- Harvard T.H. Chan School of Public Health
- SPH Theses and Dissertations
- Communities & Collections
- By Issue Date
- FAS Department
- Quick submit
- Waiver Generator
- DASH Stories
- Accessibility
- COVID-related Research

Terms of Use
- Privacy Policy
- By Collections
- By Departments
Malaria Vector Control in Sub-Saharan Africa: Impact and Economic Evaluation of Larviciding
Citable link to this page
Collections.
- SPH Theses and Dissertations [256]
Contact administrator regarding this item (to report mistakes or request changes)
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2023
Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks
- Golla Madhu ORCID: orcid.org/0000-0002-4170-3146 1 ,
- Ali Wagdy Mohamed ORCID: orcid.org/0000-0002-5895-2632 2 , 3 ,
- Sandeep Kautish ORCID: orcid.org/0000-0001-5120-5741 4 ,
- Mohd Asif Shah ORCID: orcid.org/0000-0002-0351-9559 5 , 6 , 7 &
- Irfan Ali ORCID: orcid.org/0000-0002-1790-5450 8
Scientific Reports volume 13 , Article number: 13377 ( 2023 ) Cite this article
2910 Accesses
2 Citations
1 Altmetric
Metrics details
- Epidemiology
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period, and delaying exact treatment might result in the development of further complications. The most prevalent method now available for detecting malaria is the microscope. Under a microscope, blood smears are typically examined for malaria diagnosis. Despite its advantages, this method is time-consuming, subjective, and requires highly skilled personnel. Therefore, an automated malaria diagnosis system is imperative for ensuring accurate and efficient treatment. This research develops an innovative approach utilizing an urgent, inception-based capsule network to distinguish parasitized and uninfected cells from microscopic images. This diagnostic model incorporates neural networks based on Inception and Imperative Capsule networks. The inception block extracts rich characteristics from images of malaria cells using a pre-trained model, such as Inception V3, which facilitates efficient representation learning. Subsequently, the dynamic imperative capsule neural network detects malaria parasites in microscopic images by classifying them into parasitized and healthy cells, enabling the detection of malaria parasites. The experiment results demonstrate a significant improvement in malaria parasite recognition. Compared to traditional manual microscopy, the proposed system is more accurate and faster. Finally, this study demonstrates the need to provide robust and efficient diagnostic solutions by leveraging state-of-the-art technologies to combat malaria.
Similar content being viewed by others

Reducing data dimension boosts neural network-based stage-specific malaria detection
Katharina Preißinger, Miklós Kellermayer, … János Török
Enhancing parasitic organism detection in microscopy images through deep learning and fine-tuned optimizer
Yogesh Kumar, Pertik Garg, … Muhammad Fazal Ijaz
Classification for avian malaria parasite Plasmodium gallinaceum blood stages by using deep convolutional neural networks
Veerayuth Kittichai, Morakot Kaewthamasorn, … Siridech Boonsang
Introduction
Malaria is a life-threatening disease that involves the Plasmodium parasite, which poses a high death rate. It is transmitted to humans by biting an infected female mosquito with the parasite. Malaria is predominantly a tropical disease since mosquitoes thrive in tropical areas, and it is both preventable and treated. According to the latest Global Malaria Report, there are projected to be around 241 million malaria cases and 627 thousand fatalities worldwide by 2022 1 . Moreover, research by the World Health Organization (WHO) suggests that concerns related to COVID-19 could triple the number of malaria cases 2 , 3 . In response to this global epidemic, the WHO has enacted policies to prevent, treat, eradicate, and monitor malaria 4 . Malaria, a preventable disease, can be controlled and prevented if adequate processes and protocols are used, including early diagnosis of the malarial parasite 4 . Several laboratory techniques, including polymerase chain reaction (PCR), microscopy, and rapid diagnostic test (RDT) are commonly used for investigating malaria using thick or thin blood smears 5 , 6 , 7 , 8 . However, conventional methods tend to rely heavily on manually examining blood smears under a microscope. These methods are time-consuming, subjective, and require highly trained personnel. Additionally, the reliance on clinical experts raises concerns about the consistency and accuracy of the diagnosis. To address these deficiencies, computer-aided diagnostic (CAD) methods for malaria evaluation are being developed to reduce mortality rate 9 . Therefore, automated and accurate diagnostic systems are needed to improve malaria detection. Artificial intelligence has gained more and more attention in the scientific community. It has contributed to improving detection through various diagnostic processes. Most medical imaging analyses now incorporate CAD procedures that leverage deep learning techniques for effective model learning.
However, despite advancements, malaria remains endemic in some areas where the disease is common. Early screening plays a crucial role in detecting malaria and saving lives. Consequently, this motivates us to create faster and more accurate malaria diagnosis procedures. Recently, deep learning architectures have received much attention in terms of research and are the most important method to detect disease automatically and more accurately. These generic deep networks have played a vital role in image classification, detection, and recognition 10 , 11 . In a similar vein, data-driven deep learning (DL) algorithms have surpassed manually constructed feature extraction techniques 12 . A convolutional neural network (CNN) is a type of deep learning model that employs different mechanisms, such as local receptive fields, shared weights, and clustering layers, to leverage information. Its purpose is not limited to extracting features but also extends to generating predictive targets and furnishing actionable predictive models that can effectively aid physicians 10 , 13 . Deep neural networks have shown outstanding performance in computer vision tasks in recent years. This is done using methods like the ResNet-32 network model to identify ductal carcinomas 14 precisely. Despite their effectiveness, CNN suffers from limitations in the modeling of spatial relationships and the lack of an internal representation of the geometrical restrictions on the image data. When these flaws are applied to microscopic cell images, the diagnostic model may be misclassified. The need for a more precise and efficient model arises to improve the performance of detecting and classifying malaria parasites. These challenges have prompted us to develop a rapid and more accurate diagnosis procedure for malaria. The specific hypotheses tested in this study include:
Hypothesis 1
Using the inception neural network will enable the extraction of rich and discriminative features from microscopic images of malaria cells, improving parasite detection and classification accuracy.
Hypothesis 2
The incorporation of the imperative capsule neural network will enhance the modeling of spatial relationships within the images, allowing for a more precise classification of malaria parasites.
By testing these hypotheses, the study aims to demonstrate the superiority of the proposed approach over traditional manual microscopy and other existing methods for malaria diagnosis.
This paper is organized as follows: The relevant research is presented in Section “ Related works ”, and the proposed inception-based imperative capsule neural network is discussed in Section “ Materials and methods ”. Part “ Experimental results ” summarizes and describes the outcomes of this network. Part “ Conclusions ” concludes with the article's conclusions and suggested recommendations for further study.
Related works
Several researchers have demonstrated promising results in medical applications by using data-driven machine learning (ML) and deep learning (DL) models. This study examines contemporary deep-learning applications that elicit key decision-making factors in the diagnosis process. Liang et al. 15 presented a 16-layer CNN to classify the parasitized and uninfected cells in thin blood smears. Features are extracted using a pre-trained AlexNet 16 , and a support vector machine (SVM) is trained on these features, and the model has an average accuracy of 97.37%. However, the transfer learning method achieves only 91.99% accuracy. Bibin et al. 17 proposed and tested a six-layer deep belief network to detect malaria parasites in cell images. Based on their findings, the study achieved 96.4% classification accuracy on a custom dataset using training or test randomization. Dong et al. 18 presented SVM and CNN-based approaches for classifying malaria parasites from cell images. This study attained an accuracy of more than 95% using pre-trained deep learning models such as those used in LeNet 19 , AlexNet 16 , and GoogLeNet 20 . Rajaraman et al. 21 proposed a deep-learning model for malaria parasite detection and classification. The method visualizes the activation maps of each layer and understands the probabilities of the different layers to understand the modeling process. As a result, it obtains an accuracy of 98.61%. Mahdi Postchi et al. 22 surveyed the latest advancements in image analysis and machine-learning techniques for diagnosing malaria through microscopy. Although many machine learning models using traditional features have been developed for image classification and decision-making, these models may lack generalization ability. Sivaramakrishnan et al. 23 suggested a customized CNN model and evaluated the effectiveness of pre-trained and deep-learning CNN models as feature extractors for microscopic images to differentiate between healthy and parasitic blood cells. The model uses surface features to achieve more outstanding results than deep features and applies a level-set-based algorithm to detect and segment red blood cells. This model achieved 98.6% (cell-level) accuracy. Yang et al. 24 presented a fivefold cross-validation for two-step CNN models. In the first step, the model uses an intensity-based iterative Global Mini-mum Screening method to recognize parasites, and then a CNN uses a custom CNN to classify the presence of parasites. The success rate of this method is 93.46%. Vijayalakshmi et al. 25 presented a transfer learning method with a classification accuracy of 93.13% to discriminate between illustrations of malaria-diseased cells and healthy using the VGG16 model and a support vector machine. Madhu et al. 26 proposed an improved dynamic routing process to classify malaria-infected cells from healthy cells using a fully trained capsule network, and the model achieved an accuracy of 98.82%. Loddo et al. 27 used the DenseNet-201 neural network to categorize Plasmodium falciparum life stages into four groups and used two different datasets to assess the robustness of the model. The binary classification accuracy rate was 97.68%, and the multi-classification accuracy rate was 99.40%. Meng et al. 28 proposed a neighborhood correlation graph convolutional network to identify multistage malaria parasites. The model has excellent recognition ability for multistage malaria parasites, outperforming the comparison method by at least 8.67%. Madhu et al. 29 proposed an automated diagnostic model based on deep Siamese capsule arrays for uniquely detecting and classifying malaria parasites. When simplified on the largest test sample (test = 40%), the model achieved an accuracy of 96.61% and 98%, respectively. Ha et al. 30 presented a semi-supervised graph learning framework to solve the problem of identifying apicomplexan parasites. Hybrid graph learning is also used in this approach to explore the relationships between different parasites with and without labels.
In malaria, the Plasmodium parasite causes an acute fever that is carried by female Anopheles mosquitoes. It produces life-threatening sickness if left untreated for a long time, and delaying exact treatment might lead to the development of additional comorbidities. A microscope is currently the most prevalent method for detecting malaria. Consequently, an automated approach to diagnosing malaria is required. This study proposes the development of an urgent, inception-based capsule network for classifying parasitized and uninfected cells from micrographs. These diagnostic models contain neural networks based on the Inception and Imperative Capsule architectures. Using a trained model, such as Inception V3, the first block collects rich characteristics from images of malaria cells. In the second block, a dynamic imperative capsule neural network classifies malaria cells into infected and uninfected red blood cells. The experiment's findings indicate a considerable improvement in recognizing malaria parasites, which contributes to better illness diagnosis and prevention.
By observing the existing challenges, this study aims to develop an automatic diagnostic prototype for classifying malaria parasites from microscopic cell images using the Inception neural network with the Imperative Capsule neural network. The preliminary results of this study are presented as follows:
To develop an innovative approach employing an urgent, inception-based capsule network to recognize parasitized and uninfected cells from microscopic images.
The Inception block extracts rich features from malaria cell images using a pre-trained model, such as Inception V3, which facilitates efficient representation learning to recognize the parasites.
The dynamic imperative capsule neural network is utilized to classify microscopic images into parasitized and healthy cells, enabling the detection of malaria parasites.
To compute routing by agreement among low-level and higher-level capsules that can be used to predict malaria cells and classify them into parasitized and uninfected cells using L2-Norm.
This study underscores the importance of leveraging state-of-the-art technologies to combat malaria by providing a robust and efficient diagnostic solution.
Materials and methods
Dataset collection.
Images of thin blood smears containing two distinct strains of malaria—one infected and the other not—were used in the study. These samples were gathered from patients and healthy controls who had Plasmodium falciparum infections, and they were stored at the National Institutes of Health (NIH) repository, which is open to the public for study 23 . The collection includes 13,779 images of parasites and 13,779 images of uninfected cells, totaling 27,558 images of labeled and segmented cells from thin Giemsa-stained blood smear slides. Figure 1 offers some parasitic and uninfected cell images to visualize their physical traits.
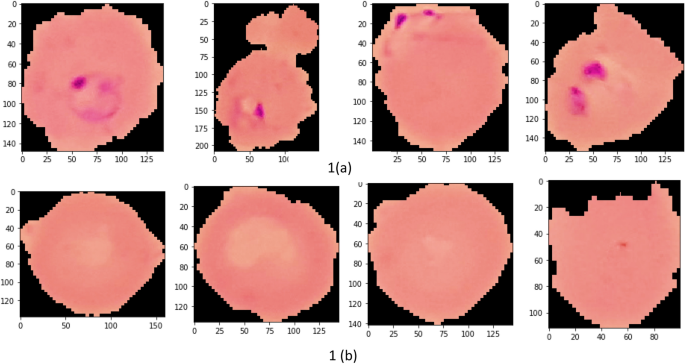
Illustration of sample malaria cell images: ( a ) Infected images; ( b ) Uninfected images (without parasites).
k-fold cross-validation (CV) test
The dataset contains 27,558 blood cell images with malaria-positive and negative samples, which were evaluated in our study for data sample training and testing, and used k-folds (k = 10, 20, 30, 40, 50) Cross-validation to evaluate the proposed model. As shown in Table 1 , the dataset is split into training and testing subsets.
Inception neural network and the imperative capsule neural network
Geoffrey Hinton et al. 31 motivated this research by addressing the limitations of traditional CNNs by proposing inception-based capsule neural networks, which require small data but have higher computational complexity.
This research develops an inception-based imperative capsule neural network for malaria detection, and its basic architecture is shown in Fig. 2 , which is similar to the architecture advocated for image classification problems by Sabour et al. 31 . According to Fig. 2 , input is first routed through fully connected inception blocks, which receive the parasitized and uninfected portions of the cell images as input and extract features on the parasitized and uninfected portions of the cell images. The inception block's output is used as the primary capsule layer's input. The primary and higher capsule layers utilize an imperative routing mechanism to learn the captured features by discerning the spatial orientation of the parasites on the extracted features. After multiple iterations, the resulting output is a feature vector with a length equivalent to the probability of the interval [0, 1], which preserves the object's pose information, minimizing the information loss caused by the feature vector extraction. This feature vector is then used to classify a test sample as infected or healthy cells, aiding in its classification.
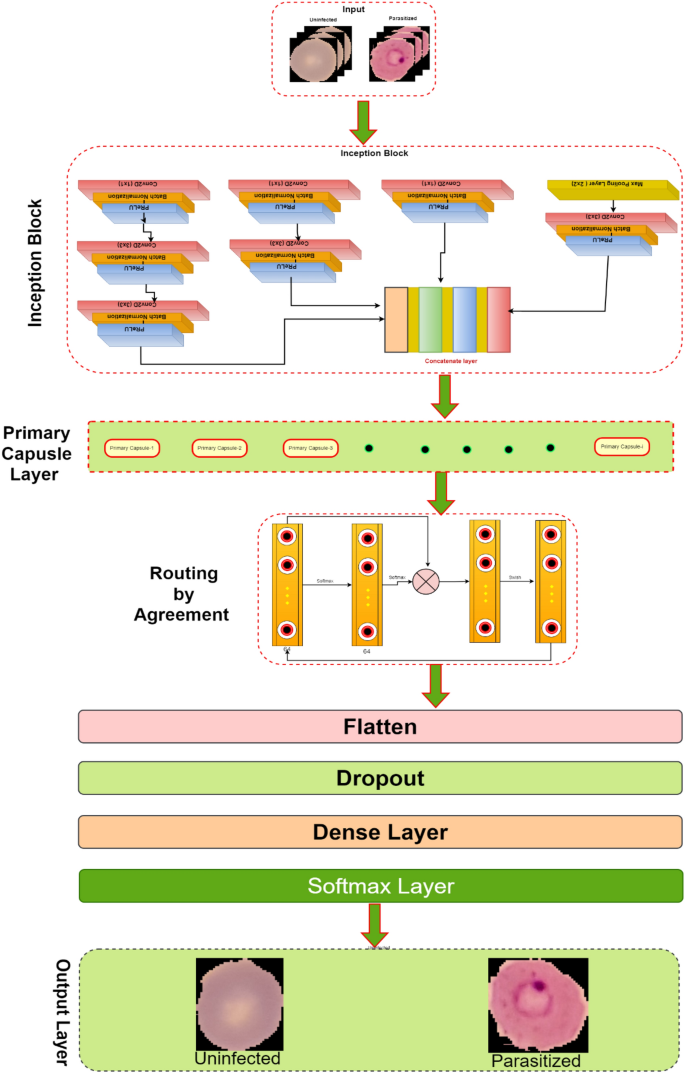
The proposed architecture of Inception-based capsule neural network.
Inception neural network block
In 2015, Google introduced a module for GoogleNet 32 , also known as Inception V3, a convolutional neural network that helps us with image analysis and object detection.
Convolutional layers are frequently employed in convolutional neural networks (CNNs) to extract information from images of malaria blood cells. The CNN's initialization block, which is made up of parallel convolutional layers with filters and kernels of various sizes, extracts feature from various scales to obtain multi-view information on parasites and healthy cells. The structure of the inception block, which is used to extract characteristics at various scales, is shown in Fig. 3 . To extract features at various sizes, this block has four parallel convolutional layers with various kernels (1 × 1, 3 × 3, and 3 × 3). A max-pooling layer with a kernel size of 2 × 2, a convolution layer with a kernel size of 1 × 1, and a batch normalizing layer make up the final parallel convolutional layer. Each parallel layer's computational cost and channel count can be decreased by using a 1 × 1 convolutional layer, and the model's computational cost can be decreased by employing a 3 × 3 max-pooling layer. The output feature maps of each of the four simultaneous convolutional layers are combined after computation to produce new feature maps that are used as the input for the capsule network.
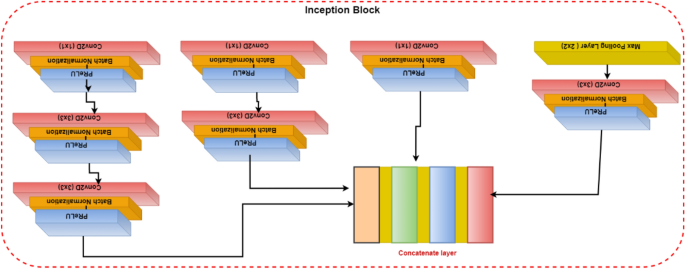
Illustration of the inception block.
