Epidemiology, Risk Factors and Complications]. Mattuizzi A. Gynecol Obstet Fertil Senol. 2020 Jan; 48(1):70-80. Epub 2019 Nov 1.
Recent Activity
- Breech Presentation - StatPearls Breech Presentation - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Position and Presentation of the Fetus
- IN THIS TOPIC
- Search Please fill out this field.
- Newsletters
- Labor & Delivery
What Causes Breech Presentation?
Learn more about the types, causes, and risks of breech presentation, along with how breech babies are typically delivered.
What Is Breech Presentation?
Types of breech presentation, what causes a breech baby, can you turn a breech baby, how are breech babies delivered.
FatCamera/Getty Images
Toward the end of pregnancy, your baby will start to get into position for delivery, with their head pointed down toward the vagina. This is otherwise known as vertex presentation. However, some babies turn inside the womb so that their feet or buttocks are poised to be delivered first, which is commonly referred to as breech presentation, or a breech baby.
As you near the end of your pregnancy journey, an OB-GYN or health care provider will check your baby's positioning. You might find yourself wondering: What causes breech presentation? Are there risks involved? And how are breech babies delivered? We turned to experts and research to answer some of the most common questions surrounding breech presentation, along with what causes this positioning in the first place.
During your pregnancy, your baby constantly moves around the uterus. Indeed, most babies do somersaults up until the 36th week of pregnancy , when they pick their final position in the womb, says Laura Riley , MD, an OB-GYN in New York City. Approximately 3-4% of babies end up “upside-down” in breech presentation, with their feet or buttocks near the cervix.
Breech presentation is typically diagnosed during a visit to an OB-GYN, midwife, or health care provider. Your physician can feel the position of your baby's head through your abdominal wall—or they can conduct a vaginal exam if your cervix is open. A suspected breech presentation should ultimately be confirmed via an ultrasound, after which you and your provider would have a discussion about delivery options, potential issues, and risks.
There are three types of breech babies: frank, footling, and complete. Learn about the differences between these breech presentations.
Frank Breech
With frank breech presentation, your baby’s bottom faces the cervix and their legs are straight up. This is the most common type of breech presentation.
Footling Breech
Like its name suggests, a footling breech is when one (single footling) or both (double footling) of the baby's feet are in the birth canal, where they’re positioned to be delivered first .
Complete Breech
In a complete breech presentation, baby’s bottom faces the cervix. Their legs are bent at the knees, and their feet are near their bottom. A complete breech is the least common type of breech presentation.
Other Types of Mal Presentations
The baby can also be in a transverse position, meaning that they're sideways in the uterus. Another type is called oblique presentation, which means they're pointing toward one of the pregnant person’s hips.
Typically, your baby's positioning is determined by the fetus itself and the shape of your uterus. Because you can't can’t control either of these factors, breech presentation typically isn’t considered preventable. And while the cause often isn't known, there are certain risk factors that may increase your risk of a breech baby, including the following:
- The fetus may have abnormalities involving the muscular or central nervous system
- The uterus may have abnormal growths or fibroids
- There might be insufficient amniotic fluid in the uterus (too much or too little)
- This isn’t your first pregnancy
- You have a history of premature delivery
- You have placenta previa (the placenta partially or fully covers the cervix)
- You’re pregnant with multiples
- You’ve had a previous breech baby
In some cases, your health care provider may attempt to help turn a baby in breech presentation through a procedure known as external cephalic version (ECV). This is when a health care professional applies gentle pressure on your lower abdomen to try and coax your baby into a head-down position. During the entire procedure, the fetus's health will be monitored, and an ECV is often performed near a delivery room, in the event of any potential issues or complications.
However, it's important to note that ECVs aren't for everyone. If you're carrying multiples, there's health concerns about you or the baby, or you've experienced certain complications with your placenta or based on placental location, a health care provider will not attempt an ECV.
The majority of breech babies are born through C-sections . These are usually scheduled between 38 and 39 weeks of pregnancy, before labor can begin naturally. However, with a health care provider experienced in delivering breech babies vaginally, a natural delivery might be a safe option for some people. In fact, a 2017 study showed similar complication and success rates with vaginal and C-section deliveries of breech babies.
That said, there are certain known risks and complications that can arise with an attempt to deliver a breech baby vaginally, many of which relate to problems with the umbilical cord. If you and your medical team decide on a vaginal delivery, your baby will be monitored closely for any potential signs of distress.
Ultimately, it's important to know that most breech babies are born healthy. Your provider will consider your specific medical condition and the position of your baby to determine which type of delivery will be the safest option for a healthy and successful birth.
ACOG. If Your Baby Is Breech .
American Pregnancy Association. Breech Presentation .
Gray CJ, Shanahan MM. Breech Presentation . [Updated 2022 Nov 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
Mount Sinai. Breech Babies .
Takeda J, Ishikawa G, Takeda S. Clinical Tips of Cesarean Section in Case of Breech, Transverse Presentation, and Incarcerated Uterus . Surg J (N Y). 2020 Mar 18;6(Suppl 2):S81-S91. doi: 10.1055/s-0040-1702985. PMID: 32760790; PMCID: PMC7396468.
Shanahan MM, Gray CJ. External Cephalic Version . [Updated 2022 Nov 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
Fonseca A, Silva R, Rato I, Neves AR, Peixoto C, Ferraz Z, Ramalho I, Carocha A, Félix N, Valdoleiros S, Galvão A, Gonçalves D, Curado J, Palma MJ, Antunes IL, Clode N, Graça LM. Breech Presentation: Vaginal Versus Cesarean Delivery, Which Intervention Leads to the Best Outcomes? Acta Med Port. 2017 Jun 30;30(6):479-484. doi: 10.20344/amp.7920. Epub 2017 Jun 30. PMID: 28898615.
Related Articles
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
What Is Breech?
When a fetus is delivered buttocks or feet first
- Types of Presentation
Risk Factors
Complications.
Breech concerns the position of the fetus before labor . Typically, the fetus comes out headfirst, but in a breech delivery, the buttocks or feet come out first. This type of delivery is risky for both the pregnant person and the fetus.
This article discusses the different types of breech presentations, risk factors that might make a breech presentation more likely, treatment options, and complications associated with a breech delivery.
Verywell / Jessica Olah
Types of Breech Presentation
During the last few weeks of pregnancy, a fetus usually rotates so that the head is positioned downward to come out of the vagina first. This is called the vertex position.
In a breech presentation, the fetus does not turn to lie in the correct position. Instead, the fetus’s buttocks or feet are positioned to come out of the vagina first.
At 28 weeks of gestation, approximately 20% of fetuses are in a breech position. However, the majority of these rotate to the proper vertex position. At full term, around 3%–4% of births are breech.
The different types of breech presentations include:
- Complete : The fetus’s knees are bent, and the buttocks are presenting first.
- Frank : The fetus’s legs are stretched upward toward the head, and the buttocks are presenting first.
- Footling : The fetus’s foot is showing first.
Signs of Breech
There are no specific symptoms associated with a breech presentation.
Diagnosing breech before the last few weeks of pregnancy is not helpful, since the fetus is likely to turn to the proper vertex position before 35 weeks gestation.
A healthcare provider may be able to tell which direction the fetus is facing by touching a pregnant person’s abdomen. However, an ultrasound examination is the best way to determine how the fetus is lying in the uterus.
Most breech presentations are not related to any specific risk factor. However, certain circumstances can increase the risk for breech presentation.
These can include:
- Previous pregnancies
- Multiple fetuses in the uterus
- An abnormally shaped uterus
- Uterine fibroids , which are noncancerous growths of the uterus that usually appear during the childbearing years
- Placenta previa, a condition in which the placenta covers the opening to the uterus
- Preterm labor or prematurity of the fetus
- Too much or too little amniotic fluid (the liquid that surrounds the fetus during pregnancy)
- Fetal congenital abnormalities
Most fetuses that are breech are born by cesarean delivery (cesarean section or C-section), a surgical procedure in which the baby is born through an incision in the pregnant person’s abdomen.
In rare instances, a healthcare provider may plan a vaginal birth of a breech fetus. However, there are more risks associated with this type of delivery than there are with cesarean delivery.
Before cesarean delivery, a healthcare provider might utilize the external cephalic version (ECV) procedure to turn the fetus so that the head is down and in the vertex position. This procedure involves pushing on the pregnant person’s belly to turn the fetus while viewing the maneuvers on an ultrasound. This can be an uncomfortable procedure, and it is usually done around 37 weeks gestation.
ECV reduces the risks associated with having a cesarean delivery. It is successful approximately 40%–60% of the time. The procedure cannot be done once a pregnant person is in active labor.
Complications related to ECV are low and include the placenta tearing away from the uterine lining, changes in the fetus’s heart rate, and preterm labor.
ECV is usually not recommended if the:
- Pregnant person is carrying more than one fetus
- Placenta is in the wrong place
- Healthcare provider has concerns about the health of the fetus
- Pregnant person has specific abnormalities of the reproductive system
Recommendations for Previous C-Sections
The American College of Obstetricians and Gynecologists (ACOG) says that ECV can be considered if a person has had a previous cesarean delivery.
During a breech delivery, the umbilical cord might come out first and be pinched by the exiting fetus. This is called cord prolapse and puts the fetus at risk for decreased oxygen and blood flow. There’s also a risk that the fetus’s head or shoulders will get stuck inside the mother’s pelvis, leading to suffocation.
Complications associated with cesarean delivery include infection, bleeding, injury to other internal organs, and problems with future pregnancies.
A healthcare provider needs to weigh the risks and benefits of ECV, delivering a breech fetus vaginally, and cesarean delivery.
In a breech delivery, the fetus comes out buttocks or feet first rather than headfirst (vertex), the preferred and usual method. This type of delivery can be more dangerous than a vertex delivery and lead to complications. If your baby is in breech, your healthcare provider will likely recommend a C-section.
A Word From Verywell
Knowing that your baby is in the wrong position and that you may be facing a breech delivery can be extremely stressful. However, most fetuses turn to have their head down before a person goes into labor. It is not a cause for concern if your fetus is breech before 36 weeks. It is common for the fetus to move around in many different positions before that time.
At the end of your pregnancy, if your fetus is in a breech position, your healthcare provider can perform maneuvers to turn the fetus around. If these maneuvers are unsuccessful or not appropriate for your situation, cesarean delivery is most often recommended. Discussing all of these options in advance can help you feel prepared should you be faced with a breech delivery.
American College of Obstetricians and Gynecologists. If your baby is breech .
TeachMeObGyn. Breech presentation .
MedlinePlus. Breech birth .
Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term . Cochrane Database Syst Rev . 2015 Apr 1;2015(4):CD000083. doi:10.1002/14651858.CD000083.pub3
By Christine Zink, MD Dr. Christine Zink, MD, is a board-certified emergency medicine with expertise in the wilderness and global medicine. She completed her medical training at Weill Cornell Medical College and residency in emergency medicine at New York-Presbyterian Hospital. She utilizes 15-years of clinical experience in her medical writing.
- MSD careers
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Fetal Presentation, Position, and Lie (Including Breech Presentation)
, MD, Children's Hospital of Philadelphia
Variations in Fetal Position and Presentation
- 3D Models (0)
- Calculators (0)
- Lab Test (0)

Presentation refers to the part of the fetus’s body that leads the way out through the birth canal (called the presenting part). Usually, the head leads the way, but sometimes the buttocks (breech presentation), shoulder, or face leads the way.
Position refers to whether the fetus is facing backward (occiput anterior) or forward (occiput posterior). The occiput is a bone at the back of the baby's head. Therefore, facing backward is called occiput anterior (facing the mother’s back and facing down when the mother lies on her back). Facing forward is called occiput posterior (facing toward the mother's pubic bone and facing up when the mother lies on her back).
Lie refers to the angle of the fetus in relation to the mother and the uterus. Up-and-down (with the baby's spine parallel to mother's spine, called longitudinal) is normal, but sometimes the lie is sideways (transverse) or at an angle (oblique).
For these aspects of fetal positioning, the combination that is the most common, safest, and easiest for the mother to deliver is the following:
Head first (called vertex or cephalic presentation)
Facing backward (occiput anterior position)
Spine parallel to mother's spine (longitudinal lie)
Neck bent forward with chin tucked
Arms folded across the chest
If the fetus is in a different position, lie, or presentation, labor may be more difficult, and a normal vaginal delivery may not be possible.
Variations in fetal presentation, position, or lie may occur when
The fetus is too large for the mother's pelvis (fetopelvic disproportion).
The fetus has a birth defect Overview of Birth Defects Birth defects, also called congenital anomalies, are physical abnormalities that occur before a baby is born. They are usually obvious within the first year of life. The cause of many birth... read more .
There is more than one fetus (multiple gestation).

Position and Presentation of the Fetus
Some variations in position and presentation that make delivery difficult occur frequently.
Occiput posterior position
In occiput posterior position (sometimes called sunny-side up), the fetus is head first (vertex presentation) but is facing forward (toward the mother's pubic bone—that is, facing up when the mother lies on her back). This is a very common position that is not abnormal, but it makes delivery more difficult than when the fetus is in the occiput anterior position (facing toward the mother's spine—that is facing down when the mother lies on her back).
Breech presentation
In breech presentation, the baby's buttocks or sometimes the feet are positioned to deliver first (before the head).
When delivered vaginally, babies that present buttocks first are more at risk of injury or even death than those that present head first.
The reason for the risks to babies in breech presentation is that the baby's hips and buttocks are not as wide as the head. Therefore, when the hips and buttocks pass through the cervix first, the passageway may not be wide enough for the head to pass through. In addition, when the head follows the buttocks, the neck may be bent slightly backwards. The neck being bent backward increases the width required for delivery as compared to when the head is angled forward with the chin tucked, which is the position that is easiest for delivery. Thus, the baby’s body may be delivered and then the head may get caught and not be able to pass through the birth canal. When the baby’s head is caught, this puts pressure on the umbilical cord in the birth canal, so that very little oxygen can reach the baby. Brain damage due to lack of oxygen is more common among breech babies than among those presenting head first.
Breech presentation is more likely to occur in the following circumstances:
Labor starts too soon (preterm labor).
Sometimes the doctor can turn the fetus to be head first before labor begins by doing a procedure that involves pressing on the pregnant woman’s abdomen and trying to turn the baby around. Trying to turn the baby is called an external cephalic version and is usually done at 37 or 38 weeks of pregnancy. Sometimes women are given a medication (such as terbutaline ) during the procedure to prevent contractions.
Other presentations
In face presentation, the baby's neck arches back so that the face presents first rather than the top of the head.
In brow presentation, the neck is moderately arched so that the brow presents first.
Usually, fetuses do not stay in a face or brow presentation. These presentations often change to a vertex (top of the head) presentation before or during labor. If they do not, a cesarean delivery is usually recommended.
In transverse lie, the fetus lies horizontally across the birth canal and presents shoulder first. A cesarean delivery is done, unless the fetus is the second in a set of twins. In such a case, the fetus may be turned to be delivered through the vagina.

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada)—dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge .
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Edition
- IN THIS TOPIC
- Help & Feedback
- About epocrates
Breech presentation
Highlights & basics, diagnostic approach, risk factors, history & exam, differential diagnosis.
- Tx Approach
Emerging Tx
Complications.
PATIENT RESOURCES
Patient Instructions
Breech presentation refers to the baby presenting for delivery with the buttocks or feet first rather than head.
Associated with increased morbidity and mortality for the mother in terms of emergency cesarean section and placenta previa; and for the baby in terms of preterm birth, small fetal size, congenital anomalies, and perinatal mortality.
Incidence decreases as pregnancy progresses and by term occurs in 3% to 4% of singleton term pregnancies.
Treatment options include external cephalic version to increase the likelihood of vaginal birth or a planned cesarean section, the optimal gestation being 37 and 39 weeks, respectively.
Planned cesarean section is considered the safest form of delivery for infants with a persisting breech presentation at term.
Quick Reference
Key Factors
buttocks or feet as the presenting part
Fetal head under costal margin, fetal heartbeat above the maternal umbilicus.
