Good Manufacturing Practice (GMP) Resources
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
GMP covers all aspects of production from the starting materials, premises, and equipment to the training and personal hygiene of staff. Detailed written procedures are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process - every time a product is made.

GMP Resources
- What is GMP?
- GMP Regulations and Preambles
- GMP Guidelines
- GMP Resources by Country
Training Options
Classroom training.
- Applying the GMPs
- GMP Auditing for the Pharmaceutical Industry
- GMP Fundamentals for the Pharmaceutical Industry
- Q7A: Implementing Good Manufacturing Practices
Online Training
USFDA's Systems-Based GMP Inspection Approach
GMP compliance is widely-accepted as the best way to conduct business, putting product quality first. Representing the “original” GMP Institute, ISPE’s GMP courses combine a convenient format with an effective, interactive learning experience. To maximize and customize your professional development. Complete each of the individual US FDA's GMP Inspection Approach online courses for an overview of all the Systems.
- Quality Systems
- Facilities and Equipment Systems
- Materials Systems
- Production Systems
- Packaging and Labeling Systems
- Laboratory Control Systems
Online Webinars
- Industry Overview: Drug Dosage Forms
- Regulatory Compliance: Standards Practices and Guides
- Regulatory Compliance: Government Regulations
- Risk Management and Quality Management Systems (QMS)
Pharmaceutical Engineering Articles

