Persuasive Essay Guide
Persuasive Essay About Covid19

How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and get in touch with our persuasive essay writing service today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2021
Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation
- Sarah Kreps 1 ,
- Nabarun Dasgupta 2 ,
- John S. Brownstein 3 , 4 ,
- Yulin Hswen 5 &
- Douglas L. Kriner ORCID: orcid.org/0000-0002-9353-2334 1
npj Vaccines volume 6 , Article number: 73 ( 2021 ) Cite this article
19k Accesses
72 Citations
43 Altmetric
Metrics details
- Health care
- Translational research
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still undermine efforts to combat the pandemic. Employing a survey of 1096 adult Americans recruited via the Lucid platform, we examined the relationships between vaccine attributes, proposed policy interventions such as financial incentives, and misinformation on public vaccination preferences. Higher degrees of vaccine efficacy significantly increased individuals’ willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use Authorization to fast-track the vaccine decreased willingness. The vaccine manufacturer had no influence on public willingness to vaccinate. We also found no evidence that belief in misinformation about COVID-19 treatments was positively associated with vaccine hesitancy. The findings have implications for public health strategies intending to increase levels of community vaccination.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

Increase in concerns about climate change following climate strikes and civil disobedience in Germany
Johannes Brehm & Henri Gruhl

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker
Introduction
In less than a year, an array of vaccines was developed to bring an end to the SARS-CoV-2 pandemic. As impressive as the speed of development was the efficacy of vaccines such as Moderna and Pfizer, which are over 90%. Despite the growing availability and efficacy, however, vaccine hesitancy remains a potential impediment to widespread community uptake. While previous surveys indicate that overall levels of vaccine acceptance may be around 70% in the United States 1 , the case of Israel may offer a cautionary tale about self-reported preferences and vaccination in practice. Prospective studies 2 of vaccine acceptance in Israel showed that about 75% of the Israeli population would vaccinate, but Israel’s initial vaccination surge stalled around 42%. The government, which then augmented its vaccination efforts with incentive programs, attributed unexpected resistance to online misinformation 3 .
Research on vaccine hesitancy in the context of viruses such as influenza and measles, mumps, and rubella, suggests that misinformation surrounding vaccines is prevalent 4 , 5 . Emerging research on COVID-19 vaccine preferences, however, points to vaccine attributes as dominant determinants of attitudes toward vaccination. Higher efficacy is associated with greater likelihood of vaccinating 6 , 7 , whereas an FDA Emergency Use Authorization 6 or politicized approval timing 8 is associated with more hesitancy. Whether COVID-19 misinformation contributes to vaccine preferences or whether these attributes or policy interventions such as incentives play a larger role has not been studied. Further, while previous research has focused on a set of attributes that was relevant at one particular point in time, the evidence and context about the available vaccines has continued to shift in ways that could shape public willingness to accept the vaccine. For example, governments, employers, and economists have begun to think about or even devise ways to incentivize monetarily COVID-19 vaccine uptake, but researchers have not yet studied whether paying people to receive the COVID-19 vaccine would actually affect likely behavior. As supply problems wane and hesitancy becomes a limiting factor, understanding whether financial incentives can overcome hesitancy becomes a crucial question for public health. Further, as new vaccines such as Johnson and Johnson are authorized, knowing whether the vaccine manufacturer name elicits or deters interest in individuals is also important, as are the corresponding efficacy rates of different vaccines and the extent to which those affect vaccine preferences. The purpose of this study is to examine how information about vaccine attributes such as efficacy rates, the incidence of side effects, the nature of the governmental approval process, identity of the manufacturers, and policy interventions, including economic incentives, affect intention to vaccinate, and to examine the association between belief in an important category of misinformation—false claims concerning COVID-19 treatments—and willingness to vaccinate.
General characteristics of study population
Table 1 presents sample demographics, which largely reflect those of the US population as a whole. Of the 1335 US adults recruited for the study, a convenience sample of 1100 participants consented to begin the survey, and 1096 completed the full questionnaire. The sample was 51% female; 75% white; and had a median age of 43 with an interquartile range of 31–58. Comparisons of the sample demographics to those of other prominent social science surveys and U.S. Census figures are shown in Supplementary Table 1 .
Vaccination preferences
Each subject was asked to evaluate a series of seven hypothetical vaccines. For each hypothetical vaccine, our conjoint experiment randomly assigned values of five different vaccine attributes—efficacy, the incidence of minor side effects, government approval process, manufacturer, and cost/financial inducement. Descriptions of each attribute and the specific levels used in the experiment are summarized in Table 2 . After seeing the profile of each vaccine, the subject was asked whether she would choose to receive the vaccine described, or whether she would choose not to be vaccinated. Finally, subjects were asked to indicate how likely they would be to take the vaccine on a seven-point likert scale.
Across all choice sets, in 4419 cases (58%) subjects said they would choose the vaccine described in the profile rather than not being vaccinated. As shown in Fig. 1 , several characteristics of the vaccine significantly influenced willingness to vaccinate.
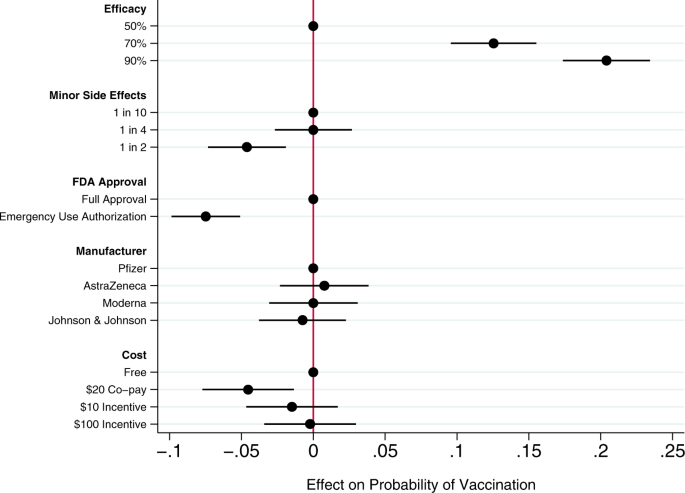
Circles present the estimated effect of each attribute level on the probability of a subject accepting vaccination from the attribute’s baseline level. Horizontal lines through points indicate 95% confidence intervals. Points without error bars denote the baseline value for each attribute. The average marginal component effects (AMCEs) are the regression coefficients reported in model 1 of Table 3 .
Efficacy had the largest effect on individual vaccine preferences. An efficacy rate of 90% increased uptake by about 20% relative to the baseline at 50% efficacy. Even a high incidence of minor side effects (1 in 2) had only a modest negative effect (about 5%) on willingness to vaccinate. Whether the vaccine went through full FDA approval or received an Emergency Use Authorization (EUA), an authority that allows the Food and Drug Administration mechanisms to accelerate the availability and use of treatments or medicines during medical emergencies 9 , significantly influenced willingness to vaccinate. An EUA decreased the likelihood of vaccination by 7% compared to a full FDA authorization; such a decline would translate into about 23 million Americans. While a $20 co-pay reduced the likelihood of vaccination relative to a no-cost baseline, financial incentives did not increase willingness to vaccinate. Lastly, the manufacturer had no effect on vaccination attitudes, despite the public pause of the AstraZeneca trial and prominence of Johnson & Johnson as a household name (our experiment was fielded before the pause in the administration of the Johnson & Johnson shot in the United States).