Other Factors
subcostal tenderness
Pelvic or bladder pain.
Diagnostics Tests
1st Tests to Order

transabdominal/transvaginal ultrasound
Treatment options.
presumptive
<37 weeks' gestation
specialist evaluation
corticosteroid
magnesium sulfate
≥37 weeks' gestation not in labor
unsuccessful ECV with persistent breech
Classifications
Types of breech presentation
Baby's buttocks lead the way into the birth canal
Hips are flexed, knees are extended, and the feet are in close proximity to the head
65% to 70% of breech babies are in this position.
Baby presents with buttocks first
Both the hips and the knees are flexed; the baby may be sitting cross-legged.
One or both of the baby's feet lie below the breech so that the foot or knee is lowermost in the birth canal
This is rare at term but relatively common with premature fetuses.
Common Vignette
Other Presentations
Epidemiology
33% of births less than 28 weeks' gestation
14% of births at 29 to 32 weeks' gestation
9% of births at 33 to 36 weeks' gestation
6% of births at 37 to 40 weeks' gestation.
Pathophysiology
- Natasha Nassar, PhD
- Christine L. Roberts, MBBS, FAFPHM, DrPH
- Jonathan Morris, MBChB, FRANZCOG, PhD
- John W. Bachman, MD
- Rhona Hughes, MBChB
- Brian Peat, MD
- Lelia Duley, MBChB
- Justus Hofmeyr, MD

Clinical exam
Palpation of the abdomen to determine the position of the baby's head
Palpation of the abdomen to confirm the position of the fetal spine on one side and fetal extremities on the other
Palpation of the area above the symphysis pubis to locate the fetal presenting part
Palpation of the presenting part to confirm presentation, to determine how far the fetus has descended and whether the fetus is engaged.
Ultrasound examination
Premature fetus.
Prematurity is consistently associated with breech presentation. [ 6 ] [ 9 ] This may be due to the smaller size of preterm infants, who are more likely to change their in utero position.
Increasing duration of pregnancy may allow breech-presenting fetuses time to grow, turn spontaneously or by external cephalic version, and remain cephalic-presenting.
Larger fetuses may be forced into a cephalic presentation in late pregnancy due to space or alignment constraints within the uterus.
small for gestational age fetus
Low birth-weight is a risk factor for breech presentation. [ 9 ] [ 11 ] [ 12 ] [ 13 ] [ 14 ] Term breech births are associated with a smaller fetal size for gestational age, highlighting the association with low birth-weight rather than prematurity. [ 6 ]
nulliparity
Women having a first birth have increased rates of breech presentation, probably due to the increased likelihood of smaller fetal size. [ 6 ] [ 9 ]
Relaxation of the uterine wall in multiparous women may reduce the odds of breech birth and contribute to a higher spontaneous or external cephalic version rate. [ 10 ]
fetal congenital anomalies
Congenital anomalies in the fetus may result in a small fetal size or inappropriate fetal growth. [ 9 ] [ 12 ] [ 14 ] [ 15 ]
Anencephaly, hydrocephaly, Down syndrome, and fetal neuromuscular dysfunction are associated with breech presentation, the latter due to its effect on the quality of fetal movements. [ 9 ] [ 14 ]
previous breech delivery
The risk of recurrent breech delivery is 8%, the risk increasing from 4% after one breech delivery to 28% after three. [ 16 ]
The effects of recurrence may be due to recurring specific causal factors, either genetic or environmental in origin.
uterine abnormalities
Women with uterine abnormalities have a high incidence of breech presentation. [ 14 ] [ 17 ] [ 18 ] [ 19 ]
female fetus
Fifty-four percent of breech-presenting fetuses are female. [ 14 ]
abnormal amniotic fluid volume
Both oligohydramnios and polyhydramnios are associated with breech presentation. [ 1 ] [ 12 ] [ 14 ]
Low amniotic fluid volume decreases the likelihood of a fetus turning to a cephalic position; an increased amniotic fluid volume may facilitate frequent change in position.
placental abnormalities
An association between placental implantation in the cornual-fundal region and breech presentation has been reported, although some studies have not found it a risk factor. [ 8 ] [ 20 ] [ 21 ] [ 22 ] [ 10 ] [ 14 ]
The association with placenta previa is also inconsistent. [ 8 ] [ 9 ] [ 22 ] Placenta previa is associated with preterm birth and may be an indirect risk factor.
Pelvic or vaginal examination reveals the buttocks and/or feet, felt as a yielding, irregular mass, as the presenting part. [ 26 ] In cephalic presentation, a hard, round, regular fetal head can be palpated. [ 26 ]
The Leopold maneuver on examination suggests breech position by palpation of the fetal head under the costal margin. [ 26 ]
The baby's heartbeat should be auscultated using a Pinard stethoscope or a hand-held Doppler to indicate the position of the fetus. The fetal heartbeat lies above the maternal umbilicus in breech presentation. [ 1 ]
Tenderness under one or other costal margin as a result of pressure by the harder fetal head.
Pain due to fetal kicks in the maternal pelvis or bladder.
breech position
Visualizes the fetus and reveals its position.
Used to confirm a clinically suspected breech presentation. [ 28 ]
Should be performed by practitioners with appropriate skills in obstetric ultrasound.
Establishes the type of breech presentation by imaging the fetal femurs and their relationship to the distal bones.
Transverse lie
Differentiating Signs/Symptoms
Fetus lies horizontally across the uterus with the shoulder as the presenting part.
Similar predisposing factors such as placenta previa, abnormal amniotic fluid volume, and uterine anomalies, although more common in multiparity. [ 1 ] [ 2 ] [ 29 ]
Differentiating Tests
Clinical examination and fetal auscultation may be indicative.
Ultrasound confirms presentation.
Treatment Approach
Breech presentation <37 weeks' gestation.
The UK Royal College of Obstetricians and Gynaecologists (RCOG) recommends that corticosteroids should be offered to women between 24 and 34+6 weeks' gestation, in whom imminent preterm birth is anticipated. Corticosteroids should only be considered after discussion of risks/benefits at 35 to 36+6 weeks. Given within 7 days of preterm birth, corticosteroids may reduce perinatal and neonatal death and respiratory distress syndrome. [ 32 ] The American College of Obstetricians and Gynecologists (ACOG) recommends a single course of corticosteroids for pregnant women between 24 and 33+6 weeks' gestation who are at risk of preterm delivery within 7 days, including those with ruptured membranes and multiple gestations. It may also be considered for pregnant women starting at 23 weeks' gestation who are at risk of preterm delivery within 7 days. A single course of betamethasone is recommended for pregnant women between 34 and 36+6 weeks' gestation at risk of preterm birth within 7 days, and who have not received a previous course of prenatal corticosteroids. Regularly scheduled repeat courses or serial courses (more than two) are not currently recommended. A single repeat course of prenatal corticosteroids should be considered in women who are less than 34 weeks' gestation, who are at risk of preterm delivery within 7 days, and whose prior course of prenatal corticosteroids was administered more than 14 days previously. Rescue course corticosteroids could be provided as early as 7 days from the prior dose, if indicated by the clinical scenario. [ 33 ]
Magnesium sulfate given before anticipated early preterm birth reduces the risk of cerebral palsy in surviving infants. Physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, and concurrent tocolysis. [ 34 ]
Breech presentation from 37 weeks' gestation, before labor
ECV is the initial treatment for a breech presentation at term when the patient is not in labor. It involves turning a fetus presenting by the breech to a cephalic (head-down) presentation to increase the likelihood of vaginal birth. [ 35 ] [ 36 ] Where available, it should be offered to all women in late pregnancy, by an experienced clinician, in hospitals with facilities for emergency delivery, and no contraindications to the procedure. [ 35 ] There is no upper time limit on the appropriate gestation for ECV, with success reported at 42 weeks.
There is no general consensus on contraindications to ECV. Contraindications include multiple pregnancy (except after delivery of a first twin), ruptured membranes, current or recent (<1 week) vaginal bleeding, rhesus isoimmunization, other indications for cesarean section (e.g., placenta previa or uterine malformation), or abnormal electronic fetal monitoring. [ 35 ] One systematic review of relative contraindications for ECV highlighted that most contraindications do not have clear empirical evidence. Exceptions include placental abruption, severe preeclampsia/HELLP syndrome, or signs of fetal distress (abnormal cardiotocography and/or Doppler flow). [ 36 ]
The procedure involves applying external pressure and firmly pushing or palpating the mother's abdomen to coerce the fetus to somersault (either forward or backward) into a cephalic position. [ 37 ]
The overall ECV success rate varies but, in a large series, 47% of women following an ECV attempt had a cephalic presentation at birth. [ 35 ] [ 38 ] Various factors influence the success rate. One systematic review found ECV success rates to be 68% overall, with the rate significantly higher for women from African countries (89%) compared with women from non-African countries (62%), and higher among multiparous (78%) than nulliparous women (48%). [ 39 ] Overall, the ECV success rates for nulliparous and multiparous non-African women were 43% and 73%, respectively, while for nulliparous and multiparous African women rates were 79% and 91%, respectively. Another study reported no difference in success rate or rate of cesarean section among women with previous cesarean section undergoing ECV compared with women with previous vaginal birth. However, numbers were small and further studies in this regard are required. [ 40 ]
Women's preference for vaginal delivery is a major contributing factor in their decision for ECV. However, studies suggest women with a breech presentation at term may not receive complete and/or evidence-based information about the benefits and risks of ECV. [ 41 ] [ 42 ] Although up to 60% of women reported ECV to be painful, the majority highlighted the benefits outweigh the risks (71%) and would recommend ECV to their friends or be willing to repeat for themselves (84%). [ 41 ] [ 42 ]
Cardiotocography and ultrasound should be performed before and after the procedure. Tocolysis should be used to facilitate the maneuver, and Rho(D) immune globulin should be administered to women who are Rhesus negative. [ 35 ] Tocolytic agents include adrenergic beta-2 receptor stimulants such as albuterol, terbutaline, or ritodrine (widely used with ECV in some countries, but not yet available in the US). One Cochrane review of tocolytic beta stimulants demonstrates that these are less likely to be associated with failed ECV, and are effective in increasing cephalic presentation and reducing cesarean section. [ 43 ] There is no current evidence to recommend one beta-2 adrenergic receptor agonist over another. Until these data are available, adherence to a local protocol for tocolysis is recommended. The Food and Drug Administration has issued a warning against using injectable terbutaline beyond 48 to 72 hours, or acute or prolonged treatment with oral terbutaline, in pregnant women for the prevention or prolonged treatment of preterm labor, due to potential serious maternal cardiac adverse effects and death. [ 44 ] Whether this warning applies to the subcutaneous administration of terbutaline in ECV is still unclear; however, studies currently support this use. The European Medicines Agency (EMA) recommends that injectable beta agonists should be used for up to 48 hours between the 22nd and 37th week of pregnancy only. They should be used under specialist supervision with continuous monitoring of the mother and unborn baby owing to the risk of adverse cardiovascular effects in both the mother and baby. The EMA no longer recommends oral or rectal formulations for obstetric indications. [ 45 ]
If ECV is successful, pregnancy care should continue as usual for any cephalic presentation. One systematic review assessing the mode of delivery after a successful ECV found that these women were at increased risk for cesarean section and instrumental vaginal delivery compared with women with spontaneous cephalic pregnancies. However, they still had a lower rate of cesarean section following ECV (i.e., 47%) compared with the cesarean section rate for those with a persisting breech (i.e., 85%). With a number needed to treat of three, ECV is still considered to be an effective means of preventing the need for cesarean section. [ 46 ]
Planned cesarean section should be offered as the safest mode of delivery for the baby, even though it carries a small increase in serious immediate maternal complications compared with vaginal birth. [ 24 ] [ 25 ] [ 31 ] In the US, most unsuccessful ECV with persistent breech will be delivered via cesarean section.
A vaginal mode of delivery may be considered by some clinicians as an option, particularly when maternal request is provided, senior and experienced staff are available, there is no absolute contraindication to vaginal birth (e.g., placenta previa, compromised fetal condition), and with optimal fetal growth (estimated weight above the tenth centile and up to 3800 g). Other factors that make planned vaginal birth higher risk include hyperextended neck on ultrasound and footling presentation. [ 24 ]
Breech presentation from 37 weeks' gestation, during labor
The first option should be a planned cesarean section.
There is a small increase in the risk of serious immediate maternal complications compared with vaginal birth (RR 1.29, 95% CI 1.03 to 1.61), including pulmonary embolism, infection, bleeding, damage to the bladder and bowel, slower recovery from the delivery, longer hospitalization, and delayed bonding and breast-feeding. [ 23 ] [ 31 ] [ 47 ] [ 48 ] [ 49 ] [ 50 ] [ 51 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 56 ] [ 57 ] [ 58 ] Consider using antimicrobial triclosan-coated sutures for wound closure to reduce the risk of surgical site infection. [ 59 ]
The long-term risks include potential compromise of future obstetric performance, increased risk of repeat cesarean section, infertility, uterine rupture, placenta accreta, placental abruption, and emergency hysterectomy. [ 60 ] [ 61 ] [ 62 ] [ 63 ]
Planned cesarean section is safer for babies, but is associated with increased neonatal respiratory distress. The risk is reduced when the section is performed at 39 weeks' gestation. [ 64 ] [ 65 ] [ 66 ] For women undergoing a planned cesarean section, RCOG recommends an informed discussion about the potential risks and benefits of a course of prenatal corticosteroids between 37 and 38+6 weeks' gestation. Although prenatal corticosteroids may reduce admission to the neonatal unit for respiratory morbidity, it is uncertain if there is any reduction in respiratory distress syndrome, transient tachypnea of the newborn, or neonatal unit admission overall. In addition, prenatal corticosteroids may result in harm to the neonate, including hypoglycemia and potential developmental delay. [ 32 ] ACOG does not recommend corticosteroids in women >37 weeks' gestation. [ 33 ]
Undiagnosed breech in labor generally results in cesarean section after the onset of labor, higher rates of emergency cesarean section associated with the least favorable maternal outcomes, a greater likelihood of cord prolapse, and other poor infant outcomes. [ 23 ] [ 67 ] [ 49 ] [ 68 ] [ 69 ] [ 70 ] [ 71 ]
This mode of delivery may be considered by some clinicians as an option for women who are in labor, particularly when delivery is imminent. Vaginal breech delivery may also be considered, where suitable, when delivery is not imminent, maternal request is provided, senior and experienced staff are available, there is no absolute contraindication to vaginal birth (e.g., placenta previa, compromised fetal condition), and with optimal fetal growth (estimated weight above the tenth centile and up to 3800 g). Other factors that make planned vaginal birth higher risk include hyperextended neck on ultrasound and footling presentation. [ 24 ]
Findings from one systematic review of 27 observational studies revealed that the absolute risks of perinatal mortality, fetal neurologic morbidity, birth trauma, 5-minute Apgar score <7, and neonatal asphyxia in the planned vaginal delivery group were low at 0.3%, 0.7%, 0.7%, 2.4%, and 3.3%, respectively. However, the relative risks of perinatal mortality and morbidity were 2- to 5-fold higher in the planned vaginal than in the planned cesarean delivery group. Authors recommend ongoing judicious decision-making for vaginal breech delivery for selected singleton, term breech babies. [ 72 ]
ECV may also be considered an option for women with breech presentation in early labor, when delivery is not imminent, provided that the membranes are intact.
A woman presenting with a breech presentation <37 weeks is an area of clinical controversy. Optimal mode of delivery for preterm breech has not been fully evaluated in clinical trials, and the relative risks for the preterm infant and mother remain unclear. In the absence of good evidence, if diagnosis of breech presentation prior to 37 weeks' gestation is made, prematurity and clinical circumstances should determine management and mode of delivery.