There is a paradigm shift occurring in the biomanufacturing space around the advancement of personalized medicine that is creating new challenges for biomanufacturing facility design, both in terms of process technology and facility development approach. Advanced therapy medicinal products are based on genes, cells, or tissues delivered to patients to provide a therapeutic benefit, based on a...
View more articles
Books, Manuals, and Guidance Documents
- See all Guidance Documents
- See all Publications
Community of Practice
Join an ISPE community of practice to participate in discussions on specific topics with your peers. Learn more about Communities of Practice .
- Process/Product Development
GMP Regulation Handbooks
- 21 CFR Part 11: Electronic Signatures
- 21 CFR Part 111: Dietary Supplements
- 21 CFR Part 210 & 211: Pharmaceutical
- ICH Q7A: Active Pharmaceutical Ingredients
- ICH Q8R2: Pharmaceutical Development
- ICH Q9: Quality Risk Management
- ICH Q10: Quality Systems
- ICH Q11: Development and Manufacture of Drug Substances
Initiatives & Programs A to Z
- Advancing Pharmaceutical Quality
- Drug Shortages
- Enabling Pharmaceutical Innovation
- Facility of the Year
- Good Manufacturing Practice
- ISPE Foundation
- ISPE in Europe
- Pharma 4.0™
- Product Quality Lifecycle Implementation
- Regulatory Digest Newsletter
- Women in Pharma®
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Development & Approval Process | Drugs
- Pharmaceutical Quality Resources
Facts About the Current Good Manufacturing Practice (CGMP)
Pharmaceutical Quality affects every American. The Food and Drug Administration (FDA) regulates the quality of pharmaceuticals very carefully. The main regulatory standard for ensuring pharmaceutical quality is the Current Good Manufacturing Practice (CGMP) regulations for human pharmaceuticals. Consumers expect that each batch of medicines they take will meet quality standards so that they will be safe and effective. Most people, however, are not aware of CGMP, or how FDA assures that drug manufacturing processes meet these basic objectives. Recently, FDA has announced a number of regulatory actions taken against drug manufacturers based on the lack of CGMP. This paper discusses some facts that may be helpful in understanding how CGMP establishes the foundation for drug product quality.
What is CGMP?
CGMP refers to the Current Good Manufacturing Practice regulations enforced by the FDA. CGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This formal system of controls at a pharmaceutical company, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures, and errors. This assures that drug products meet their quality standards.
The CGMP requirements were established to be flexible in order to allow each manufacturer to decide individually how to best implement the necessary controls by using scientifically sound design, processing methods, and testing procedures. The flexibility in these regulations allows companies to use modern technologies and innovative approaches to achieve higher quality through continual improvement. Accordingly, the "C" in CGMP stands for "current," requiring companies to use technologies and systems that are up-to-date in order to comply with the regulations. Systems and equipment that may have been "top-of-the-line" to prevent contamination, mix-ups, and errors 10 or 20 years ago may be less than adequate by today's standards.
It is important to note that CGMP regulations for drugs contain the minimum requirements. Many pharmaceutical manufacturers are already implementing comprehensive, modern quality systems and risk management approaches that exceed these minimum standards.
Why is CGMP so important?
A consumer usually cannot detect (through smell, touch, or sight) that a drug product is safe or if it will work. While CGMP requires testing, testing alone is not adequate to ensure quality. In most instances testing is done on a small sample of a batch (for example, a drug manufacturer may test 100 tablets from a batch that contains 2 million tablets), so that most of the batch can be used for patients rather than destroyed by testing. Therefore, it is important that drugs are manufactured under conditions and practices required by the CGMP regulations to assure that quality is built into the design and manufacturing process at every step. Facilities that are in good condition, equipment that is properly maintained and calibrated, employees who are qualified and fully trained, and processes that are reliable and reproducible, are a few examples of how CGMP requirements help to assure the safety and efficacy of drug products.
How does FDA determine if a company is complying with CGMP regulations?
FDA inspects pharmaceutical manufacturing facilities worldwide, including facilities that manufacture active ingredients and the finished product. Inspections follow a standard approach and are conducted by highly trained FDA staff. FDA also relies upon reports of potentially defective drug products from the public and the industry. FDA will often use these reports to identify sites for which an inspection or investigation is needed. Most companies that are inspected are found to be fully compliant with the CGMP regulations.
If a manufacturer is not following CGMP, are drug products safe for use
If a company is not complying with CGMP regulations, any drug it makes is considered “adulterated” under the law. This kind of adulteration means that the drug was not manufactured under conditions that comply with CGMP. It does not mean that there is necessarily something wrong with the drug.
For consumers currently taking medicines from a company that was not following CGMP, FDA usually advises these consumers not to interrupt their drug therapy, which could have serious implications for their health. Consumers should seek advice from their health care professionals before stopping or changing medications. Regulatory actions against companies with poor CGMP are often intended to prevent the possibility of unsafe and/or ineffective drugs. In rare cases, FDA regulatory action is intended to stop the distribution or manufacturing of violative product. The impact of CGMP violations depends on the nature of those violations and on the specific drugs involved. A drug manufactured in violation of CGMP may still meet its labeled specifications, and the risk that the drug is unsafe or ineffective could be minimal. Thus, FDA’s advice will be specific to the circumstances, and health care professionals are best able to balance risks and benefits and make the right decision for their patients.
What can FDA do to protect the public when there are CGMP violations?
If the failure to meet CGMP results in the distribution of a drug that does not offer the benefit as labeled because, for example, it has too little active ingredient, the company may subsequently recall that product. This protects the public from further harm by removing these drugs from the market. While FDA cannot force a company to recall a drug, companies usually will recall voluntarily or at FDA’s request. If a company refuses to recall a drug, FDA can warn the public and can seize the drug.
FDA can also bring a seizure or injunction case in court to address CGMP violations even where there is no direct evidence of a defect affecting the drug’s performance. When FDA brings a seizure case, the agency asks the court for an order that allows federal officials to take possession of “adulterated” drugs. When FDA brings an injunction case, FDA asks the court to order a company to stop violating CGMP. Both seizure and injunction cases often lead to court orders that require companies to take many steps to correct CGMP violations, which may include repairing facilities and equipment, improving sanitation and cleanliness, performing additional testing to verify quality, and improving employee training. FDA can also bring criminal cases because of CGMP violations, seeking fines and jail time.
How would a new drug company learn about CGMP and about FDA’s expectations on complying with them?
FDA publishes regulations and guidance documents for industry in the Federal Register . This is how the federal government notifies the public of what we are doing and why. FDA’s website, www.fda.gov also contains links to the CGMP regulations, guidance documents , and various resources to help drug companies comply with the law. FDA also conducts extensive public outreach through presentations at national and international meetings and conferences, to discuss and explain the CGMP requirements and the latest policy documents.