Model 2 of Table 3 presents an expanded model specification to investigate the association between misinformation and willingness to vaccinate. The primary additional independent variable of interest is a misinformation index that captures the extent to which each subject believes or rejects eight claims (five false; three true) about COVID-19 treatments. Additional analyses using alternate operationalizations of the misinformation index yield substantively similar results (Supplementary Table 4 ). This model also includes a number of demographic control variables, including indicators for political partisanship, gender, educational attainment, age, and race/ethnicity, all of which are also associated with belief in misinformation about the vaccine (Supplementary Table 2 ). Finally, the model also controls for subjects’ health insurance status, past experience vaccinating against seasonal influenza, attitudes toward the pharmaceutical industry, and beliefs about vaccine safety generally.
Greater levels of belief in misinformation about COVID-19 treatments were not associated with greater vaccine hesitancy. Instead, the relevant coefficient is positive and statistically significant, indicating that, all else being equal, individuals who scored higher on our index of misinformation about COVID-19 treatments were more willing to vaccinate than those who were less susceptible to believing false claims.
Strong beliefs that vaccines are safe generally was positively associated with willingness to accept a COVID-19 vaccine, as were past histories of frequent influenza vaccination and favorable attitudes toward the pharmaceutical industry. Women and older subjects were significantly less likely to report willingness to vaccinate than men and younger subjects, all else equal. Education was positively associated with willingness to vaccinate.
This research offers a comprehensive examination of attitudes toward COVID-19 vaccination, particularly the role of vaccine attributes, potential policy interventions, and misinformation. Several previous studies have analyzed the effects of vaccine characteristics on willingness to vaccinate, but the modal approach is to gauge willingness to accept a generic COVID-19 vaccine 10 , 11 . Large volumes of research show, however, that vaccine preferences hinge on specific vaccine attributes. Recent research considering the influence of attributes such as efficacy, side effects, and country of origin take a step toward understanding how properties affect individuals’ intentions to vaccinate 6 , 7 , 8 , 12 , 13 , but evidence about the attributes of actual vaccines, debates about how to promote vaccination within the population, and questions about the influence of misinformation have moved quickly 14 .
Our conjoint experiment therefore examined the influence of five vaccine attributes on vaccination willingness. The first category of attributes involved aspects of the vaccine itself. Since efficacy is one of the most common determinants of vaccine acceptance, we considered different levels of efficacy, 50%, 70%, and 90%, levels that are common in the literature 7 , 15 . Evidence from Phase III trials suggests that even the 90% efficacy level in our design, which is well above the 50% threshold from the FDA Guidance for minimal effectiveness for Emergency Use Authorization 16 , has been exceeded by both Pfizer’s and Moderna’s vaccines 17 , 18 . The 70% efficacy threshold is closer to the initial reports of the efficacy of the Johnson & Johnson vaccine, whose efficacy varied across regions 19 . Our analysis suggests that efficacy levels associated with recent mRNA vaccine trials increases public vaccine uptake by 20% over a baseline of a vaccine with 50% efficacy. A 70% efficacy rate increases public willingness to vaccinate by 13% over a baseline vaccine with 50% efficacy.
An additional set of epidemiological attributes consisted of the frequency of minor side effects. While severe side effects were plausible going into early clinical trials, evidence clearly suggests that minor side effects are more common, ranging from 10% to 100% of people vaccinated depending on the number of doses and the dose group (25–250 mcg) 20 . Since the 100 mcg dose was supported in Phase III trials 21 , we include the highest adverse event probability—approximating 60% as 1 in 2—and 1 in 10 as the lowest likelihood, approximating the number of people who experienced mild arthralgia 20 . Our findings suggest that a the prevalence of minor side effects associated with recent trials (i.e. a 1 in 2 chance), intention to vaccinate decreased by about 5% versus a 1 in 10 chance of minor side effects baseline. However, at a 25% rate of minor side effects, respondents did not indicate any lower likelihood of vaccination compared to the 10% baseline. Public communications about how to reduce well-known side effects, such as pain at the injection site, could contribute to improved acceptance of the vaccine, as it is unlikely that development of vaccine-related minor side effects will change.
We then considered the effect of EUA versus full FDA approval. The influenza H1N1 virus brought the process of EUA into public discourse 22 , and the COVID-19 virus has again raised the debate about whether and how to use EUA. Compared to recent studies also employing conjoint experimental designs that showed just a 3% decline in support conditional on EUA 6 , we found decreases in support of more than twice that with an EUA compared to full FDA approval. Statements made by the Trump administration promising an intensely rapid roll-out or isolated adverse events from vaccination in the UK may have exacerbated concerns about EUA versus full approval 8 , 23 , 24 , 25 . This negative effect is even greater among some subsets of the population. As shown in additional analyses reported in the Supplementary Information (Supplementary Fig. 5 ), the negative effects are greatest among those who believe vaccines are generally safe. Among those who believe vaccines generally are extremely safe, the EUA decreased willingness to vaccinate by 11%, all else equal. This suggests that outreach campaigns seeking to assure those troubled by the authorization process used for currently available vaccines should target their efforts on those who are generally predisposed to believe vaccines are safe.
Next, we compared receptiveness as a function of the manufacturer: Moderna, Pfizer, Johnson and Johnson, and AstraZeneca, all firms at advanced stages of vaccine development. Vaccine manufacturers in the US have not yet attempted to use trade names to differentiate their vaccines, instead relying on the association with manufacturer reputation. In other countries, vaccine brand names have been more intentionally publicized, such as Bharat Biotech’s Covaxin in India and Gamaleya Research Institute of Epidemiology and Microbiology Sputnik V in Russia. We found that manufacturer names had no impact on willingness to vaccinate. As with hepatitis and H. influenzae vaccines 26 , 27 , interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research suggests that at least as far as public receptiveness goes, interchangeability would not introduce concerns. We found no significant differences in vaccination uptake across any of the manufacturer treatments. Future research should investigate if a manufacturer preference develops as new evidence about efficacy and side effects becomes available, particularly depending on whether future booster shots, if needed, are deemed interchangeable with the initial vaccination.
Taking up the question of how cost and financial incentives shape behavior, we looked at paying and being paid to vaccinate. While existing research suggests that individuals are often willing to pay for vaccines 28 , 29 , some economists have proposed that the government pay individuals up to $1,000 to take the COVID-19 vaccine 30 . However, because a cost of $300 billion to vaccinate the population may be prohibitive, we posed a more modest $100 incentive. We also compared this with a $10 incentive, which previous studies suggest is sufficient for actions that do not require individuals to change behavior on a sustained basis 31 . While having to pay a $20 co-pay for the vaccine did deter individuals, the additional economic incentives had no positive effect although they did not discourage vaccination 32 . Consistent with past research 31 , 33 , further analysis shows that the negative effect of the $20 co-pay was concentrated among low-income earners (Supplementary Fig. 7 ). Financial incentives failed to increase vaccination willingness across income levels.