Primary Options
12 mg intramuscularly every 24 hours for 2 doses
6 mg intramuscularly every 12 hours for 4 doses
The UK Royal College of Obstetricians and Gynaecologists recommends that corticosteroids should be offered to women between 24 and 34+6 weeks' gestation, in whom imminent preterm birth is anticipated. Corticosteroids should only be considered after discussion of risks/benefits at 35 to 36+6 weeks. Given within 7 days of preterm birth, corticosteroids may reduce perinatal and neonatal death and respiratory distress syndrome. [ 32 ]
The American College of Obstetricians and Gynecologists recommends a single course of corticosteroids for pregnant women between 24 and 33+6 weeks' gestation who are at risk of preterm delivery within 7 days, including those with ruptured membranes and multiple gestations. It may also be considered for pregnant women starting at 23 weeks' gestation who are at risk of preterm delivery within 7 days. A single course of betamethasone is recommended for pregnant women between 34 and 36+6 weeks' gestation at risk of preterm birth within 7 days, and who have not received a previous course of prenatal corticosteroids. Regularly scheduled repeat courses or serial courses (more than two) are not currently recommended. A single repeat course of prenatal corticosteroids should be considered in women who are less than 34 weeks' gestation, who are at risk of preterm delivery within 7 days, and whose prior course of prenatal corticosteroids was administered more than 14 days previously. Rescue course corticosteroids could be provided as early as 7 days from the prior dose, if indicated by the clinical scenario. [ 33 ]
consult specialist for guidance on dose
external cephalic version (ECV)
There is no upper time limit on the appropriate gestation for ECV; it should be offered to all women in late pregnancy by an experienced clinician in hospitals with facilities for emergency delivery and no contraindications to the procedure. [ 35 ] [ 36 ]
ECV involves applying external pressure and firmly pushing or palpating the mother's abdomen to coerce the fetus to somersault (either forward or backward) into a cephalic position. [ 37 ]
There is no general consensus on contraindications to ECV. Contraindications include multiple pregnancy (except after delivery of a first twin), ruptured membranes, current or recent (<1 week) vaginal bleeding, rhesus isoimmunization, other indications for cesarean section (e.g., placenta previa or uterine malformation), or abnormal electronic fetal monitoring. [ 35 ] One systematic review of relative contraindications for ECV highlighted that most contraindications do not have clear empirical evidence. Exceptions include placental abruption, severe preeclampsia/HELLP syndrome, or signs of fetal distress (abnormal cardiotocography and/or Doppler flow). [ 36 ]
Cardiotocography and ultrasound should be performed before and after the procedure.
If ECV is successful, pregnancy care should continue as usual for any cephalic presentation. A systematic review assessing the mode of delivery after a successful ECV found that these women were at increased risk for cesarean section and instrumental vaginal delivery compared with women with spontaneous cephalic pregnancies. However, they still had a lower rate of cesarean section following ECV (i.e., 47%) compared with the cesarean section rate for those with a persisting breech (i.e., 85%). With a number needed to treat of 3, ECV is still considered to be an effective means of preventing the need for cesarean section. [ 46 ]
tocolytic agents
see local specialist protocol for dosing guidelines
Tocolytic agents include adrenergic beta-2 receptor stimulants such as albuterol, terbutaline, or ritodrine (widely used with external cephalic version [ECV] in some countries, but not yet available in the US). They are used to delay or inhibit labor and increase the success rate of ECV. There is no current evidence to recommend one beta-2 adrenergic receptor agonist over another. Until these data are available, adherence to a local protocol for tocolysis is recommended.
The Food and Drug Administration has issued a warning against using injectable terbutaline beyond 48-72 hours, or acute or prolonged treatment with oral terbutaline, in pregnant women for the prevention or prolonged treatment of preterm labor, due to potential serious maternal cardiac adverse effects and death. [ 44 ] Whether this warning applies to the subcutaneous administration of terbutaline in ECV is still unclear; however, studies currently support this use. The European Medicines Agency (EMA) recommends that injectable beta agonists should be used for up to 48 hours between the 22nd and 37th week of pregnancy only. They should be used under specialist supervision with continuous monitoring of the mother and unborn baby owing to the risk of adverse cardiovascular effects in both the mother and baby. The EMA no longer recommends oral or rectal formulations for obstetric indications. [ 45 ]
A systematic review found there was no evidence to support the use of nifedipine for tocolysis. [ 73 ]
There is insufficient evidence to evaluate other interventions to help ECV, such as fetal acoustic stimulation in midline fetal spine positions, or epidural or spinal analgesia. [ 43 ]
Rho(D) immune globulin
300 micrograms intramuscularly as a single dose
Nonsensitized Rh-negative women should receive Rho(D) immune globulin. [ 35 ]
The indication for its administration is to prevent rhesus isoimmunization, which may affect subsequent pregnancy outcomes.
Rho(D) immune globulin needs to be given at the time of external cephalic version and should be given again postpartum to those women who give birth to an Rh-positive baby. [ 74 ]
It is best administered as soon as possible after the procedure, usually within 72 hours.
Dose depends on brand used. Dose given below pertains to most commonly used brands. Consult specialist for further guidance on dose.
elective cesarean section/vaginal breech delivery
Mode of delivery (cesarean section or vaginal breech delivery) should be based on the experience of the attending clinician, hospital policies, maternal request, and the presence or absence of complicating factors. In the US, most unsuccessful external cephalic version (ECV) with persistent breech will be delivered via cesarean section.
Cesarean section, at 39 weeks or greater, has been shown to significantly reduce perinatal mortality and neonatal morbidity compared with vaginal breech delivery (RR 0.33, 95% CI 0.19 to 0.56). [ 31 ] Although safer for these babies, there is a small increase in serious immediate maternal complications compared with vaginal birth (RR 1.29, 95% CI 1.03 to 1.61), as well as long-term risks for future pregnancies, including pulmonary embolism, bleeding, infection, damage to the bladder and bowel, slower recovery from the delivery, longer hospitalization, and delayed bonding and breast-feeding. [ 23 ] [ 31 ] [ 47 ] [ 48 ] [ 49 ] [ 50 ] [ 51 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 56 ] [ 57 ] [ 58 ] Consider using antimicrobial triclosan-coated sutures for wound closure to reduce the risk of surgical site infection. [ 59 ]
Vaginal delivery may be considered by some clinicians as an option, particularly when maternal request is provided, when senior and experienced staff are available, when there is no absolute contraindication to vaginal birth (e.g., placenta previa, compromised fetal condition), and with optimal fetal growth (estimated weight above the tenth centile and up to 3800 g). Other factors that make planned vaginal birth higher risk include hyperextended neck on ultrasound and footling presentation. [ 24 ]
For women undergoing a planned cesarean section, the UK Royal College of Obstetricians and Gynaecologists recommends an informed discussion about the potential risks and benefits of a course of prenatal corticosteroids between 37 and 38+6 weeks' gestation. Although prenatal corticosteroids may reduce admission to the neonatal unit for respiratory morbidity, it is uncertain if there is any reduction in respiratory distress syndrome, transient tachypnea of the newborn, or neonatal unit admission overall. In addition, prenatal corticosteroids may result in harm to the neonate, including hypoglycemia and potential developmental delay. [ 32 ] The American College of Obstetricians and Gynecologists does not recommend corticosteroids in women >37 weeks' gestation. [ 33 ]
It is best administered as soon as possible after delivery, usually within 72 hours.
Administration of postpartum Rho (D) immune globulin should not be affected by previous routine prenatal prophylaxis or previous administration for a potentially sensitizing event. [ 74 ]
≥37 weeks' gestation in labor: no imminent delivery
planned cesarean section
For women with breech presentation in labor, planned cesarean section at 39 weeks or greater has been shown to significantly reduce perinatal mortality and neonatal morbidity compared with vaginal breech delivery (RR 0.33, 95% CI 0.19 to 0.56). [ 31 ]
Although safer for these babies, there is a small increase in serious immediate maternal complications compared with vaginal birth (RR 1.29, 95% CI 1.03 to 1.61), as well as long-term risks for future pregnancies, including pulmonary embolism, infection, bleeding, damage to the bladder and bowel, slower recovery from the delivery, longer hospitalization, and delayed bonding and breast-feeding. [ 23 ] [ 31 ] [ 47 ] [ 48 ] [ 49 ] [ 50 ] [ 51 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 56 ] [ 57 ] [ 58 ] Consider using antimicrobial triclosan-coated sutures for wound closure to reduce the risk of surgical site infection. [ 59 ]
Continuous cardiotocography monitoring should continue until delivery. [ 24 ] [ 25 ]
vaginal breech delivery
Mode of delivery (cesarean section or vaginal breech delivery) should be based on the experience of the attending clinician, hospital policies, maternal request, and the presence or absence of complicating factors.
This mode of delivery may be considered by some clinicians as an option, particularly when maternal request is provided, when senior and experienced staff are available, when there is no absolute contraindication to vaginal birth (e.g., placenta previa, compromised fetal condition), and with optimal fetal growth (estimated weight above the tenth centile and up to 3800 g). Other factors that make planned vaginal birth higher risk include hyperextended neck on ultrasound and footling presentation. [ 24 ]
For women with persisting breech presentation, planned cesarean section has, however, been shown to significantly reduce perinatal mortality and neonatal morbidity compared with vaginal breech delivery (RR 0.33, 95% CI 0.19 to 0.56). [ 31 ]
ECV may also be considered an option for women with breech presentation in early labor, provided that the membranes are intact.
There is no upper time limit on the appropriate gestation for ECV. [ 35 ]
Involves applying external pressure and firmly pushing or palpating the mother's abdomen to coerce the fetus to somersault (either forward or backward) into a cephalic position. [ 37 ]
Relative contraindications include placental abruption, severe preeclampsia/HELLP syndrome, and signs of fetal distress (abnormal cardiotocography and/or abnormal Doppler flow). [ 35 ] [ 36 ]
Rho(D) immune globulin needs to be given at the time of ECV and should be given again postpartum to those women who give birth to an Rh-positive baby. [ 74 ]
≥37 weeks' gestation in labor: imminent delivery
cesarean section
For women with persistent breech presentation, planned cesarean section has been shown to significantly reduce perinatal mortality and neonatal morbidity compared with vaginal breech delivery (RR 0.33, 95% CI 0.19 to 0.56). [ 31 ] Although safer for these babies, there is a small increase in serious immediate maternal complications compared with vaginal birth (RR 1.29, 95% CI 1.03 to 1.61), as well as long-term risks for future pregnancies, including pulmonary embolism, infection, bleeding, damage to the bladder and bowel, slower recovery from the delivery, longer hospitalization, and delayed bonding and breast-feeding. [ 23 ] [ 31 ] [ 47 ] [ 48 ] [ 49 ] [ 50 ] [ 51 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 56 ] [ 57 ] [ 58 ] Consider using antimicrobial triclosan-coated sutures for wound closure to reduce the risk of surgical site infection. [ 59 ]
This mode of delivery may be considered by some clinicians as an option, particularly when delivery is imminent, maternal request is provided, when senior and experienced staff are available, when there is no absolute contraindication to vaginal birth (e.g., placenta previa, compromised fetal condition), and with optimal fetal growth (estimated weight above the tenth centile and up to 3800 g). Other factors that make planned vaginal birth higher risk include hyperextended neck on ultrasound and footling presentation. [ 24 ]
It is best administered as soon as possible after the delivery, usually within 72 hours.
External cephalic version before term
Moxibustion, postural management, follow-up overview, perinatal complications.
Compared with cephalic presentation, persistent breech presentation has increased frequency of cord prolapse, abruptio placentae, prelabor rupture of membranes, perinatal mortality, fetal distress (heart rate <100 bpm), preterm delivery, lower fetal weight. [ 10 ] [ 11 ] [ 67 ]
complications of cesarean section
There is a small increase in the risk of serious immediate maternal complications compared with vaginal birth (RR 1.29, 95% CI 1.03 to 1.61), including pulmonary embolism, infection, bleeding, damage to the bladder and bowel, slower recovery from the delivery, longer hospitalization, and delayed bonding and breast-feeding. [ 23 ] [ 31 ] [ 47 ] [ 48 ] [ 49 ] [ 50 ] [ 51 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 56 ] [ 57 ] [ 58 ]
The long-term risks include potential compromise of future obstetric performance, increased risk of repeat cesarean section, infertility, uterine rupture, placenta accreta, placental abruption, and emergency hysterectomy. [ 60 ] [ 61 ] [ 62 ] [ 63 ] The evidence suggests that using sutures, rather than staples, for wound closure after cesarean section reduces the incidence of wound dehiscence. [ 59 ]
Emergency cesarean section, compared with planned cesarean section, has demonstrated a higher risk of severe obstetric morbidity, intra-operative complications, postoperative complications, infection, blood loss >1500 mL, fever, pain, tiredness, and breast-feeding problems. [ 23 ] [ 48 ] [ 50 ] [ 70 ] [ 81 ]
Key Articles
Impey LWM, Murphy DJ, Griffiths M, et al; Royal College of Obstetricians and Gynaecologists. Management of breech presentation: green-top guideline no. 20b. BJOG. 2017 Jun;124(7):e151-77. [Full Text]
Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database Syst Rev. 2015 Jul 21;(7):CD000166. [Abstract] [Full Text]
Royal College of Obstetricians and Gynaecologists. External cephalic version and reducing the incidence of term breech presentation. March 2017 [internet publication]. [Full Text]
Cluver C, Gyte GM, Sinclair M, et al. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015 Feb 9;(2):CD000184. [Abstract] [Full Text]
de Hundt M, Velzel J, de Groot CJ, et al. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014 Jun;123(6):1327-34. [Abstract]
Referenced Articles
1. Cunningham F, Gant N, Leveno K, et al. Williams obstetrics. 21st ed. New York: McGraw-Hill; 1997.
2. Kish K, Collea JV. Malpresentation and cord prolapse. In: DeCherney AH, Nathan L, eds. Current obstetric and gynecologic diagnosis and treatment. New York: McGraw-Hill Professional; 2002.
3. Scheer K, Nubar J. Variation of fetal presentation with gestational age. Am J Obstet Gynecol. 1976 May 15;125(2):269-70. [Abstract]
4. Nassar N, Roberts CL, Cameron CA, et al. Diagnostic accuracy of clinical examination for detection of non-cephalic presentation in late pregnancy: cross sectional analytic study. BMJ. 2006 Sep 16;333(7568):578-80. [Abstract] [Full Text]
5. Roberts CL, Peat B, Algert CS, et al. Term breech birth in New South Wales, 1990-1997. Aust N Z J Obstet Gynaecol. 2000 Feb;40(1):23-9. [Abstract]
6. Roberts CL, Algert CS, Peat B, et al. Small fetal size: a risk factor for breech birth at term. Int J Gynaecol Obstet. 1999 Oct;67(1):1-8. [Abstract]
7. Brar HS, Platt LD, DeVore GR, et al. Fetal umbilical velocimetry for the surveillance of pregnancies complicated by placenta previa. J Reprod Med. 1988 Sep;33(9):741-4. [Abstract]
8. Kian L. The role of the placental site in the aetiology of breech presentation. J Obstet Gynaecol Br Commonw. 1963 Oct;70:795-7. [Abstract]
9. Rayl J, Gibson PJ, Hickok DE. A population-based case-control study of risk factors for breech presentation. Am J Obstet Gynecol. 1996 Jan;174(1 Pt 1):28-32. [Abstract]
10. Westgren M, Edvall H, Nordstrom L, et al. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985 Jan;92(1):19-22. [Abstract]
11. Brenner WE, Bruce RD, Hendricks CH. The characteristics and perils of breech presentation. Am J Obstet Gynecol. 1974 Mar 1;118(5):700-12. [Abstract]
12. Hall JE, Kohl S. Breech presentation. Am J Obstet Gynecol. 1956 Nov;72(5):977-90. [Abstract]
13. Morgan HS, Kane SH. An analysis of 16,327 breech births. JAMA. 1964 Jan 25;187:262-4. [Abstract]
14. Luterkort M, Persson P, Weldner B. Maternal and fetal factors in breech presentation. Obstet Gynecol. 1984 Jul;64(1):55-9. [Abstract]
15. Braun FH, Jones KL, Smith DW. Breech presentation as an indicator of fetal abnormality. J Pediatr. 1975 Mar;86(3):419-21. [Abstract]
16. Albrechtsen S, Rasmussen S, Dalaker K, et al. Reproductive career after breech presentation: subsequent pregnancy rates, interpregnancy interval, and recurrence. Obstet Gynecol. 1998 Sep;92(3):345-50. [Abstract]
17. Zlopasa G, Skrablin S, Kalafatić D, et al. Uterine anomalies and pregnancy outcome following resectoscope metroplasty. Int J Gynaecol Obstet. 2007 Aug;98(2):129-33. [Abstract]
18. Acién P. Breech presentation in Spain, 1992: a collaborative study. Eur J Obstet Gynecol Reprod Biol. 1995 Sep;62(1):19-24. [Abstract]
19. Michalas SP. Outcome of pregnancy in women with uterine malformation: evaluation of 62 cases. Int J Gynaecol Obstet. 1991 Jul;35(3):215-9. [Abstract]
20. Fianu S, Vaclavinkova V. The site of placental attachment as a factor in the aetiology of breech presentation. Acta Obstet Gynecol Scand. 1978;57(4):371-2. [Abstract]
21. Haruyama Y. Placental implantation as the cause of breech presentation [in Japanese]. Nihon Sanka Fujinka Gakkai Zasshi. 1987 Jan;39(1):92-8. [Abstract]
22. Filipov E, Borisov I, Kolarov G. Placental location and its influence on the position of the fetus in the uterus [in Bulgarian]. Akush Ginekol (Sofiia). 2000;40(4):11-2. [Abstract]
23. Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. BMJ. 2001 May 5;322(7294):1089-93. [Abstract] [Full Text]
24. Impey LWM, Murphy DJ, Griffiths M, et al; Royal College of Obstetricians and Gynaecologists. Management of breech presentation: green-top guideline no. 20b. BJOG. 2017 Jun;124(7):e151-77. [Full Text]
25. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG committee opinion no. 745: mode of term singleton breech delivery. Obstet Gynecol. 2018 Aug;132(2):e60-3. [Abstract] [Full Text]
26. Beischer NA, Mackay EV, Colditz P, eds. Obstetrics and the newborn: an illustrated textbook. 3rd ed. London: W.B. Saunders; 1997.