Capsule networks block
To classify the items in the MNIST dataset, Sabour et al. 31 presented a capsule network (CapsNet). It uses a neural network to produce an output vector that includes both a scalar and a vector encoding the features of the objects in the image. In our experiment, these capsule networks are trained by carefully adjusting the number of rounds in the dynamic routing algorithm. Using Parametric ReLU (PReLU), it is possible to investigate the behavior of nonlinear activations during dynamic routing 33 . The presence of features in the form of vectors containing low-level entity instantiation parameters is estimated using the principal capsule layer. CapsNet transforms the scalar output using feature detectors in this layer, then passes the vector output of the capsules to the following layer using a modified routing method 31 . Because parameter tuning is critical for better network learning and faster convergence, proper initialization is used to start the routing procedure with kernel initializer before the primary capsule layer; the dynamic routing algorithm is activated with Glorot-normalization 34 . Each capsule, \(i\) has an activity vector \({u}_{i}\in R\) in the layer of \(l,\) which captures information about the features extracted from an entity (i.e., blood cell image). The output of the activity vector \({u}_{i}\) of the \(i\) th level capsule is fed as data into the next level layer, i.e., \(l+1\) layer. The \({j}{\text{th}}\) layer capsules of layer \(l+1\) will get data from \({u}_{i}\) and compute the product weight matrix \({W}_{ij}^{T}\) . The results are stored in the form of \({\widehat{u}}_{(j|i)}.\) This vector is the layer of capsules \(i\) at level \(l\) layer, which is the transformation of the entity represented by capsule \(j\) at the level of \(l+1\) . Then apply the transformation matrix \({W}_{ij}^{T}\) to capsule output \({u}_{i}\) of the previous layer, as shown in Eq. ( 1 ).
In Eq. ( 1 ), capsule \(i\) is the primary capsule layer, \(j\) is the higher-level capsule layer, and \({u}_{i}\) is the output of the capsule network of the upper layer and \({W}_{ij}^{T}\) is the learnable weighted matrix between the \({i}{\text{th}}\) capsule to \({j}{\text{th}}\) capsule. Which is multiplied by each output vector and the coupling coefficient \({C}_{ij}\) is added to the linear sum stage. Then the capsules are in the higher level, which is filled with the sum of the output vector in the lower-level layer, and we add it with a coupling coefficient \({C}_{ij}\) which is computed during the routing method shown in Eq. ( 2 ).
In dynamic routing, the coupling coefficient is determined by Eq. ( 2 ). In the process of calculating \({S}_{j}\) in forward propagation, \({W}_{ij}^{T}\) is set to a random value, \({a}_{ij}\) is initialized to zero, \({u}_{i}\) is the output of the previous layer, and then compute a weighted sum \({S}_{j}\) with weights \({C}_{ij}\) (the sum of these coefficients is equal to one) and it is denoted as follows:
The squashing function map of \({S}_{j}\) yields the output vector \({v}_{j},\) which is obtained is defined as follows:
The squashing function, defined by Eq. ( 4 ), ensures that short vectors are reduced to fewer dimensions near zero while long vectors are scaled to unit length, thus introducing nonlinearity to the capsule network. The total input Sj processed by the jth dimensional capsule array contributes to the coupling coefficient Cij. An activation function PReLU is applied to update the coupling coefficients, instead of the squashing function, by operating on Sj. During the iterative learning phase, these coupling coefficients are updated using Eq. ( 5 ), which proceeds as follows:
In Eq. ( 5 ), \({a}_{ij}\) is a parameter used as a weighted proxy, which means that it gives higher weights to appropriate predictions, and it starts at zero and is modified as the training progress.
However, it is initialized with the current input weights to improve the learning method by reducing the computational cost and improving the predictive ability. The number of routing iterations (n = 3) is used as a hyperparameter allowing one to choose a specific number of iterations during the training (here, epochs = 100) period, and the details of this network parameters are shown in Table 2 . The learning period is evaluated by evaluating the convergence, and our model is repeated for only three iterations. Figure 4 depicts the comprehensive learning curves for iterations over 100 epochs.
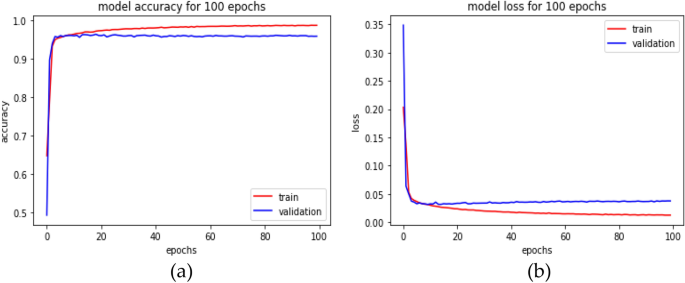
An inception-based capsule network with a router in 3 iterations, depicted as ( a ) accuracy curves and ( b ) loss decay curves.
PReLU activations are utilized during the routing by agreement process to improve the understanding of feature invariance in the captured images of malaria cells. In a conventional capsule network, the squash activation function is typically used as a non-linearity. However, using PReLU as a non-linearity is believed to lead to better generalization and convergence over time. The last layer of the network comprises two capsules (parasitized and uninfected cells) reflecting the probability of the interval [0, 1] and the position information of the object, preserving the pose information to reduce information loss caused by the extracted feature vector. This enables the classification of test samples into either parasitized or uninfected cells, thus aiding in cell feeding.
Loss function
Our current loss function 31 also includes the mean squared error rate (MSE) alongside the marginal loss. Change the settings for faster convergence and add proper model regularization and noise addition when training the classification model with a value set to 0.45.
In Eq. ( 6 ), \({m}^{+}\) and \({m}^{-}\) are the category prediction values, \(\sigma \) is the balance coefficient, \({T}_{x} \mathrm{is \, the \, label \, of \, category}, \) and classification probability vector \(\Vert {v}_{x}\Vert \) is the size. For this study, the default values are set as \({m}^{+}=0.85 \& {m}^{-}=0.15\) , \(\sigma =0.45\) . The total loss function, in this case, refers to the loss of capsules representing both malaria-parasitized and uninfected classes.
Experimental results
This section describes the proposed model's implementation in-depth and thoroughly analyses how well it performs under various restrictions. The proposed network was evaluated against front-line classification models created by several authors, which were pre-trained using NIH malaria datasets 23 and other private datasets to assess whether red blood cells are parasitized or not. According to Table 3 , the proposed model for malaria parasite identification and classification performed well on the NIH malaria dataset, along with the comparison findings. It is important to note that most models typically exhibit low performance on this dataset. Although their weights can handle common classification datasets, they frequently fall short because of ineffective feature extraction brought on by too much depth. Instead, the Inception-based capsule network model classifies parasitized and uninfected cells accurately during the diagnostic process by utilizing external knowledge to produce rich characteristics. On international benchmarks, the suggested model performs noticeably better.
As stated in the Table 4 , our model is assessed for layer-wise testing cell images, varying from training to 80% and testing to 20%.
In this analysis, experiments are conducted on various distributions, and the suggested network's implementation, as shown in Table 4 , achieves an accuracy of 99.35% and an AUC score of at least 99.73% at a test ratio of 20%. Table 4 shows the models' overall generality as measured by various standard classification metrics, including accuracy score, AUC–ROC, sensitivity, and specificity. Limiting diagnostic power does not assess the likelihood that a certain patient will acquire a disease, but it does affect diagnostic accuracy, even though they choose sensitivity and specificity. Table 5 displays the effectiveness of the suggested capsule array at various nonlinearity levels. Compared to the performance of cutting-edge pre-trained models, the generalization distribution for the training and test samples is 80% to 20%.
The performance metrics for every deep learning architecture are compiled in Table 5 . The proposed malaria detection algorithm outperforms the compared deep learning models in terms of performance. The results showed an accuracy of more than 99.35%, an AUC score of 99.73%, and an F1 score of 99.36%. The accuracy score is a well-known metric with a domain that is invariant to general utility; hence it is imperative to note. As a result, the effectiveness of the suggested model is assessed using various measuring techniques. The model was created to be assessed by segregating partition samples that vary from 10 to 50%, ensuring that the model is adequately generalized. Figure 5 displays the predicted results of the suggested model on images of malarial cells. The true value is shown on the x-axis, and the model forecast is shown on the y-axis.
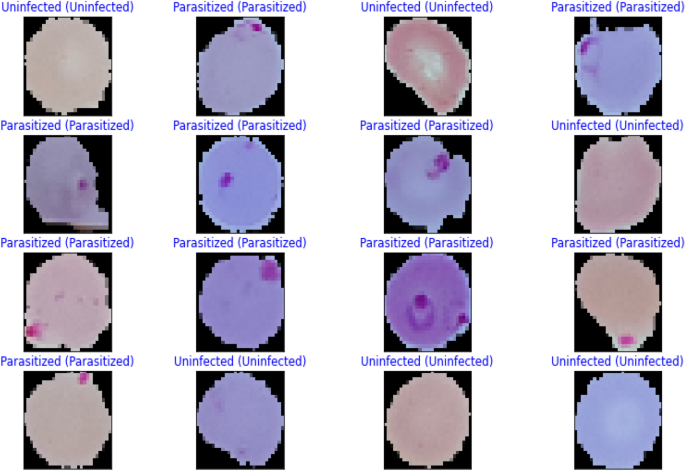
Illustration of some prediction results of the proposed model.
Time complexity analysis
According to our study, the learning model was trained for 100 epochs to assess the time complexity of the model. The results show that our model takes around 33.8667 min for training and 3 s for complete testing, which is less than all the compared models. This study addresses the urgent need for automated malaria detection and classification. It proposes a novel approach based on integrating inception and imperative capsule neural networks. This research has the potential to significantly improve malaria diagnosis, contributing to more effective disease management and prevention. Additionally, the study contributes to the growing field of deep learning in medical image analysis. It showcases the applicability of advanced neural network architectures to address critical healthcare challenges.
Conclusions
This research develops a deep-learning approach by combining the imperative capsule neural network with the inception neural network to distinguish between malaria-parasitized and uninfected cells. This enhances the classification accuracy of identifying malaria parasites from photographs of blood cells. With well-chosen parameters, the capsule model can efficiently finish the procedure for classifying uninfected cells or parasites into different categories. Models with different loss parameters are compared to the proposed model, and the results show that the model's performance can be increased by adjusting the loss parameters. The proposed network achieves higher classification accuracy while analyzing blood cell images for malaria than competing deep learning methods. Under the worst-case scenario (50/50 split), the model obtains an accuracy of 98.10% on the test, while on the 20% split, it achieves an accuracy of 99.355%. These experimental results are helpful since the developed model is robust and flexible and has outperformed competing models. In the work's future scope, the model may be utilized to recognize parasite species and stages in thin blood smears. This research opens opportunities for future advancements in malaria diagnosis and surveillance, including using mobile and portable imaging devices for point-of-care testing.
Data availability
The data that support the findings of this study are openly available in the National Library of Medicine (NLM)—Malaria Data: https://lhncbc.nlm.nih.gov/LHC-research/LHC-projects/image-processing/malaria-datasheet.html and reference number Ref. 23 .
https://www.who.int/news-room/fact-sheets/detail/malaria .
Alnussairi, M. H. D. & İbrahim, A. A. Malaria parasite detection using deep learning algorithms based on (CNNs) technique. Comput. Electr. Eng. 103 , 108316 (2022).
Article Google Scholar
Chakradeo, K., Delves, M. & Titarenko, S. Malaria parasite detection using deep learning methods. Int. J. Comput. Inf. Eng. 15 (2), 175–182 (2021).
Google Scholar
Fact Sheet about MALARIA. https://www.who.int/news-room/fact-sheets/detail/malaria . Accessed 26 Nov 2022.
Devi, S. S., Roy, A., Singha, J., Sheikh, S. A. & Laskar, R. H. Malaria infected erythrocyte classification based on a hybrid classifier using microscopic images of thin blood smear. Multimed. Tools Appl. 77 (1), 631–660 (2018).
Mfuh, K. O. et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 18 (1), 1–8 (2019).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 , 36–55 (2018).
Article PubMed PubMed Central Google Scholar
Hanscheid, T. & Valadas, E. Malaria diagnosis. Am. J. Trop. Med. Hyg. 61 , 179. https://doi.org/10.4269/ajtmh.1999.61.179 (1999).
Article CAS PubMed Google Scholar
Alonso-Ramírez, A. A. et al. Classifying parasitized and uninfected malaria red blood cells using convolutional-recurrent neural networks. IEEE Access 10 , 97348–97359 (2022).
Krizhevsky, A., Ilya Sutskever, S. & Geoffrey, E. H. ImageNet classification with deep convolutional neural networks. Commun. ACM 60 (6), 84–90 (2017).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition , 779–788 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 (7553), 436–444 (2015).
Article ADS CAS PubMed Google Scholar
Razzak, M. I., Naz, S. & Zaib, A. Deep Learning for Medical Image Processing: Overview, Challenges, and the Future 323–350 (Springer, 2018).
Praveen, S. P., Srinivasu, P. N., Shafi, J., Wozniak, M. & Ijaz, M. F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 12 (1), 20804 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Liang, Z. et al . CNN-based image analysis for malaria diagnosis. in IEEE International Conference on Bioinformatics and Biomedicine, IEEE , 493–496 (2016).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. in Proceedings of the 25th International Conference on Neural Information Processing Systems, Volume 1 (NIPS'12) , 1097–1105 (2012).
Bibin, D., Nair, M. S. & Punitha, P. Malaria parasite detection from peripheral blood smear images using deep belief networks. IEEE Access 5 , 9099–9108 (2017).
Dong, Y. et al . Evaluations of deep convolutional neural networks for automatic identification of malaria-infected cells. in EMBS International Conference on Biomedical & Health Informatics, IEEE , 101–104 (2017).
Lecun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86 (11), 2278–2324 (1998).
Szegedy, C. et al . Going deeper with convolutions. in Proceedings of the 2015 (CVPR) , 1–9 (2015).
Sivaramakrishnan, R., Antani, S. & Jaeger, S. Visualizing deep learning activations for improved malaria cell classification. Med. Inf. Healthc. 1 , 40–47 (2017).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 (6), 36–55 (2018).
Sivaramakrishnan, R. et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ 6 , e4568 (2018).
Yang, F. et al. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 24 (5), 1427–1438 (2019).
Article PubMed Google Scholar
Vijayalakshmi, A. & Rajesh Kanna, B. Deep learning approach to detect malaria from microscopic images. Multimed. Tools Appl. 79 (21), 1–21 (2020).
Madhu, G. et al. Imperative dynamic routing between capsules network for malaria classification. Comput. Mater. Contin. 68 (1), 903–919 (2021).
Loddo, A., Fadda, C. & Di Ruberto, C. An empirical evaluation of convolutional networks for malaria diagnosis. J. Imaging 8 , 3. https://doi.org/10.3390/jimaging8030066 (2022).
Meng, X., Ha, Y. & Tian, J. Neighbor correlated graph convolutional network for multi-stage malaria parasite recognition. Multimed. Tools Appl. 81 , 11393–11414. https://doi.org/10.1007/s11042-022-12098-6 (2022).
Madhu, G. et al. DSCN-net: A deep Siamese capsule neural network model for automatic diagnosis of malaria parasites detection. Multimed. Tools Appl. 81 , 34105–34127. https://doi.org/10.1007/s11042-022-13008-6 (2022).
Ha, Y., Meng, X., Du, Z., Tian, J. & Yuan, Y. Semi-supervised graph learning framework for apicomplexan parasite classification. Biomed. Signal Process. Control 81 , 104502. https://doi.org/10.1016/j.bspc.2022.104502 (2022).
Sabour, S., Frosst, N. & Hinton, G. E. Dynamic routing between capsules. Adv. Neural Inf. Process. Syst. 1 , 3856–3866 (2017).
Szegedy, C. et al . Going deeper with convolutions. in Proc. CVPR 2015 , 1–9 (2015).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. in Proceedings of the IEEE International Conference on Computer Vision , 1026–1034 (2015).
Glorot, X. & Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. in Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics , 249–256 (2010).
Das, D. K., Maiti, A. K. & Chakraborty, C. Automated system for characterization and classification of malaria-infected stages using light microscopic images of thin blood smears. J. Microsc. 257 (3), 238–252 (2015).
Díaz, G., González, F. A. & Romero, E. A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J. Biomed. Inform. 42 (2), 296–307 (2009).
Gopakumar, G. P. et al. Convolutional neural network-based malaria diagnosis from focus stack of blood smear images acquired using custom-built slide scanner. J. Biophoton. 11 (3), e201700003 (2018).
Rahman, A. et al . Improving malaria parasite detection from red blood cell using deep convolutional neural networks. (2019). arXiv:1907.10418 .
Download references
Author information
Authors and affiliations.
Department of Information Technology, VNR Vignana Jyothi Institute of Engineering and Technology, Hyderabad, Telangana, 500090, India
Golla Madhu
Operations Research Department, Faculty of Graduate Studies for Statistical Research, Cairo University, Giza, 12613, Egypt
Ali Wagdy Mohamed
Applied Science Research Center, Applied Science Private University, Amman, Jordan
LBEF Campus (Asia Pacific University of Technology & Innovation, Malaysia), Kathmandu, 44600, Nepal
Sandeep Kautish
College of Business and Economics, Kabridahar University, Po Box 250, Kabridahar, Ethiopia
Mohd Asif Shah
School of Business, Woxsen University, Kamkole, Sadasivpet, Hyderabad, 502345, Telangana, India
Division of Research and Development, Lovely Professional University, Phagwara, 144001, Punjab, India
Department of Statistics & Operations Research, Aligarh Muslim University, Aligarh, 202002, India
You can also search for this author in PubMed Google Scholar
Contributions
G.M. conceived and designed the experiments, performed the experiments, and prepared figures and/or tables. A.W.M., S.K., M.A.S. and I.A. supervised the study, analyzed the results, and provided insightful suggestions for the manuscript. All authors have read and authored or reviewed drafts of the paper and approved the final draft.
Corresponding author
Correspondence to Mohd Asif Shah .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Madhu, G., Mohamed, A.W., Kautish, S. et al. Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks. Sci Rep 13 , 13377 (2023). https://doi.org/10.1038/s41598-023-40317-z
Download citation
Received : 13 April 2023
Accepted : 08 August 2023
Published : 17 August 2023
DOI : https://doi.org/10.1038/s41598-023-40317-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- français
- español
- português
Related Links
Global technical strategy for malaria 2016–2030, 2021 update.

View Statistics
Description, more languages, collections.