Foundations of Good Manufacturing Practices
Good Manufacturing Practices (GMP) are critical to ensuring a medical product is quality assured and fit for its intended use. Achieving both protects the public from substandard products and helps to maintain and/or improve the health and well-being of patients.
Basic GMP principles are specified by the World Health Organization (WHO) and the Pharmaceutical Inspection Cooperation Scheme (PIC/S). Many countries have their own GMP guidelines, which are often based on the WHO standard. Manufacturers must comply with the regulatory requirements of the countries where they produce and market their products.
This free online course provides an overview of essential GMP principles and requirements. It covers key principles of the WHO and PIC/S standards and will equip participants with a broad, foundational understanding of GMP.
Developed by the Promoting the Quality of Medicines (PQM) program, which was funded by the U.S. Agency for International Development, this course is readily accessible for anyone who works in medical products production and quality assurance. This includes manufacturers and regulatory authorities.
The self-paced course includes ten modules on GMP as it relates to:
- Medicinal products (finished dosage form)
- Active pharmaceutical ingredients (APIs)
- Quality risk management
- Deviations, root cause analysis (RCA) tools and corrective and preventive action (CAPA)
- Qualification of facilities, equipment, and utilities
- Data integrity and computer system validation
- Heating, ventilation, and air conditioning (HVAC) systems
- Water systems
The course is self-paced, with each module taking approximately 30-45 minutes to complete. Enroll here
Important message
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

49 templates

18 templates

32 templates

42 templates

40 templates

16 templates
Good Manufacturing Practices Campaign
Good manufacturing practices campaign presentation, free google slides theme and powerpoint template.
From pharmaceuticals to food products, having good manufacturing practices help manufacturers adhere to strict standards and regulations that minimize the risk of contamination and errors during the production process. This editable template has our seal of approval (actually, it has some icons that look like seals of approval), so its manufacturing must have been adhered to those practices! Make the most of the colorful elements and push your campaign so that it reaches as many people as possible.
Features of this template
- 100% editable and easy to modify
- 20 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.


Premium template
Unlock this template and gain unlimited access

Register for free and start editing online

cGMP: Introduction to Good Manufacturing Practice (GMP)

Benefits of Being Certified from Biopharma Institute: Throughout our training students will be engaging in active learning using interactive eLearning modules validated by 3rd party organizations for relevancy, compliance, and regulatory content. Courses are developed by subject matter experts (SMEs) and instructional design professionals with the goal to promote the students' retaining of key knowledge. The programs further offer access to regulatory references, real-life case studies, and introduce other important information necessary to assist with learning. Since 2003, Biopharma Institute has been aiding both professionals and their corporate employers with fulfilling training requirements. Biopharma Institute's certifications are recognized by a wide spectrum of industries, regulators, and companies around the world. Furthermore, our training solutions are customizable to any organization's training needs. Today, Biopharma Institute offers over 300 courses training on clinical research, drug manufacturing (cGMP), regulatory affairs, validation systems, pharmacovigilance, good laboratory practice (GLP), and data integrity. We supply our training to clinical research, pharmaceutical, and medical device industries.
Employee / Corporate Training: For corporations seeking a method to train employees, economically, without having to set aside time for travel, webinars, or live, in-person training; Biopharma Institute is the training partner you can rely on. Our training provides an effective, engaging, self-paced learning experience which fits conveniently into the day-to-day activities of most types of learners. Our eLearning modules are SCORM-compliant and can be delivered from most corporate learning management systems (LMS). We catering to both small-scale and large-scale training requests, with the same goal in mind: To make this a good experience for everyone involved, from the students to those managing the group training.
Search Courses:
Export catalog.

- GMP: Good Manufacturing Practice
- GMP Auditing: FDA Inspection Readiness
- GLP: Good Laboratory Practice
- GCP: ICH Good Clinical Practice
- GDocP: Data Integrity & Documentation
- GPP: Good Pharmacoepidemiology Practice
- GVP: Good Pharmacovigilance Practice
- GDP: Good Distribution Practice
- Quality Assurance (QA) - Pharma & Device
- QRM: Quality Risk Management
- Drug Safety & Pharmacovigilance
- Regulatory Affairs (Pharma & Device)
- Validation: Process & Equipment
- CSV: Computer System Validation
- CSA: Computer Software Assurance
- OSHA / HR EEO for Employees
- Change Control Training
- Equipment Qualification & Commissioning
- FDA 21 CFR Part 211: GMP Drug Mfg.
- FDA 21 CFR Part 117: GMP Food Mfg.
- FDA 21 CFR Part 820: GMP Device Mfg.
- FDA 21 CFR Part 11: Electronic Records
- EU MDR: Medical Device Regulation
- MDSAP: Device Single Audit Program
- ISO 14971:2019 - Medical Devices
- ISO 13485:2016 - Medical Devices
- ISO 14155:2020 - Medical Devices
- GMP: Refresher Training
- GMP: For Beginners
- Clinical Research Training Programs
- CRA: Clinical Research Associate
- CRC: Clinical Research Coordinator
- Clinical Trial Training: Sponsors & CROs
- HIPAA & GDRP in Clincal Research
- Pharmaceutical Microbiology
- Contamination Control & Aseptic Techniques
- Pharmaceutical Sales & Marketing
5% Discount on Enrollment Through April 2024
Discount Code: ENROLLNOW5

More Information:
More information, phone support.
Copyright 2024 - Biopharma Institute. All Rights Reserved.