27. Royal College of Obstetricians and Gynaecologists. Antepartum haemorrhage: green-top guideline no. 63. November 2011 [internet publication]. [Full Text]
28. American College of Obstetricians and Gynecologists. Practice bulletin no. 175: ultrasound in pregnancy. Obstet Gynecol. 2016 Dec;128(6):e241-56. [Abstract]
29. Enkin M, Keirse MJNC, Neilson J, et al. Guide to effective care in pregnancy and childbirth. 3rd ed. Oxford: Oxford University Press; 2000.
30. Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD000083. [Abstract] [Full Text]
31. Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database Syst Rev. 2015 Jul 21;(7):CD000166. [Abstract] [Full Text]
32. Stock SJ, Thomson AJ, Papworth S, et al. Antenatal corticosteroids to reduce neonatal morbidity and mortality: Green-top Guideline No. 74. BJOG. 2022 Jul;129(8):e35-60. [Abstract] [Full Text]
33. American College of Obstetricians and Gynaecologists Committee on Obstetric Practice. Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. August 2017 (reaffirmed 2020) [internet publication]. [Full Text]
34. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee opinion no. 455: magnesium sulfate before anticipated preterm birth for neuroprotection. March 2010 (reaffirmed 2020) [internet publication]. [Full Text]
35. Royal College of Obstetricians and Gynaecologists. External cephalic version and reducing the incidence of term breech presentation. March 2017 [internet publication]. [Full Text]
36. Rosman AN, Guijt A, Vlemmix F, et al. Contraindications for external cephalic version in breech position at term: a systematic review. Acta Obstet Gynecol Scand. 2013 Feb;92(2):137-42. [Abstract]
37. Hofmeyr GJ. Effect of external cephalic version in late pregnancy on breech presentation and caesarean section rate: a controlled trial. Br J Obstet Gynaecol. 1983 May;90(5):392-9. [Abstract]
38. Beuckens A, Rijnders M, Verburgt-Doeleman GH, et al. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016 Feb;123(3):415-23. [Abstract]
39. Nassar N, Roberts CL, Barratt A, et al. Systematic review of adverse outcomes of external cephalic version and persisting breech presentation at term. Paediatr Perinat Epidemiol. 2006 Mar;20(2):163-71. [Abstract]
40. Sela HY, Fiegenberg T, Ben-Meir A, et al. Safety and efficacy of external cephalic version for women with a previous cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2009 Feb;142(2):111-4. [Abstract]
41. Pichon M, Guittier MJ, Irion O, et al. External cephalic version in case of persisting breech presentation at term: motivations and women's experience of the intervention [in French]. Gynecol Obstet Fertil. 2013 Jul-Aug;41(7-8):427-32. [Abstract]
42. Nassar N, Roberts CL, Raynes-Greenow CH, et al. Evaluation of a decision aid for women with breech presentation at term: a randomised controlled trial [ISRCTN14570598]. BJOG. 2007 Mar;114(3):325-33. [Abstract] [Full Text]
43. Cluver C, Gyte GM, Sinclair M, et al. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015 Feb 9;(2):CD000184. [Abstract] [Full Text]
44. US Food & Drug Administration. FDA Drug Safety Communication: new warnings against use of terbutaline to treat preterm labor. Feb 2011 [internet publication]. [Full Text]
45. European Medicines Agency. Restrictions on use of short-acting beta-agonists in obstetric indications - CMDh endorses PRAC recommendations. October 2013 [internet publication]. [Full Text]
46. de Hundt M, Velzel J, de Groot CJ, et al. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014 Jun;123(6):1327-34. [Abstract]
47. Lydon-Rochelle M, Holt VL, Martin DP, et al. Association between method of delivery and maternal rehospitalisation. JAMA. 2000 May 10;283(18):2411-6. [Abstract]
48. Yokoe DS, Christiansen CL, Johnson R, et al. Epidemiology of and surveillance for postpartum infections. Emerg Infect Dis. 2001 Sep-Oct;7(5):837-41. [Abstract]
49. van Ham MA, van Dongen PW, Mulder J. Maternal consequences of caesarean section. A retrospective study of intra-operative and postoperative maternal complications of caesarean section during a 10-year period. Eur J Obstet Gynecol Reprod Biol. 1997 Jul;74(1):1-6. [Abstract]
50. Murphy DJ, Liebling RE, Verity L, et al. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001 Oct 13;358(9289):1203-7. [Abstract]
51. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001 Jul;15(3):232-40. [Abstract]
52. Wilson PD, Herbison RM, Herbison GP. Obstetric practice and the prevalence of urinary incontinence three months after delivery. Br J Obstet Gynaecol. 1996 Feb;103(2):154-61. [Abstract]
53. Persson J, Wolner-Hanssen P, Rydhstroem H. Obstetric risk factors for stress urinary incontinence: a population-based study. Obstet Gynecol. 2000 Sep;96(3):440-5. [Abstract]
54. MacLennan AH, Taylor AW, Wilson DH, et al. The prevalence of pelvic disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000 Dec;107(12):1460-70. [Abstract]
55. Thompson JF, Roberts CL, Currie M, et al. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002 Jun;29(2):83-94. [Abstract]
56. Australian Institute of Health and Welfare. Australia's mothers and babies 2015 - in brief. October 2017 [internet publication]. [Full Text]
57. Mutryn CS. Psychosocial impact of cesarean section on the family: a literature review. Soc Sci Med. 1993 Nov;37(10):1271-81. [Abstract]
58. DiMatteo MR, Morton SC, Lepper HS, et al. Cesarean childbirth and psychosocial outcomes: a meta-analysis. Health Psychol. 1996 Jul;15(4):303-14. [Abstract]
59. National Institute for Health and Care Excellence. Caesarean birth. Mar 2021 [internet publication]. [Full Text]
60. Greene R, Gardeit F, Turner MJ. Long-term implications of cesarean section. Am J Obstet Gynecol. 1997 Jan;176(1 Pt 1):254-5. [Abstract]
61. Coughlan C, Kearney R, Turner MJ. What are the implications for the next delivery in primigravidae who have an elective caesarean section for breech presentation? BJOG. 2002 Jun;109(6):624-6. [Abstract]
62. Hemminki E, Merilainen J. Long-term effects of cesarean sections: ectopic pregnancies and placental problems. Am J Obstet Gynecol. 1996 May;174(5):1569-74. [Abstract]
63. Gilliam M, Rosenberg D, Davis F. The likelihood of placenta previa with greater number of cesarean deliveries and higher parity. Obstet Gynecol. 2002 Jun;99(6):976-80. [Abstract]
64. Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. Br J Obstet Gynaecol. 1995 Feb;102(2):101-6. [Abstract]
65. Annibale DJ, Hulsey TC, Wagner CL, et al. Comparative neonatal morbidity of abdominal and vaginal deliveries after uncomplicated pregnancies. Arch Pediatr Adolesc Med. 1995 Aug;149(8):862-7. [Abstract]
66. Hook B, Kiwi R, Amini SB, et al. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics. 1997 Sep;100(3 Pt 1):348-53. [Abstract]
67. Nassar N, Roberts CL, Cameron CA, et al. Outcomes of external cephalic version and breech presentation at term: an audit of deliveries at a Sydney tertiary obstetric hospital, 1997-2004. Acta Obstet Gynecol Scand. 2006;85(10):1231-8. [Abstract]
68. Nwosu EC, Walkinshaw S, Chia P, et al. Undiagnosed breech. Br J Obstet Gynaecol. 1993 Jun;100(6):531-5. [Abstract]
69. Flamm BL, Ruffini RM. Undetected breech presentation: impact on external version and cesarean rates. Am J Perinatol. 1998 May;15(5):287-9. [Abstract]
70. Cockburn J, Foong C, Cockburn P. Undiagnosed breech. Br J Obstet Gynaecol. 1994 Jul;101(7):648-9. [Abstract]
71. Leung WC, Pun TC, Wong WM. Undiagnosed breech revisited. Br J Obstet Gynaecol. 1999 Jul;106(7):638-41. [Abstract]
72. Berhan Y, Haileamlak A. The risks of planned vaginal breech delivery versus planned caesarean section for term breech birth: a meta-analysis including observational studies. BJOG. 2016 Jan;123(1):49-57. [Abstract] [Full Text]
73. Wilcox C, Nassar N, Roberts C. Effectiveness of nifedipine tocolysis to facilitate external cephalic version: a systematic review. BJOG. 2011 Mar;118(4):423-8. [Abstract]
74. Qureshi H, Massey E, Kirwan D, et al. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med. 2014 Feb;24(1):8-20. [Abstract] [Full Text]
75. Hutton EK, Hofmeyr GJ, Dowswell T. External cephalic version for breech presentation before term. Cochrane Database Syst Rev. 2015 Jul 29;(7):CD000084. [Abstract] [Full Text]
76. Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012 May 16;(5):CD003928. [Abstract] [Full Text]
77. Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD000051. [Abstract] [Full Text]
78. Hannah ME, Whyte H, Hannah WJ, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. Am J Obstet Gynecol. 2004 Sep;191(3):917-27. [Abstract]
79. Eide MG, Oyen N, Skjaerven R, et al. Breech delivery and Intelligence: a population-based study of 8,738 breech infants. Obstet Gynecol. 2005 Jan;105(1):4-11. [Abstract]
80. Whyte H, Hannah ME, Saigal S, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. Am J Obstet Gynecol. 2004 Sep;191(3):864-71. [Abstract]
81. Brown S, Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol. 1998 Feb;105(2):156-61. [Abstract]
Published by
American College of Obstetricians and Gynecologists
2016 (reaffirmed 2022)
Royal College of Obstetricians and Gynaecologists (UK)
National Institute for Health and Care Excellence (UK)
Topic last updated: 2024-03-05
Natasha Nassar , PhD
Associate Professor
Menzies Centre for Health Policy
Sydney School of Public Health
University of Sydney
Christine L. Roberts , MBBS, FAFPHM, DrPH
Research Director
Clinical and Population Health Division
Perinatal Medicine Group
Kolling Institute of Medical Research
Jonathan Morris , MBChB, FRANZCOG, PhD
Professor of Obstetrics and Gynaecology and Head of Department
Peer Reviewers
John W. Bachman , MD
Consultant in Family Medicine
Department of Family Medicine
Mayo Clinic
Rhona Hughes , MBChB
Lead Obstetrician
Lothian Simpson Centre for Reproductive Health
The Royal Infirmary
Brian Peat , MD
Director of Obstetrics
Women's and Children's Hospital
North Adelaide
South Australia
Lelia Duley , MBChB
Professor of Obstetric Epidemiology
University of Leeds
Bradford Institute of Health Research
Temple Bank House
Bradford Royal Infirmary
Justus Hofmeyr , MD
Head of the Department of Obstetrics and Gynaecology
East London Private Hospital
East London
South Africa
The evolution of fetal presentation during pregnancy: a retrospective, descriptive cross-sectional study
Affiliations.
- 1 1st Department of Obstetrics and Gynecology, Medical University of Warsaw, Warsaw, Poland.
- 2 Teaching Department of Obstetrics and Gynecology in Ruda Slaska, Medical University of Silesia, Ruda Slaska, Poland.
- PMID: 25753199
- DOI: 10.1111/aogs.12626
We investigated changes in the frequencies of four primary types of singleton fetal lie/presentation for each gestational week from 18 to 39 weeks in a retrospective, cross-sectional study which analyzed ultrasound examination records of fetal positions, in the outpatient prenatal diagnosis clinics in two cities in Poland. We calculated the prevalence and 95% confidence intervals for each type of lie/presentation. We then identified the gestational age after which no statistically significant changes in terms of prevalence were observed, by comparing the results at each week with the prevalence of cephalic presentation at 39(+0) weeks, used as reference. A total of 18 019 ultrasound examinations were used. From 22 to 36 weeks of gestation, the prevalence of cephalic presentation increased from 47% (45-50%) to 94% (91-96%), before and after which times plateaus were noted. Spontaneous change from breech to cephalic is unlikely to occur after 36 weeks of gestation.
Keywords: Fetal lie; breech; cephalic; external version; fetal presentation.
© 2015 Nordic Federation of Societies of Obstetrics and Gynecology.
Publication types
- Research Support, Non-U.S. Gov't
- Cross-Sectional Studies
- Gestational Age
- Labor Presentation*
- Retrospective Studies
- Ultrasonography, Prenatal
- Open access
- Published: 25 March 2024
Reproductive factors and subsequent pregnancy outcomes in patients with prior pregnancy loss
- Xin Yang 1 ,
- Fangxiang Mu 1 ,
- Jian Zhang 1 ,
- Liwei Yuan 1 ,
- Wei Zhang 1 ,
- Yanting Yang 1 &
- Fang Wang 1
BMC Pregnancy and Childbirth volume 24 , Article number: 219 ( 2024 ) Cite this article
149 Accesses
Metrics details
At present, individualized interventions can be given to patients with a clear etiology of pregnancy loss to improve the subsequent pregnancy outcomes, but the current reproductive status of the patient cannot be changed. The aim of this study was to investigate the association between female reproductive status and subsequence pregnancy outcome in patients with prior pregnancy loss (PL).
A prospective, dynamic population cohort study was carried out at the Second Hospital of Lanzhou University. From September 2019 to February 2022, a total of 1955 women with at least one previous PL were enrolled. Maternal reproductive status and subsequent reproductive outcomes were recorded through an electronic medical record system and follow-up. Logistic regression was used to evaluate the association between reproductive status and the risk of subsequent reproductive outcomes.