- Publications
Show Statistical Information
- 1. Headquarters
- Open access
- Published: 21 July 2011
Mathematical models of malaria - a review
- Sandip Mandal 1 ,
- Ram Rup Sarkar 1 &
- Somdatta Sinha 1
Malaria Journal volume 10 , Article number: 202 ( 2011 ) Cite this article
83k Accesses
220 Citations
17 Altmetric
Metrics details
Mathematical models have been used to provide an explicit framework for understanding malaria transmission dynamics in human population for over 100 years. With the disease still thriving and threatening to be a major source of death and disability due to changed environmental and socio-economic conditions, it is necessary to make a critical assessment of the existing models, and study their evolution and efficacy in describing the host-parasite biology. In this article, starting from the basic Ross model, the key mathematical models and their underlying features, based on their specific contributions in the understanding of spread and transmission of malaria have been discussed. The first aim of this article is to develop, starting from the basic models, a hierarchical structure of a range of deterministic models of different levels of complexity. The second objective is to elaborate, using some of the representative mathematical models, the evolution of modelling strategies to describe malaria incidence by including the critical features of host-vector-parasite interactions. Emphasis is more on the evolution of the deterministic differential equation based epidemiological compartment models with a brief discussion on data based statistical models. In this comprehensive survey, the approach has been to summarize the modelling activity in this area so that it helps reach a wider range of researchers working on epidemiology, transmission, and other aspects of malaria. This may facilitate the mathematicians to further develop suitable models in this direction relevant to the present scenario, and help the biologists and public health personnel to adopt better understanding of the modelling strategies to control the disease
Malaria is an ancient disease having a huge social, economic, and health burden. It is predominantly present in the tropical countries. Even though the disease has been investigated for hundreds of years, it still remains a major public health problem with 109 countries declared as endemic to the disease in 2008. There were 243 million malaria cases reported, and nearly a million deaths - primarily of children under 5 years [ 1 ]. With no effective vaccine in sight and many of the older anti-malarial drugs losing effectiveness due to the parasite evolving drug resistance, prevention (using bed nets) is still the only advisory given to afflicted persons. Malaria has also gained prominence in recent times since climate change or global warming is predicted to have unexpected effects on its incidence. Both increase and fluctuation in temperature affects the vector and parasite life cycle. This can cause reduced prevalence of the disease in some areas, while it may increase in others. Thus climate change can affect malaria prevalence pattern by moving away from lower latitudes to regions where populations have not developed immunity to the disease [ 2 – 8 ].
Malaria is caused by the protozoan parasites of genus Plasmodium . In humans it is caused by Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale , and Plasmodium vivax . Of these, P. falciparum is the most common cause of infection in Africa and South East Asia, and is responsible for ~80% of all malaria cases and ~90% of deaths [ 1 ]. In India, P. vivax , has been the primary pathogen responsible for malaria, even though P. falciparum cases are on the rise in recent times [ 9 ]. The parasite requires two hosts to complete its life cycle - the vector female Anopheles mosquito and human. The bites/bloodmeals of infected mosquitoes are the mode of transmission of the parasite between the human hosts. Grassi and Ross discovered the mosquito's role in the parasite life cycle and transmission in 1897 [ 1 ], and the genomes of Anopheles mosquito and P. falciparum were sequenced in 2002 [ 10 , 11 ]. During the interim 105 years, much scientific research was undertaken and progress made in the understanding of the host-parasite-vector interactions and their biology. However, the complexities in the life cycle of the parasite, highly complex environmental and social interactions, evolutionary pressure of drugs and control measures contributing to drug resistance of parasite, unforeseen effects of climate change, and migration of population between endemic and non endemic areas continued to contribute to the huge burden of morbidity and mortality accompanying the disease. These have also thrown up new challenges to researchers and public health professionals.
Among all areas in Biology, researchers in infectious disease were one of the foremost to realize the important role of mathematics and mathematical models in providing an explicit framework for understanding the disease transmission dynamics within and between hosts and parasites. In a mathematical expression or a model, several known clinical and biological information are included in a simplified form by selecting features that seem to be important to the question being investigated in disease progression and dynamics. Therefore, a model is an "approximation" of the complex reality, and its structure depends upon the processes being studied and aimed for extrapolation. Based on the questions being asked, these studies can help fit empirical observations, and can be applied to make theoretical predictions on lesser known or unknown situations. For example, mathematical models have been widely used by epidemiologists as tools to predict the occurrence of epidemics of infectious diseases, and also as a tool for guiding research for eradication of malaria at the present time [ 12 , 13 ].
Malaria is one of the oldest diseases studied for a long time from all angles, and vast literature exists describing a host of modelling approaches. Different approaches are helpful in guiding different stages of the disease through synthesizing available information and extrapolating it. It is felt that a combination of different approaches, rather than a single type of modelling, may have long term usefulness in eradication and control [ 13 ]. In the recent years, global eradication and control efforts [ 14 , 15 ] have led to a surge of activities leading to many studies and publications. It is a formidable task to review all types of models in one article. In this article a historical path has been considered, and an attempt is made to take into account some of those mathematical models, which are primarily focused on the transmission dynamics of the infection in the host and vector populations, using the epidemiological compartment modelling approach [ 16 , 17 ]. The modelling methodology is predominantly deterministic and differential equation based.
This in no way undermines the importance of other models that are concerned with the "within host" biology, or population genetic models that have an increasing impact in eradication and control. To study the infection phenomena inside the individual host, "within host" models consider the interaction of the parasite with the immune cells in an individual host [ 18 – 21 ]. Population genetic models study evolution and spread of the parasite in a complex landscape of varying host immunity, host death, drugs, and mosquito availability [ 22 , 23 ]. These are connected to the parasitological status of a population, which is related to the different classes in the epidemiological compartment models. As mentioned earlier, only few recent papers are referred on these topics, and interested readers may get further leads from them. Different modelling methodologies have also been adopted in addition to differential equation-based models. Few examples are, individual-based models [ 24 ], habitat-based models [ 25 ], integrated models [ 26 , 27 ], and others [ 17 , 28 – 34 ]. In spite of the wide range of these models and methodologies, the major modelling approach still remains the transmission of infection through the epidemiological compartments of human and vector populations. Further, with the recent concern with climate change [ 5 , 6 ], the importance of the power of prediction of mathematical models in understanding the infectious disease transmission, highlights the requirement of a consolidated review on this modelling strategy and evolution of the models employed till date.
Sir Ronald Ross, while working at the Indian Medical Service in 1890's, demonstrated the life-cycle of the malaria parasite in mosquito, and was one among the first to publish a series of papers using mathematical functions to study transmission of Malaria in early 1900 [ 35 – 39 ]. He developed a simple model, now known as the classical "Ross model" [ 36 ], which explained the relationship between the number of mosquitoes and incidence of malaria in humans. A commonly adopted method of parsimony in developing mathematical models is to accept the simplest possible theoretical description consistent with the data available at a given time. However, these simple models often have limited predictability and are not satisfactory when new data becomes available, and more complexities of interactions are considered. Therefore, subsequently several models have been developed by researchers who extended Ross's model by considering different factors, such as latent period of infection (Table 1 ) in mosquitoes and human [ 12 , 40 ], age-related differential susceptibility to malaria in human population [ 12 , 41 , 42 ], acquired immunity [ 41 , 43 , 44 ], and spatial and genetic heterogeneity of host and parasite [ 45 – 49 ].
With all these models at hand, it is not a trivial matter to infer the crucial features of the disease, and get a coherent understanding of the development of the models from interactions among the vector, parasite and host. In this review, the emphasis is more on the evolution of different mathematical models (mainly differential equation based) of malaria with a brief discussion on stochastic models and data based statistical models. The first aim of this paper is to develop a hierarchical structure of the range of deterministic models of different levels of complexity, starting from the basic Ross Model. The second objective is to elaborate on the evolution of modelling strategies in different steps, using some of the key mathematical models that describe malaria incidence by including specific properties of host-vector-parasite interactions. To reach a wide range of researchers working on the epidemiology, transmission, and other aspects of malaria, the models have been critically analysed, so that it will be useful in understanding and classifying the numerous between-host models in this area. This may help mathematicians to further develop suitable models, and biologists and public health professionals to adopt better strategies for controlling the disease.
Model basics
In epidemiological compartment models of infectious diseases, transmission of infectious agents in the host population is the fundamental process to be described. When a pathogen appears in a host community, it partitions individuals in the community into categories depending on parasite density inside them and the type of infection. These categories or compartments are represented by standard notation of S-E-I-R after the pioneering work of Kermack and McKendrik [ 16 ]. In a simple form they are as follows: the first group consists of the fraction of host population that is Susceptible ( S ) to infection; then comes the Exposed ( E ) class - the fraction of population whose individuals are infected by the pathogen, but not capable of passing on the infection to others during a latent period (Table 1 ). The next is I class or Infectious individuals, who give rise to more infected individuals through interaction with the Susceptibles. Finally, those individuals who recover from the infection make up the R class.
There may be variations in the compartment structure depending on the type of disease. For example, the I class of individuals may not recover at all and die; R can consist of individuals, who recover with temporary or permanent immunity, thereby further subdividing the epidemiological compartments. Using these notations, eight classes of compartmental models are possible - SI, SIS, SEI, SEIS, SIR, SIRS, SEIR and SEIRS [ 50 ]. For example, in an SEIRS model, a fraction of the susceptible ( S ) population gets exposed ( E ) to infection, a part of which then becomes infectious ( I ). Some from the I class recover from the disease, and become part of the R class with temporary immunity. When immunity is lost, they become susceptible to pathogen attack again, and enter the S class. The Plasmodium parasite requires both human and mosquito for its life cycle to complete, and the infection is transferred between susceptible human individuals through the bite of infected mosquitoes, which acquire infection through a blood meal from infected humans. In malaria models, therefore, these compartments have been applied to both human (host) and vector (mosquito).
Epidemiological compartments, for an SEIRS model, separating different stages of infection and parasite density in the host population, are shown in Figure 1 . Different stages of infection, which are significant to the dynamics of transmission, are shown in the transfer diagram at the top of Figure 1 . The level of infectious agent that replicates inside a host may develop from small inoculums to a higher level, and later decline and/or disappear altogether as it passes through these compartments (shown by blue colour scale in Figure 1 ). Latent period, Incubation period and Symptomatic period (Table 1 for definitions) are also shown in Figure 1 . In many cases of infection, the incubation period and latent period are not the same [ 12 ]. Appearance of symptoms is important for case diagnosis and treatment. But Asymptomatic infections are commonly observed in humans and require clinical validation. The status of the clinical markers (presence/absence indicated by +/-) for diagnosis of each compartment is denoted by P (PCR), Sc (Sero-conversion) and C (Cellular immunity) [ 51 – 53 ], and are shown in the bottom panel of Figure 1 (Table 2 for details). Starting from the basic Ross model many transmission models of malaria have been developed by considering regulation of the passage of the human host and mosquito vector through these epidemiological compartments as a function of the host and parasite-specific factors, their interactions, and external environmental variables.
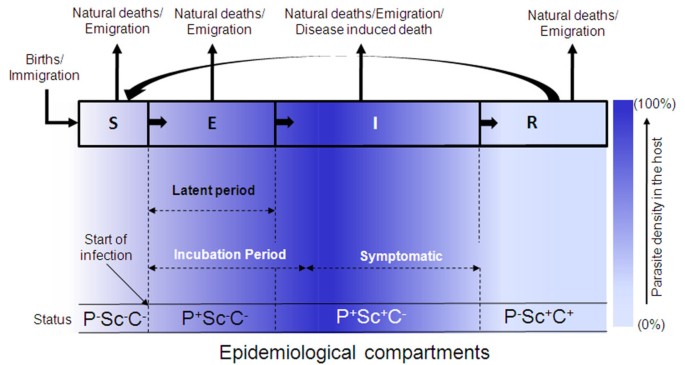
Epidemiological Compartments separating different stages of infection and parasite density in a population . S, E, I and R represent Susceptible, Exposed, Infected and Recovered fraction of the population respectively. Arrows on the top indicate different ways of population loss and transfer of population from one compartment to another. Different periods (Latent, Incubation, Symptomatic) characteristic of infection are shown by dotted arrows. The bottom panel shows the status of clinical markers for each compartment - PCR (P), Sero-conversion (Sc) and Cellular immunity (C) (positive or negative). Colour Bar indicates the density of parasites in host in different compartments (0-100%). See text and Table 2 for details.
Hierarchy of malaria models
Even for the restricted set of deterministic Ordinary Differential Equation (ODE) models of epidemiological compartments being considered in this article, summarising hundred years of extensive theoretical work on malaria modelling with the incorporation of ever-increasing complexities, have the possibility of unintentional bias, omissions, and under-representations. Keeping this in mind, an attempt has been made here to elaborate the evolution of these models by considering some representative mathematical models that include the increasing complexities of host-vector-parasite interactions. The word "hierarchy of models" used here (shown here as a tree in Figure 2 ), is based on the undisputed fact that the start of the tree is the model by Ronald Ross [ 36 – 39 ], and its highly significant improvement, with focus on application in mosquito eradication, by George Macdonald [ 40 ]. The rest of the models have been grouped here purely on the basis of increasing complexity of the epidemiological compartments in the host and vector populations, and hence the temporal order is not conserved. The three basic models, based on which other models were developed, are shown as the grey trunk of the tree.
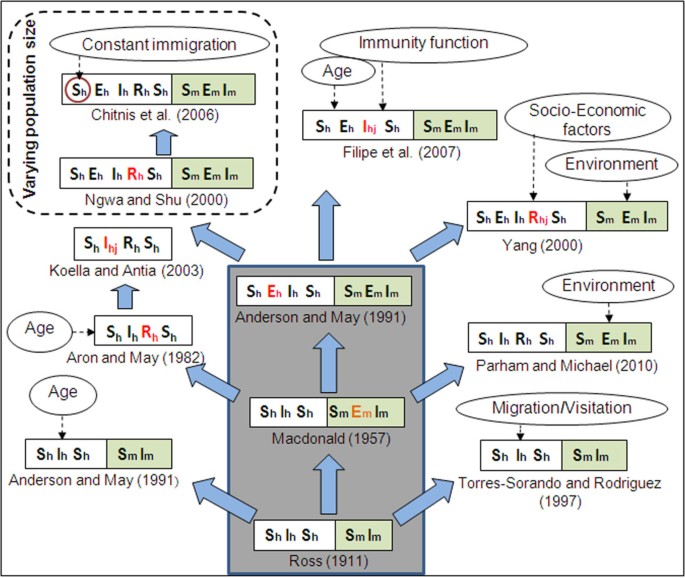
Evolution and grouping of different types of SEIR malaria models . Subscripts 'h' and 'm' stands for human and mosquito. Double-folded boxes are for both human & mosquito population, and single fold boxes are only for human. First time addition of a new compartment is shown in red. The subscript 'j' (= 1, 2, 3) indicates further subdivision of the corresponding compartment. Three models inside the big grey box are considered as the Basic malaria models in this paper. Dotted arrows show the incorporation of complex factors in different models or specific compartment (red circle). Total population size is constant for all models, except the ones inside the dashed box.
The epidemiological compartments are kept in double-fold boxes in Figure 2 . The human classes ( S h , E h , I h , R h ) are in the left fold and the mosquito classes ( S m , E m , I m ) in the right. In general, the human classes in malaria infection end with the susceptible ( S h ) class, but the mosquito populations die of infection and hence it can only go up to the infected class ( I m ). In models where there is only a single-fold box (for the human classes), the effect of vector is introduced through their vectorial capacity of infection (Table 1 for definition). The newly introduced compartment over the earlier one is shown in red. The effects of different complex factors, such as age, immunity, environment and socio-economic, in different models or specific compartments (red) are shown by dotted arrows.
Ronald Ross in his first mathematical model of malaria used the word "pathometry" to mean "quantitative study of a disease either in the individual or in the community" [ 36 ]. Ross, through his model, showed that reduction of mosquito numbers "below a certain figure" ( Transmission threshold ) was sufficient to counter malaria - a concept far ahead of his time. After about 40 years, George Macdonald [ 40 ], in the 1950s, reasserted the usefulness of mathematical epidemiology based on 20 years of fieldwork. He modified Ross's model by integrating biological information of latency in the mosquito due to malaria parasite development, and implicated the survivorship of adult female mosquito as the weakest element in the malaria cycle. This provided a rationale for a massive World Health Organization (WHO)-coordinated campaign, which focused on using the insecticide dichlorodiphenyltrichloroethane (DDT) that killed mosquitoes, for the elimination of malaria transmission among 500 million people in Africa [ 54 , 55 ]. Latency of infection in humans was introduced by Anderson and May [ 12 ] in Macdonald's model making the additional "Exposed" class in humans.
Researchers have modified the basic Ross model to explain the effect of age structure of prevalence [ 12 ], migration and visitation of people [ 48 ]. Several models were also put forward after Macdonald's model by combining additional complexities of human immunity, parasite diversity, and resistance, to explain large amounts of epidemiological data collected in Africa and other parts of the world [ 17 , 56 , 57 ]. They were fairly successful in describing region-specific incidence data. Thus, all other models shown in Figure 2 , and discussed in this paper, are developed from these three basic models by incorporating different factors to make them biologically more realistic in explaining disease prevalence and prediction. The only new class that is added in humans is the recovered ( R h ) class, which incorporates a time dependent immunity developed on recovery from infection, before being transferred to the susceptible ( S h ) class again. The major advantage of these early models was to provide a suitable control strategy through the Transmission threshold criterion, which is based on the reproductive capacity of the parasite, and termed as basic reproductive number, R 0 (Table 3 for details). Even though the concept of threshold was first introduced by Ross, it originated from Fisher's " net reproductive value " for a parasite [ 58 ]. From its inception the concept of R 0 is widely discussed in any study on population biology of a parasite [ 59 – 61 ]. The basic results of all these models can also be described by estimating the basic reproductive number ( R 0 ).
In the following sections, starting from the details of the basic models, the representative mathematical models in each group along with their underlying features are discussed, and their specific contributions are reviewed.
Important features and comparative analysis of mathematical models
Basic models.
The three basic models, shown in the grey trunk of the tree in Figure 2 , are given in Table 4 . The first column of the Table gives the mathematical model; the basic reproductive number, R 0 - is given in the second column; and, the parameters (with range of values used in literature) are described in the last column. These basic models used the simplest scenario by incorporating only two critical features for predicting malaria progression in the host and vector populations - epidemiological compartments in the populations, and latency periods of pathogen in the mosquito and human. The population size of human is kept constant (unity) in all three basic models.