Good Manufacturing Practices
Aug 16, 2012
430 likes | 983 Views
Good Manufacturing Practices. Guilin, PRC Dr AJ van Zyl for Quality Assurance and Safety: Medicines Medicines Policy and Standards Health Technology and Pharmaceuticals Cluster World Health Organization. Program. Good Manufacturing Practices Presentation on GMP (Production focus)
Share Presentation
- volumetric solution preparation
- quality control laboratories
- computer system
- organization structure
- working standard

Presentation Transcript
Good Manufacturing Practices Guilin, PRC Dr AJ van Zyl for Quality Assurance and Safety: Medicines Medicines Policy and Standards Health Technology and Pharmaceuticals Cluster World Health Organization
Program • Good Manufacturing Practices • Presentation on GMP (Production focus) • Presentation on GMP (QC focus) • Product specific focus • Group session
Guidelines and references GMP: World Health Organization WHO Technical Report Series, No. 908, 2003, Annex 4. Good Manufacturing Practices for pharmaceutical products: main principles WHO Technical Report Series, No. Annex 3. WHO Technical Report Series, No. Annex 3.
Good Manufacturing Practices (GMP) Introduction General considerations Glossary 1. Quality assurance 2. Good manufacturing practices for pharmaceutical products (GMP) 3. Sanitation and hygiene 4. Qualification and validation 5. Complaints 6. Product recalls 7. Contract production and analysis
Good Manufacturing Practices (GMP) 8. Self-inspection and quality audits 9. Personnel 10. Training 11. Personal hygiene 12. Premises Quality control areas 13. Equipment 14. Materials Reagents and culture media Reference standards 15. Documentation
Good Manufacturing Practices (GMP) 16. Good practices in production 17. Good practices in quality control Control of starting materials and intermediate, bulk and finished products Test requirements Batch record review Stability studies
Good Practices for Quality Control Laboratories (GPQCL) Part One. Management and infrastructure 1. Organization and management 2. Quality system 3. Control of documentation 4. Records 5. Data processing equipment 6. Personnel 7. Premises 8. Equipment, instruments and other devices
Good Practices for Quality Control Laboratories (GPQCL) Part Two. Materials and set-up of equipment, instruments and other devices 9. Specifications archive 10. Reagents 11. Reference materials 12. Calibration, validation and verification of equipment, instruments and other devices 13. Traceability
GPQCL Part Three. Working procedures 14. Incoming sample 15. Analytical worksheet 16. Testing 17. Evaluation of test results 18. Retained samples Part Four. Safety in pharmaceutical control laboratories 19. General rules
Good Manufacturing Practices (GMP) • Where to start the inspection? • Laboratory layout – overview of the QC laboratory, activities, personnel • Chemical analysis, instrumentation, micro • Product to be inspected • API, excipients, bulk, FP • Specification, test methods, parameters and acceptance criteria • Utilities (HVAC, water, etc)
Good Manufacturing Practices (GMP) • Where to start the inspection? (2) • Packaging material control • Data verification (e.g. stability) • Other documents: • SOPs • Reports and records • Validation and qualification • OOS • Trend analysis
(GMP) - QC • "On site" inspection • Sample inward register • Artesunate and selected excipients • Date of entry • Date received and cross references • Purchase order and delivery note • Approved supplier and manufacturer • Number of containers and batch number(s) • Damaged containers • Samples taken • Control number (AR) allocated
(GMP) - QC • Documents requested: • Select specific batches for verification • Artesunate specification and excipients specifications • Standard Test Methods • Analytical reports for the batches selected • Pharmacopoeia • SOPs (e.g. sampling, pooling of samples and sampling plan), reagents and volumetric solution preparation, reference standard control . . .
(GMP) - QC • General information: • How are the samples taken? • Size of the sample? • Pooling for composite samples? • Sample containers? • Sampling tools? • Sample storage • Work allocation • Current specifications
(GMP) - QC • Data verification: • Analytical reports against the specifications • COAs (suppliers, own) and raw data • 1. Number of samples verified (cross check) • 2. Tests as per specification with acceptance criteria • 3. Individual tests/parameters • 4. Source or raw data in analyst work books or data sheets • 5. Issuing of the sheets/books
(GMP) - QC • Artesunate:
(GMP) - QC • Artesunate: Identification (and assay?) • Each sample, from each container • Method used – "in-house" or pharmacopoeia • Validated method • Reference standard (RS) used • Official RS or Working standard • Preparation • Control • Records • Storage, container etc • Chromatograms or spectra • Date, traceability (batch numbers, time, analyst, equipment)
(GMP) - QC • Artesunate (cont): • Chromatograms or spectra (cont) • Manual integration, Peak symmetry, system suitability • Equipment logbook • date used, analyst, calibrated (records), maintenance (record), qualification reports, computer system validated, access control, changes, column used, column performance, column log, column washing and storage, analyst information, qualification, training, signature
(GMP) - QC • Artesunate: • Follow the same procedure for each test or selected tests • Excipients: • Follow the same procedure for the excipients • Also micro tests as applicable • Finished product: • Follow the same procedure • Release and rejection procedure
(GMP) - QC • Other checks may include: • Environment of the laboratory • Glassware calibration and use • Equipment and instruments • Other products and materials tested • Impact • Glass ware • Cleaning, condition, storage • SOPs • Specifications archive • Analytical method validation • Analyst performance
(GMP) - QC • Documentation review may include: • OOS • SOP, reports, investigations • CAPA • Water system • Water system qualification • Water sampling and testing • Cleaning validation • Environmental control • Particulate matter • Micro • Air samples, settle plates, contact plates, personnel
(GMP) - QC • Primary packaging material, e.g. • Aluminium foil and PVC, PVDC • HDPE containers • Cotton wool • Dessicant • Procedure for receiving, sampling, testing • Where? Who? How? How many? Supplier? Batch control? AR numbers? Traceability? • Specifications, test methods, release reports
(GMP) - QC • Printed packaging material e.g. • Labels and cartons • Leaflets • Procedure for receiving, sampling, testing • Where? Who? How? How many? Supplier? Batch control? AR numbers? Traceability? • Specifications, test methods, release reports
(GMP) - QC • Retention samples • APIs • Excipients • Packaging materials • Finished Products • Area • Environmental conditions • Period kept • Packaging • Quantity
(GMP) - QC • Stability testing • Conditions e.g. Zone II, Zone IV (now a and b) • Periods • Accelerated • Real time • Procedure and plan with intervals • Compliance with program • Verify source data • Incubators or chambers • Qualification (DQ, IQ, OQ, PQ) • Packaging
(GMP) - QC • Stability • P
(GMP) - QC • Micro lab • Personnel • Organization structure, job descriptions and responsibilities • Qualifications and experience • Activities • Excipients • Environmental monitoring • Water monitoring • Instruments and equipment • Status (qualification, calibration, SOPs and records)
(GMP) - QC • Micro lab • Media • Storage • Preparation procedure and records • Positive and negative control • Microbiologist training • Waste materials • Cleaning
(GMP) - QC • Micro lab • P
- More by User