Our study also yields important insights into the relationship between one prominent category of COVID-19 misinformation and vaccination preferences. We find that susceptibility to misinformation about COVID-19 treatments—based on whether individuals can distinguish between factual and false information about efforts to combat COVID-19—is considerable. A quarter of subjects scored no higher on our misinformation index than random guessing or uniform abstention/unsure responses (for the full distribution, see Supplementary Fig. 2 ). However, subjects who scored higher on our misinformation index did not exhibit greater vaccination hesitancy. These subjects actually were more likely to believe in vaccine safety more generally and to accept a COVID-19 vaccine, all else being equal. These results run counter to recent findings of public opinion in France where greater conspiracy beliefs were negatively correlated with willingness to vaccinate against COVID-19 34 and in Korea where greater misinformation exposure and belief were negatively correlated with taking preventative actions 35 . Nevertheless, the results are robust to alternate operationalizations of belief in misinformation (i.e., constructing the index only using false claims, or measuring misinformation beliefs as the number of false claims believed: see Supplementary Table 4 ).
We recommend further study to understand the observed positive relationship between beliefs in COVID-19 misinformation about fake treatments and willingness to receive the COVID-19 vaccine. To be clear, we do not posit a causal relationship between the two. Rather, we suspect that belief in misinformation may be correlated with an omitted factor related to concerns about contracting COVID-19. For example, those who believe COVID-19 misinformation may have a higher perception of risk of COVID-19, and therefore be more willing to take a vaccine, all else equal 36 . Additional analyses reported in the Supplementary Information (Supplementary Fig. 6 ) show that the negative effect of an EUA on willingness to vaccinate was concentrated among those who scored low on the misinformation index. An EUA had little effect on the vaccination preferences of subjects most susceptible to misinformation. This pattern is consistent with the possibility that these subjects were more concerned with the disease and therefore more likely to vaccinate, regardless of the process through which the vaccine was brought to market.
We also observe that skepticism toward vaccines in general does not correlate perfectly with skepticism toward the COVID-19 vaccine. Therefore, it is important not to conflate people who are wary of the COVID-19 vaccine and those who are anti-vaccination, as even medically informed individuals may be hesitant because of the speed at which the COVID-19 vaccine was developed. For example, older people are more likely to believe vaccines are safe but less willing to receive the COVID-19 vaccine in our survey, perhaps following the high rates of vaccine skepticism among medical staff expressing concerns regarding the safety of a rapidly-developed vaccine 2 . This inverse relationship between age and willingness to vaccinate is also surprising. Most opinion surveys find older adults are more likely to vaccinate than younger adults 37 . However, most of these survey questions ask about willingness to take a generic vaccine. Two prior studies, both recruiting subjects from the Lucid platform and employing conjoint experiments to examine the effects of vaccine attributes on public willingness to vaccinate, also find greater vaccine hesitancy among older Americans 6 , 7 . Future research could explore whether these divergent results are a product of the characteristics of the sample or of the methodological design in which subjects have much more information about the vaccines when indicating their vaccination preferences.
An important limitation of our study is that it necessarily offers a snapshot in time, specifically prior to both the election and vaccine roll-out. We recommend further study to understand more how vaccine perceptions evolve both in terms of the perceived political ownership of the vaccine—now that President Biden is in office—and as evidence has emerged from the millions of people who have been vaccinated. Similarly, researchers should consider analyzing vaccine preferences in the context of online vaccine controversies that have been framed in terms of patient autonomy and right to refuse 38 , 39 . Vaccination mandates may evoke feelings of powerlessness, which may be exacerbated by misinformation about the vaccines themselves. Further, researchers should more fully consider how individual attributes such as political ideology and race intersect with vaccine preferences. Our study registered increased vaccine hesitancy among Blacks, but did not find that skepticism was directly related to misinformation. Perceptions and realities of race-based maltreatment could also be moderating factors worth exploring in future analyses 40 , 41 .
Overall, we found that the most important factor influencing vaccine preferences is vaccine efficacy, consistent with a number of previous studies about attitudes toward a range of vaccines 6 , 42 , 43 . Other attributes offer potential cautionary flags and opportunities for public outreach. The prospect of a 50% likelihood of mild side effects, consistent with the evidence about current COVID-19 vaccines being employed, dampens likelihood of uptake. Public health officials should reinforce the relatively mild nature of the side effects—pain at the injection site and fatigue being the most common 44 —and especially the temporary nature of these effects to assuage public concerns. Additionally, in considering policy interventions, public health authorities should recognize that a $20 co-pay will likely discourage uptake while financial incentives are unlikely to have a significant positive effect. Lastly, belief in misinformation about COVID-19 does not appear to be a strong predictor of vaccine hesitancy; belief in misinformation and willingness to vaccinate were positively correlated in our data. Future research should explore the possibility that exposure to and belief in misinformation is correlated with other factors associated with vaccine preferences.
Survey sample and procedures
This study was approved by the Cornell Institutional Review Board for Human Participant Research (protocol ID 2004009569). We conducted the study on October 29–30, 2020, prior to vaccine approval, which means we captured sentiments prospectively rather than based on information emerging from an ongoing vaccination campaign. We recruited a sample of 1096 adult Americans via the Lucid platform, which uses quota sampling to produce samples matched to the demographics of the U.S. population on age, gender, ethnicity, and geographic region. Research has shown that experimental effects observed in Lucid samples largely mirror those found using probability-based samples 45 . Supplementary Table 1 presents the demographics of our sample and comparisons to both the U.S. Census American Community Survey and the demographics of prominent social science surveys.
After providing informed consent on the first screen of the online survey, participants turned to a choice-based conjoint experiment that varied five attributes of the COVID-19 vaccine. Conjoint analyses are often used in marketing to research how different aspects of a product or service affect consumer choice. We build on public health studies that have analyzed the influence of vaccine characteristics on uptake within the population 42 , 46 .
Conjoint experiment
We first designed a choice-based conjoint experiment that allowed us to evaluate the relative influence of a range of vaccine attributes on respondents’ vaccine preferences. We examined five attributes summarized in Table 2 . Past research has shown that the first two attributes, efficacy and the incidence of side effects, are significant drivers of public preferences on a range of vaccines 47 , 48 , 49 , including COVID-19 6 , 7 , 13 , 50 . In this study, we increased the expected incidence of minor side effects from previous research 6 to reflect emerging evidence from Phase III trials. The third attribute, whether the vaccine received full FDA approval or an EUA, examines whether the speed of the approval process affects public vaccination preferences 6 . The fourth attribute, the manufacturer of the vaccine, allows us to examine whether the highly public pause in the AstraZeneca trial following an adverse event, and the significant differences in brand familiarity between smaller and less broadly known companies like Moderna and household name Johnson & Johnson affects public willingness to vaccinate. The fifth attribute examines the influence of a policy tool—offsetting the costs of vaccination or even incentivizing it financially—on public willingness to vaccinate.