Among all patients, the rates of subsequent infertility, early PL, late PL, and live birth were 20.82%, 24.33%, 1.69% and 50.77% respectively. In logistic regression, we found that age (OR 1.08, 95% CI 1.04–1.13) and previous cesarean delivery history (OR 2.46, 95% CI 1.27–4.76) were risk factors for subsequent infertility in patients with PL. Age (OR 1.06, 95% CI 1.03–1.10), age at first pregnancy (OR 1.06, 95% CI 1.03–1.10), BMI (OR 1.06, 95% CI 1.02–1.11), previous PL numbers (OR 1.18, 95% CI 1.04–1.57) and without pre-pregnancy intervention (OR 1.77, 95% CI 1.35–2.24) were risk factors for non-live birth. Age (OR 1.06, 95% CI 1.03–1.09), age at first pregnancy (OR 1.06, 95% CI 1.02–1.09), BMI (OR 1.07, 95% CI 1.02–1.11), previous PL numbers (OR 1.15, 95% CI 1.02–1.31) and without pre-pregnancy intervention (OR 2.16, 95% CI 1.65–2.84) were risk factors for PL.
Conclusions
The reproductive status of people with PL is strongly correlated with the outcome of subsequent pregnancies. Active pre-pregnancy intervention can improve the subsequent pregnancy outcome.
Trial registration
This study was registered in the Chinese Clinical Trial Registry with the registration number of ChiCTR2000039414 (27/10/2020).
Peer Review reports
Pregnancy loss (PL) is defined as the spontaneous demise of a pregnancy before the fetus reaches viability, which is a significant negative life event and impacts 10–15% of clinically recognized pregnancies. Recurrent pregnancy loss (RPL) refers to two or more consecutive PL episodes with the same sexual partner, accounting for approximately 1–2% [ 1 , 2 ]. There are many reasons for the occurrence of RPL, including genetic abnormalities (fetal genetic abnormalities and parental genetic abnormalities), reproductive tract anatomical abnormalities, immune diseases, endocrine diseases, antiphospholipid syndrome, thrombotic disorders, and infections, but about 40-50% of the etiologies remain unexplained, Molecular mechanisms have not been fully explored, and these are defined as unexplained recurrent pregnancy loss (URPL) [ 3 , 4 ]. In addition, PL was defined as primary if there without a previous ongoing pregnancy (viable pregnancy) beyond 24 weeks gestation, otherwise it was defined as secondary [ 1 ]. PL is a serious adverse event in life that greatly affects the physical and mental health of women. Women who experience PL have increased rates of anxiety and depression and other psychological disorders. It is reported that in RPL, the incidence of anxiety and depression in women can be as high as 47.7% and 51.7%, respectively [ 5 ]. At the same time, anxiety, and depression symptoms in women in early pregnancy are also risk factors for RPL [ 6 ].
In addition to influencing the etiology of pregnancy loss, personal factors (age, first pregnancy age, BMI) and reproductive status (total pregnancy number, pregnancy loss number, pregnancy type, induced abortion, live birth, ectopic pregnancies, molar pregnancy and, etc.) of the patient greatly influence the reproductive outcome [ 7 ]. Studies have found that age, the number of previous pregnancy loss and BMI are important influencing factors in pregnancy loss. The relationship between age and reproductive outcomes is well established, age-adjusted odds ratios for pregnancy loss were found to increase after each pregnancy loss and to be as high as 63% among women who had experienced six or more miscarriages [ 8 ]. However, the relationship between BMI and pregnancy outcomes remains controversial. Zhang et al. found that BMI ≥ 24.0 was associated with an increased risk of RPL. However, Lo and colleagues demonstrated that maternal obesity (BMI ≥ 30 kg/m2) significantly increased the risk of the disease miscarriage in couples with URPL, while there was no increased risk in women with overweight. Maconochie et al. found underweight (BMI < 18.5) was significantly associated with sporadic first trimester miscarriage, However, Lo et al. found that no increased risk of subsequent PL in women who are underweight as compared to women with normal BMI [ 9 , 10 ].
Some differences were also found between primary and secondary PL, with secondary PL and ≥ 4 prior PL strongly associated with HLA-DRB1*03, and secondary PL of a boy from a previous birth has a negative impact on the outcome of subsequent pregnancies [ 11 , 12 ]. Notably, patients with secondary PL had higher levels of tumor necrosis factor-α (TNF-α) in peripheral blood than primary PL, while high plasma TNF-α levels are reported to increase the risk of miscarriage in women with RPL [ 13 ]. This may indicate a higher risk of miscarriage in patients with secondary PL. It is also controversial whether previous induced abortion have an effect on subsequent PL. Infante-Rivard et al. found that induced abortion was a risk factor for subsequent PL, while Chung et al. found no statistical difference between induced abortion and PL risk [ 14 , 15 ].
At present, some studies have found that reproductive history does not compromise subsequent live birth and perinatal outcomes in patients undergoing first frozen embryo transfer in in-vitro fertilization [ 16 ]. Whereas, a registry-based cohort study revealed that obstetric complications (still birth, ectopic pregnancies, and pregnancy losses) had a negative effect on the chance of live birth in the next pregnancy, and the identical pregnancy outcomes immediately preceding the next pregnancy had a larger impact than the total number of any outcome [ 17 ]. However, no studies have comprehensively evaluated reproductive factors and pregnancy outcomes in patients with prior PL.
Currently, individualized interventions can be given to patients with a clear etiology of PL to improve the outcome of subsequent pregnancies, but the current reproductive status of the patient cannot be changed. Therefore, this study aims to explore the relationship between reproductive factors and pregnancy outcomes in patients with prior PL.
Study population
A prospective, dynamic population cohort study was carried out at a university-affiliated fertility center. The cohort began in September 2019 and enrolled 1955 patients through February 2022. Written informed consent was obtained at the time of recruitment. Inclusion criteria: patients who had experienced at least one PL (diagnosis of PL according to the ESHRE, which spontaneous abortions prior to 24 weeks of gestation including biochemical pregnancy, and early PL was defined as PL before 10 weeks of gestational age [ 1 ]) and aged 18–42 years. Exclusion criteria: Patients who did not undergo any clinical examination after presentation and patients with severe psychiatric disorders who were not able to voluntarily enroll for subsequent follow-up. Patients are carefully asked for their reproductive history and personal demographic information when they join. If a patient had experienced a pregnancy loss and was currently non-pregnant at the time of presentation, an individualized pre-pregnancy intervention was given based on the results of the clinical examination. Pre-pregnancy interventions include improvements in thyroid function, correction of prothrombotic status, treatment of immune system disorders such as antiphospholipid antibody syndrome, folic acid supplementation, and advice on maintaining a healthy lifestyle. If a patient had experienced a pregnancy loss and was already pregnant at the time of presentation, pre-pregnancy intervention was lacking. During pregnancy, patients receive individualized treatment based on clinical symptoms and laboratory test results, including progesterone supplementation, aspirin, low molecular weight heparin, hydroxychloroquine, etc.
Data collection
The population data was obtained from the Reproductive Medicine Middle School at the Second Hospital of Lanzhou University. Demographic information included age (< 25, 25–29, 30–34, ≥ 35), age at first pregnancy, BMI (< 18.5, 18.5–23.9, 24.0-27.9, ≥ 28), education and ethnicity. Pregnancy status data included the patient’s total number of previous pregnancies, the history of induced abortion, live birth (delivery method), birth defects, ectopic pregnancy, hydatidiform mole, previous PL numbers and pregnancy loss type (primary or secondary). Age at menarche, menstrual cycle, dysmenorrhea status and history of pelvic surgery were also collected. Each patient was followed up every 6 months after the first visit to track the patient’s pregnancy status, most recently in August 2022. At follow-up, we collected the outcome of the next pregnancy, the gestational age, delivery method, gender, birth weight of the live birth and whether the newborn was admitted to a neonatology department. Whether the mother had gestational diabetes mellitus, gestational hypertension, intrauterine cholestasis during pregnancy, and premature rupture. In addition, there are some patients in the follow-up process, both spouses want to have children, have normal sexual life, more than a year without contraception, but still do not conceive, we defined it as infertility [ 18 ]. We obtained information through a medical records registry and telephone follow-up.
Statistical analysis
Descriptive statistics were used to describe the proportion and mean ± standard deviation of the demographic characteristics. Independent sample t test was used to compare the differences between the two groups, and one-way analysis of variance (ANOVA) was used to compare the differences among the three groups. Categorical data were compared with the chi-square test or Fisher’s exact test. The P < 0.1 of the variables were included in the Logistic regression analysis to estimate the odds ratio (OR) between research factors and risk of pregnancy outcome.
Characteristics of participants
From all participants, 1955 patients were enrolled into our database between September 2019 to February 2022. Table 1 shows that the average age is 30.51 ± 4.41 years and the average of first pregnancy age is 26.41 ± 3.74 years. The proportion of overweight [(BMI 24.0-27.9 kg/m 2 )/ obesity (BMI ≥ 28 kg/m 2 ) was diagnosed according to the Working Group on Obesity in China [ 19 ])] was 26.13%. Only one PL accounted for 40.87% and the RPL accounted for 59.13%. Primary PL accounted for 78.31%.
The total number of cumulative pregnancies (defined as the total number of pregnancies at the time of the first visit for all patients, excluding the current already pregnant at the time of the first visit) was 4606, of which 3696 were PLs, 445 were live births, 251 were induced abortions, 101 were ectopic pregnancies, 20 were hydatidiform moles, 75 were birth defects, and 18 were others (Supplementary Fig. 1 ).
At the time of the first visit, 1,593 patients were currently non-pregnant, preparing for their next pregnancy and seeking help. There were also 362 patients who had also experienced at least one previous PL but sought treatment after their current pregnancy was confirmed, who were already pregnant at the time of the first visit.
Reproductive status in different age, BMI, pregnancy loss numbers groups in the study
The survey showed that in different age groups (< 25, 25–29, 30–34, ≥ 35), the BMI, total pregnancy numbers, PL numbers and first pregnancy age were increased with age and the difference was statistically significant ( P < 0.001). With the increase of age, the proportion of the types of secondary PL and the proportion of those who experienced induced abortion, live birth, cesarean section and pelvic surgery are increased ( P < 0.001). The rate of ectopic pregnancies was higher in the 30–35 age group. With the increase of age, the proportion of women with regular periods increases, while the number of women with moderate or severe dysmenorrhea decreases (Supplementary Table 1 ). In different BMI groups (< 18.5 kg/m 2 , 18.5–23.9 kg/m 2 , 24.0-27.9 kg/m 2 , ≥ 28 kg/m 2 ), there were differences in patients age and first pregnancy age. In addition, with the increase of BMI, the age of menarche was slightly earlier ( P = 0.003). And the incidence of pelvic surgery was lowest in the normal-weight group ( P < 0.001) (Supplementary Table 2 ). In different PL numbers groups (1, 2, 3, ≥ 4), the total pregnancy numbers and age were increased with the number of PL, the first pregnancy age was decreased with the number of PL ( P < 0.001). With the increase of the number of PL, the proportion of secondary PL, live birth and regular menstruation are increased ( P < 0.05) (Supplementary Table 3 ).
The follow-up results of 1955 patients
Figure 1 . shows that during follow-up, 74 cases were refused to accept follow-up. Of the remaining 1881 patients, 1532 were non-pregnant at the time of consultation and 349 were already pregnant at the time of consultation. In a follow-up study of 1,532 non-pregnant women, we found that 644 patients who were not pregnant, of whom 319 patients had been diagnosed as infertile for more than 1 year without contraception. A total of 888 women experienced a second pregnancy, of which 174 had early PL, 6 had late PL, and 445 had a live birth. In the follow-up study of 349 pregnant women, we found that there were 127 women experienced their next early PL, 15 had late PL, and 183 had a live birth. Among all patients, the incidence of subsequent infertility was 20.82% (319/1532), the incidence of early PL was 24.33% [(174 + 127)/ (888 + 349)], and the incidence of late PL was 1.69% [(6 + 15)/ (888 + 349)]. The live birth rate was 50.77% [(445 + 183)/ (888 + 349)].
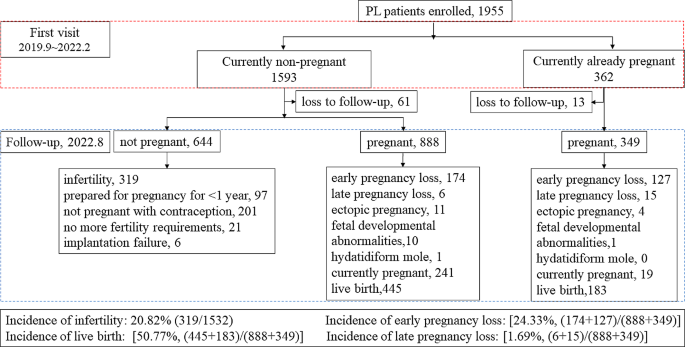
Flow diagram of the patients selected for the study
Maternal and infant complications in patients with live birth in a subsequent pregnancy
Fig. 2 . shows that, in the study, 628 confirmed live births were reported as of August 2022, of which preterm birth occurred in 68 patients, accounting for 10.83%. A total of 567 women reported their mode of delivery, including 223 (39.33%) vaginal delivery and 344 (60.67%) cesarean section. There were 43 cases of cesarean section due to patients’ request which called non-iatrogenic cesarean Sect. (43/567, 7.58%) and 301 cases of cesarean section due to medical reasons which called iatrogenic cesarean Sect. (301/567, 53.09%). The gender of the newborns was reported in 562 cases, including 275 singleton boys and 275 singleton girls. 461 cases reported whether they had gestational diabetes mellitus, of which 55 cases were diagnosed with gestational diabetes mellitus, accounting for 11.93%; 476 cases reported whether they had gestational hypertension, and 33 cases (6.93%) were diagnosed. 447 cases were reported whether they had intrahepatic cholestasis of pregnancy, and 12 cases (2.68%) were diagnosed. 479 cases reported whether they had premature rupture, and 63 cases were confirmed, accounting for 13.15%. 298 cases reported whether they had postpartum hemorrhage, and 6 cases were confirmed, accounting for 2.01%.
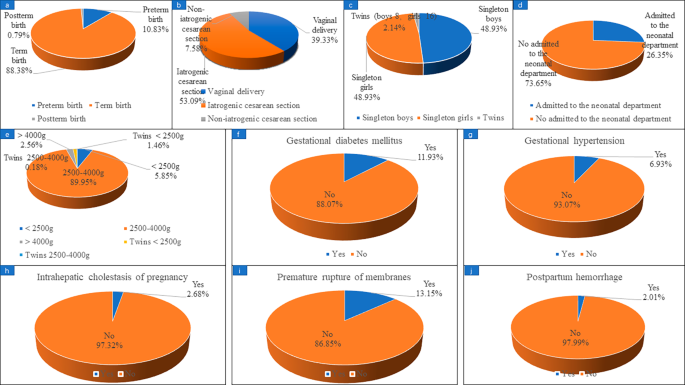
Maternal and infant complications in patients with live birth in subsequent pregnancy. (a) preterm birth; (b) delivery method; (c) gender of newborn; (d) newborns admitted to the neonatal department; (e) neonatal weight; (f) gestational diabetes mellitus; (g) gestational hypertension; (h) intrahepatic cholestasis of pregnancy; (i) premature rupture; (j) postpartum hemorrhage
Whether the previous pregnancy status affects the patient’s subsequent pregnancy?
During follow-up, 319 patients were diagnosed with infertility after their last pregnancy loss, and 1237 patients were able to achieve a successful pregnancy. There was a significant difference in age between the infertility group and the successful pregnancy group (31.02 ± 4.79 vs. 30.16 ± 4.13, P < 0.001). There were also statistical differences between the infertility and successful pregnancy groups in the type of PL, the previous live birth and the delivery method, the previous birth defects. The age of first pregnancy and BMI were different, but not statistically significant. There were no statistical differences in the total pregnancy numbers, the previous PL numbers, the history of induced abortion, ectopic pregnancy, hydatidiform mole, menarche age, menstrual cycle, dysmenorrhea or not, previous pelvic surgery, the last pregnancy termination method between the infertility group and the successful pregnancy group (Table 2 ). The P < 0.1 of the variables were included in the logistic regression and found that, increasing age (OR 1.08, 95% CI 1.04–1.13) and previous cesarean delivery history (OR 2.46, 95% CI 1.27–4.76) were risk factors for subsequent infertility in patients with PL (Table 3 ).