Good Manufacturing Practices: WHO Inspections
Good Manufacturing Practices: WHO Inspections. Tony Gould (Slides provided by Dr Andre van Z yl). Inspections. To get started: Risk assessment (SOP) Scheduling Preparation Announce inspection Provide tentative inspection plan Inspect, prepare inspection report Review corrective action.
1.03k views • 38 slides

Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP) and Good Tissue Practices (GTP)
Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP) and Good Tissue Practices (GTP). Richard E. Giles, Ph.D. Associate Professor Regulatory Affairs Institutional Compliance Office. What? GLPs, GMPs & GTPs General Definitions Scope & Details Who? Users Oversight Why?
2.65k views • 58 slides
![good manufacturing practice presentation GOOD MANUFACTURING PRACTICES [GMP]](https://cdn4.slideserve.com/650827/good-manufacturing-practices-gmp-dt.jpg)
GOOD MANUFACTURING PRACTICES [GMP]
GOOD MANUFACTURING PRACTICES [GMP]. WHAT IS GMP ?. G OOD M ANUFACTURING P RACTICE. What is GMP?.
2.78k views • 179 slides

Quality and Good Manufacturing Practices
Quality and Good Manufacturing Practices. Quality and Good Manufacturing Practices 6 November 2007 Jiaxing, People's Republic of China . Quality and Good Manufacturing Practices. GMP: Validation Presented by: Dr A J van Zyl Technical Officer: WHO HTP/PSM/QSM [email protected].
880 views • 56 slides