Attribute levels and attribute order were randomly assigned across participants. A sample choice set is presented in Supplementary Fig. 1 . After viewing each profile individually, subjects were asked: “If you had to choose, would you choose to get this vaccine, or would you choose not to be vaccinated?” Subjects then made a binary choice, responding either that they “would choose to get this vaccine” or that they “would choose not to be vaccinated.” This is the dependent variable for the regression analyses in Table 3 . After making a binary choice to take the vaccine or not be vaccinated, we also asked subjects “how likely or unlikely would you be to get the vaccine described above?” Subjects indicated their vaccination preference on a seven-point scale ranging from “extremely likely” to “extremely unlikely.” Additional analyses using this ordinal dependent variable reported in Supplementary Table 3 yield substantively similar results to those presented in Table 3 .
To determine the effect of each attribute-level on willingness to vaccinate, we followed Hainmueller, Hopkins, and Yamamoto and employed an ordinary least squares (OLS) regression with standard errors clustered on respondent to estimate the average marginal component effects (AMCEs) for each attribute 51 . The AMCE represents the average difference in a subject choosing a vaccine when comparing two different attribute values—for example, 50% efficacy vs. 90% efficacy—averaged across all possible combinations of the other vaccine attribute values. The AMCEs are nonparametrically identified under a modest set of assumptions, many of which (such as randomization of attribute levels) are guaranteed by design. Model 1 in Table 3 estimates the AMCEs for each attribute. These AMCEs are illustrated in Fig. 1 .
Analyzing additional correlates of vaccine acceptance
To explore the association between respondents’ embrace of misinformation about COVID-19 treatments and vaccination willingness, the survey included an additional question battery. To measure the extent of belief in COVID-19 misinformation, we constructed a list of both accurate and inaccurate headlines about the coronavirus. We focused on treatments, relying on the World Health Organization’s list of myths, such as “Hand dryers are effective in killing the new coronavirus” and true headlines such as “Avoiding shaking hands can help limit the spread of the new coronavirus 52 .” Complete wording for each claim is provided in Supplementary Appendix 1 . Individuals read three true headlines and five myths, and then responded whether they believed each headline was true or false, or whether they were unsure. We coded responses to each headline so that an incorrect accuracy assessment yielded a 1; a correct accuracy assessment a -1; and a response of unsure was coded as 0. From this, we created an additive index of belief in misinformation that ranged from -8 to 8. The distribution of the misinformation index is presented in Supplementary Fig. 2 . A possible limitation of this measure is that because the survey was conducted online, some individuals could have searched for the answers to the questions before responding. However, the median misinformation index score for subjects in the top quartile in terms of time spent taking the survey was identical to the median for all other respondents. This may suggest that systematic searching for correct answers is unlikely.
To ensure that any association observed between belief in misinformation and willingness to vaccinate is not an artifact of how we operationalized susceptibility to misinformation, we also constructed two alternate measures of belief in misinformation. These measures are described in detail in the Supplementary Information (see Supplementary Figs. 3 and 4 ). Additional regression analyses using these alternate measures of misinformation beliefs yield substantively similar results (see Supplementary Table 4 ). Additional analyses examining whether belief in misinformation moderates the effect of efficacy and an FDA EUA on vaccine acceptance are presented in Supplementary Fig. 6 .
Finally, model 2 of Table 3 includes a range of additional control variables. Following past research, it includes a number of demographic variables, including indicator variables identifying subjects who identify as Democrats or Republicans; an indicator variable identifying females; a continuous variable measuring age (alternate analyses employing a categorical variable yield substantively similar results); an eight-point measure of educational attainment; and indicator variables identifying subjects who self-identify as Black or Latinx. Following previous research 6 , the model also controlled for three additional factors often associated with willingness to vaccinate: an indicator variable identifying whether each subject had health insurance; a variable measuring past frequency of influenza vaccination on a four-point scale ranging from “never” to “every year”; beliefs about the general safety of vaccines measured on a four-point scale ranging from “not at all safe” to “extremely safe”; and a measure of attitudes toward the pharmaceutical industry ranging from “very positive” to “very negative.”
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data and statistical code to reproduce the tables and figures in the manuscript and Supplementary Information are published at the Harvard Dataverse via this link: 10.7910/DVN/ZYU6CO.
Hamel, L., Kirzinger, A., Munana, C. & Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020 | KFF (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
Article CAS PubMed Google Scholar
Scharf, I. & Ben Zion, I. As Vaccinations Lag, Israel Combats Online Misinformation. AP2 (21AD).
Nyhan, B. & Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33 , 459–464 (2015).
Article PubMed Google Scholar
Hussain, A., Ali, S., Ahmed, M. & Hussain, S. The anti-vaccination movement: a regression in modern medicine. Cureus 10 , e2919 (2018).
PubMed PubMed Central Google Scholar
Kreps, S. et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. open 3 , (2020).
Motta, M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med . 113642, https://doi.org/10.1016/j.socscimed.2020.113642 (2021).
Bokemper, S. E., Huber, G. A., Gerber, A. S., James, E. K. & Omer, S. B. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine , https://doi.org/10.1016/j.vaccine.2020.12.048 (2021).
Quinn, S. C., Jamison, A. M. & Freimuth, V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am. J. Public Health e1–e4 (2020).
Malik, A. A., McFadden, S. A. M., Elharake, J. & Omer, S. B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 26 , 100495 (2020).
Article PubMed PubMed Central Google Scholar
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Lazarus, J. V. et al . A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med . 1–4 (2020).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Heal ., https://doi.org/10.1016/s2468-2667(21)00012-8 (2021).
Wood, S. & Schulman, K. Beyond politics—promoting Covid-19 vaccination in the United States. N. Engl. J. Med . NEJMms2033790, https://doi.org/10.1056/NEJMms2033790 (2021).
Marshall, H. S., Chen, G., Clarke, M. & Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine 34 , 671–677 (2016).
Administration, F. and D. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry . (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med ., https://doi.org/10.1056/nejmoa2035389 (2020).
Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. (2020). Available at: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial . (Accessed 28 Feb 2021).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383 , 1920–1931 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383 , 2427–2438 (2020).
Quinn, S. C., Kumar, S., Freimuth, V. S., Kidwell, K. & Musa, D. Public willingness to take a vaccine or drug under emergency use authorization during the 2009 H1N1 pandemic. Biosecurity Bioterrorism 7 , 275–290 (2009).
Holden Thorp, H. A dangerous rush for vaccines. Science 369 , 885 (2020).
Limaye, R. J., Sauer, M. & Truelove, S. A. Politicizing public health: the powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother ., https://doi.org/10.1080/21645515.2020.1846400 (2020).
Feuer, W. Trump Says ‘No President’s Ever Pushed’ the FDA Like Jim, Vaccine Coming ‘Very Shortly’. CNBC (2020).
Soysal, A., Gokçe, I., Pehlivan, T. & Bakir, M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur. J. Pediatr. 166 , 533–539 (2007).