Whether the previous pregnancy status affects the live birth in subsequent pregnancy?
Of the 1237 women who had subsequent pregnancies, 977 had final pregnancy outcomes, including 628 live births and 349 non-live births. We found that the age, age at first pregnancy, BMI, and previous pregnancy loss numbers were lower in the live birth group than in the non-live birth group. Pre-pregnancy intervention increased live births compared to without pre-pregnancy intervention. Total pregnancy numbers were different but not statistically significant between the live birth group and the non-live birth group. There were no statistical differences in the total pregnancy numbers, the pregnancy interval, the pregnancy type, the history of induced abortion, ectopic pregnancy, hydatidiform mole, menarche age, menstrual cycle, dysmenorrhea or not, previous pelvic surgery, the last pregnancy termination method between the live birth group and the non-live birth group (Table 2 ). In logistic regression analysis, we found that age (OR 1.06, 95% CI 1.03–1.10), age at first pregnancy (OR 1.06, 95% CI 1.03–1.10), BMI (OR 1.06, 95% CI 1.02–1.11), previous pregnancy loss numbers (OR 1.18, 95% CI 1.04–1.57) and without pre-pregnancy intervention (OR 1.77, 95% CI 1.35–2.24) were risk factors for non-live birth (Table 4 ).
Whether the previous pregnancy status affects the pregnancy loss in subsequent pregnancy?
Of the 1237 women who had subsequent pregnancies, 322 had confirmed subsequent pregnancy losses and 756 had pregnancies that were > 24 W, which was considered an ongoing pregnancy. We found that age, age at first pregnancy, BMI, and previous pregnancy loss numbers were higher in the pregnancy loss group than in the ongoing pregnancy group. Pre-pregnancy intervention decreased pregnancy loss compared to without pre-pregnancy intervention. There were no statistical differences in the total pregnancy numbers, the pregnancy interval, the pregnancy type, the history of induced abortion, ectopic pregnancy, hydatidiform mole, menarche age, menstrual cycle, dysmenorrhea or not, previous pelvic surgery, the last pregnancy termination method between the pregnancy loss group and the ongoing pregnancy group (Table 2 ). In logistic regression analysis, we found that age (OR 1.06, 95% CI 1.03–1.09), age at first pregnancy (OR 1.06, 95% CI 1.02–1.09), BMI (OR 1.07, 95% CI 1.02–1.11), previous pregnancy loss numbers (OR 1.15, 95% CI 1.02–1.31) and without pre-pregnancy intervention (OR 2.16, 95% CI 1.65–2.84) were risk factors for PL (Table 5 ).
The incidence of PL has been increasing in recent years, but few studies have summarized the reproductive status of patients with previous PL. Our study summarized the distribution of pregnancies in 1955 pregnancy loss patients and followed them for subsequent pregnancy outcomes. We found that patients with PL also had other adverse pregnancy events, such as birth defects (3.73%), ectopic pregnancy (4.65%) and hydatidiform mole (1.02%). But none of this have an effect on subsequent pregnancies in our analysis. Of the 1955 women with PL, 20.46% had a previous live birth, of which 32.91% were delivered by cesarean section, which increased the risk of subsequent infertility in women with PL, but had no effect on the ongoing pregnancy and live birth in subsequent pregnancies. In recent years, the relationship between cesarean scar uterus and subsequent secondary infertility has been gradually recognized, but the specific mechanism is not clear [ 20 , 21 ]. Nobuta et al. found that a cause of secondary infertility in women with cesarean scar syndrome may be chronic inflammation of the uterine cavity [ 22 ]. We also found that prior induced abortion, mode of termination of the last pregnancy, age at menarche, menstrual cycle, and level of dysmenorrhea had no effect on subsequent pregnancy outcomes. However, previous studies have found that the risk of spontaneous abortion decreases with the increase in the number of induced abortions among female workers in the Jinchang Cohort [ 7 ]. This is not consistent with our results. The possible reason is that the reference population was derived from all female workers in the Jinchang cohort in China, most of whom had normal reproductive function. In contrast, all the patients in our study were women of childbearing age who had experienced at least one pregnancy loss.
Our study found that age is an important risk factor in the occurrence of infertility after PL, also resulting in an increased risk of pregnancy loss and a decreased live birth in subsequent pregnancies. The association between female age and RPL has been consistently demonstrated in several studies. The age-related risk of pregnancy loss followed a J-shaped curve, with the lowest risk at ages 25 to 29 years, an increase in risk among women 30 to 35 years of age, and then a sharp rise in risk among women 40 to 44 years of age [ 8 ].
Age at first pregnancy, BMI, and the number of previous PL were also key indicators of subsequent pregnancy failure. Based on a computer-simulated fertility model, couples should start trying to conceive when the woman is 31 or less to have at least a 90% chance of having a two-child family, and if IVF is not feasible, couples should start planning no later than 27. In order to achieve a one-child family, couples should start trying before the age of 32, or 35 if IVF is an option [ 23 ].
Our study found that approximately 26.13% (140/658) of prior PL patients were overweight/obesity, which is higher than the pre-pregnancy overweight/obesity rates found in a birth cohort in Shanghai (19.06% (106/556)) [ 24 ]. But in the USA, a 2009–2010 survey indicated that 55.8% of women of childbearing age were overweight or obese, defined as having a BMI of 25 or higher, significantly higher than our research found [ 25 ]. There are also variations in the threshold of BMI for pregnancy. Zhang et al. reported that, a BMI of 24.0 kg/m 2 or greater was associated with an increased risk of RPL, but Lo and colleagues demonstrated that maternal obesity (BMI ≥ 30.0 kg/m 2 ) significantly increased the risk of miscarriage in couples with unexplained RPL and there was no increased risk in women with overweight and underweight [ 10 , 26 ]. This suggests that BMI reference ranges should be tailored to patient geographic region and disease status.
The impact of the number of previous PL on the chance of live birth has been investigated in several cohort studies. The risk of PL during a second pregnancy is associated with the number of PL. The risk is about 20% after one PL, 28% after two PLs, and 43% after three or more PLs [ 27 , 28 ]. In a nested cohort, it was demonstrated that the number of prior miscarriages was a determinant both for time to live birth and cumulative incidence of live birth [ 29 , 30 ]. It is worth noting that for secondary URPL, only consecutive PL after the birth influenced the subsequent prognosis, while the number of losses prior to the birth did not affect the prognosis in the next pregnancy [ 31 ].
Finally, we found that individualized pre-pregnancy intervention increased the rate of live birth and decreased the rate of PL in subsequent pregnancies. These individualized pre-pregnancy interventions were based on patient clinical examination findings, including treatment for endocrine abnormalities, prethrombotic state, immune disorders, antiphospholipid antibody syndrome, and lifestyle modification before subsequence pregnancy. Study found that a combination of heparin and aspirin treatment can improve the APS and recurrent pregnancy loss of the pregnancy outcomes of women but add corticosteroids (e.g., prednisone), cannot improve live birth rates, and increase the risk of obstetric diseases, such as premature delivery, preeclampsia, gestational diabetes, enter the neonatal intensive care unit [ 32 , 33 ]. Patients with RPL who have overt hypothyroidism before or during the first trimester should be treated with levothyroxine (thyroid hormone replacement therapy). However, levothyroxine did not improve pregnancy outcomes in patients with subclinical hypothyroidism [ 34 ]. For immune diseases, the treatment of intravenous immune globulin (IVIG) is still controversial [ 35 , 36 ]. At present, there are still some controversies and uncertainties in the treatment of PL patients, and further standardized treatment is needed. In addition, RPL is an independent risk factor for women’s long-term increased incidence of malignant tumors (such as breast cancer and cervical cancer) and cardiovascular diseases [ 37 ]. Therefore, we should give individualized pre-pregnancy intervention to patients with PL not only to improve the subsequent pregnancy outcome, but also to potentially reduce the risk of long-term complications.
Our study still has some limitations. We did not capture complications for all patients who had live births. Due to the individualization of pre-pregnancy treatment, the diagnosis and treatment process were not recorded in detail. However, we are in the process of establishing pregnancy-loss specific cohorts, and the management of future patients will be more careful.
Maternal age and a history of cesarean section in a previous pregnancy are key factors for subsequent failure to achieve a successful pregnancy in patients with PL. Maternal age, age at first pregnancy, BMI, number of previous PL and pre-pregnancy treatment are the key factors affecting subsequent PL.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. ESHRE guideline: recurrent pregnancy loss. Hum Reprod open. 2018;2018(2):hoy004. https://doi.org/10.1093/hropen/hoy004 .
Article PubMed PubMed Central Google Scholar
Ruderman RS, Yilmaz BD, McQueen DB. Treating the couple: how recurrent pregnancy loss impacts the mental health of both partners. Fertil Steril. 2020;114(6):1182. https://doi.org/10.1016/j.fertnstert.2020.09.165 .
Article PubMed Google Scholar
Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, Al-Memar M, Brewin J, Abrahams VM, Maheshwari A, Christiansen OB, Stephenson MD, Goddijn M, Oladapo OT, Wijeyaratne CN, Bick D, Shehata H, Small R, Bennett PR, Regan L, Rai R, Bourne T, Kaur R, Pickering O, Brosens JJ, Devall AJ, Gallos ID, Quenby S. Recurrent miscarriage: evidence to accelerate action. Lancet (London England). 2021;397(10285):1675–82. https://doi.org/10.1016/s0140-6736(21)00681-4 .
Article CAS PubMed Google Scholar
Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Reviews Disease Primers. 2020;6(1):98. https://doi.org/10.1038/s41572-020-00228-z .
Voss P, Schick M, Langer L, Ainsworth A, Ditzen B, Strowitzki T, Wischmann T, Kuon RJ. Recurrent pregnancy loss: a shared stressor—couple-orientated psychological research findings. Fertil Steril. 2020;114(6):1288–96. https://doi.org/10.1016/j.fertnstert.2020.08.1421 .
Wang Y, Meng Z, Pei J, Qian L, Mao B, Li Y, Li J, Dai Z, Cao J, Zhang C, Chen L, Jin Y, Yi B. Anxiety and depression are risk factors for recurrent pregnancy loss: a nested case-control study. Health Qual Life Outcomes. 2021;19(1):78. https://doi.org/10.1186/s12955-021-01703-1 .
Hu X, Miao M, Bai Y, Cheng N, Ren X. Reproductive factors and risk of spontaneous abortion in the Jinchang Cohort. Int J Environ Res Public Health. 2018;15(11). https://doi.org/10.3390/ijerph15112444 .
Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ (Clinical research ed) 2019,364l869. https://doi.org/10.1136/bmj.l869 .
Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG: Int J Obstet Gynecol. 2007;114(2):170–86. https://doi.org/10.1111/j.1471-0528.2006.01193.x .
Article CAS Google Scholar
Lo W, Rai R, Hameed A, Brailsford SR, Al-Ghamdi AA, Regan L. The effect of body mass index on the outcome of pregnancy in women with recurrent miscarriage. J Fam Commun Med. 2012;19(3):167–71. https://doi.org/10.4103/2230-8229.102316 .
Article Google Scholar
Kruse C, Steffensen R, Varming K, Christiansen OB. A study of HLA-DR and -DQ alleles in 588 patients and 562 controls confirms that HLA-DRB1*03 is associated with recurrent miscarriage. Hum Reprod (Oxford England). 2004;19(5):1215–21. https://doi.org/10.1093/humrep/deh200 .
Nielsen HS, Andersen AM, Kolte AM, Christiansen OB. A firstborn boy is suggestive of a strong prognostic factor in secondary recurrent miscarriage: a confirmatory study. Fertil Steril. 2008;89(4):907–11. https://doi.org/10.1016/j.fertnstert.2007.04.029 .
Piosik ZM, Goegebeur Y, Klitkou L, Steffensen R, Christiansen OB. Plasma TNF-α levels are higher in early pregnancy in patients with secondary compared with primary recurrent miscarriage. Am J Reproductive Immunol (New York NY: 1989). 2013;70(5):347–58. https://doi.org/10.1111/aji.12135 .
Infante-Rivard C, Gauthier R. Induced abortion as a risk factor for subsequent fetal loss. Epidemiol (Cambridge Mass). 1996;7(5):540–2.
Chung CS, Smith RG, Steinhoff PG, Mi MP. Induced abortion and spontaneous fetal loss in subsequent pregnancies. Am J Public Health. 1982;72(6):548–54. https://doi.org/10.2105/ajph.72.6.548 .
Article CAS PubMed PubMed Central Google Scholar
Chen D, Xu Q, Mao X, Zhang J, Wu L. Reproductive history does not compromise subsequent live birth and perinatal outcome following in-vitro fertilization: analysis of 25 329 first frozen-thawed embryo transfer cycles without preimplantation genetic testing for aneuploidy. Ultrasound Obstet Gynecology: Official J Int Soc Ultrasound Obstet Gynecol. 2023;62(3):430–8. https://doi.org/10.1002/uog.26220 .
Kolte AM, Westergaard D, Lidegaard Ø, Brunak S, Nielsen HS. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod (Oxford England). 2021;36(4):1065–73. https://doi.org/10.1093/humrep/deaa326 .
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Human reproduction (Oxford, England) 2017,32(9):1786–1801. https://doi.org/10.1093/humrep/dex234 .
Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci: BES. 2002;15(1):83–96.
PubMed Google Scholar
Bi B, Gao S, Ruan F, Shi Y, Jiang Y, Liu S, Lv W. Analysis on clinical association of uterine scar diverticulum with subsequent infertility in patients underwent cesarean section. Medicine. 2021;100(41):e27531. https://doi.org/10.1097/md.0000000000027531 .
Ahamed FM, Solkar S, Stevikova M, Moya BP. Link between cesarean section scar defect and secondary infertility: case reports and review. JBRA Assist Reprod. 2023;27(1):134–41. https://doi.org/10.5935/1518-0557.20220009 .
Nobuta Y, Tsuji S, Kitazawa J, Hanada T, Nakamura A, Zen R, Amano T, Murakami T. Decreased fertility in women with cesarean scar syndrome is Associated with chronic inflammation in the uterine cavity. Tohoku J Exp Med. 2022;258(3):237–42. https://doi.org/10.1620/tjem.2022.J082 .
Habbema JD, Eijkemans MJ, Leridon H, te Velde ER. Realizing a desired family size: when should couples start? Hum Reprod (Oxford England). 2015;30(9):2215–21. https://doi.org/10.1093/humrep/dev148 .
Wang Z, Niu J, Ji H, Miao M, Yang L, Chen X, Li X, Song X, Chen A, Liang H, Yuan W. Association of pre-pregnancy body mass index and gestational weight gain with neonatal anogenital distance in a Chinese birth cohort. Reproductive Health. 2022;19(1):152. https://doi.org/10.1186/s12978-022-01458-y .
Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. https://doi.org/10.1001/jama.2012.39 .
Zhang BY, Wei YS, Niu JM, Li Y, Miao ZL, Wang ZN. Risk factors for unexplained recurrent spontaneous abortion in a population from southern China. Int J Gynaecol Obstet. 2010;108(2):135–8. https://doi.org/10.1016/j.ijgo.2009.09.019 .
Greenberg T, Tzivian L, Harlev A, Serjienko R, Mazor M, Bashiri A. Index pregnancy versus post-index pregnancy in patients with recurrent pregnancy loss. J maternal-fetal Neonatal Medicine: Official J Eur Association Perinat Med Federation Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2015;28(1):63–7. https://doi.org/10.3109/14767058.2014.900752 .
Coccia ME, Rizzello F. Two-year outcome after recurrent first trimester miscarriages: prognostic value of the past obstetric history. Arch Gynecol Obstet. 2017;295(1):261–2. https://doi.org/10.1007/s00404-016-4213-8 .
Kling C, Magez J, Hedderich J, von Otte S, Kabelitz D. Two-year outcome after recurrent first trimester miscarriages: prognostic value of the past obstetric history. Arch Gynecol Obstet. 2016;293(5):1113–23. https://doi.org/10.1007/s00404-015-4001-x .