Good Manufacturing Practices and Enforcement Actions
2. 2. Introduction. Good Manufacturing Practices (GMP) ensure quality, safety, and consistencyGMP is the law (Code of Federal Regulations)Laws are upheld and enforced by the FDA.Enforcement is facilitated by facility inspections. 3. 3. Food and Cosmetic Act. A drug is deemed adulterated if
489 views • 47 slides

GOOD MANUFACTURING PRACTICES (GMP)
GOOD MANUFACTURING PRACTICES (GMP). Rowan Chemical Engineering 2003. Delivering products free of all possible contamination. Guarantee high quality products to the consumer. OBJECTIVE. What are GMPs about?. P. M. G. TOOLS & MATERIALS. DRUGS & MEDICATIONS . HAIR PROTECTION. CONTRACTORS.
2.5k views • 22 slides

PART II GOOD MANUFACTURING PRACTICES (GMP)
PART II GOOD MANUFACTURING PRACTICES (GMP). GMP. Prerequisite programs which will provide the basic environmental and operating conditions that are necessary for the production of safe and wholesome food. . SSOP Sanitation Standard Operating Procedures.
1.78k views • 123 slides

Good Manufacturing Practices for BioTechnology
Good Manufacturing Practices for BioTechnology . Steven S. Kuwahara, Ph.D. GXP BioTechnology, LLC PMB 506, 1669-2 Hollenbeck Ave. Sunnyvale, CA 94087-5042 Tel. & FAX (408) 530-9338 E-Mail: [email protected]. History of U.S. Food and Drug Law.
715 views • 34 slides

Current Good Manufacturing Practices (cGMPs)
Current Good Manufacturing Practices (cGMPs). Assures that feed… has the identity and strength, which it purports meets the quality, purity, and safety requirements, which it is represented to possess Minimum requirements for manufacture of medicated animal feed .
561 views • 22 slides

Current Good Manufacturing Practices (cGMP’s)
Current Good Manufacturing Practices (cGMP’s). Biotechnology. using living cells and materials produced by cells to create pharmaceutical, diagnostic, agricultural, environmental, and other products to benefit society. How Do Biotech Products Differ from Chemical Drugs?.
2.76k views • 31 slides

Good Manufacturing Practices (GMPs) (Continued)
Good Manufacturing Practices (GMPs) (Continued). Equipment 110.40. Designed for ease of sanitation noncorrosive food surfaces Properly maintained Installation facilitates cleaning Must not contribute to adulteration lubricants, fuel, metal fragments, other contaminates
324 views • 6 slides

Developed by. Good Manufacturing Practices. Agenda. Good Manufacturing Practices Contamination General Employee Hygiene Food Handling Practices. Good Manufacturing Practices. Deal with contamination by people by food materials by packaging materials by hazardous materials
2.14k views • 33 slides

Good Manufacturing Practices Working Group
Good Manufacturing Practices Working Group.
283 views • 12 slides

GOOD MANUFACTURING PRACTICES 2214
GOOD MANUFACTURING PRACTICES 2214. Steven C Seideman Extension Food Processing Specialist Cooperative Extension Service University of Arkansas. INTRODUCTION.
984 views • 68 slides

Good Manufacturing Practices. What is it? How is it carried out ?. The Early Beginnings. 1900’s house-calls Home remedies, ointments and miracle elixirs Entertainment and music No regulations until 1902. Food and Drug Administration Assures Safety in Drugs it Approves.
724 views • 13 slides

Good Manufacturing Practices and Quality Control
Good Manufacturing Practices and Quality Control. PHARMACEUTICAL QUALITY MANAGEMENT. PRINCIPLE. The holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are: i - Fit for their intended use
846 views • 46 slides

Good Manufacturing Practices (GMPs)
Good Manufacturing Practices (GMPs). GMP’s. 21 CFR - Code of Federal Regulations Part 110 , Subparts: General provisions Buildings and facilities Equipment Production and process control Defect action levels. General Provisions-Definitions Only important ones are given below.
341 views • 11 slides

Good Manufacturing Practices Introduction - GMP
Good Manufacturing Practices Introduction - GMP. Xin Chen and Chris Lepine. Some questions to consider:. What are some good rules for manufacturing companies? Do you usually trust people that you do not know with your life? How well do people regulate themselves?. >.
3.51k views • 15 slides