Greenberg, D. P. & Feldman, S. Vaccine interchangeability. Clin. Pediatrics 42 , 93–99 (2003).
Article Google Scholar
Bishai, D., Brice, R., Girod, I., Saleh, A. & Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. PharmacoEconomics 25 , 143–154 (2007).
Cameron, M. P., Newman, P. A., Roungprakhon, S. & Scarpa, R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 , 3712–3717 (2013).
Litan, R. Want herd immunity? Pay people to take the vaccine. Brookings (2020).
Kane, R. L., Johnson, P. E., Town, R. J. & Butler, M. A structured review of the effect of economic incentives on consumers’ preventive behavior. Am. J. Preventive Med. 27 , 327–352 (2004).
Volpp, K. G., Loewenstein, G. & Buttenheim, A. M. Behaviorally informed strategies for a National COVID-19 vaccine promotion program. JAMA - J. Am. Med. Assoc. 325 , 125–126 (2020).
Google Scholar
Nowalk, M. P. et al. Improving influenza vaccination rates in the workplace. A randomized trial. Am. J. Prev. Med. 38 , 237–246 (2010).
Bertin, P., Nera, K. & Delouvée, S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front. Psychol. 11 , 2471 (2020).
Lee, J. J. et al. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: cross-sectional online study. J. Med. Internet Res. 22 , e22205 (2020).
Reyna, V. F. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 30 , 3790–3797 (2012).
Lin, C., Tu, P. & Beitsch, L. M. Confidence and receptivity for covid‐19 vaccines: a rapid systematic review. Vaccines 9 , 1–32 (2021).
Broniatowski, D. A. et al. Facebook pages, the ‘Disneyland’ measles outbreak, and promotion of vaccine refusal as a civil right, 2009–2019. Am. J. Public Health 110 , S312–S318 (2020).
Moon, K., Riege, A., Gourdon-Kanhukamwe, A. & Vallée-Tourangeau, G. The Moderating Effect of Autonomy on Promotional Health Messages Encouraging Flu Vaccination Uptake Among Healthcare Professionals . (PsyArXiv, 2020), https://doi.org/10.31234/OSF.IO/AJV4Q .
Ferdinand, K. C., Nedunchezhian, S. & Reddy, T. K. The COVID-19 and influenza “Twindemic”: barriers to influenza vaccination and potential acceptance of SARS-CoV2 vaccination in African Americans. J. Natl. Med. Assoc. 112 , 681–687 (2020).
PubMed Google Scholar
Jaiswal, J., LoSchiavo, C. & Perlman, D. C. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 24 , 2776–2780 (2020).
Determann, D. et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE 9 , e102505 (2014).
Simpson, C. R., Ritchie, L. D., Robertson, C., Sheikh, A. & McMenamin, J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study. Lancet Infect. Dis. 12 , 696–702 (2012).
Remmel, A. COVID vaccines and safety: what the research says. Nature 590 , 538–540 (2021).
Coppock, A. & McClellan, O. A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Polit. 6 , 1–14 (2019).
de Bekker-Grob, E. W. et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine 36 , 1467–1476 (2018).
Determann, D. et al. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets–A discrete choice experiment in four European countries, 2013. Eurosurveillance 21 , 1–13 (2016).
de Bekker-Grob, E. W. et al. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 28 , 6692–6697 (2010).
Guo, N., Zhang, G., Zhu, D., Wang, J. & Shi, L. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine 35 , 2848–2854 (2017).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. USA 118 , 2021726118 (2021).
Hainmueller, J., Hopkins, D. J. & Yamamoto, T. Causal inference in conjoint analysis: Understanding multidimensional choices via stated preference experiments. Polit. Anal. 22 , 1–30 (2014).
Organization, W. H. Coronavirus disease (COVID-19) advice for the public: Mythbusters. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters . (Accessed 14 Jan 2021).
Download references
Acknowledgements
S.K. and D.K. would like to thank the Cornell Atkinson Center for Sustainability for financial support.
Author information
Authors and affiliations.
Department of Government, Cornell University, Ithaca, NY, USA
Sarah Kreps & Douglas L. Kriner
Injury Prevention Research Center, University of North Carolina, Chapel Hill, NC, USA
Nabarun Dasgupta
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
John S. Brownstein
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, USA
Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
Yulin Hswen
You can also search for this author in PubMed Google Scholar
Contributions
S.K. and D.K. designed the experiment/survey instrument and conducted the statistical analysis. S.K., N.D., J.B., Y.H., and D.K. all contributed to the conceptual design of the research and to the writing of the paper.
Corresponding author
Correspondence to Douglas L. Kriner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kreps, S., Dasgupta, N., Brownstein, J.S. et al. Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation. npj Vaccines 6 , 73 (2021). https://doi.org/10.1038/s41541-021-00335-2
Download citation
Received : 25 January 2021
Accepted : 06 April 2021
Published : 14 May 2021
DOI : https://doi.org/10.1038/s41541-021-00335-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Progress with covid vaccine development and implementation.
- Richard W. Titball
- David I. Bernstein
- Alan D. T. Barrett
npj Vaccines (2024)
A synthesis of evidence for policy from behavioural science during COVID-19
- Kai Ruggeri
- Friederike Stock
- Robb Willer
Nature (2024)
The rapid progress in COVID vaccine development and implementation
- Nicolas V. J. Fanget
npj Vaccines (2022)
COVID-19 Vaccine Acceptability and Financial Incentives among Unhoused People in Los Angeles County: a Three-Stage Field Survey
- Allison D. Rosen
- Jacqueline Beltran
- Chelsea L. Shover
Journal of Urban Health (2022)
The shot, the message, and the messenger: COVID-19 vaccine acceptance in Latin America
- Pablo Argote
- Elena Barham
- Oscar Pocasangre
npj Vaccines (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

Should COVID-19 vaccines be mandatory? Two experts discuss
Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Disclosure statement
Alberto Giubilini receives funding from the Arts and Humanities Research Council/UK Research and Innovation (AHRC/UKRI) and has previously received funding from the Wellcome Trust.
Vageesh Jain is affiliated with Public Health England under an honorary contract as a speciality registrar.
University College London provides funding as a founding partner of The Conversation UK.
University of Oxford provides funding as a member of The Conversation UK.
View all partners

To be properly protective, COVID-19 vaccines need to be given to most people worldwide. Only through widespread vaccination will we reach herd immunity – where enough people are immune to stop the disease from spreading freely. To achieve this, some have suggested vaccines should be made compulsory , though the UK government has ruled this out . But with high rates of COVID-19 vaccine hesitancy in the UK and elsewhere , is this the right call? Here, two experts to make the case for and against mandatory COVID-19 vaccines.
Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford
COVID-19 vaccination should be mandatory – at least for certain groups. This means there would be penalties for failure to vaccinate, such as fines or limitations on freedom of movement.
The less burdensome it is for an individual to do something that prevents harm to others, and the greater the harm prevented, the stronger the ethical reason for mandating it.