Ross introduced the first deterministic differential equation model of malaria by dividing the human population into susceptible ( S h ) and infected ( I h ) compartments, with the infected class returning to susceptible class again leading to the SIS structure. The mosquito population also has only two compartments ( S m , I m ), but they do not recover from infection due to their short life span, and thereby follow the SI structure. Time evolution of the fraction of individuals in the infected classes ( I h , I m ) is studied using two differential equations - one each for the human and mosquito (Table 4 ). It is clear that the parameters, m, a, b , and c , that contribute to the increase of R 0 in this model, are related to mosquitoes and humans, and any change in them can significantly affect malaria transmission. Increasing mosquito mortality and reducing mosquito biting rate can reduce R 0 . The Ross model outlines the basic features of malaria transmission, and puts the main burden of transmission on mosquito-specific features, thereby paving the way for mosquito-based malaria control programmes.
The malaria parasite spends approximately 10 days inside a mosquito during its life cycle. The simple Ross model did not consider this latency period of the parasite in mosquitoes and their survival during that period. This resulted in the model predicting a rapid progress of the epidemic in human, and a higher equilibrium prevalence of infectious mosquitoes. Macdonald considered this latency period ( t m ), and introduced the Exposed ( E m ) class in the mosquitoes [ 40 ]. Therefore, in this model (Table 4 ), the mosquito population is divided into three compartments ( SEI ), and the model studies the time evolution of the exposed ( E m ) and infected ( I m ) classes in mosquito. The R 0 for this model is consequently scaled down with increasing latency period.
In a natural extension to the Ross and Macdonald's models, Anderson and May considered the ~21 days latency period of the parasite in humans, and introduced the Exposed ( E h ) class in human population in their model [ 12 ]. This divided the host population into three compartments ( S h , E h , I h ), along with that in the mosquito population ( S m , E m , I m ). This, therefore, is a SEIS model for the human population, and the model consists of four differential equations (Table 4 ) describing the time evolution of both the exposed and infected classes for humans and mosquitoes ( E h , I h , E m , I m ). The R 0 for this model is further reduced due to inclusion of human latency period. A comparative study of Ross (RR), Macdonald (MC), and Anderson-May (AM) models for the prevalence of infected humans and mosquitoes ( I h , I m ) is shown in Figure 3a . The figure shows that inclusion of the latency periods of parasites in humans and mosquitoes not only reduces the long term prevalence of both I h and I m (RR being the highest and AM the lowest); the rates of progression to these final infected populations are also reduced. Even with this minimal complexity, these basic models can give some idea of the effect of different types of interventions on disease transmission dynamics, which is discussed in the next section.
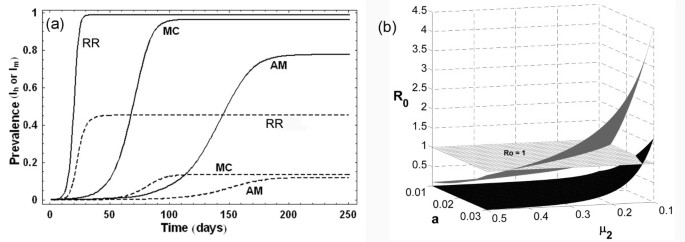
(a) Prevalence curves of human (I h : solid line) and mosquito (I m : dashed line) populations in Ross (RR), Macdonald (MC) and Anderson-May (AM) models . Parameters used are: a = 0.2 day -1 , b = 0.5, c = 0.5, m = 20, r = 0.01 day -1 , μ 1 = 0.017 year -1 , μ 2 = 0.12 day -1 , τ m = 10 days, τ h = 21 days . (b) Variation of basic reproductive number ( R 0 ) with mosquito biting rate ( a ) and mosquito mortality rate ( μ 2 ) in Ross model (grey surface) and Anderson-May model (black surface). The surface of R 0 = 1 is shown as gridded white plane.
Predicting the effects of interventions in the basic models
The parameters mosquito density ( m ), biting rate ( a ) and mosquito mortality rate ( μ 2 ) are important in regulating the fraction of human population that will enter into the exposed ( E ) and infected ( I ) classes. The most important fact for any epidemiologist or public health person is to have an idea about the relative effects of interventions in these parameters to the intensity of transmission, the measure of which is R 0 . Given the expressions of R 0 in all three models in Table 4 it is clear that the square dependence of the biting rate ' a ' implies that halving the biting rate is more effective than halving the coefficients ' b ' or ' c ' in all three models Thus, reducing the biting rate (by using bed nets, or any other method) will be an effective method of controlling the transmission. But this is not so obvious for all parameters. For example, the relative effect of reducing the adult mosquito mortality ( μ 2 ) in comparison to biting rate ( a ) is different in these models due to the presence of the exponential function of μ 2 . Figure 3b shows this with the Ross and Anderson-May models, where the variation of R 0 is plotted with changes in two parameters, biting rate ( a ) and adult mosquito mortality ( μ 2 ) with other parameters as per Table 4 . Due to higher disease prevalence predicted by Ross model (see Figure 3a ), the R 0 surface is also higher compared to that of the Anderson-May model. The surface of R 0 = 1 shows that onset of epidemic happens at higher values of parameters in Ross model compared to Anderson-May model. These results indicate that compared to the reduction in biting rate ' a ', reducing the length of life of adult mosquitoes is most effective in decreasing malaria cases, in the latter two of the three basic models. As mentioned before, these model results provided rationale for control of malaria transmission through the mosquitoes, using insecticides (DDT) and insecticide-impregnated bed nets, since they affect m, a , and μ 2 . Thus, even at this low level of complexity, these models had been successful in describing the factors that influence the transmission of the disease, which were useful in control and eradication of malaria from many countries of the world.
Complex models
Over and above the simple scenario described in the basic models, many other factors such as, host factors, demographic heterogeneity, geographic distribution of populations, rules of social interactions, climate and environmental influences, and the ecology of the area play important roles in the development of malaria in space and time. Age-specific host immunity, parasite diversity, DDT and drug resistance dynamics, vector population dynamics, effect of global warming are also interacting factors and variables that influence disease dynamics at different scales. There has not been A MODEL that has been able to incorporate all factors and variables because of the overwhelming complexity of the system. Also, a model's utility may not always lie in its mathematical analysis or incorporating finer details. The ability to base it on relevant details and ask specific questions that can be tested, are the hallmark of useful models. Along with fitting the past data and predicting the future, it should also be able to point to areas where data needs to be generated in order to increase our conceptual grasp. Such improvements in modelling generally occur in multiple steps, one leading to the other, as more information become available. The next section elaborates on some representative next-generation mathematical models that evolved from the above-mentioned basic models, and includes the increasing complexities of host-vector-parasite interactions. Specifically, the factors considered here are - (i) Age and immunity, (ii) Host-Pathogen variability and resistant Strains, (iii) Environmental factors, (iv) Social and economic factors, and (v) Migration and visitation.
Age and immunity
Malaria burden differs depending on age and gender in humans. In African children, most malaria deaths occur under the age of 5-years. As a result of continuous exposure and the ability to develop a degree of immunity to the disease, older Africans have reduced risk. Outside Africa, where continuous exposure does not occur, the disease burden extends into adulthood [ 1 ]. Age and immunity, therefore, are known to be important inter-related factors for transmission of malaria in a population. The importance of incorporation of immunity in malaria models is aptly described by Koella [ 56 ] - " Incorporating immunity into malaria models is important for two reasons. First, the neglect of immunity leads to unrealistic predictions. Incorporating immunity can help to make models more realistic. Secondly, modelling immunity, and in particular the effect of vaccines, can help to predict the outcome of vaccination programmes" . A number of epidemiological studies [ 41 , 43 , 44 , 57 , 62 ] have focused on this important aspect by including immunity and age structure of the human community in the models. In this scenario, the infection moves differentially within different age groups based on their immune status, and also with time.
Age structure was included by Anderson and May [ 12 ] in the simple Ross model by considering the human population density in the I h class as a function of age ( α ) and time ( t ) as,
Immunity can be included in a model in two ways - by considering a separate Immune class ( R h ) in humans, and by incorporating an Immunity function in existing models. Some models (Dietz et al [ 57 ], Aron [ 43 ], Ngwa and Shu [ 64 ], Ngwa [ 65 ], Chitnis et al [ 32 , 66 ], Yang [ 67 , 68 ]) have introduced a separate immune class in their models, whereas, some others (Fillipe et al [ 44 ] and [ 69 – 72 ]) have used complex immunity functions in their model. Assuming that the malaria immunity is not permanent, Dietz et al [ 57 ] first proposed a model considering seven compartments of human. The effect of mosquito was introduced through vectorial capacity . In this model a person may either recover from the infected class ( I h ) and directly return to the susceptible class ( S h ), or become re-infected through a temporary immune class ( R h ). The model has shown a good fit to the data obtained from northern Nigeria. The changes on each compartment in this model were presented using difference equations. The differential equation based models that incorporate immune classes are discussed below.
Immune class
Generally immunity is modelled by considering the fact that individuals are born susceptible to become infected at a rate of h infections per year, but they subsequently recover and acquire immunity at a slow rate. If immunity is temporary and lasts only for τ years (in absence of new infections), then they again become susceptible to infection. Immunity is also boosted by new infections. In this simple scenario [ 73 ], the average per capita rate of loss of immunity γ ( h, τ ) is given by
The range for h is estimated between 0 to the order of 10 3 [ 74 , 75 ], and the upper limit of τ has not been reported so far [ 56 ]. The variation of γ ( h, τ ) with force of infection ( h ) and period of immunity ( τ ), in absence of new infections, is shown in Figure 4a for a small parameter range to highlight the relative effects of changes in h and τ , as the major variation of γ is observed only for low values of h . Due to continuous exposure, in high endemic zones where force of infection ( h ) is very high, the rate of loss of immunity is nearly zero. Figure 4a clearly shows that the rate of loss of immunity (γ) is faster at low τ values, whereas for the force of infection ( h ), it decreases slowly. For a fixed τ, γ decreases monotonically with force of infection ( h ), implying reduced rate of transformation from recovered class to susceptible class due to increase in force of infection. It is further reduced if the period of immunity ( τ ) is increased. Some of the models that have considered the immune class are discussed below.
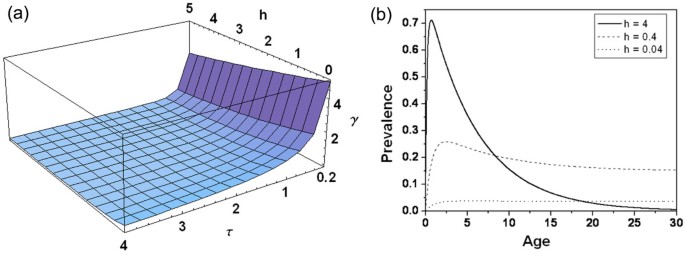
(a) Variation in loss of immunity ( γ ) with force of infection ( h ), and immunity period ( τ ) in absence of new infections . (b) Age-Prevalence curve simulated from Aron-May model for three different levels of force of infection ( h ). Other parameter values are r = 0.8, q = 0.2 , and τ = 5 .
In a population that has reached its equilibrium pattern of infection, time can be represented through the age (α) of the cohort. Considering this, Aron and May [ 41 ] proposed an age-specific immunity model with a new compartment - Immune ( R h ) - in humans. This model, thus, consists of three compartments in humans: Susceptible ( S h ), Infected ( I h ) and Immune ( R h ), and is a SIRS model. This model is shown in a single-fold box for host in Figure 2 , because the effect of mosquito is introduced only through the force of infection, h . The infected individuals can recover at a rate r to become susceptible again, or may acquire immunity at a slow rate of q . This simple model of immunity, incorporates the immunity factor by adding an extra term γ ( h, τ ) R h - of people who lose immunity - in the susceptible class, and subtracting the same from the immune class in equation ( 2 ).
Solution of equation 2 shows how the prevalence of infection varies with the age of human. Figure 4b shows the prevalence of infection ( I h ) with age at three different forces of infection ( h ). At higher infection ( h = 4 ), I h rises rapidly with age in young infants and children, attains a peak, and then declines in the older children to reach a low level in adults. Prevalence in adults decreases due to the increase in immunity. For low h ( h = 0.04 ), this dependence on age is negligible. This model predicts that the prevalence rises quickly in early childhood and declines slowly into adulthood in highly endemic areas, due to slow acquisition of immunity with age/time. Interestingly, the prevalence among adults is highest when h is in an intermediate value. The adult crossover of the age-prevalence curve with increasing h resembles the pattern of acute infection described by Boyd in tropical Africa [ 63 ].
Inclusion of the "Recovered" class with immunity of the host has been the source of many later models that considered other variations in host-pathogen interactions [ 12 , 56 ]. A few models are mentioned below, which along with introducing the "Recovered" class in humans in the Anderson-May (AM) model, also differ in some of the critical assumptions from the models discussed so far. One of the features that has been consistently followed in all the models discussed above is the constancy of population size. Mortality and migrations are major factors in changing the population size in an area and the inclusion of varying population size in the model makes them more realistic.
Ngwa and Shu proposed an immunity model in which disease related death rate is considered to be significantly high, and the total population is not constant (shown inside the dashed box in Figure 2 ). The Ngwa-Shu model [ 64 ] model consists of four compartments in humans - Susceptible ( S h ), Exposed ( E h ), Infected ( I h ) and Immune ( R h ) - and three compartments in mosquitoes - Susceptible ( S m ), Exposed ( E m ), and Infected ( I m ) (see Additional file 1 Table S1). Mathematical analysis of the model shows that the Basic Reproductive Number, R 0 , can describe the malaria transmission dynamics of the disease, where a globally stable disease-free state exists if R 0 < 1, while for R 0 > 1, the endemic equilibrium becomes globally stable. This model explicitly shows the role of inclusion of demographic effects (net population growth) in predicting the number of fatalities that may arise as a result of the disease. In a similar theme, Chitins et al [ 32 , 66 ] included constant immigration of susceptible human population, (see Additional file 1 Table S2). Considering immigration of people and excluding direct human recovery from the infectious to susceptible class (as is considered in other models here), they showed that the population approaches the locally asymptotically stable endemic equilibrium point, or stable disease-free equilibrium point, depending on the initial size of the susceptible class.
Immunity can be described as a continuum of different levels of protection rather than a single class, as anti-malarial immunity develops slowly among people exposed to continuous and intense malaria transmission. Yang [ 67 ] divided the immune class ( R h ) in human population into immune ( R h1 ), partially immune ( R h2 ) and non-immune but with immunologic memory ( R h3 ), with each class having differential immunity (see Additional file 1 Table S3). The mathematical analysis of Yang model shows that the effects of these three types of immune responses lead to delay in the reappearance of the individuals, who already had experienced malaria, to the susceptible population. Hence the community under high threat of malaria (high R 0 ) shows low prevalence of individuals with asexual blood-stage infection and without infectious gametocytes, whereas, the same community is relatively free of severe infection due to the increase in immunity by re-infection.
Immunity functions
Due to lack of confirmed markers of immunological protection, different processes that determine the immunity acquisition to clinical disease and to asymptomatic carriage of malaria parasites are poorly understood. The models discussed in the earlier section consider the immune individuals as a separate class, with no consideration of the types of processes that drive acquisition of immunity and its role in disease progression. In an insightful approach, Filipe et al [ 44 ] introduced three age-specific "immunity-functions" in their SEI model for the human host, in which the infected humans are divided into three classes - infected with severe disease ( I h1 ), asymptomatic patent infection ( I h2 ), and infected with undetectable parasite density ( I h3 ). The effect of mosquito density is incorporated through the force of infection ( h ). The dynamics of transmission of infection in this model is given in Additional file 1 (Table S4).
The three immunity functions (IF) introduced in the Filipe model are - (i) Reducing the susceptibility to clinical disease, ϕ (IF1), (ii) speeding up of the clearance of detectable parasites, r A (IF2), and (iii) increasing tolerance to sub-patent infections, r ij (IF3). These functions depend on age and disease transmission intensity (i.e., Entomological Inoculation Rate , see Table 1 ) in a complex manner. They base their model assumptions on the fact that the rates at which both types of immunity - clinical and anti-parasite - develop are different. Details of the immunity functions are given in Additional file 2 . All these processes have widely varied time scales, which make the disease transmission in this age-structured population quite complex. The first two types of immune functions reproduced the epidemiological age-prevalence curves seen in empirical data better. The third one i.e. the tolerance to sub-patent infections, is not required to explain the empirical data.
Susceptibility to clinical disease (effect of IF1) develops early in life and then decreases with age, and is inversely proportional to the inoculation rate (Figure 5a ). Anti-parasite immunity, on the other hand, develops later in life and results in more rapid clearance of parasite. Figure 5b shows that the rate of recovery from detectable parasite (effect of IF2) increases with age and force of infection ( h ). These figures clearly show that these two distinct acquired immunity processes are required to demonstrate: (i) reduction in clinical susceptibility and (ii) a parasite immunity process that develops substantially in later life, and, which increases the rate of natural recovery. In addition, this also explains the duration of clinical and parasite immunity from the age-prevalence pattern. To show the efficacy of different intervention strategies more complex models have been developed in this area [ 69 – 72 ]
Response of immunity functions: (a) susceptibility ( ϕ ) to develop clinical disease; and (b) rate of clearance of detectable parasites ( r A ), to variation in force of infection ( h ) and age .
Host-pathogen variability and resistant strain models
The basic models assume homogeneity in the host and parasite populations in terms of their response to the process of transmission of infection. They consider all the individual hosts and parasites in the population to have an equal chance of developing disease or becoming immune or transmitting infection. However modern application of molecular typing methods has shown that there exist diversity among host and parasites in responding to infection. Further, long term and indiscriminate use of insecticides (DDT) and drugs (quinine and chloroquine) brought forth the hitherto neglected issue of heterogeneity in vector and parasite phenotypes and genotypes. The evolutionary consequences of these interventions had serious negative impact in malaria control. Most models of population heterogeneity and resistance consider within-host processes. Many mathematical models have been developed with pathogen population structure and heterogeneous host population to describe variable antigenic response, immune selection, pathogen strain structure [ 76 – 78 ]. Inclusion of evolution of drug resistance, along with other factors, in the models can help in the design of rational strategies for the control of drug resistance [ 79 – 86 ].
Several resistant-strain models have been developed based on evolution of drug resistance through host immunity [ 82 , 85 ], and by considering the practical implications of the artemisinin combination therapy (ACT) drug policies adopted by many countries [ 84 ]. Population genetic considerations of the cost of resistance (Table 1 ), are also included in this type of models [ 81 , 87 ]. More recent work elaborates the complexity of the process of drug resistance by considering the interaction of several environmental, pharmacological and genetic factors [ 86 ]. These models are important as they address phenomena critical to public health, i.e., the evolution of drug resistance in malaria parasites.
In general, these resistant-Strain models divide the infected host population ( I h ) into two compartments, i.e., infected by drug-sensitive strain and drug-resistant strain of the parasite.