Kaandorp SP, van Mens TE, Middeldorp S, Hutten BA, Hof MH, van der Post JA, van der Veen F, Goddijn M. Time to conception and time to live birth in women with unexplained recurrent miscarriage. Hum Reprod (Oxford England). 2014;29(6):1146–52. https://doi.org/10.1093/humrep/deu052 .
Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod (Oxford England). 2016;31(11):2428–34. https://doi.org/10.1093/humrep/dew169 .
Lu C, Liu Y, Jiang HL. Aspirin or heparin or both in the treatment of recurrent spontaneous abortion in women with antiphospholipid antibody syndrome: a meta-analysis of randomized controlled trials. J maternal-fetal Neonatal Medicine: Official J Eur Association Perinat Med Federation Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2019;32(8):1299–311. https://doi.org/10.1080/14767058.2017.1404979 .
Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;2005(2):Cd002859. https://doi.org/10.1002/14651858.CD002859.pub2 .
Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, Bender-Atik R, Agrawal R, Bhatia K, Edi-Osagie E, Ghobara T, Gupta P, Jurkovic D, Khalaf Y, MacLean M, McCabe C, Mulbagal K, Nunes N, Overton C, Quenby S, Rai R, Raine-Fenning N, Robinson L, Ross J, Sizer A, Small R, Tan A, Underwood M, Kilby MD, Boelaert K, Daniels J, Thangaratinam S, Chan SY, Coomarasamy A. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380(14):1316–25. https://doi.org/10.1056/NEJMoa1812537 .
Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13(3):176–89. https://doi.org/10.1038/nri3401 .
Christiansen OB, Larsen EC, Egerup P, Lunoee L, Egestad L, Nielsen HS. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double-blind, placebo-controlled trial. BJOG: Int J Obstet Gynecol. 2015;122(4):500–8. https://doi.org/10.1111/1471-0528.13192 .
Charach R, Sheiner E, Beharier O, Sergienko R, Kessous R. Recurrent pregnancy loss and future risk of female malignancies. Arch Gynecol Obstet. 2018;298(4):781–7. https://doi.org/10.1007/s00404-018-4868-4 .
Download references
Acknowledgements
Not applicable.
This work was supported by the Special Fund for Doctoral Student Training of The Second Hospital of Lanzhou University in 2019 (Grant No. YJS-BD-19) and the innovation and development project of medical postgraduate training of Lanzhou University (Grant No.820809059).
Author information
Authors and affiliations.
Reproductive Medicine Center, Second Hospital of Lanzhou University, No.82, Cuiying Road, Chengguan District, Lanzhou, 730030, Gansu Province, China
Xin Yang, Fangxiang Mu, Jian Zhang, Liwei Yuan, Wei Zhang, Yanting Yang & Fang Wang
You can also search for this author in PubMed Google Scholar
Contributions
Xin Yang: data analysis and drafted the manuscript. Fangxiang Mu: collect basic clinical characteristics data of PL patients. Jian Zhang and Liwei Yuan: follow-up the pregnancy outcomes in their next pregnancy. Wei Zhang and Yanting Yang: data verification. Fang Wang: provide research proposals and funding support. All authors reviewed the manuscript.
Corresponding author
Correspondence to Fang Wang .
Ethics declarations
Ethics approval and consent to participation.
The study was approved by the Ethics Committee of Lanzhou University Second Hospital (Ethical Approval Number: 2019A-231). All subjects gave informed consent before participation. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Competing interests.
The authors declare no potential conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yang, X., Mu, F., Zhang, J. et al. Reproductive factors and subsequent pregnancy outcomes in patients with prior pregnancy loss. BMC Pregnancy Childbirth 24 , 219 (2024). https://doi.org/10.1186/s12884-024-06422-1
Download citation
Received : 08 December 2022
Accepted : 14 March 2024
Published : 25 March 2024
DOI : https://doi.org/10.1186/s12884-024-06422-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pregnancy loss
- Reproductive status
- Pregnancy outcome
- Logistic regression
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Read our research on: Abortion | Podcasts | Election 2024
Regions & Countries
What the data says about abortion in the u.s..
Pew Research Center has conducted many surveys about abortion over the years, providing a lens into Americans’ views on whether the procedure should be legal, among a host of other questions.
In a Center survey conducted nearly a year after the Supreme Court’s June 2022 decision that ended the constitutional right to abortion , 62% of U.S. adults said the practice should be legal in all or most cases, while 36% said it should be illegal in all or most cases. Another survey conducted a few months before the decision showed that relatively few Americans take an absolutist view on the issue .
Find answers to common questions about abortion in America, based on data from the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, which have tracked these patterns for several decades:
How many abortions are there in the U.S. each year?
How has the number of abortions in the u.s. changed over time, what is the abortion rate among women in the u.s. how has it changed over time, what are the most common types of abortion, how many abortion providers are there in the u.s., and how has that number changed, what percentage of abortions are for women who live in a different state from the abortion provider, what are the demographics of women who have had abortions, when during pregnancy do most abortions occur, how often are there medical complications from abortion.
This compilation of data on abortion in the United States draws mainly from two sources: the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, both of which have regularly compiled national abortion data for approximately half a century, and which collect their data in different ways.
The CDC data that is highlighted in this post comes from the agency’s “abortion surveillance” reports, which have been published annually since 1974 (and which have included data from 1969). Its figures from 1973 through 1996 include data from all 50 states, the District of Columbia and New York City – 52 “reporting areas” in all. Since 1997, the CDC’s totals have lacked data from some states (most notably California) for the years that those states did not report data to the agency. The four reporting areas that did not submit data to the CDC in 2021 – California, Maryland, New Hampshire and New Jersey – accounted for approximately 25% of all legal induced abortions in the U.S. in 2020, according to Guttmacher’s data. Most states, though, do have data in the reports, and the figures for the vast majority of them came from each state’s central health agency, while for some states, the figures came from hospitals and other medical facilities.
Discussion of CDC abortion data involving women’s state of residence, marital status, race, ethnicity, age, abortion history and the number of previous live births excludes the low share of abortions where that information was not supplied. Read the methodology for the CDC’s latest abortion surveillance report , which includes data from 2021, for more details. Previous reports can be found at stacks.cdc.gov by entering “abortion surveillance” into the search box.
For the numbers of deaths caused by induced abortions in 1963 and 1965, this analysis looks at reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. In computing those figures, we excluded abortions listed in the report under the categories “spontaneous or unspecified” or as “other.” (“Spontaneous abortion” is another way of referring to miscarriages.)
Guttmacher data in this post comes from national surveys of abortion providers that Guttmacher has conducted 19 times since 1973. Guttmacher compiles its figures after contacting every known provider of abortions – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, and it provides estimates for abortion providers that don’t respond to its inquiries. (In 2020, the last year for which it has released data on the number of abortions in the U.S., it used estimates for 12% of abortions.) For most of the 2000s, Guttmacher has conducted these national surveys every three years, each time getting abortion data for the prior two years. For each interim year, Guttmacher has calculated estimates based on trends from its own figures and from other data.
The latest full summary of Guttmacher data came in the institute’s report titled “Abortion Incidence and Service Availability in the United States, 2020.” It includes figures for 2020 and 2019 and estimates for 2018. The report includes a methods section.
In addition, this post uses data from StatPearls, an online health care resource, on complications from abortion.
An exact answer is hard to come by. The CDC and the Guttmacher Institute have each tried to measure this for around half a century, but they use different methods and publish different figures.
The last year for which the CDC reported a yearly national total for abortions is 2021. It found there were 625,978 abortions in the District of Columbia and the 46 states with available data that year, up from 597,355 in those states and D.C. in 2020. The corresponding figure for 2019 was 607,720.
The last year for which Guttmacher reported a yearly national total was 2020. It said there were 930,160 abortions that year in all 50 states and the District of Columbia, compared with 916,460 in 2019.
- How the CDC gets its data: It compiles figures that are voluntarily reported by states’ central health agencies, including separate figures for New York City and the District of Columbia. Its latest totals do not include figures from California, Maryland, New Hampshire or New Jersey, which did not report data to the CDC. ( Read the methodology from the latest CDC report .)
- How Guttmacher gets its data: It compiles its figures after contacting every known abortion provider – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, then provides estimates for abortion providers that don’t respond. Guttmacher’s figures are higher than the CDC’s in part because they include data (and in some instances, estimates) from all 50 states. ( Read the institute’s latest full report and methodology .)
While the Guttmacher Institute supports abortion rights, its empirical data on abortions in the U.S. has been widely cited by groups and publications across the political spectrum, including by a number of those that disagree with its positions .
These estimates from Guttmacher and the CDC are results of multiyear efforts to collect data on abortion across the U.S. Last year, Guttmacher also began publishing less precise estimates every few months , based on a much smaller sample of providers.
The figures reported by these organizations include only legal induced abortions conducted by clinics, hospitals or physicians’ offices, or those that make use of abortion pills dispensed from certified facilities such as clinics or physicians’ offices. They do not account for the use of abortion pills that were obtained outside of clinical settings .
(Back to top)
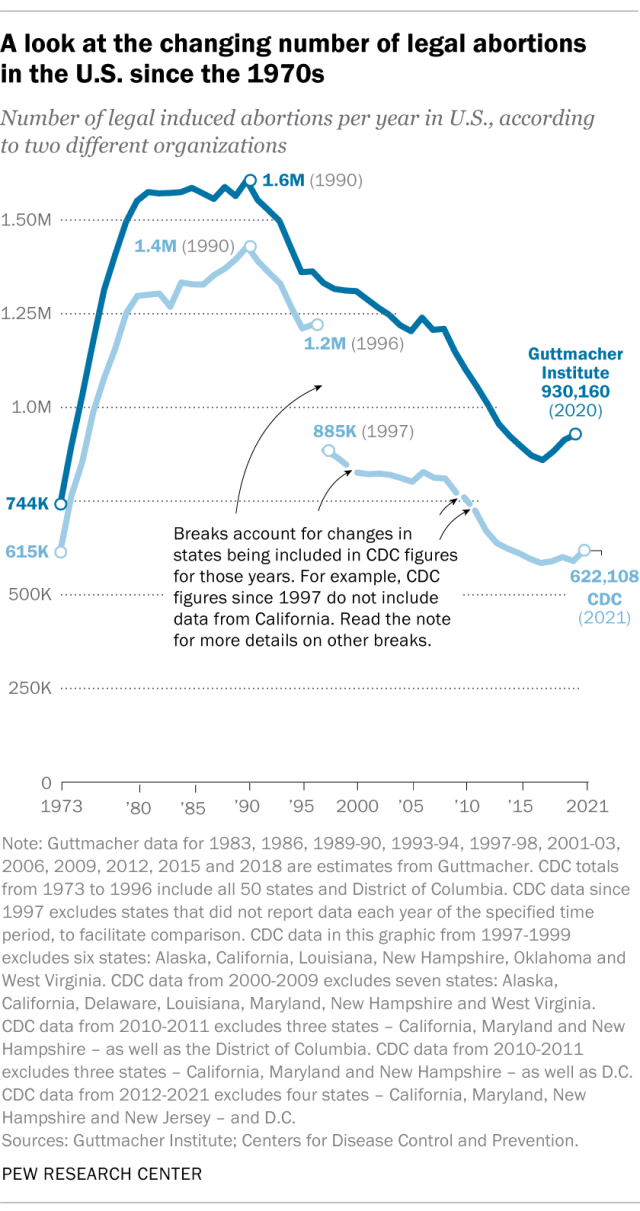
The annual number of U.S. abortions rose for years after Roe v. Wade legalized the procedure in 1973, reaching its highest levels around the late 1980s and early 1990s, according to both the CDC and Guttmacher. Since then, abortions have generally decreased at what a CDC analysis called “a slow yet steady pace.”
Guttmacher says the number of abortions occurring in the U.S. in 2020 was 40% lower than it was in 1991. According to the CDC, the number was 36% lower in 2021 than in 1991, looking just at the District of Columbia and the 46 states that reported both of those years.
(The corresponding line graph shows the long-term trend in the number of legal abortions reported by both organizations. To allow for consistent comparisons over time, the CDC figures in the chart have been adjusted to ensure that the same states are counted from one year to the next. Using that approach, the CDC figure for 2021 is 622,108 legal abortions.)
There have been occasional breaks in this long-term pattern of decline – during the middle of the first decade of the 2000s, and then again in the late 2010s. The CDC reported modest 1% and 2% increases in abortions in 2018 and 2019, and then, after a 2% decrease in 2020, a 5% increase in 2021. Guttmacher reported an 8% increase over the three-year period from 2017 to 2020.
As noted above, these figures do not include abortions that use pills obtained outside of clinical settings.
Guttmacher says that in 2020 there were 14.4 abortions in the U.S. per 1,000 women ages 15 to 44. Its data shows that the rate of abortions among women has generally been declining in the U.S. since 1981, when it reported there were 29.3 abortions per 1,000 women in that age range.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher’s data, the CDC’s figures also suggest a general decline in the abortion rate over time. In 1980, when the CDC reported on all 50 states and D.C., it said there were 25 abortions per 1,000 women ages 15 to 44.
That said, both Guttmacher and the CDC say there were slight increases in the rate of abortions during the late 2010s and early 2020s. Guttmacher says the abortion rate per 1,000 women ages 15 to 44 rose from 13.5 in 2017 to 14.4 in 2020. The CDC says it rose from 11.2 per 1,000 in 2017 to 11.4 in 2019, before falling back to 11.1 in 2020 and then rising again to 11.6 in 2021. (The CDC’s figures for those years exclude data from California, D.C., Maryland, New Hampshire and New Jersey.)
The CDC broadly divides abortions into two categories: surgical abortions and medication abortions, which involve pills. Since the Food and Drug Administration first approved abortion pills in 2000, their use has increased over time as a share of abortions nationally, according to both the CDC and Guttmacher.
The majority of abortions in the U.S. now involve pills, according to both the CDC and Guttmacher. The CDC says 56% of U.S. abortions in 2021 involved pills, up from 53% in 2020 and 44% in 2019. Its figures for 2021 include the District of Columbia and 44 states that provided this data; its figures for 2020 include D.C. and 44 states (though not all of the same states as in 2021), and its figures for 2019 include D.C. and 45 states.
Guttmacher, which measures this every three years, says 53% of U.S. abortions involved pills in 2020, up from 39% in 2017.
Two pills commonly used together for medication abortions are mifepristone, which, taken first, blocks hormones that support a pregnancy, and misoprostol, which then causes the uterus to empty. According to the FDA, medication abortions are safe until 10 weeks into pregnancy.
Surgical abortions conducted during the first trimester of pregnancy typically use a suction process, while the relatively few surgical abortions that occur during the second trimester of a pregnancy typically use a process called dilation and evacuation, according to the UCLA School of Medicine.
In 2020, there were 1,603 facilities in the U.S. that provided abortions, according to Guttmacher . This included 807 clinics, 530 hospitals and 266 physicians’ offices.