Being vaccinated dramatically reduces the risk of seriously harming or killing others. Vaccines such as the Pfizer , AstraZeneca or Moderna ones with 90-95% efficacy at preventing people from getting sick are also likely to be effective at stopping the virus from spreading, though possibly to a lower degree. Such benefits would come at a very minimal cost to individuals.
Lockdown is mandatory. Exactly like mandatory vaccination, it protects vulnerable people from COVID-19. But, as I have argued in detail elsewhere, unlike mandatory vaccination, lockdown entails very large individual and societal costs. It is inconsistent to accept mandatory lockdown but reject mandatory vaccination. The latter can achieve a much greater good at a much smaller cost.
Also, mandatory vaccination ensures that the risks and burdens of reaching herd immunity are distributed evenly across the population. Because herd immunity benefits society collectively, it’s only fair that the responsibility of reaching it is shared evenly among society’s individual members.
Of course, we might achieve herd immunity through less restrictive alternatives than making vaccination mandatory – such as information campaigns to encourage people to be vaccinated. But even if we reach herd immunity, the higher the uptake of vaccines, the lower the risk of falling below the herd immunity threshold at a later time. We should do everything we can to prevent that emergency from happening – especially when the cost of doing so is low.
Fostering trust and driving uptake by making people more informed is a nice narrative, but it’s risky. Merely giving people information on vaccines does not always result in increased willingness to vaccinate and might actually lower confidence in vaccines. On the other hand, we’ve seen mandatory vaccination policies in Italy recently successfully boost vaccine uptake for other diseases.
Mandatory seatbelt policies have proven very successful in reducing deaths from car accidents, and are now widely endorsed despite the (very small) risks that seatbelts entail. We should see vaccines as seatbelts against COVID-19. In fact, as very special seatbelts, which protect ourselves and protect others.

Vageesh Jain, NIHR Academic Clinical Fellow in Public Health Medicine, UCL
Mandatory vaccination does not automatically increase vaccine uptake. An EU-funded project on epidemics and pandemics, which took place several years before COVID-19, found no evidence to support this notion. Looking at Baltic and Scandinavian countries, the project’s report noted that countries “where a vaccination is mandatory do not usually reach better coverage than neighbour or similar countries where there is no legal obligation”.
According to the Nuffield Council of Bioethics, mandatory vaccination may be justified for highly contagious and serious diseases. But although contagious, Public Health England does not classify COVID-19 as a high-consequence infectious disease due to its relatively low case fatality rate.
COVID-19 severity is strongly linked with age, dividing individual perceptions of vulnerability within populations. The death rate is estimated at 7.8% in people aged over 80, but at just 0.0016% in children aged nine and under. In a liberal democracy, forcing the vaccination of millions of young and healthy citizens who perceive themselves to be at an acceptably low risk from COVID-19 will be ethically disputed and is politically risky.
Public apprehensions for a novel vaccine produced at breakneck speed are wholly legitimate. A UK survey of 70,000 people found 49% were “very likely” to get a COVID-19 vaccine once available. US surveys are similar . This is not because the majority are anti-vaxxers.
Despite promising headlines, the trials and pharmaceutical processes surrounding them have not yet been scrutinised. With the first trials only beginning in April , there is limited data on long-term safety and efficacy. We don’t know how long immunity lasts for. None of the trials were designed to tell us if the vaccine prevents serious disease or virus transmission.
To disregard these ubiquitous concerns would be counterproductive. As a tool for combating anti-vaxxers – estimated at around 58 million globally and making up a small minority of those not getting vaccinated – mandatory vaccines are also problematic. The forces driving scientific and political populism are the same . Anti-vaxxers do not trust experts, industry and especially not the government. A government mandate will not just be met with unshakeable defiance, but will also be weaponised to recruit others to the anti-vaxxer cause.
In the early 1990s, polio was endemic in India , with between 500 and 1,000 children getting paralysed daily. By 2011, the virus was eliminated. This was not achieved through legislation. It was down to a consolidated effort to involve communities, target high-need groups, understand concerns, inform, educate, remove barriers, invest in local delivery systems and link with political and religious leaders.
Mandatory vaccination is rarely justified. The successful roll-out of novel COVID-19 vaccines will require time, communication and trust. We have come too far, too fast, to lose our nerve now.
- Mandatory vaccination
- Coronavirus
- Vaccine hesitancy
- Coronavirus insights

Sydney Horizon Educators (Identified)

Senior Disability Services Advisor

Deputy Social Media Producer

Associate Professor, Occupational Therapy

GRAINS RESEARCH AND DEVELOPMENT CORPORATION CHAIRPERSON
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
An Overview of the Vaccine Debate
Looking at Both Sides of the Argument
There is a wealth of research demonstrating the efficacy and safety of vaccines —including how some have virtually eradicated infectious diseases that once killed millions. However, this has done little to sway those who believe that untold harms are being hidden from the American public.
The vaccine debate—including the argument as to whether vaccines are safe, effective, or could cause conditions like autism —has received a lot of attention from the media in recent years. With so much conflicting information being publicized, it can be a challenge to discern what is true and what is not. Therefore, it is important to learn the facts before making health decisions.
Claims and Controversy
Those who are part of the anti-vaccination movement include not only non-medical professionals but several scientists and healthcare providers who hold alternative views about vaccines and vaccination in general.
Some notable examples include:
- British healthcare provider Andrew Wakefield, who in 1998 published research linking the MMR vaccine and autism . That study has since been retracted, and he was later removed from the medical registry in the United Kingdom for falsifying scientific data.
- Pediatrician Bob Sears, who wrote the bestseller "The Vaccine Book: Making the Right Decision for your Child ," which suggested that many essential childhood vaccines were "optional." However, he was subsequently put on probation by the Medical Review Board of California in 2018 for alleged medical negligence and the inappropriate writing of medical exemptions for vaccinations.
- Dr. Jane M. Orient, director of the Association of American Healthcare Providers and Surgeons, who was among the leading opponents of the COVID-19 vaccine and one of the leading proponents of using hydroxychloroquine to treat COVID-19 during the pandemic.
These opposing views and claims, along with other information promoted by the news and social media, have led some people to question whether they know everything they need to know about vaccines.
Common Concerns Regarding Vaccines
The arguments made against vaccines are not new and have been made well before the first vaccine was developed for smallpox back in the 18th century.
The following are some of the common arguments against vaccines:
- Vaccines contain "toxic" ingredients that can lead to an assortment of chronic health conditions such as autism.
- Vaccines are a tool of "Big Pharma," in which manufacturers are willing to profit off of harm to children.
- Governments are "pharma shills," meaning they are bought off by pharmaceutical companies to hide cures or approve drugs that are not safe.
- A child’s immune system is too immature to handle vaccines , leading the immune system to become overwhelmed and trigger an array of abnormal health conditions.
- Natural immunity is best , suggesting that a natural infection that causes disease is "better" than receiving a vaccine that may cause mild side effects.
- Vaccines are not tested properly , suggesting a (highly unethical) approach in which one group of people is given a vaccine, another group is not, and both are intentionally inoculated with the same virus or bacteria.