The model, proposed by Koella and Antia [ 82 ], further divides the host population infected by drug-sensitive strain into two compartments - treated and untreated . So this model consists of five compartments of human: susceptible ( S h ), sensitive, infected, and treated ( I h1 ), sensitive, infected, and untreated ( I h2 ), infected with the resistant strain ( I h3 ), and the recovered ( R h ). The role of mosquito vector is included through inoculation rates of sensitive and resistant parasites. The formulation of the model is described in Additional file 1 (Table S5). The primary prediction of this model indicates that there is a threshold proportion of people ( f c ) among the infected and treated ( I h1 ) classes, below which resistance cannot spread, and above which resistance will eventually become fixed in the population. The threshold level f c is defined as:
where, ∈ is termed as the "Effectiveness of treatment", i.e., the ratio of the duration of infection for the untreated and treated sensitive parasites, and Γ is the cost of resistance . Thus, these two parameters (Γ and ∈ ) regulate whether drug sensitive or resistant parasite will be dominant in the population. The model also shows that, in the absence of drug or treatment, the fitness of resistant parasite reduces with respect to sensitive parasite; otherwise both the parasites have identical properties. In this case, sensitive and resistant parasites cannot co-exist.
Environmental factors
The epidemiology of the host, vector, and pathogen for malaria necessitates consideration of the conditions that increase the mosquito population density. Modelling the dynamics of mosquito populations to increase understanding of malaria transmission across a range of environmental conditions, including climate change, is an important and emerging research area. The basic reproductive numbers ( R 0 ) for the basic models depend crucially on the parameters related to mosquito density. Environmental factors, such as temperature, humidity, rainfall and wind patterns have great impact on mosquito reproduction, development and longevity and the parasite survival in its life cycle in mosquito. It is known that mosquito breeding is influenced by temperature - a change in temperature from 12°C to 31°C reduces the number of days required for breeding from 65 days to 7.3 days [ 88 ]. The sporogony of the parasites in vector is completed in 55 days at 16°C, which reduces to 7 days at 28°C [ 89 ]. With recent surge of interest on the effects of global warming on malaria incidence, modelling the effects of environmental factors in malaria transmission has become quite relevant and topical [ 6 – 8 , 90 , 91 ]. As humidity is conducive to mosquito growth, rainfall and stagnant water bodies also influence mosquito density.
The environmental impact on the transmission of malaria is therefore studied primarily by modifying the mosquito population dynamics. Influence of temperature and humidity change on the rate of transformation from juveniles to adults in the susceptible class of adult mosquitoes has been modelled [ 88 ]. In addition, several mathematical studies have been performed to simulate the effect of environmental variability in the abundance of mosquito populations such as, random fluctuation in the form of colour noise in infected mosquito dynamics of Ross model [ 92 ], periodic or noisy form of the force of infection [ 12 , 41 , 92 ]. Several studies have also included the effect of environmental fluctuations in diverse ways [ 2 , 5 , 67 , 68 , 93 , 94 ] with the aim to develop realistic and validated malaria modelling frameworks that are able to identify the crucial linkages between pathogen transmission processes and climactic factors.
In a recent study, Parham and Michael proposed a model [ 5 ], to study the dynamics of the mosquito population by considering simultaneous effects of rainfall and temperature. The model consists of three compartments in humans ( S h , I h , R h ) with fixed duration of latency, and three compartments in mosquitoes ( S m , E m , I m ) (see Additional file 1 Table S6). Different environmental factors are introduced in this model through parameters related to mosquitoes. The birth rate of adult mosquito is considered to be a function of rainfall and temperature, whereas, mosquito mortality rate, biting rate, duration of sporogonic cycle and survival probability of infected mosquitoes over the incubation period of the parasite are considered to be dependent on temperature variation. The major finding of this model is that changes in rainfall patterns not only influence vector abundance, but also strongly govern malaria endemicity, invasion and extinction. However, when sufficient rainfall exists to sustain vector development and survival, then the temperature affects the pathogen life cycle, and has stronger influence on the rate of disease spread.

Social and economic factors
Malaria risk is highly dependent on the socioeconomic conditions of the host population. It is now fairly evident that " as a general rule of thumb, where malaria prospers most, human societies have prospered least " [ 95 ]. Poverty is largely concentrated in the tropical and subtropical zones, and that is where most malaria transmission is observed. The extent of the correlation suggests that malaria and poverty are intimately related. In most endemic areas of malaria, changes in social and economic conditions are considered to be far more important than temperature shift [ 68 ]. The economic and social burdens from factors such as fertility, population growth, premature mortality, misdiagnosis, inflicted by the disease on the society have been studied by many authors [ 95 – 100 ]. Given the nature of the factors, most investigations are case studies, and there are only few differential equation based models that incorporate socio-economic structure.
Using a mathematical model (see Additional file 1 Table S3), Yang showed how the basic reproductive number ( R 0 ) of malaria transmission changes with global warming and local social and economic conditions [ 68 ]. In this model good, intermediate and poor economic conditions among human community have been considered and each condition is further divided into three temperature zones. A host of factors control disease transmission rates in this model such as, differential immunity, endemicity, resistance, economic conditions and temperature dependence of mosquito development. These lead to different R 0 for three temperature zones with different socio-economic structures. These modelling results point out the requirement of proper management of the surrounding environment, along with good health care system, in disease transmission. From the point of view of designing field research, it is shown in a mosquito based model [ 101 ], that the effectiveness of malaria control through different types of intervention methods (insecticide-treated nets and indoor residual spraying) can have differential protection, with the former being more protective. The socio-economic scenario for large scale deployment of interventions at the population level has also been addressed using modelling studies [ 102 ].
Migration and visitation
One of the important reasons for the failure of strategies to eradicate infectious disease is because of their neglect of the mobility patterns of the host. The importance of the role of human migration is evident in the recent increase in malaria incidence not only in the endemic zones, but also in zones where malaria had been eradicated [ 17 , 103 – 105 ]. Mainly two types of mobility patterns that can spread the infection to newer areas, are considered - migration , i.e., when the people move from one region to another with no returns; and visitation , when the people return to their original region after visiting other regions.
The elaboration of different approaches to model malaria transmission in populations, as described in Figure 2 , is given in the earlier sections. As mentioned earlier, only few models have been discussed above as examples to demonstrate the manner in which different factors and variables relevant to the host-vector-parasite biology have been incorporated in the basic models. Because of the overwhelming complexity of the disease system and its nonlinear interdependence on the environmental and socio-economic factors, there has not been one consensus general model where all factors are included. Linking the within-host and between-host dynamics of malaria has been one approach towards a comprehensive model [ 26 , 27 ]. Some effort has also been directed to develop software that include many factors for simulating the disease output [ 23 , 106 ].
Stochastic models
Plasmodium life-cycle and mosquito population density are highly dependent on different internal processes and external environmental factors, which are probabilistic in nature. In many of the models referred above, stochasticity has been included in different ways. Even when the main structure of the compartments is similar to the differential equation based models, stochasticity has been included through individual variability in individual based models [ 24 , 27 ], and probabilistic variation in different variables and parameters of transmission processes and environmental factors [ 27 , 107 – 110 ]. Models integrating stochasticity with other factors such as, spatial contact structure and temporal forcing, also explain many interesting features of disease transmission [ 111 , 112 ]. Though not discussed in detail here, these models are powerful tools to explain complex interactions characterized by realistic descriptions.
Data based statistical modelling
One of the major uses of a model is to fit past data and predict the future trend. This capability of a model improves the credibility of the underlying hypothesis of the model. In general epidemiological data represent the disease prevalence in an area over a period of time (time series data), and is given by the number of cases and number of infected persons. As has been enumerated in the earlier sections, this number, representing the prevalence of the disease, is the result of a large number of interacting nonlinear processes in host-pathogen interaction - both deterministic and probabilistic. These processes may have variable contribution to the development of the infection depending on the type of disease - a few may have larger contribution in the process, and others can be peripheral. Statistical modelling involves developing relationships between these factors in the form of mathematical equations. Statistical models that fit curves of past temporal prevalence of a disease, do not make any assumptions about the internal mechanisms that a mathematical model provides. This modelling approach involves application of a variety of elaborate statistical methodologies and tests, and has been extensively used in describing and predicting ( forecasting ) malaria incidence in different regions.
Several results from the models described in the previous sections have been validated with experimental data. Incorporating age and immunity, Aron [ 73 ] verified the results with the Garki project data [ 62 ]. To show the effect of socio economic conditions and environmental factors, Yang tested his model results with malaria data from three regions - (i) disease free but potentially under risk (Southeast Brazil: upper bound of temperature 20°C), (ii) disease at low endemic levels (Amazon region and Southeast Asia), and (iii) disease at higher endemic levels (Africa: lower bound of temperature 31°C) [ 68 ]. Filipe et al used the data from Northern Tanzania and north and south bank of river Gambia, to verify their model findings [ 44 ]. This kind of statistical modelling remains a highly preferred approach in quantitative malaria research to understand the disease incidence and the role of different factors [ 113 ].
One useful statistical approach to understand how disease prevalence changes based on other variables such as, temperature or rainfall, is the method of Regression modelling [ 114 ]. It involves techniques for modelling and analysis of the relationship between a dependent variable and one or more independent variables. Different variants of this method has been used to model malaria transmission in Africa through the Malaria Risk in Africa (MARA) project and in Europe [ 115 ], understanding the effect of childhood population data, climate averages and Normalized Difference Vegetation Index (NDVI) in Mali from 1960 [ 116 ], role of temperature and rainfall from meteorological stations and community-based parasitological survey in the prediction of malaria risk [ 117 ], effect of vector abundance, population immunity, on malaria incidence [ 118 ], predict the seasonal pattern of malaria in Kenya using NDVI [ 119 ] and to show evidence and causality between health and poverty in malaria prevalence [ 120 ]. Researchers have also used elaborate time series analysis models to show seasonality patterns in the malaria incidence [ 121 – 124 ].
With the availability of world-wide datasets on population distribution, global circulation, environmental factors, and parasitological prevalence, epidemiologists have now increasingly been interested in global modelling perspectives [ 89 ]. Such activities require, along with mathematical models, in depth statistical modelling techniques such as, bayesian inference using Markov chain Monte Carlo method, and multivariate statistical modelling techniques to generate maximum likelihood predictions for posterior probability of parasite distributions on the world map [ 6 , 125 – 127 ]. The most reassuring result from these global studies is that contrary to prevailing forecasts of global malaria expansion due to climate change, other natural and anthropogenic forces acting on the disease have actually resulted in a net reduction in transmission.
The above theoretical exercises depend crucially on the quality and availability of data and the methodologies used to build the models. They yield limited predictability and understanding due to factors such as, lack of information or excessive complexity. These models also evolve based on newer data and increased understanding of processes involved. Thus the data based analysis in the statistical modelling approach continues to be a major area in malaria research.
Summary and outlook
A model is a mathematical abstraction of reality. The level of abstraction depends on the questions asked and the scale at which the underlying causative processes are studied. For example, in inter-host transmission of an infection, most molecular events in host-pathogen interactions (e.g., types of immune cells involved, parasite development inside the host, signaling pathways) are not considered. Many of these processes are condensed into a single parameter in the immune function or inoculation rate. In intra-host models, on the other hand, how the titers of the infective agent or related molecules change in an individual is studied, because that is what decides the diseased state of the host individual. In epidemiological models, intra-host processes between host-parasite-vector are neglected, but the host and vector population are subdivided in terms of the infection/diseased state (i.e., Susceptible, Exposed, Infected, and Immune/Recovered). These models aim to match their results with the available epidemiological data, where incidence of the infectious disease (and death due to it) determines the status of an epidemic in the population. Here, from the public health point of view, one is more interested in knowing if the infection will die out, or persist in a population through the important parameter R 0 . Yet, as more molecular studies are coming to the fore and both detection of infection and mode of infection propagation (genes, proteins, pathways, immune interactions) are elucidated, the epidemiological models also would need to consider these processes for inclusion as parameters or subclasses. This is clearly visible in the later models, where both the infected and recovered classes are divided into subclasses (such as, asymptomatic ), which have different time scales and/or transmission modes. In this review, efforts have been taken to group the epidemiological models of malaria in terms of the complexity of infection processes included in its description, which makes them more realistic. The age-specific distribution of infection due to differential immunity across age is one such case. The assumption is that more realistic models would enhance the understanding of the infection transmission process at the population level, which, in turn, may help in better prediction of intervention strategies. The specific models discussed here are only indicative and not exhaustive. Pure mathematical analysis of the models, even though not so popular among the biologists, is important. They allow clear understanding of the logic of the system behaviour in terms of the relationship among the parameters and variables, which are representative to real biological processes. It will be useful to develop connections between mathematical analysis and their real world implications, since such analyses may help us to understand hitherto unknown scenario, such as the effect of temperature, seasonal forcing, excessive rainfall, correlation between different variables and parameter changes. Among the innumerable statistical models based on malaria incidence data, only a few approaches have been described here. The results of these models are mostly data specific and applicable primarily to the particular data set studied. They are highly useful for prediction in that specific context, but may not work in other places/scenarios. Models that incorporate the essentials of host-parasite-vector interaction, proper clinical population subdivisions for disease transmission and also describe multiple data sets from different ecological regions, promise to be an ideal combination of both approaches. It is now clear that the role of indirect factors such as, social structure, economic status, play an overwhelming role in the transmission and persistence of malaria in a region. It also underlies the failure of several control measures where local heterogeneity was not considered. It is the need of the hour to include factors such as the role of heterogeneity in host population due to social status, local differences in ecology due to poverty, differential effects of disease transmission in populations residing in habitats of different temperature, in the mathematical models. Such a description has the possibility of yielding understanding of malaria transmission for populations with societal differences and climate change. Mathematical models have the ability to address several multiplicative, feedback and nonlinear effects that enhance or suppress the effects of factors such as, exposure, immunity, spatiotemporal heterogeneities, control measures and environment, in order to capture key linkages to the complex transmission dynamics. They can also include stochasticity in different variables and parameters to simulate realistic scenario. This comparative analysis of different mathematical models of malaria would contribute to consolidate our understanding about the evolution of these models, and may also help in developing new models by incorporating features discussed above to improve predictions and deciding realistic control measures.
World Health Organization (WHO) and WHO Global Malaria Programme. http://www.who.int/malaria/about_us/en/index.html , [ http://www.who.int/topics/malaria/en/ ]
Martens WJM, Niessen LW, Rotmans J, Jetten TH, McMichael AJ: Potential Impact of global climate change on malaria risk. Environ Health Perspect. 1995, 103: 458-464. 10.1289/ehp.95103458.
PubMed Central CAS PubMed Google Scholar
Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW: Climate change and the resurgence of malaria in East African highlands. Nature. 2002, 415: 905-909. 10.1038/415905a.
Tanser FC, Sharp B, le Sueur D: Potential effect of climate change of malaria transmission in Africa. Lancet. 2003, 362: 1792-1798. 10.1016/S0140-6736(03)14898-2.
PubMed Google Scholar
Parham PE, Michael E: Modeling the effects of weather and climate change on malaria transmission. Environ Health Perspect. 2010, 118: 620-626.
PubMed Central PubMed Google Scholar
Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI: Climate change and the global malaria recession. Nature. 2010, 465: 342-346. 10.1038/nature09098.
Evengård B, Sauerborn R: Climate change influences infectious diseases both in the Arctic and the tropics: joining the dots. Global Health Action. 2009, 2:
Google Scholar
Peterson AT: Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009, 9: 59-10.1186/1471-2334-9-59.
Pattanayak S, Sharma VP, Kalra NL, Orlov VS, Sharma RS: Malaria paradigms in India and control strategies. Indian J Malar. 1994, 31: 141-199.
CAS Google Scholar
Holt RA, Subramanian GM, Halpern A: The genome sequence of the malaria mosquito Anopheles gambiae . Science. 2002, 298: 129-149. 10.1126/science.1076181.
CAS PubMed Google Scholar
Gardner MJ, Hall N, Fung E: Genome sequence of the human malaria parasite Plasmodium falciparum . Nature. 2002, 419: 498-511. 10.1038/nature01097.
Anderson RM, May RM: Infectious diseases of humans: dynamics and control. 1991, London: Oxford University Press
The malERA Consultative Group on Modeling: A Research agenda for malaria eradication: modeling. PLoS Med. 2011, 8: e1000403-
PubMed Central Google Scholar
Roll Back Malaria Partnership: The global malaria action plan, for a malaria free world. 2008, Geneva, Switzerland, [ http://www.rbm.who.int/gmap/index.html ]
Alonso PL, Brown G, Arevalo M, Binka F, Chitnis C, Collins F, Doumbo O, Greenwood B, Hall L, Levine M, Mendis K, Newmann R, Plowe C, Rodriguez MH, Sinden R, Slusker L, Tanner M: A research agenda to underpin malaria eradication. PLoS Med. 2011, 8: e1000406-10.1371/journal.pmed.1000406.
Kermack WO, McKendrick AG: Contribution to the mathematical theory to epidemics. Proc R Soc Lond Series A. 1922, 115: 100-121.
Bailey NTJ: The Biomathematics of malaria. 1982, London: Charles Griffin and Co Ltd
Hoshen MB, Heinrich R, Stein WD, Ginsburg H: Mathematical modelling of the within-host dynamics of Plasmodium falciparum . Parasitology. 2001, 121: 227-235.
Dietz K, Raddatz G, Molineaux L: Mathematical model of the first wave of Plasmodium falciparum asexual parasitemia in non-immune and vaccinated individuals. Am J Trop Med Hyg. 2006, 75: 46-55.
Mideo N, Day T, Read AF: Modelling malaria pathogenesis. Cell Microbiol. 2008, 10: 1947-1955. 10.1111/j.1462-5822.2008.01208.x.
McQueen PG, McKenzie FE: Host control of malaria infections: constraints on immune and erythropoeitic response kinetics. PLoS Comput Biol. 2008, 4: 1-15. 10.1371/journal.pcbi.0040001.
Mackinnon MJ, Marsh K: The Selection Landscape of Malaria Parasites. Science. 2010, 328: 866-871. 10.1126/science.1185410.
Antao T, Hastings IM: ogaraK: a population genetics simulator for malaria. Bioinformatics. 2011, 27: 1335-1336. 10.1093/bioinformatics/btr139.
Gu W, Killeen GF, Mbogo CM, Regens JL, Githure JI, Beier JC: An individual-based model of Plasmodium falciparum malaria transmission on the coast of Kenya. Trans R Soc Trop Med Hyg. 2003, 97: 43-50. 10.1016/S0035-9203(03)90018-6.
Gu W, Novak RJ: Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence and prevalence of malaria. Am J Trop Med Hyg. 2005, 73: 546-552.