551 views • 33 slides

PART II GOOD MANUFACTURING PRACTICES (GMP). GMP. Prerequisite programs which will provide the basic environmental and operating conditions that are necessary for the production of safe and wholesome food. SSOP Sanitation Standard Operating Procedures.
1.65k views • 123 slides

Good Manufacturing Practices in Employee Training
A GMP Manufacturing Facility is a production facility or a clinical trial materials plant for the manufacture of pharmaceutical products. This is a one-stop solution, where you get all - raw material, manufactured, packaging service. A&C provides the manufacturing facility. For more information visit our website.
196 views • 6 slides
Home Good Manufacturing Practices PowerPoint Template Good Manufacturing Practices Template for Presentation
Good Manufacturing Practices Template for Presentation
Return to Good Manufacturing Practices PowerPoint Template .
Download unlimited PowerPoint templates, charts and graphics for your presentations with our annual plan.
Template Tags:
Download unlimited content, our annual unlimited plan let you download unlimited content from slidemodel. save hours of manual work and use awesome slide designs in your next presentation..

IMAGES
VIDEO
COMMENTS
Good manufacturing practice (GMP) May 16, 2016 •. 518 likes • 277,120 views. Sagar Savale. Good Manufacturing Practice is a set of regulations, codes, and guidelines for the manufacture of drug substances and drug products, medical devices, in vivo and in vitro diagnostic products, and foods. Read more. Health & Medicine. 1 of 21. Download Now.
Presentation Series:Day In the Life. 5 Modules. Introduction. Consumer Safety Officer. ... Covers Current Good Manufacturing Practices (cGMPs) Two types: Comprehensive: Level 2.
Good Manufacturing Practices or GMP is a system that consists of processes, procedures and documentation that ensures manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards. Implementing GMP can help cut down on losses and waste, avoid recall ...
Design, delivery, and maintenance of manufacturing support facilities, utilities, process equipment, and automation control so that they perform as intended to meet business objectives, such as capacity, yield, operational efficiency, and reliability. Development of a production process that can repeatedly produce a quality product.
This document discusses Good Manufacturing Practices (GMP) for pharmaceutical products as outlined by the World Health Organization (WHO). It provides definitions and explanations of key GMP concepts including quality assurance, quality management, and ensuring consistent production of pharmaceuticals according to appropriate quality standards ...
Good Manufacturing Practice (GMP) Resources. Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product.
The main regulatory standard for ensuring pharmaceutical quality is the Current Good Manufacturing Practice (CGMP) regulations for human pharmaceuticals. Consumers expect that each batch of ...
Ultratech4. good manufacturing practices presentation. Education. 1 of 22. Download Now. Download to read offline. good manufacturing practices presentation - Download as a PDF or view online for free.
Good Manufacturing Practices (GMP) are critical to ensuring a medical product is quality assured and fit for its intended use. Achieving both protects the public from substandard products and helps to maintain and/or improve the health and well-being of patients. Basic GMP principles are specified by the World Health Organization (WHO) and the ...
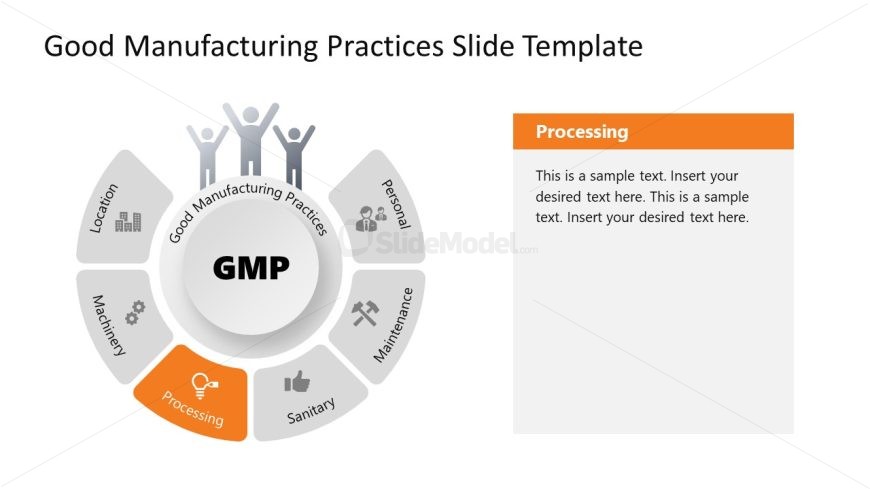
Present the major components of standard GMP guidelines using a 100% editable Good Manufacturing Practices PowerPoint Template. This PPT template carries a circular diagram with six segments to mention the components, i.e., personnel, maintenance, sanitary, processing, machinery, and location. The first slide of the template shows this diagram with a central core element to mention the title.
Good Manufacturing Practices or GMP is a system that consists of processes, procedures and documentation that ensures manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards. Implementing GMP can help cut down on losses and waste, avoid recall ...