- Infectious diseases have declined due in part to improved hygiene and sanitation , suggesting that hand-washing and other sanitary interventions are all that are needed to prevent epidemics.
- Vaccines cause the body to "shed" virus , a claim that is medically true, although the amount of shed virus is rarely enough to cause infection.
The impact of anti-vaccination claims has been profound. For example, it has led to a resurgence of measles in the United States and Europe, despite the fact that the disease was declared eliminated in the U.S. back in 2000.
Studies have suggested that the anti-vaccination movement has cast doubt on the importance of childhood vaccinations among large sectors of the population. The added burden of the COVID-19 pandemic has led to further declines in vaccination rates.
There is also concern that the same repercussions may affect COVID-19 vaccination rates—both domestically and abroad. Ultimately, vaccine rates must be high for herd immunity to be effective.
According to a study from the Centers for Disease Control and Prevention (CDC), the rate of complete recommended vaccination among babies age 5 months has declined from 66.6% in 2016 to 49.7% by May 2020. Declines in vaccination coverage were seen in other age groups as well.
Benefits of Vaccination
Of the vaccines recommended by the CDC, the benefits of immunization are seen to overwhelmingly outweigh the potential risks. While there are some people who may need to avoid certain vaccines due to underlying health conditions, the vast majority can do so safely.
According to the U.S. Department of Health and Human Services, there are five important reasons why your child should get the recommended vaccines:
- Immunizations can save your child’s life . Consider that polio once killed up to 30% of those who developed paralytic symptoms. Due to polio vaccination, the disease is no longer a public health concern in the United States.
- Vaccination is very safe and effective . Injection site pain and mild, flu-like symptoms may occur with vaccine shots. However, serious side effects , such as a severe allergic reaction, are very rare.
- Immunization protects others . Because respiratory viruses can spread easily among children, getting your child vaccinated not only protects your child but prevents the further spread of disease.
- Immunizations can save you time and money . According to the non-profit Borgen Project, the average cost of a measles vaccination around the world is roughly $1.76, whereas the average cost of treating measles is $307. In the end, the cost of prevention is invariably smaller than the cost of treatment.
- Immunization protects future generations . Smallpox vaccinations have led to the eradication of smallpox . Rubella (German measles) vaccinations have helped eliminate birth defects caused by infection of pregnant mothers in the developed world. With persistence and increased community uptake, measles could one day be declared eliminated (again) as well.
A Word From Verywell
If you have any questions or concerns about vaccinations, do not hesitate to speak with your healthcare provider or your child's pediatrician.
If a vaccine on the immunization schedule has been missed, speak to a healthcare provider before seeking the vaccination on your own (such as at a pharmacy or clinic). In some cases, additional doses may be needed.
Vaccines Healthcare Provider Discussion Guide
Get our printable guide for your next healthcare provider's appointment to help you ask the right questions.
Sign up for our Health Tip of the Day newsletter, and receive daily tips that will help you live your healthiest life.
Thank you, {{form.email}}, for signing up.
There was an error. Please try again.
Eggerton L. Lancet retracts 12-year-old article linking autism to MMR vaccines . CMAJ . 2010 Mar 9; 182(4):e199-200. doi:10.1503/cmaj.109-3179
Park A. Doctor behind vaccine-autism link loses license . Time .
Offit PA, Moser CA. The problem with Dr Bob's alternative vaccine schedule . Pediatrics. 2009 Jan;123 (1):e164-e169. doi:10.1542/peds.2008-2189
Before the Medical Board of California, Department of Consumer Affairs, State of California. In the Matter of the Accusation Against Robert William Sears, M.D., Case No. 800-2015-012268 .
Stolberg SG. Anti-vaccine doctor has been invited to testify before Senate committee . The New York Times.
Wolfe RM, Sharp LK. Anti-vaccinationists past and present . BMJ. 2002;325(7361):430-2. doi:10.1136/bmj.325.7361.430
Agley J, Xiao Y. Misinformation about COVID-19: Evidence for differential latent profiles and a strong association with trust in science . BMC Public Health. 2021;21:89. doi:10.1186/s12889-020-10103-x
Centers for Disease Control and Prevention. Measles history .
Hussain A, Ali S, Ahmed M, Hussain S. The anti-vaccination movement: a regression in modern medicine . Cureus . 2018;10(7): e2919. doi:10.7759/cureus.2919
Bramer CA, Kimmins LM, Swanson R, et al. Decline in child vaccination coverage during the COVID-19 pandemic — Michigan Care Improvement Registry, May 2016–May 2020 . MMWR. 2020 May;69(20):630-1. doi:10.15585/mmwr.mm6920e1
Centers for Disease Control and Prevention. Why vaccinate .
Centers for Disease Control and Prevention. Poliomyelitis .
Centers for Disease Control and Prevention. Making the vaccine decision .
Borgen Project. What is the cost of measles in the developed world? .
By Vincent Iannelli, MD Vincent Iannelli, MD, is a board-certified pediatrician and fellow of the American Academy of Pediatrics. Dr. Iannelli has cared for children for more than 20 years.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 107, Issue 3
- Should children be vaccinated against COVID-19?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-2388-4318 Petra Zimmermann 1 , 2 , 3 ,
- http://orcid.org/0000-0002-2395-4574 Laure F Pittet 3 , 4 , 5 ,
- http://orcid.org/0000-0003-1756-5668 Adam Finn 6 , 7 ,
- http://orcid.org/0000-0001-7361-719X Andrew J Pollard 8 , 9 ,
- http://orcid.org/0000-0003-3446-4594 Nigel Curtis 3 , 4 , 10
- 1 Faculty of Science and Medicine , University of Fribourg , Fribourg , Switzerland
- 2 Department of Paediatrics , Fribourg Hospital HFR , Fribourg , Switzerland
- 3 Infectious Diseases Research Group , Murdoch Children’s Research Institute , Parkville , Victoria , Australia
- 4 Department of Paediatrics , The University of Melbourne , Parkville , Victoria , Australia
- 5 Pediatric Infectious Diseases Unit , Geneva University Hospitals and Faculty of Medicine , Geneva , Switzerland
- 6 Bristol Vaccine Centre, School of Clinical Sciences and School of Cellular & Molecular Medicine , University of Bristol , Bristol , UK
- 7 Bristol Royal Hospital for Children , University Hospitals Bristol NHS Foundation Trust , Bristol , UK
- 8 Oxford Vaccine Group, Department of Paediatrics , University of Oxford , Oxford , UK
- 9 NIHR Oxford Biomedical Research Centre , Oxford , UK
- 10 Infectious Diseases Unit , The Royal Children’s Hospital Melbourne , Parkville , Victoria , Australia
- Correspondence to Dr Petra Zimmermann, Faculty of Science and Medicine, University of Fribourg, Fribourg 1700, Switzerland; petra.zimmermann{at}unifr.ch
Whether all children under 12 years of age should be vaccinated against COVID-19 remains an ongoing debate. The relatively low risk posed by acute COVID-19 in children, and uncertainty about the relative harms from vaccination and disease mean that the balance of risk and benefit of vaccination in this age group is more complex. One of the key arguments for vaccinating healthy children is to protect them from long-term consequences. Other considerations include population-level factors, such as reducing community transmission, vaccine supply, cost, and the avoidance of quarantine, school closures and other lockdown measures. The emergence of new variants of concern necessitates continual re-evaluation of the risks and benefits. In this review, we do not argue for or against vaccinating children against COVID-19 but rather outline the points to consider and highlight the complexity of policy decisions on COVID-19 vaccination in this age group.