McKenzie FE, Bossert WH: An integrated model of Plasmodium falciparum dynamics. J Theor Biol. 2005, 232: 411-426.
Smith T, Maire N, Ross A, Penny M, Chitnis N, Schapira A, Studer A, Genton B, Lengeler C, Tediosi F, De Savigny D, Tanner M: Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology. 2008, 135: 1507-1516. 10.1017/S0031182008000371.
Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC: A simplified model for predicting malaria entomological inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000, 62: 535-544.
McKenzie FE: Why model malaria?. Parasitol Today. 2000, 16: 458-464. 10.1016/S0169-4758(00)01804-4.
Gu W, Mbogo CM, Githure JI, Regens JL, Killeen GF, Swalm CM, Yan G, Beiser JC: Low recovery rates stabilize malaria endemicity in areas of low transmission in coastal Kenya. Acta Trop. 2003, 86: 71-81. 10.1016/S0001-706X(03)00020-2.
Smith DL, Dushoff J, McKenzie FE: The risk of mosquito-borne infection in a heterogeneous environment. PloS Biol. 2004, 2: 1957-1964.
Chitnis N, Cushing JM, Hyman JM: Bifurcation analysis of a mathematical model for malaria transmission. SIAM J Appl Math. 2006, 67: 24-45. 10.1137/050638941.
Ngwa GA: On the population dynamics of the malaria vector. Bull Math Biol. 2006, 68: 2161-2189. 10.1007/s11538-006-9104-x.
Parham PE, Ferguson NM: Space and contact networks: capturing the locality of disease transmission. J R Soc Interface. 2006, 3: 483-493. 10.1098/rsif.2005.0105.
Ross R: The prevention of malaria. 1911, London: John Murray
Ross R: Some a priori pathometric equations. Br Med J. 1915, 1: 546-447. 10.1136/bmj.1.2830.546.
Ross R: An application of the theory of probabilities to the study of a priori pathometry - I. Proc R Soc. 1916, A92: 204-230.
Ross R: An application of the theory of probabilities to the study of a priori pathometry - II. Proc R Soc. 1916, A93: 212-225.
Ross R, Hudson HP: An application of the theory of probabilities to the study of a priori pathometry - III. Proc R Soc. 1916, A93: 225-240.
Macdonald G: The epidemiology and control of malaria. 1957, London: Oxford University Press
Aron JL, May RM: The population dynamics of malaria. Population Dynamics of Infectious Disease. Edited by: Anderson RM. 1982, London: Chapman and Hall, 139-179.
Dietz K: Mathematical models for transmission and control of malaria. Principles and Practice of Malariology. Edited by: Wernsdorfer W, McGregor Y. 1988, Edinburgh: Churchill Livingston, 1091-1133.
Aron JL: Mathematical modeling of immunity to malaria. Math Biosci. 1988, 90: 385-396. 10.1016/0025-5564(88)90076-4.
Filipe JAN, Riley EM, Darkeley CJ, Sutherland CJ, Ghani AC: Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comp Biol. 2007, 3: 2569-2579.
Hasibeder G, Dey C: Population dynamics of mosquito-borne disease: persistence in a completely heterogeneous environment. Theor Popul Biol. 1988, 33: 31-53. 10.1016/0040-5809(88)90003-2.
Gupta S, Swinton J, Anderson RM: Theretical studies of the effects of heterogeneity in the parasite population on the transmission dynamics of malaria. Proc R Soc Lond B. 1994, 256: 231-238. 10.1098/rspb.1994.0075.
Gupta S, Hill AVS: Dynamic interactions in malaria: host heterogeneity meets parasite polymorphism. Proc R Soc Lond B. 1995, 261: 271-277. 10.1098/rspb.1995.0147.
Torres-Sorando L, Rodriguez DJ: Models of spatio-temporal dynamics in malaria. Ecol Model. 1997, 104: 231-240. 10.1016/S0304-3800(97)00135-X.
Rodriguez DJ, Torres-Sorando L: Models of infectious diseases in spatially heterogeneous environments. Bull Math Biol. 2001, 63: 547-571. 10.1006/bulm.2001.0231.
Hethcote HW: A thousand and one epidemic models. Frontiers in mathematical biology. Lecture notes in Biomathematics. Edited by: Simon A Levin. 1984, Springer, 100: 504-515.
Tang YW, Procop GW, Persing DH: Molecular diagnostics of infectious diseases. Clin Chem. 1997, 43: 2021-2038.
Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, da Silva J: PCR as a confirmatory technique for laboratory diagnosis of malaria. J Clin Microbiol. 2006, 44: 1087-1089. 10.1128/JCM.44.3.1087-1089.2006.
Nothdurft HD, Jelinek T, Bluml A, Von Sonnenburg F, Loscher T: Seroconversion to circumsporozoite antigen of Plasmodium falciparum demonstrates a high risk of malaria transmission in travelers to East Africa. Clin Infect Dis. 1999, 28: 641-642. 10.1086/515155.
Macdonald G: Epidemiological basis of malaria control. Bull World Health Organ. 1956, 15: 613-626.
Pampana E: A Textbook of Malaria Eradication. 1969, London: Oxford University Press
Koella JC: On the use of mathematical models of malaria transmission. Acta Trop. 1991, 49: 1-25. 10.1016/0001-706X(91)90026-G.
Dietz K, Molineaux L, Thomas A: A malaria model tested in the African savannah. Bull World Health Organ. 1974, 50: 347-357.
Fisher RA: The genetical theory of natural selection. 1930, Oxford: Clarendon Press
Macdonald G: The analysis of equilibrium in malaria. Trop Dis Bull. 1952, 49: 813-829.
Dietz K: Transmission and control of arbovirus diseases. Epidemiology. Edited by: Ludwig D, Cooke KL. 1975, Philadelphia:SIAM, 104-121.
Heffernan JM, Smith RJ, Wahl LM: Perspectives on the basic reproductive ratio. J Royal Soc Interface. 2005
Molineaux L, Gramiccia G: The Garki project: research on the epidemiology and control of malaria in the Sudan savanna of West Africa. 1980, Geneva: World Health Organization
Boyd MF: Epidemiology of malaria: factors related to the intermediate host. Malariology. Edited by: Boyd MF. 1949, Philadelphia: WB Saunders Company, 551-607.
Ngwa GA, Shu WS: A mathematical model for endemic malaria with variable human and mosquito populations. Math Comput Model. 2000, 32: 747-763. 10.1016/S0895-7177(00)00169-2.
Ngwa GA: Modelling the dynamics of endemic malaria in growing populations. Discrete Contin Dyn Syst - Ser B. 2004, 4: 1173-1202.
Chitnis N: Using mathematical models in controlling the spread of malaria. PhD thesis. 2005, University of Arizona, Program in Applied Mathematics
Yang HM: Malaria transmission model for different levels of acquired immunity and temperature-dependent parameters (vector). Revista de Saúde Pública. 2000, 34: 223-231.
Yang HM, Ferreira MU: Assessing the effects of global warming and local social and economic conditions on the malaria transmission. Revista de Saúde Pública. 2000, 34: 214-222.
Okell LC, Drakeley CJ, Bousema T, Whitty CJ, Ghani AC: Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008, 5: e226-10.1371/journal.pmed.0050226.
Ghani AC, Sutherland CJ, Riley EM, Drakeley CJ, Griffin JT, Gosling RD, Filipe JAN: Loss of population levels of immunity to malaria as a result of exposure-reducing interventions: Consequences for interpretation of disease trends. PLoS ONE. 2009, 4: e4383-10.1371/journal.pone.0004383.
Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basáñez MG, Ghani AC: Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010, 7: e1000324-10.1371/journal.pmed.1000324.
Cairns M, Ghani A, Okell L, Gosling R, Carneiro I, Anto F, Asoala V, Owusu-Agyei S, Greenwood B, Chandramohan D, Milligan P: Modelling the Protective Efficacy of alternative delivery schedules for intermittent preventive treatment of malaria in infants and children. PLoS ONE. 2011, 6: e18947-10.1371/journal.pone.0018947.
Aron JL: Dynamics of acquired immunity boosted by exposure to infection. Math Biosci. 1983, 64: 249-259. 10.1016/0025-5564(83)90007-X.
Shililu , Ghebremeskel T, Mengistu S, Fekadu H, Zerom M, Mbogo C, Githure J, Novak R, Brantly E, Beier JC: High seasonal variation in entomologic inoculation rates in Eritrea, a semi-arid region of unstable malaria in Africa. Am J Trop Med Hyg. 2003, 69: 607-613.
Kelly-Hope L, McKenzie FE: The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009, 8: 19-10.1186/1475-2875-8-19.
Gupta S, Galvani A: The effects of host heterogeneity on pathogen population structure. Phil Trans R Soc Lond B. 1999, 354: 711-719. 10.1098/rstb.1999.0424.
Gupta S, Anderson RM: Population structure of pathogens: the role of immune selection. Parasitol Today. 1999, 15: 497-501. 10.1016/S0169-4758(99)01559-8.
Recker M, Nee S, Bull PC, Kinyanjui S, Marsh K, Newbold C, Gupta S: Transient cross-reactive immune responses can orchestrate antigenic variation in malaria. Nature. 2004, 429: 555-558. 10.1038/nature02486.
Hastings IM: A model for the origins and spread of drug-resistant malaria. Parasitology. 1997, 115: 133-141. 10.1017/S0031182097001261.
Dye C, Williams BG: Multigenic drug resistance among inbred malaria parasites. Proc R Soc London Ser B Biol Sci. 1997, 264: 61-67. 10.1098/rspb.1997.0009.
Koella JC: Costs and benefits of resistance against antimalarial drugs. Parasitol Today. 1998, 14: 360-364. 10.1016/S0169-4758(98)01297-6.
Koella JC, Antia R: Epidemiological models for the spread of anti-malarial resistance. Malar J. 2003, 2: 3-10.1186/1475-2875-2-3.
Mackinnon MJ: Drug resistance models for malaria. Acta Tropica. 2005, 94: 207-217.
Pongtavornpinyo W, Yeung S, Hastings I, Dondorp A, Day N, White N: Spread of antimalarial drug resistance: Mathematical model with practical implications for ACT drug policies. Malar J. 2008, 7: 229-10.1186/1475-2875-7-229.
Chiyaka C, Garira W, Dube S: Effects of treatment and drug resistance on the transmission dynamics of malaria in endemic areas. Theor Popul Biol. 2009, 75: 14-29. 10.1016/j.tpb.2008.10.002.
Antao T, Hastings IM: Environmental, pharmacological and genetic influences on the spread of drug-resistant malaria. Proc R Soc B. 2011, 278: 1705-1712. 10.1098/rspb.2010.1907.
Boëte C, Koella JC: A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J. 2002, 1: 3-10.1186/1475-2875-1-3.
Li J, Welch RM, Nair US, Sever TL, Irwin DE, Cordon-Rosales C, Padilla N: Dynamic Malaria Models with Environmental Changes. Proceedings of the Thirty- Fourth Southeastern Symposium on System Theory. 2002, Huntsville, AL, 396-400.
Martens WJM, Niessen L, Rotmans J, Jetten TH, McMichael J: Climate change and vector-borne disease: a global modelling perspective. Global Environ Change. 1995, 5: 195-209. 10.1016/0959-3780(95)00051-O.
Paaijmans KP, Read AF, Thomas MB: Understanding the link between malaria risk and climate. Proc Nat Acad Sci USA. 2009, 106: 13844-13849. 10.1073/pnas.0903423106.
Jetten TH, Martens WJM, Takken W: Model simulations to estimate malaria risk under climate change. J Med Entomol. 1996, 33: 361-371.
Chattopadhyay J, Sarkar RR, Chaki S, Bhattacharya S: Effects of environmental fluctuations on the occurrence of malignant malaria-a model based study. Ecol Model. 2004, 177: 179-192. 10.1016/j.ecolmodel.2004.03.001.
Hoshen MB, Morse AP: A weather-driven model of malaria transmission. Malar J. 2004, 3: 32-10.1186/1475-2875-3-32.
Yé Y, Hoshen M, Kyobutungi C, Louis VR, Sauerborn R: Local scale prediction of Plasmodium falciparum malaria transmission in an endemic region using temperature and rainfall. Global Health Action. 2009, 2:
Sachs J, Malaney P: The economic and social burden of malaria. Nature. 2002, 415: 680-685. 10.1038/415680a.
Wyse APP, Bevilacqua L, Rafikov M: Simulating malaria model for different treatment intensities in a variable environment. Ecol model. 2007, 206: 322-330. 10.1016/j.ecolmodel.2007.03.038.
Amexo M, Tolhurst R, Barnish G, Bates I: Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004, 364: 1896-1898. 10.1016/S0140-6736(04)17446-1.
van Lieshout M, Kovats RS, Livermore MTJ, Martens P: Climate change and malaria: analysis of the SRES climate and socio-economic scenarios. Glob Environ Change. 2004, 14: 87-99. 10.1016/j.gloenvcha.2003.10.009.
Martens P, Kovats RS, Nijhof S, de Vires P, Livermore MTJ, Bradley DJ, Cox J, McMichael AJ: Climate change and future populations at risk from malaria. Global Environmental Change. 1999, 9: S89-S107.
Laxminaraya R: Act now or latter? Economics of malaria resistance. Am J Trop Med Hyg. 2004, 71: 187-195.
Chitnis N, Schapira A, Smith T, Steketee R: Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am J Trop Med Hyg. 2010, 83: 230-240. 10.4269/ajtmh.2010.09-0179.
Killeen GF, Smith TA: Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg. 2007, 101: 867-880. 10.1016/j.trstmh.2007.04.022.
Aragón LE: Expansión de la frontera, expansión de la enfermeded: movilidad geográfica y salud en la Amazonia. Enfoque Integral de la Salud Humana en la Amazonia. Edited by: Yarzabal L, Espinal C, Aragón LE. 1992, Imprenta UCV, Caracas, 429-456.
Sandia-Mago A: Venezuela: malaria y movilidad humana estacional de las comunidades indigenas del rio riecito del estado Apure. Fermentum. 1994, 3: 102-123.
Martens P, Hall L: Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000, 6: 103-109. 10.3201/eid0602.000202.
Martens P: Health and climate change. Modelling the impacts of global warming and ozone depletion. 1998, London: Earthscan Publications
Smith TA: Estimation of heterogeneity in malaria transmission by stochastic modelling of apparent deviations from mass action kinetics. Malar J. 2008, 7: 12-10.1186/1475-2875-7-12.
Saul A: Transmission dynamics of Plasmodium falciparum . Parasitology Today. 1996, 12: 74-79. 10.1016/0169-4758(96)80659-4.
Craig MH, Snow RW, Le Sueur D: Climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999, 15: 105-111. 10.1016/S0169-4758(99)01396-4.
Gaudart J, Touré O, Dessay N, Dicko A, Ranque S, Forest L, Demongeot J, Doumbo OK: Modelling malaria incidence with environmental dependency in a locality of Sudanese savannah area, Mali. Malar J. 2009, 8: 61-10.1186/1475-2875-8-61.
Dangerfield CE, Ross JV, Keeling MJ: Integrating stochasticity and network structure into an epidemic model. J R Soc Interface. 2009, 6: 761-774.
Parham PE, Michael E: Outbreak properties of epidemic models: The roles of temporal forcing and stochasticity on pathogen invasion dynamics. J Theor Biol. 2011, 271: 1-9. 10.1016/j.jtbi.2010.11.015.
Abeku TA: Forecasting malaria incidence from historical morbidity patterns in epidemic- prone areas of Ethiopia-simple seasonal adjustment method performs best. Trop Med Int Health. 2002, 7: 851-857. 10.1046/j.1365-3156.2002.00924.x.
Kirk RE: Statistics: An Introduction. 2008, Belmont, CA: Thomson Learning, Inc., 5
Kuhn KG, Campbell-Lendrum DH, Davies CR: A continental risk map of malaria mosquito (Diptera: Culicidae) vectors in Europe. J Med Entomol. 2002, 39: 621-630. 10.1603/0022-2585-39.4.621.
Kleinschmidt J, Bagayoko M, Clarke GPY, Craig M, Le D: A spatial statistical approach to malaria mapping. Sauer-International Epidemiological Association. 2000, 29: 355-361.
Ye Y, Louis VR, Simboro S, Sauerborn R: Effect of meteorological factors on clinical malaria risk among children: an assessment using village-based meteorological stations and community-based parasitological survey. BMC Public Health. 2007, 7: 101-10.1186/1471-2458-7-101.
Lindblade KA, Walker ED, Wilson ML: Early warning of malaria epidemics in African highlands using Anopheles (Diptera: Culicidae)-Indoor resting density. J Med Entomol. 2000, 37: 664-674. 10.1603/0022-2585-37.5.664.
Hay SI, Snow RW, Rogers DJ: From predicting mosquito habitat to malaria seasons using remotely sensed data - practice, problems and perspectives. Parasitol Today. 1998, 14: 306-313. 10.1016/S0169-4758(98)01285-X.
Khanum S, Singh A: Health, Poverty and Human security: Illustrations from malaria in SEA. Regional Health Forum. 2007, s11: 33-44.
Briet O, Vounatsou P, Gunawardene DM, Galppaththy GNL, Amerasinghe PH: Models for short-term malaria prediction in Sri Lanka. Malar J. 2008, 7: 76-10.1186/1475-2875-7-76.
Abellana R, Ascaso C, Aponte J, Saute F, Nhalungo D, Nhacolo A, Alonso P: Spatio-seasonal modeling of the incidence rate of malaria in Mozambique. Malar J. 2008, 7: 228-10.1186/1475-2875-7-228.
Cancre N, Tall A, Rogier C, Faye J, Sarr O, Trape JF, Spiegel A, Bois F: Bayesian analysis of an epidemiological model of Plasmodium falciparum malarial infection in Ndiop, Senegal. Am J Epidemiol. 2000, 152: 760-770. 10.1093/aje/152.8.760.
Chatterjee C, Sarkar RR: Multi-step polynomial regression method to model and forecast malaria incidence. PLoS ONE. 2009, 4: e4726-10.1371/journal.pone.0004726.
Smith DL, McKenzie FE, Snow RW, Hay SI: Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007, 5: e42-10.1371/journal.pbio.0050042.
Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith DL, Moyeed RA, Snow RW: World malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009, 6: e1000048-
Rogers DJ, Randolph SE: The global spread of malaria in a future, warmer world. Science. 2000, 289: 1763-1766.
Download references
Acknowledgements
The authors are grateful to the anonymous referees for valuable comments and suggestions in improving the content and C. Suguna for critcally reading the manuscript. Department of Science and Technology, India is acknowledged for financial support (SR/SO/AS-25/2008).