Free Google Slides theme and PowerPoint template. From pharmaceuticals to food products, having good manufacturing practices help manufacturers adhere to strict standards and regulations that minimize the risk of contamination and errors during the production process. This editable template has our seal of approval (actually, it has some icons ...
Alternatively, call toll-free from the US or Canada: +1 (888) 424-6576. International callers may dial: +1 (201) 301-8370. For quotes or invoices please provide the course (s) and number of students. This cGMP: Introduction to Good Manufacturing Practice (GMP) course includes sections on: Introduction to current good manufacturing practices ...
Good Manufacturing Practices and Enforcement Actions. 2. 2. Introduction. Good Manufacturing Practices (GMP) ensure quality, safety, and consistencyGMP is the law (Code of Federal Regulations)Laws are upheld and enforced by the FDA.Enforcement is facilitated by facility inspections. 3. 3. Food and Cosmetic Act. A drug is deemed adulterated if
HR Industrial Industry Manufacturing Practice Process Management. Return to Good Manufacturing Practices PowerPoint Template. Our annual unlimited plan let you download unlimited content from SlideModel. Save hours of manual work and use awesome slide designs in your next presentation. Subscribe Now. Presentation Template for Good Manufacturing ...
Good Manufacturing Practices (GMP) Introduction General considerations Glossary 1. Quality assurance 2. Good manufacturing practices for pharmaceutical products (GMP) 3. Sanitation and hygiene 4. Qualification and validation 5. Complaints 6. Product recalls 7. Contract production and analysis.
2. Good Manufacturing Practice (GMP) "A GMP is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product". GMP covers all aspects of production from the starting materials, premises and equipment to the ...
Download our 100% customizable Good Manufacturing Practices PPT template to highlight the process of manufacturing items in compliance with quality standards. High-resolution slides! Related Products. Industry Challenges. $5.00. Add to Wish List Add to Compare. Industry Environment. $5.00. Add to Wish List Add ...
5. Practice Makes Perfect. Be the first to add your personal experience. 6. Feedback Loop. Be the first to add your personal experience. 7. Here's what else to consider. Be the first to add your ...
See other industries within the Manufacturing sector: Aerospace Product and Parts Manufacturing , Agriculture, Construction, and Mining Machinery Manufacturing , Alumina and Aluminum Production and Processing , Animal Food Manufacturing , Animal Slaughtering and Processing , Apparel Accessories and Other Apparel Manufacturing , Apparel Knitting Mills , Architectural and Structural Metals ...
This presentation provides a 45-minute overview on the whats, the whys and the hows of recycling your waste. When: Wednesday, February 21, 2024, 11:00 AM until 12:00 PM Eastern Time (US & Canada) (UTC-05:00) Where: Magnolia Manor- Multipurpose B. 2120 Del Webb Gardens Drive....
Industry: Basic Chemical Manufacturing , Steel Product Manufacturing from Purchased Steel , Nonferrous Metal (except Aluminum) Production and Processing , Architectural and Structural Metals Manufacturing , Forging and Stamping See All Industries, Warehousing and Storage , Fuels and radioactive compounds, Steel pipe and tubes, Primary nonferrous Metals, nec, Miscellaneous Metalwork, Metal ...
HR Industrial Industry Manufacturing Practice Process Management. Return to Good Manufacturing Practices PowerPoint Template. Our annual unlimited plan let you download unlimited content from SlideModel. Save hours of manual work and use awesome slide designs in your next presentation. Subscribe Now. Good Manufacturing Practices Slide Template.
Industry: Iron and Steel Mills and Ferroalloy Manufacturing , Clay Product and Refractory Manufacturing , Cement and Concrete Product Manufacturing , Ingots, steel, Brick and structural clay tile See All Industries, Concrete products, nec, Bars and bar shapes, steel, hot-rolled, Sheet or strip, steel, hot-rolled Galvanized pipes, plates, sheets, etc.: iron and steel See Fewer Industries