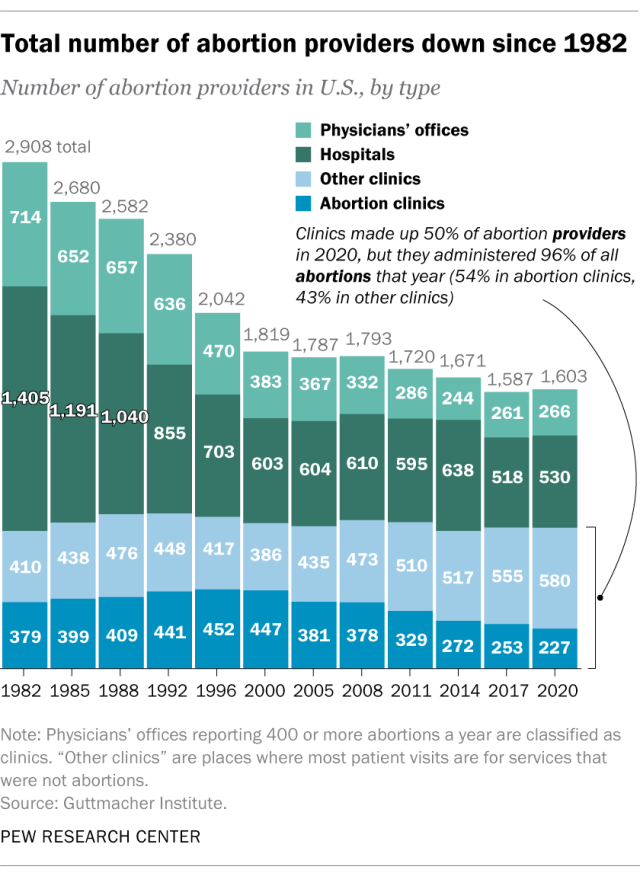
While clinics make up half of the facilities that provide abortions, they are the sites where the vast majority (96%) of abortions are administered, either through procedures or the distribution of pills, according to Guttmacher’s 2020 data. (This includes 54% of abortions that are administered at specialized abortion clinics and 43% at nonspecialized clinics.) Hospitals made up 33% of the facilities that provided abortions in 2020 but accounted for only 3% of abortions that year, while just 1% of abortions were conducted by physicians’ offices.
Looking just at clinics – that is, the total number of specialized abortion clinics and nonspecialized clinics in the U.S. – Guttmacher found the total virtually unchanged between 2017 (808 clinics) and 2020 (807 clinics). However, there were regional differences. In the Midwest, the number of clinics that provide abortions increased by 11% during those years, and in the West by 6%. The number of clinics decreased during those years by 9% in the Northeast and 3% in the South.
The total number of abortion providers has declined dramatically since the 1980s. In 1982, according to Guttmacher, there were 2,908 facilities providing abortions in the U.S., including 789 clinics, 1,405 hospitals and 714 physicians’ offices.
The CDC does not track the number of abortion providers.
In the District of Columbia and the 46 states that provided abortion and residency information to the CDC in 2021, 10.9% of all abortions were performed on women known to live outside the state where the abortion occurred – slightly higher than the percentage in 2020 (9.7%). That year, D.C. and 46 states (though not the same ones as in 2021) reported abortion and residency data. (The total number of abortions used in these calculations included figures for women with both known and unknown residential status.)
The share of reported abortions performed on women outside their state of residence was much higher before the 1973 Roe decision that stopped states from banning abortion. In 1972, 41% of all abortions in D.C. and the 20 states that provided this information to the CDC that year were performed on women outside their state of residence. In 1973, the corresponding figure was 21% in the District of Columbia and the 41 states that provided this information, and in 1974 it was 11% in D.C. and the 43 states that provided data.
In the District of Columbia and the 46 states that reported age data to the CDC in 2021, the majority of women who had abortions (57%) were in their 20s, while about three-in-ten (31%) were in their 30s. Teens ages 13 to 19 accounted for 8% of those who had abortions, while women ages 40 to 44 accounted for about 4%.
The vast majority of women who had abortions in 2021 were unmarried (87%), while married women accounted for 13%, according to the CDC , which had data on this from 37 states.
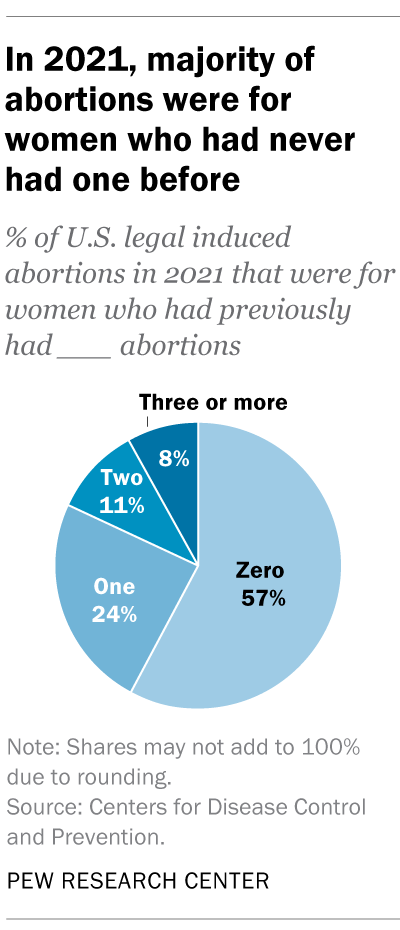
In the District of Columbia, New York City (but not the rest of New York) and the 31 states that reported racial and ethnic data on abortion to the CDC , 42% of all women who had abortions in 2021 were non-Hispanic Black, while 30% were non-Hispanic White, 22% were Hispanic and 6% were of other races.
Looking at abortion rates among those ages 15 to 44, there were 28.6 abortions per 1,000 non-Hispanic Black women in 2021; 12.3 abortions per 1,000 Hispanic women; 6.4 abortions per 1,000 non-Hispanic White women; and 9.2 abortions per 1,000 women of other races, the CDC reported from those same 31 states, D.C. and New York City.
For 57% of U.S. women who had induced abortions in 2021, it was the first time they had ever had one, according to the CDC. For nearly a quarter (24%), it was their second abortion. For 11% of women who had an abortion that year, it was their third, and for 8% it was their fourth or more. These CDC figures include data from 41 states and New York City, but not the rest of New York.
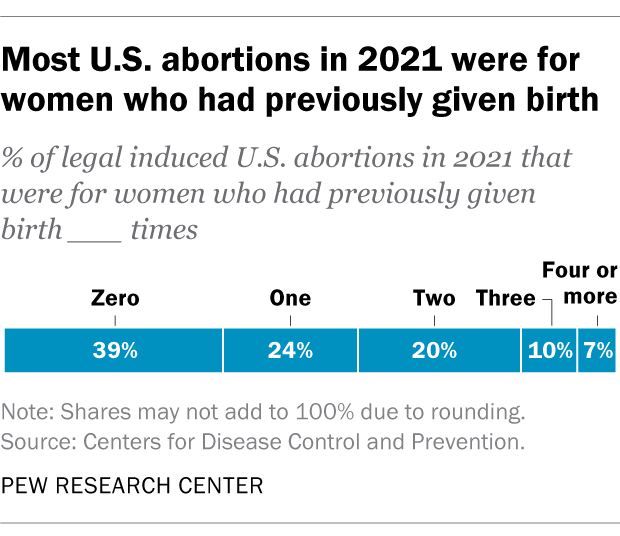
Nearly four-in-ten women who had abortions in 2021 (39%) had no previous live births at the time they had an abortion, according to the CDC . Almost a quarter (24%) of women who had abortions in 2021 had one previous live birth, 20% had two previous live births, 10% had three, and 7% had four or more previous live births. These CDC figures include data from 41 states and New York City, but not the rest of New York.
The vast majority of abortions occur during the first trimester of a pregnancy. In 2021, 93% of abortions occurred during the first trimester – that is, at or before 13 weeks of gestation, according to the CDC . An additional 6% occurred between 14 and 20 weeks of pregnancy, and about 1% were performed at 21 weeks or more of gestation. These CDC figures include data from 40 states and New York City, but not the rest of New York.
About 2% of all abortions in the U.S. involve some type of complication for the woman , according to an article in StatPearls, an online health care resource. “Most complications are considered minor such as pain, bleeding, infection and post-anesthesia complications,” according to the article.
The CDC calculates case-fatality rates for women from induced abortions – that is, how many women die from abortion-related complications, for every 100,000 legal abortions that occur in the U.S . The rate was lowest during the most recent period examined by the agency (2013 to 2020), when there were 0.45 deaths to women per 100,000 legal induced abortions. The case-fatality rate reported by the CDC was highest during the first period examined by the agency (1973 to 1977), when it was 2.09 deaths to women per 100,000 legal induced abortions. During the five-year periods in between, the figure ranged from 0.52 (from 1993 to 1997) to 0.78 (from 1978 to 1982).
The CDC calculates death rates by five-year and seven-year periods because of year-to-year fluctuation in the numbers and due to the relatively low number of women who die from legal induced abortions.
In 2020, the last year for which the CDC has information , six women in the U.S. died due to complications from induced abortions. Four women died in this way in 2019, two in 2018, and three in 2017. (These deaths all followed legal abortions.) Since 1990, the annual number of deaths among women due to legal induced abortion has ranged from two to 12.
The annual number of reported deaths from induced abortions (legal and illegal) tended to be higher in the 1980s, when it ranged from nine to 16, and from 1972 to 1979, when it ranged from 13 to 63. One driver of the decline was the drop in deaths from illegal abortions. There were 39 deaths from illegal abortions in 1972, the last full year before Roe v. Wade. The total fell to 19 in 1973 and to single digits or zero every year after that. (The number of deaths from legal abortions has also declined since then, though with some slight variation over time.)
The number of deaths from induced abortions was considerably higher in the 1960s than afterward. For instance, there were 119 deaths from induced abortions in 1963 and 99 in 1965 , according to reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. The CDC is a division of Health and Human Services.
Note: This is an update of a post originally published May 27, 2022, and first updated June 24, 2022.

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
Key facts about the abortion debate in America
Public opinion on abortion, three-in-ten or more democrats and republicans don’t agree with their party on abortion, partisanship a bigger factor than geography in views of abortion access locally, do state laws on abortion reflect public opinion, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

IMAGES
VIDEO
COMMENTS
Frank breech. When a baby's feet or buttocks are in place to come out first during birth, it's called a breech presentation. This happens in about 3% to 4% of babies close to the time of birth. The baby shown below is in a frank breech presentation. That's when the knees aren't bent, and the feet are close to the baby's head.
Fetal position refers to whether the baby is facing your spine (anterior position) or facing your belly (posterior position). Fetal position can change often: Your baby may be face up at the beginning of labor and face down at delivery. Here are the many possibilities for fetal presentation and position in the womb.
Occiput or cephalic anterior: This is the best fetal position for childbirth. It means the fetus is head down, facing the birth parent's spine (facing backward). Its chin is tucked towards its chest. The fetus will also be slightly off-center, with the back of its head facing the right or left. This is called left occiput anterior or right ...
During routine prenatal care, clinicians assess fetal lie and presentation with physical examination in the late third trimester. Ultrasonography can also be done. If breech presentation is detected, external cephalic version can sometimes move the fetus to vertex presentation before labor, usually at 37 or 38 weeks.
During pregnancy, the fetus can be positioned in many different ways inside the mother's uterus. The fetus may be head up or down or facing the mother's back or front. At first, the fetus can move around easily or shift position as the mother moves. Toward the end of the pregnancy the fetus is larger, has less room to move, and stays in one ...
The term presentation describes the leading part of the fetus or the anatomical structure closest to the maternal pelvic inlet during labor. The presentation can roughly be divided into the following classifications: cephalic, breech, shoulder, and compound. Cephalic presentation is the most common and can be further subclassified as vertex, sinciput, brow, face, and chin. The most common ...
Breech presentation refers to the fetus in the longitudinal lie with the buttocks or lower extremity entering the pelvis first. The three types of breech presentation include frank breech, complete breech, and incomplete breech. In a frank breech, the fetus has flexion of both hips, and the legs are straight with the feet near the fetal face, in a pike position.
Presentation of twins in Der Rosengarten ("The Rose Garden"), a standard medical text for midwives published in 1513. In obstetrics, the presentation of a fetus about to be born specifies which anatomical part of the fetus is leading, that is, is closest to the pelvic inlet of the birth canal. According to the leading part, this is identified as a cephalic, breech, or shoulder presentation.
Position and Presentation of the Fetus. Toward the end of pregnancy, the fetus moves into position for delivery. Normally, the position of a fetus is facing rearward (toward the woman's back) with the face and body angled to one side and the neck flexed, and presentation is head first. An abnormal position is facing forward, and abnormal ...
Introduction. Cephalic presentation is the most physiologic and frequent fetal presentation and is associated with the highest rate of successful vaginal delivery as well as with the lowest frequency of complications 1.Studies on the frequency of breech presentation by gestational age (GA) were published more than 20 years ago 2, 3, and it has been known that the prevalence of breech ...
The vertex presentation describes the orientation a fetus should be in for a safe vaginal delivery. It becomes important as you near your due date because it tells your pregnancy care provider how they may need to deliver your baby. Vertex means "crown of the head.". This means that the crown of the fetus's head is presenting towards the ...
Breech Births. In the last weeks of pregnancy, a baby usually moves so his or her head is positioned to come out of the vagina first during birth. This is called a vertex presentation. A breech presentation occurs when the baby's buttocks, feet, or both are positioned to come out first during birth. This happens in 3-4% of full-term births.
Compound presentation means that a fetal hand is coming out with the fetal head. This is a problem because: The amount of baby that must come through the birth canal at one time is increased. There is increased risk of mechanical injury to the arm and shoulder, including fractures, nerve injuries and soft tissue injury.
Abnormal Fetal Lie. If the fetal lie is abnormal, an external cephalic version (ECV) can be attempted - ideally between 36 and 38 weeks gestation. ECV is the manipulation of the fetus to a cephalic presentation through the maternal abdomen. It has an approximate success rate of 50% in primiparous women and 60% in multiparous women.
Approximately 3-4% of babies end up "upside-down" in breech presentation, with their feet or buttocks near the cervix. Breech presentation is typically diagnosed during a visit to an OB-GYN ...
At full term, around 3%-4% of births are breech. The different types of breech presentations include: Complete: The fetus's knees are bent, and the buttocks are presenting first. Frank: The fetus's legs are stretched upward toward the head, and the buttocks are presenting first. Footling: The fetus's foot is showing first.
There can be many variations in the fetal presentation which is determined by which part of the fetus is projecting towards the internal cervical os. This includes: cephalic presentation: fetal head presenting towards the internal cervical os, considered normal and occurs in the vast majority of births (~97%); this can have many variations ...
In shoulder dystocia, the fetus is positioned normally Abnormal Position and Presentation of the Fetus Position refers to whether the fetus is facing rearward (toward the woman's back—that is, face down when the woman lies on her back) or forward (face up). It's important to check the baby's... read more (head first) for delivery, but the fetus's shoulder becomes lodged against the ...
Malpresentation can mean your baby's face, brow, buttocks, foot, back, shoulder, arms or legs or the umbilical cord are against the cervix. It's safest for your baby's head to come out first. If any other body part goes down the birth canal first, the risks to you and your baby may be higher. Malpresentation increases the chance that you ...
A normal fetal lie is an ideal position for labor and baby delivery in which the baby is head-down with the chin tucked into its chest. The back of the head is positioned so that it is ready to enter the pelvis. The fetus faces the mother's back, called cephalic presentation, and the babies mostly settle in this position by 32 to 36 weeks of ...
Breech presentation is a normal finding in preterm pregnancies, when the fetus is more mobile, and should not be considered abnormal until late pregnancy. Knowledge of the fetal presentation is important at the time of delivery (regardless of gestation) and prior to delivery as the pregnancy approaches term because this is when external ...
We investigated changes in the frequencies of four primary types of singleton fetal lie/presentation for each gestational week from 18 to 39 weeks in a retrospective, cross-sectional study which analyzed ultrasound examination records of fetal positions, in the outpatient prenatal diagnosis clinics in two cities in Poland.
Pregnancy loss (PL) is defined as the spontaneous demise of a pregnancy before the fetus reaches viability, which is a significant negative life event and impacts 10-15% of clinically recognized pregnancies. ... If a patient had experienced a pregnancy loss and was already pregnant at the time of presentation, pre-pregnancy intervention was ...
The vast majority of abortions occur during the first trimester of a pregnancy. In 2021, 93% of abortions occurred during the first trimester - that is, at or before 13 weeks of gestation, according to the CDC. An additional 6% occurred between 14 and 20 weeks of pregnancy, and about 1% were performed at 21 weeks or more of gestation.
The recommended starting dose is 0.3 mg/kg by subcutaneous injection. (2.1) The recommended target dose is 0.7 mg/kg every 3 weeks by subcutaneous injection. (2.2) Dosage modifications due to increased hemoglobin (Hgb) and decreased platelets may be necessary. Check Hgb and platelets before each dose for the first 5 doses, or longer if values ...