Author information
Authors and affiliations.
Centre for Cellular and Molecular Biology (CSIR), Uppal Road, Hyderabad, 500007, India
Sandip Mandal, Ram Rup Sarkar & Somdatta Sinha
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Somdatta Sinha .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
SM identified literature sources, carried out the analysis, and wrote the manuscript. RRS contributed in designing the study and to the formatting and writing of the manuscript. SS conceived the idea for the article, designed the study, and contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
Electronic supplementary material
12936_2010_1790_moesm1_esm.doc.
Additional file 1: Description of different mathematical models of malaria. This file contains eight Tables (S1-S7i,ii), giving description of mathematical expressions and parameters used in different models. (DOC 202 KB)
12936_2010_1790_MOESM2_ESM.DOC
Additional file 2: Description of different immunity functions . This file contains details of three immunity functions used in Filipe model [ 44 ]. (DOC 62 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, authors’ original file for figure 5, authors’ original file for figure 6, authors’ original file for figure 7, authors’ original file for figure 8, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Mandal, S., Sarkar, R.R. & Sinha, S. Mathematical models of malaria - a review. Malar J 10 , 202 (2011). https://doi.org/10.1186/1475-2875-10-202
Download citation
Received : 11 September 2010
Accepted : 21 July 2011
Published : 21 July 2011
DOI : https://doi.org/10.1186/1475-2875-10-202
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Normalize Difference Vegetation Index
- Malaria Transmission
- Malaria Incidence
- Mosquito Population
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Plasmodium falciparum malaria in pregnancy and fetal, newborn, and maternal outcomes among a cohort of pregnant women in coastal Kenya, 2006 - 2009
Add to collection, downloadable content.
- March 22, 2019
- Affiliation: Gillings School of Global Public Health, Department of Epidemiology
- Plasmodium falciparum malaria in pregnancy causes adverse pregnancy outcomes, most notably reduced birth weight and maternal anemia. Preventive treatment that is safe during pregnancy has been shown to effectively reduce rates of malaria in pregnancy, yet in malaria-endemic regions rates of adverse pregnancy outcomes remain high. We sought to explore the association of malaria in pregnancy and other risk factors with poor outcomes, among a cohort of pregnant women who received the recommended preventative treatment for malaria at antenatal care. The prevalence of malaria at the first antenatal care visit was 11%, and malaria infection was associated with lower measures of fetal growth, as measured by ultrasound. Among live, term births, the mean birth weight was not significantly different for malaria-positive vs. malaria-negative women. However, among women with under-nutrition, as measured by low body-mass-index, malaria exposure was associated with significantly decreased birth weight (mean difference -370 grams, 95% CI -728, -12 g). The rates of maternal anemia (hemoglobin <11.0 g/dL) and moderate/severe anemia (hemoglobin < 9.0 g/dL) at antenatal care were 70% and 27%, respectively. Moderate/severe maternal anemia at first antenatal care was associated with malaria as diagnosed by microscopy (aRR 2.06, 95% CI 1.24, 3.44) as was high-intensity hookworm infection in multivariate regression (aRR 2.37, 95% CI 1.44, 3.91). Our findings suggest the importance of good preventative treatment for malaria in pregnancy to minimize the impact of exposure to malaria on fetal and newborn growth. However, under-nutrition has an important role and research and programs to improve maternal nutritional health may be important to important to further improving birth outcomes in low-resource settings. Furthermore, given the high prevalence of anemia seen in our study, also associated with under-nutrition, as well as hookworm, and malaria, further research is needed to optimize interventions around pregnancy to improve maternal and newborn health in malaria-endemic regions.
- Epidemiology
- https://doi.org/10.17615/1jfw-vm95
- Dissertation
- In Copyright
- Meshnick, Steven R.
- Doctor of Philosophy
- University of North Carolina at Chapel Hill
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
- Share full article
Advertisement
Supported by
Kent Campbell, Pivotal Figure in the Fight Against Malaria, Dies at 80
Among his accomplishments in a four-decade career in public health, he helped pioneer programs providing bed nets in Africa.

By Michael S. Rosenwald
Kent Campbell, an instrumental figure in the global battle against malaria — most notably in Africa, where he led an innovative program providing bed nets to protect rural villagers from the mosquitoes carrying the disease — died on Feb. 20 in Oro Valley, Ariz., a suburb of Tucson. He was 80.
His death, in a nursing care facility, was caused by complications of cancer, his children said.
As chief of the malaria branch of the Centers for Disease Control and Prevention from 1981 to 1993, and later as an adviser to UNICEF and the Bill & Melinda Gates Foundation, Dr. Campbell is credited with helping to save lives on multiple continents.
In Zambia, where he began working on a program with the Gates Foundation in 2005 distributing bed nets and newer antimalarial drugs, malaria cases were cut in half within three years. The program was later expanded to more than 40 other countries in Africa.
“His legacy in my country is as one of the people who greatly contributed to the control and prevention of malaria,” Kafula Silumbe, a Zambian public health specialist who worked closely with Dr. Campbell, said in an interview. “It was a collective effort, but he definitely was part of that initial push.”
Tall and lanky, with a Southern drawl that revealed his Tennessee upbringing, Dr. Campbell stumbled on what would become a four-decade-long career in public health.
In 1972, during his pediatric residency in Boston, he joined the C.D.C. as a conscientious objector to the Vietnam War. Not long after, he was sent to Sierra Leone to help investigate an outbreak of Lassa fever , a virulent hemorrhagic virus.
“I had never heard of Lassa fever,” he said in a video history of the C.D.C. “Probably couldn’t even spell it if I’d been asked to.”
He had little to no training in the importance or use of personal protective equipment. For relief from the intense heat, he poked holes in his breathing apparatus, which he later admitted was a bad idea.
Hoping to learn more about Lassa fever, agency officials dispatched him to Ireland to conduct serologic, or antibody-detecting, tests on nuns who had previously worked in Sierra Leone. He traveled there with his wife, Elizabeth (Knight) Campbell, whom he had married in 1966.
A few days later, he nearly collapsed from an intense headache, high fever and an excruciating sore throat.
Dr. Campbell and his wife then traveled to London so that he could be treated at a hospital with expertise in tropical diseases. The episode then took a surreal turn: When U.S. officials sent a military transport plane to retrieve the couple, they shipped inside it a spare Apollo space capsule, which the Campbells rode in as a precautionary measure.
“In retrospect, it’s not clear whether I had Lassa fever,” Dr. Campbell said. “But clearly I didn’t die.”
With a reprieve on life and a newfound appreciation for disease hunting, he stayed on with the C.D.C. He moved to El Salvador in 1973 to take on malaria, which had been essentially orphaned by global public health agencies and aid groups.
“He was indignant about the injustice and unfairness of things,” Laurie Garrett, who wrote about Dr. Campbell in her book “The Coming Plague: Newly Emerging Diseases in a World Out of Balance” (1994), said in an interview. “It just didn’t seem right to him that a scourge like malaria that was killing millions of people every single year wasn’t getting investment and concern and global attention because most of the people dying of it were poor.”
Carlos Clinton Campbell III was born on Jan. 9, 1944, in Knoxville, Tenn. His father was a life insurance salesman, and his mother, Betty Ann (Murphy) Campbell, managed the household. His parents wanted to call him Clint, but his younger sister, Ann, had trouble saying the name, and he wound up as Kent.
He took an early interest in medicine after his sister and mother both died from cancer — Ann when she was 5, their mother when he was in high school.
He studied biology at Haverford College in Pennsylvania, graduating in 1966. He earned his medical degree from Duke University in 1970 and received a master’s in public health at Harvard University after completing his pediatric residency there.
Dr. Campbell bounced around the world, from the corridors of public health to isolated villages, and back.
“He had a deceptive demeanor because of his Southern, laconic exterior,” Ms. Garrett said. “Almost every time you’d go into his office, these gigantic, long legs would go up on the desk, and he’d lean back in his chair. And because he’s so tall, he would automatically fill up, you know, 12 feet of space.”
This made him seem easygoing.
“But then, when he got going, you could feel everything boiling up to the surface,” she added. “He was incredibly impatient, and I think that drove him to ask big questions and to take bold steps to try and help things.”
Following his work at the C.D.C., Dr. Campbell helped create a college of public health at the University of Arizona and consulted for several global health organizations. In 2005, he joined PATH , a health equity nonprofit based in Seattle, as director of the malaria program in Africa funded by the Gates Foundation.
With malaria becoming resistant to the most common drug treatments, he focused on prevention.
“The vector in Africa is basically a single species that is distributed all over the continent called Anopheles gambiae,” he said in an interview with AllAfrica, a Pan-African news organization. “It is like the superstar of transmitters.”
Two years after the bed-net program began in Zambia, the country saw a 29 percent decrease in child mortality, according to PATH.
“To put that in perspective: There’s nothing matching that, which is reflective of how much death malaria caused in Zambia and how powerful bed nets are to decrease transmission,” Dr. Campbell told AllAfrica. “That’s all it really took. It was just remarkable. Clinics emptied out during the transmission season.”
He is survived by his wife; his children, Dr. Kristine Campbell and Dr. Patrick Campbell; his brothers, Robert and John Campbell; his stepsisters, Melissa Hansen and Rebecca Arrants; and four grandchildren.
Dr. Campbell retired from PATH in 2015.
“I hadn’t set out to battle this infection and disease,” he wrote of his professional career. “In reality, it chose me.”
He added, “We chose not to listen to the naysayers.”
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

An Overview of Malaria in Pregnancy
Melissa bauserman.
1 Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC
Andrea L Conroy
2 Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN
Krysten North
3 Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC
Jackie Patterson
4 Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC
5 Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC
Steve Meshnick
6 Department of Epidemiology, University of North Carolina Gilligns School of Global Public Health, Chapel Hill, NC
One hundred twenty-five million pregnant women are at risk for contracting malaria, a preventable cause of maternal and infant morbidity and death. Malaria parasites contribute to adverse pregnancy and birth outcomes due to their preferential accumulation in placental intervillous spaces. Pregnant women are particularly vulnerable to malaria infections, and malaria infections during pregnancy put their fetuses at risk. Malaria in pregnancy is associated with anemia, stillbirth, low birth weight and maternal and fetal death. We review the challenges to diagnosing malaria in pregnancy, as well as strategies to prevent and treat malaria in pregnancy. Finally, we discuss the current gaps in knowledge and potential areas for continued research.
Introduction
Globally, an estimated 125 million pregnant women reside in areas where they are at risk of contracting malaria in pregnancy (MIP), and MIP remains an important preventable cause of adverse birth outcomes. 1 Although there are five species of malaria that infect humans, two main species of Plasmodium contribute to adverse maternal and fetal outcomes in pregnancy, P. falciparum and P. vivax. In sub-Saharan Africa, where the majority of adverse birth outcomes attributable to malaria occur, P. falciparum is the dominant species. However, over half of pregnancies that are potentially exposed to malaria occur in Southeast Asia and the Western Pacific where P. falciparum and P. vivax coexist. Co-existence of P. falciparum and P. vivax also occurs in South America, where 3% of the global total of women at risk for MIP reside. 1
Over the past decade, there has been significant progress in reducing the global prevalence of P. falciparum , particularly in Africa. However, women remain at high risk of MIP, with over 50% of women in high transmission areas having P. falciparum detected in peripheral blood at presentation to antenatal care. 2 , 3 This high prevalence of disease results from an increased risk of contracting malaria among pregnant compared to non-pregnant women. Women who are younger, malnourished, primigravidae/secundigravidae, lack immunity to pregnancy-associated malaria, or living with HIV are at the highest risk of malaria-associated adverse pregnancy outcomes. 4 , 5
Pathophysiology of Malaria in Pregnancy
MIP contributes to adverse pregnancy outcomes, at least in part, due to the preferential accumulation of parasites in the placental intervillous space. Placental sequestration is common in infections with P. falciparum because malaria parasites export a protein, VAR2CSA, to the red blood cell membrane that facilitates adherence to chondroitin-sulfate A (CSA) on syndecan-1, which is anchored in placental tissue. 6 This interaction is associated with the recruitment, retention and activation of mononuclear cells in the placenta—and is thought to mediate malaria’s effect on birth outcomes. 7 Maternal antibodies against VAR2CSA are protective. 5 P. vivax can also lead to placental changes, but to date no studies have unequivocally documented the sequestration of P. vivax infected erythrocytes in the placenta. 8
A number of histological changes in P. falciparum infected placentae have been described, including the infiltration of mononuclear cells, deposition of malaria pigment, thickening of the trophoblast basement membrane, syncytial knotting, and complement deposition. 9 – 11 Inflammation in the placenta has been linked to impaired transplacental transport of glucose 12 and amino acids, 13 and disruption of the insulin-like growth hormone axis. 14 Impaired nutrient transport across the placenta may be further exacerbated by altered placental angiogenesis 15 leading to changes in both the villous architecture 16 and surface area for nutrient exchange, as well as impaired uteroplacental blood flow. 15 , 17 These histologic and functional changes likely contribute to impaired fetal growth. Longitudinal Doppler data support the idea that malaria in the first half of pregnancy can lead to changes in umbilical artery blood flow and fetal growth in pregnancy, and this is affected by both gravidity and nutritional status. 18
Risk Factors for Malaria in Pregnancy
Environmental, parasite, and maternal factors influence the severity of MIP. In areas where malaria transmission is high, the primary burden of malaria is in primigravidae, whereas, in areas of low transmission, all gravidities are at risk. In areas of high transmission, primigravidae develop antibodies to VAR2CSA protein produced by malaria parasites, and are partially protected during subsequent pregnancies; this tends not to happen in areas of low transmission. 19 Overall, pregnant women living in areas with low or unstable (episodic) transmission have little or no immunity to malaria and are at a two-to-three times higher risk of severe disease compared to non-pregnant controls. 20 P. falciparum has typically been associated with more severe MIP than P. vivax , although P. vivax is more likely to occur in a mother with little acquired immunity. 21 Maternal age and gravidity also play a role in the severity of MIP. Younger mothers are at greater risk for severe malaria infection compared to older mothers, who appear to have some protection from severe disease. 4 In high transmission areas, such as sub-Saharan Africa, primigravidae and secundigravidae are at greater risk for severe malaria infection compared to multigravidae, but this is not true in areas of low transmission, where multigravidae have not had prior malaria exposure nor developed immunity. 22
Malnourished pregnant women are at increased risk for adverse birth outcomes with MIP. 23 A meta-analysis using individual patient data from 14,633 pregnancies from Africa and the Western Pacific between 1996–2015 showed that malaria and malnutrition are common exposures, with 35% of women having either of those exposures. Pregnant women with malnutrition and malaria were at an increased risk of LBW compared to women with only 1 of those risk factors. 24 Recent data suggests reduced L-arginine intake is one mechanism through which malnutrition contributes to low birth weight with both nutritional survey data 25 and preclinical models 26 suggesting that L-arginine supplementation may reduce preterm birth 25 and increase fetal viability, placental vascularization, and birth weight. 26
Burden of Malaria
Maternal effects.
The clinical effects of malaria on pregnant women vary from no symptoms to severe anemia and death. Women living in areas of low malaria transmission who have a lower degree of acquired immunity are more likely to experience complications such as renal failure, pulmonary edema, and cerebral malaria. 27 Despite this, the overall maternal mortality rate is similar in low-transmission areas (0.6–12.5%) compared to malaria-endemic areas (0.5–23%). 4 More research is warranted on the topic of malaria-related mortality during pregnancy, as the current data are limited and inconsistent.
Maternal anemia is one of the most common symptoms of MIP. Plasmodium causes anemia through hemolysis, increased splenic clearance of erythrocytes, and reduced red blood cell production. While severe anemia during pregnancy (hemoglobin <7 g/dL) is often multifactorial with significant nutritional components, malaria can play an important role. 28 In one estimate in sub-SaharanAfrica, the population attributable fraction of malaria to severe anemia during pregnancy was 26%. 4 For endemic areas with a 5% baseline prevalence of severe anemia, epidemiological modeling predicts malaria-induced anemia to contribute to nine maternal deaths per 100,000 live births. 4 However, in a population-based study in the Democratic Republic of the Congo, malaria played little or no role as a driver of anemia during pregnancy. 29
Pregnant women are three times more likely to be affected by severe malaria. 30 The World Health Organization defines severe malaria as parasitemia with evidence of end organ dysfunction ( Table 1 ). The presenting features of severe malaria can include severe anemia, hypoglycemia, acute respiratory distress syndrome, renal failure and cerebral malaria. 30 The median mortality of severe MIP is 39% (range 8–100%). 30 Severe malaria must be treated promptly with intensive care and parenteral antimalarial medication to reduce mortality. 31
Treatment Strategies for MIP
Fetal Effects
Malaria is an important cause of stillbirth throughout endemic areas ( Figure 1 ). MIP contributes to 12–20% of stillbirths in endemic regions of sub-Saharan Africa, with lower rates if the mother undergoes treatment. 32 P. falciparum detection both in peripheral blood samples or placental samples at delivery nearly doubles the odds of stillbirth (odds ratio 1.81 and 1.5, respectively). 32 Stillbirth risk is higher in areas with low to intermediate endemicity compared to areas of high endemicity. 33 The risk of stillbirth can be modified with appropriate malaria intervention efforts. For example, the use of insecticide-treated bed nets (ITN) is associated with lower rates of placental malaria and stillbirth (risk ratio 0.67 [95% CI 0.45 to 1.00] for stillbirth). 34

IUGR = Intrauterine growth retardation. Reprinted with permission ( 4 ).
MIP increases the risk of low birth weight (LBW), and approximately 20% of cases of LBW in malaria-endemic areas are attributed to placental infection with malaria ( Figure 1 ). 35 A mother with a malaria-infected placenta is twice as likely to have a baby with LBW. 36 LBW in turn is associated with higher infant mortality rates. In Africa, LBW has been associated with a three - to 20-fold increase in the probability of infant mortality. 36 , 37 Parity is an important factor in LBW. Primigravidae with MIP have two to seven higher odds of LBW and mortality than multigravidae. 38 The timing of infection also seems to play a role in infant size. Second-trimester infection is more likely to result in LBW than third-trimester infection; but data on first-trimester infection are limited. 39
MIP causes LBW due to both intrauterine growth restriction (IUGR) and prematurity. Up to 70% of IUGR in endemic areas is due to malaria, presumably as a result of impaired oxygen and nutrient delivery to the fetus. 35 , 36 The contribution of malaria to preterm birth is also substantial, with up to 36% of prematurity in malaria-endemic areas attributable to Plasmodium infection. Prematurity may result from the host’s immune response to malaria parasites triggering early labor. 33 These adverse fetal effects are species specific, as P. ovale and P. malariae species are not associated with adverse birth outcomes.
Congenital Malaria
Congenital malaria is defined as the identification of asexual P. falciparum parasites in the cord blood or peripheral blood of an infant during the first 7 days of life. 40 The true prevalence of congenital malaria is uncertain. While the prevalence was previously thought to be between <1% and 6%, 40 more recent studies have demonstrated a prevalence rate up to 33% in high-endemicity areas; but it is not clear how many of these congenital infections persist and cause clinical illness. 41 Most descriptive reports of congenital malaria are from infants who are born in non-endemic areas to mothers with a history of travel to endemic areas. Infant symptoms include fever associated with hepatosplenomegaly, hemolytic anemia, thrombocytopenia, and feeding intolerance. 41 These infants often become symptomatic between 10 and 30 days of life, although they can present later. Their symptoms may progress rapidly and can be fatal. 27 , 42 Because, much of the literature on congenital malaria is derived from case reports, more research is needed to better understand the epidemiology and pathophysiology of congenital malaria.
Effects in Early Childhood
Offspring are affected by placental malaria into childhood, ( Figure 1 ). Prenatal malaria exposure is associated with an increased risk of early malaria infection in children as young as four to six months of age. 40 Placental parasitemia may increase the risk of malaria infections in infancy and childhood through several mechanisms. For example, MIP might interfere with maternal antibody passage to offspring, compromising the immunity of the fetus and newborn, making the offspring vulnerable to early malaria infections. 40 In utero exposure to malaria induces the development of T reg cells that lead to fetal immune tolerance to malaria antigens that persists into childhood. 43 Placental malaria has been associated with subsequent susceptibility to non-malaria infections, including measles and tetanus, suggesting additional effects on infant immunity that may be due to the obstruction of antibody passage across the placenta. 44 Acute placental malaria infection has been associated with increased one-year mortality in infants 45 and decreased length and weight gain in the first year of life. 46 , 47 MIP is also associated with anemia during infancy and the infant’s risk for anemia with maternal peripheral parasitemia at delivery is 11.8% and 9.2% with placental malaria infection. 48
The diagnosis of MIP can be challenging due to placental sequestration of parasitized erythrocytes, low circulating levels of parasites and limited resources in malaria endemic areas for advanced diagnostic techniques. Microscopic identification of malaria from the blood by an experienced and well-equipped technician remains the gold standard for malaria diagnosis. 49 However, rapid diagnostic tests (RDTs) that test for malaria antigens, like histidine-rich protein-1 (PfHRP2), are another option for malaria diagnosis. 49 – 51 RDTs are easier than microscopy to perform in low-resource settings, because they are not dependent on highly trained technicians in well-equipped laboratories. Therefore, RDTs might be the most appropriate point of care testing among symptomatic mothers in low-resource settings. 31 , 50 However, the use of RDTs might be insufficient in diagnosing MIP among mothers with asymptomatic infection, because RDTs require a higher circulating parasite burden than microscopy for detection. 52 RDTs are also insufficient to detect low amounts of circulating parasites because of placental sequestration. In research studies, the gold standard for malaria diagnosis has been placental histopathology, but it is not practical in many field sites. 53 , 54 Using microscopy of placental blood as the referent, the sensitivity of RDTs is 81% (95% CI 55–93) and the specificity is 94% (95% CI 76–99). 31 Molecular techniques, such as polymerase chain reaction (PCR) diagnosis, have increased sensitivity of diagnosis when compared to microscopy of placental blood to 94% (95% CI 86–98), but specificity of 94% (95% CI 86–98). 31 A new generation of ultrasensitive RDT’s is now being developed and might mitigate these diagnostic problems. 55
Prevention and Treatment
Prevention strategies in africa.
In malaria-endemic areas in Africa, a combination of vector control (preventing exposure to mosquitos) and chemoprevention (preventive medication-based treatment) strategies are used to prevent MIP. The World Health Organization (WHO) recommends a combined approach using insecticide treated bed nets (ITNs) to reduce exposure to mosquitoes carrying malaria and chemoprevention. 56 ITNs work by providing a physical barrier from mosquitoes, and repelling or killing susceptible mosquitoes, which reduces mosquito density and maintains the nets’ effectiveness even after the integrity of the barrier is compromised. 57 The use of ITNs in Africa have been shown to reduce LBW by 23%, miscarriages and stillbirths by 33%, and placental parasitemia by 23%. 58 Despite the proven efficacy of ITNs, uptake was estimated at only 39% in Africa between 2009 and 2011. 59 Since that time, ITN use has steadily increased, and an estimated 61% of pregnant women at risk for malaria slept under an ITN in 2017 56 , ( Figure 2 ). Malarial resistance to pyrethroids, the most commonly used insecticide in ITNs has been reported across sub-Saharan Africa and might impair future efficacy. 57

Percentage of the population in Sub-Saharan Africa at risk for malaria with access to ITN and using ITNs.
ITN: insecticide-treated bed nets Sources: World Health Organization and Malaria Atlas Project. Reprinted with permission ( 56 ).
Spraying the walls of households with insecticide to reduce human exposure to mosquitos, a procedure known as indoor residual spraying (IRS) is part of a comprehensive vector control program. Globally, IRS usage has declined and only 3% of the population at risk was protected by IRS in 2017, which might be related to a switch in insecticide from pyrethroids to more expensive chemicals. 56 IRS has been shown to improve outcomes of MIP, with women protected by IRS having lower incidence of MIP and lower risk of placental malaria. 60 , 61 When women were protected during greater than 90% of the time of their pregnancies, women had lower risk of preterm birth (risk ratio 0.35, 95% CI, 0.15–0.84). 60 IRS is an important part of a comprehensive vector control program and might contribute to improved birth outcomes in malaria-endemic regions.
Chemoprevention strategies have been successfully used to prevent adverse health outcomes associated with MIP. The most efficacious of these strategies uses a technique of intermittent preventive treatment in pregnancy (IPTp). IPTp consists of providing monthly doses of anti-malarial medication to all pregnant women starting in the second trimester. Women who receive at least 2 courses of IPTp have a relative risk reduction of 40% for moderate to severe anemia, 61% for antenatal parasitemia, 55% for placental parasitemia and 27% for low birthweight. 62 Since 2012, the WHO has recommended monthly IPTp to reduce the incidence of these complications of pregnancy. 63 However, despite WHO recommendations, in 2017, only 22% of pregnant women received three or more doses of IPTp in Sub-Saharan Africa ( Figure 3 ). 64

Percentage of pregnant women receiving IPTp, by dose in Sub-Saharan Africa, 2010–2017.
ANC: antenatal care, IPTp: intermittent preventive treatment in pregnancy, listed by dose. Sources: National Malaria Programme, World Health Organization, US Centers for Disease Control and Prevention. Reprinted with permission ( 56 ).
Sulfadoxine-pyrimethamine (SP) has been the drug of choice for IPTp in women who are HIV-negative. 65 Plasmodium resistance to SP has emerged through multiple mutations in the P. falciparum dihydrofolate reductase (Pfdhfr) and dihydropteroate synthetase (Pfdhps), with penetrance of this haplotype in some areas of greater than 90%. 66 Due to increasingly more resistant organisms, IPTp with SP is less effective at inhibiting parasite growth and preventing fetal growth restriction. 66 In light of increasing resistance, alternative strategies for chemoprevention are being tested, including IPTp with alternative medications and strategies of intermittent screening and treatment in pregnancy (ISTp). ISTp strategies use RDTs to diagnosis MIP at multiple time points and treat only when RDTs are positive. However, these strategies depend on accurate diagnosis of MIP and have not been proven to be effective alternatives to IPTp-SP even in highly resistant areas. 66 IPTp with dihydroartemisinin-piperaquine (DP) might be a promising alternative to IPTp-SP, but more research is needed in this area. 66
Prevention Strategies Outside of Africa
In South America and Asia, where malaria transmission is typically lower than in Africa, data on traditional chemoprophylaxis is limited. Prophylaxis with mefloquine or chloroquine has been efficacious for preventing MIP in pregnant women in Thailand. 67 , 68 Other chemoprophylactic strategies employed outside of Africa have included monthly IPTp-SP with azithromycin and ISTp-SP and artesunate. These strategies show promise in the reduction of low birth weight or maternal parasitemia.
Treatment of uncomplicated malaria
All MIP infections should be treated promptly to avoid complications to the mother and fetus. 31 To ensure safety of treatment in pregnancy, the WHO recommends trimester-specific and species-specific treatment strategies for uncomplicated malaria ( Table 1 ). For first trimester treatment of P. falciparum , the WHO recommends a 7-day treatment course of quinine with clindamycin, and second line treatment includes artemisinin-based combination therapy (ACT) or oral artesunate with clindamycin. 65 First trimester treatment of uncomplicated non- falciparum malaria consists of chloroquine or quinine for chloroquine-resistant infections.
Second and third trimester treatment of uncomplicated malaria follows the same guidelines as treatment for malaria in non-pregnant adults. 31 Therefore, first line treatments with ACTs can be used in MIP. ACTs include a short-acting artemisinin component and a longer acting partner drug, such as SP. 69 The potent artemisinin reduces the number of parasites quickly and the longer acting partner drug acts on the remaining parasites and provides a post-treatment prophylactic effect, preventing new infections. 69 ACTs have achieved cure rates as high as 99.2% for uncomplicated MIP without demonstrating significant safety concerns. 70
Treatment of Severe Malaria
Severe malaria has historically been attributed to infections with P. falciparum , but more recent evidence has included P. vivax as a significant contributor to severe malaria. 30 For pregnant patients with severe malaria, the WHO recommends the same treatments for both P. falciparum and P. vivax infections. The WHO also recommends treatment with primaquine after delivery to achieve cure and prevent relapses by eradicating P. vivax sequestered in the liver, ( Table 1 ). 30
Artemisinins are the most efficacious drugs for severe MIP after the first trimester. Because they are embryotoxic and teratogenic in animal studies, the WHO does not recommend their use in the first trimester for uncomplicated malaria. However, their use is recommended even in the first trimester in cases of severe malaria because of the high risk of maternal mortality. 30 Data on the use of artemisinins in the first trimester in humans show no associated increased risk of adverse pregnancy outcomes. 30 Long-term neurodevelopmental studies are needed to evaluate the safety of different drug combinations on child development.
Quinine is an alternative to artemisinin that has been used for centuries for the treatment of malaria. Although it is not considered embryotoxic or teratogenic in animal studies, it is less well-tolerated in humans. 30 Quinine can prolong the cardiac QT interval and is associated with tinnitus, headache, blurred vision, altered auditory acuity, nausea, diarrhea, and, rarely, massive hemolysis. 65 These side effects reduce compliance with treatment regimens and lead to higher levels of treatment failure. 30
An efficacious vaccine against malaria could be of particular benefit for pregnant women. One vaccine, RTS,S, is now approved by the European Medicines Agency, but is only modestly effective. 71 Efforts are also underway to develop a vaccine targeting the VAR2CSA antigen to protect women against pregnancy-associated malaria. 72
Co-Infection with HIV
Co-infections with malaria and HIV worsen morbidity and mortality for each disease, possibly due to alterations in the balance between the immune response to malaria and stimulation of viral replication. 73 MIP is associated with a two-fold higher HIV viral load. Conversely, women living with HIV experience more placental and peripheral malaria, higher parasite densities, more frequent febrile illnesses, more severe anemia and worse birth outcomes compared to non-HIV infected mothers with MIP. 73 Chemoprevention for women living with HIV in malaria-endemic areas includes daily co-trimoxazole. SP is discouraged for chemoprevention due to potential adverse drug reactions between SP and co-trimoxazole. 74 Although pregnant women living with HIV have the greatest risk of severe MIP, treatment strategies for severe malaria in conjunction with HIV treatments have not been well studied. 30 , 73 The WHO recommends against the use of zidovidine or efavirenz and the use of artesunate amodiaquine due to neutropenia and hepatotoxicity respectively. 65 Pregnant women receiving highly active antiretroviral therapy regimens should receive quinine when cardiac monitoring is available.
Conclusions
Malaria is among the most common and easily preventable causes of poor birth outcomes in the world. IPTp and ITNs distribution programs have helped decrease malaria risk among pregnant women in many parts of the world. However, greater efforts are needed especially in light of increasing drug and insecticide resistance. A better understanding of the pathogenesis of malaria during pregnancy could lead to the development of new interventions to prevent its health consequences.
Gaps in Knowledge (sidebar)
- What are the mechanisms by which malaria during pregnancy affects fetal growth?
- What is the natural history of congenital malaria?
- How can we increase uptake of IPTp and ITN’s by pregnant women?
- When is the optimal gestational age to start IPTp?
- How can we enable women to begin IPTp earlier during pregnancy?
- When malaria prevalence drops, when is it appropriate to stop IPTp?
- When high levels of resistance to SP develop, what should be done to protect pregnant women from malaria?
- Will it be possible to vaccinate primigravidae against pregnancy-associated malaria?
DISCLOSURES
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Reference List

IMAGES
VIDEO
COMMENTS
Malaria and Susceptibility to Other. thesis, p. 388. Prevalence of Malaria among Patients Attending Wolkite Health Center. Sep 2017; 1-29; Degifiebereka; DegifieBereka. (September, 2017 ...
Furthermore, we removed 50 case reports, 146 studies in malaria non-endemic areas, and 16 reports including 10 thesis and 6 NGO reports. Using the remaining 77 eligible studies, we provided an overview of each topic and complemented our narrative with WHO, CDC reports, and 10 complimentary readings. ... Malaria infection is caused by ...
First, I focus on the ecology that serves as a backdrop to transmission, and focus on the role agriculture may play. In doing so, I attempt to understand how agriculture affects both mosquito behavior, as well as malaria risk in under-5 children in the Democratic Republic of Congo (DRC), a country with one of the world's highest malaria burdens.
ii Dissertation Abstract Background: Recently, malaria has become a major global health priority.As a result there has been renewed interest in malaria control, elimination, and eradication. Zambia is one of the Elimination 8 countries and one of the President's Malaria Initiative focus
Mr AB Mapossa. PhD, Chemical Engineering. Thesis. Slow-release of mosquito repellents from microporous polyolefin strands. Prof WW Focke. Mr M Mpofu. PhD, Environmental Health. Thesis. Effectiveness of community larval source management (LSM) as an additional vector control intervention for malaria elimination.
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
Results. Eighty-one (19.0%) microscopically confirmed malaria cases were recorded, P.vivax was the most frequently detected species (n = 58; 71.6%).Interestingly, 73.2% (n = 309) of the participant did not utilize LLINs due to the fear of toxicity (37.4%, n = 158), misconception (21.6%, n = 91), and shortage (14.2%, n = 60).The data showed age, gender, marital status, family size, usage of ...
Results suggest that the larviciding intervention had a significant protective effect, decreasing by 21% the odds of being infected with malaria. Larviciding was found to be cost-effective for incidences as low as 40 infections per 1,000 individuals per year but the cost-effectiveness ratios were highly dependent on the assumed baseline malaria ...
Table 1 demonstrates characteristics of studies included by considering national population, GDP, malaria morbidity and mortality measures. All fifteen eligible studies were cross-sectional designs in which data were retrieved from HWs, health facilities, or medical records. Studies were published between 2009 and 2020 and eleven (73.3%) studies were conducted in Africa, one study was ...
The SARS-CoV-2 pandemic has disrupted malaria healthcare services. According to the latest World Malaria Report, about two-thirds of the 69,000 additional malaria deaths in 2020 compared to 2019 ...
Johns Hopkins University
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period ...
The Relationship Between Malaria Status in Under-five Children and Some Household Demographic, Socioeconomic and Environmental Factors Associated with the Disease in Sierra Leone ... thesis may be granted by the author or, in his/her absence, by the professor under whose direction it was written, or in his/her absence, by the Associate Dean ...
Abstract. Public health strategies for malaria in endemic countries aim to prevent transmission of the disease and control the vector. This historical analysis considers the strategies for vector control developed during the first four decades of the twentieth century. In 1925, policies and technological advances were debated internationally ...
Background Climate and environmental factors could be one of the primary factors that drive malaria transmission and it remains to challenge the malaria elimination efforts. Hence, this study was aimed to evaluate the effects of meteorological factors and topography on the incidence of malaria in the Boricha district in Sidama regional state of Ethiopia. Methods Malaria morbidity data recorded ...
Global technical strategy for malaria 2016-2030, 2021 update. View/ Open. 9789240031357-eng.pdf (2.100Mb) ...
Each year there are more than 200 million new cases of malaria, a preventable and treatable disease. According to the World Health Organization's (WHO) World malaria report 2019, there were no global gains in reducing new infections between 2014 and 2018, and nearly as many people died from malaria in 2018 as in the previous year. TDR's malaria research focuses on helping low- and middle ...
Malaria is a global public health burden with an estimated 229 million cases reported worldwide in 2019. About 94% of the reported cases were recorded in the African region. About 200 different species of protozoa have been identified so far and among them, at least 13 species are known to be pathogenic to humans.
Public health strategies for malaria in endemic countries aim to prevent transmission of the disease and control the vector. This historical analysis considers the strategies for vector control developed during the first four decades of the twentieth century. In 1925, policies and technological advances were debated internationally for the first time after the outbreak of malaria in Europe ...
Mathematical models have been used to provide an explicit framework for understanding malaria transmission dynamics in human population for over 100 years. With the disease still thriving and threatening to be a major source of death and disability due to changed environmental and socio-economic conditions, it is necessary to make a critical assessment of the existing models, and study their ...
Plasmodium falciparum malaria in pregnancy and fetal, newborn, and maternal outcomes among a cohort of pregnant women in coastal Kenya, 2006 - 2009. ... Deposit your senior honors thesis. Scholarly Journal, Newsletter or Book. Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book ...
Kent Campbell, an instrumental figure in the global battle against malaria — most notably in Africa, where he led an innovative program providing bed nets to protect rural villagers from the ...
The outlook for malaria control is grim. The disease, caused by mosquito-borne parasites, is present in 102 countries and is responsible for over 100 million clinical cases and 1 to 2 million deaths each year. Over the past two decades, efforts to control malaria have met with less and less success. In many regions where malaria transmission had been almost eliminated, the disease has made a ...
Introduction. Globally, an estimated 125 million pregnant women reside in areas where they are at risk of contracting malaria in pregnancy (MIP), and MIP remains an important preventable cause of adverse birth outcomes. 1 Although there are five species of malaria that infect humans, two main species of Plasmodium contribute to adverse maternal and fetal outcomes in pregnancy, P. falciparum ...