- child health
- communicable diseases
- epidemiology
Data availability statement
No data are available. N/A.
https://doi.org/10.1136/archdischild-2021-323040
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is already known on this topic?
COVID-19 is generally asymptomatic or mild in children, but can be more severe in those with certain comorbidities.
There is no consensus on whether all healthy children less than 12 years of age should be vaccinated against COVID-19.
Data from COVID-19 vaccine use in this age group will become available in the near future.
What this study adds?
The balance of risks and benefits of COVID-19 vaccination in children is more complex than in adults as the relative harms from vaccination and disease are less well established in this age group.
One of the key arguments for vaccinating children less than 12 years of age, apart from reducing acute illness, is to protect them from long-term consequences of COVID-19; other considerations include population-level factors.
The risks and benefits need continual re-evaluation with the emergence of new variants of concern, and new data on effectiveness and adverse effects.
Introduction
Whether all children should be offered vaccination against SARS-CoV-2 has been controversial in children aged 12–15 years old, and remains so for those under 12 years of age, partly because the balance of risk and benefit in this age group is more complex (see figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Summary of benefits and risks of vaccinating children against COVID-19. PIMS-TS, paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2.
The risk of severe acute COVID-19 in healthy children infected with SARS-CoV-2 is much lower than in adults. 1–10 Two longer term consequences of SARS-CoV-2 infection might therefore be more of a concern in this age group. The first is ‘paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 (PIMS-TS)’, also known as ‘multisystem inflammatory syndrome in children’, an immune-mediated disease that occurs in a small proportion of children 2–6 weeks after being infected with SARS-CoV-2. 11–20 The second is long COVID-19, the persistence of symptoms following SARS-CoV-2 infection, a heterogeneous group of conditions. 21
Aside from potential long-term consequences, other considerations in deciding on COVID-19 vaccine policy for children include safety (both common reactions and rare serious side effects), population-level factors, such as reducing community transmission, vaccine supply, cost of vaccination, the avoidance of quarantine, school closures and other lockdown measures, and the potential impact on routine immunisation programmes.
In this review, we do not argue for or against vaccinating children against COVID-19 but rather outline the points to consider to highlight the complexity of policy decisions on COVID-19 vaccination in this age group.
Benefits and risks of vaccinating children against COVID-19
The main question for implementing any vaccine is ‘do the benefits of the vaccine in preventing the harms of the disease outweigh any known or potential risks associated with vaccination?’ To date, two COVID-19 vaccines have been shown to be effective in children aged 12–17 years, and have been authorised for emergency use and subsequently recommended for this age group in many countries. 22–26 Both vaccines are currently being evaluated in children aged 6 months–12 years and it is likely that emergency authorisation will be sought in this age group soon. Nevertheless, COVID-19 vaccine trials in adolescents so far include less than 4000 participants and appropriately focus on efficacy, immunogenicity and rates of common reactions. 25 26 A phase 2/3 trial in children 5–12 years of age recently reported that a messenger RNA (mRNA) vaccine was safe, well tolerated and induced robust neutralising antibodies. 27 Results from the same trial in children under 5 years of age are expected by the end of 2021. Rare adverse effects are difficult to detect with such sample sizes, and are often seen only after large-scale use. Outside clinical trials, millions of adolescents between 12 and 18 years of age have been vaccinated, including 13 million in the USA. 28 Arguments for and against vaccinating children against COVID-19 are summarised in table 1 .
- View inline
Arguments for and against vaccinating children against COVID-19
Potential benefits of vaccinating children
Protection against covid-19.
COVID-19 is generally a mild disease in children with less than 2% of symptomatic children requiring hospital admission. 1–10 The rate of intensive care admission of hospitalised children ranges between 2% and 13%. 1 7 8 29 30 Higher rates (10%–25%, 31 32 up to 33% in some studies 33 34 ) are reported from the USA. However, these numbers often include children who are hospitalised with COVID-19 and not because of COVID-19, and therefore overestimate the severity. In children and adolescents, the risk of death from SARS-CoV-2 infection is 0.005%, 35 and in those who are hospitalised with COVID-19 it is 0%–0.7%. 1 7 8 29 30 33 34 However, again, these numbers often include children who died with a SARS-CoV-2 infection and not because of it (a recent population-based study showed that only 41% of child deaths reported from SARS-CoV-2 infections were from COVID-19). 35 Therefore, the prevention of SARS-CoV-2 infection is not as strong an argument for vaccinating all healthy children as it is for adults. Nevertheless, this might change if new variants emerge which cause more severe disease in otherwise healthy children.
There are insufficient data to estimate the risk of myocarditis in children and adolescents with COVID-19, although one report from the USA suggested a risk of 876 cases per million. 36 Another study reported an adjusted risk ratio for myocarditis from patients with COVID-19 compared with patients without COVID-19 of 36.8 in children less than 16 years of age and 7.4 in adolescents 16–24 years of age. 37 A third study reported an 8.2-fold increase in myocarditis admissions during the pandemic, but no cases among the 1371 children and adolescents less than 18 years of age. 38 Information on the long-term outcome of myocarditis resulting from SARS-CoV-2 infection (e.g., progression to fibrosis) is currently lacking.
In the USA, with the emergence of the more transmissible Delta variant, a recent rise in infections in children has led to overcrowded hospital and intensive care units. 39 For hospitalised children, intensive care admission and mortality rates are currently stable at 23% and 0.4% 29 – 1.8%, 30 respectively. Of note, this has occurred in settings with low vaccine coverage in adults and suboptimal preventive measures in place. There are no reports indicating an increase in the severity of COVID-19 in children since the Delta variant has become dominant.
At this time, COVID-19 vaccines only have ‘emergency use authorisation’ in children between 12 and 16 years of age, which is for interventions that address a serious or life-threatening condition. It has been argued that, unless children are at high risk of severe COVID-19 because of an underlying condition, it is unclear whether the benefits to the individual outweigh the risks in this age group, and approval through the standard regulatory process should be awaited. 40
There are good reasons to consider offering vaccination to children and adolescents at higher risk of being hospitalised or becoming severely unwell from a SARS-CoV-2 infection, as, in their case, the risk of harm from vaccination is estimated to be lower than the risk of harm from COVID-19. This includes children with neurodisabilities, Down’s syndrome, immunodeficiencies, malignancies, some cardiac, respiratory and renal diseases, obesity and poorly controlled diabetes. 41
The low risk of hospitalisation and death from COVID-19 might not be a good argument against vaccinating against this disease as the risk is similar or even higher than that for other diseases for which vaccines are routinely given, such as varicella, rubella, hepatitis A and influenza. 42 In addition, if a high proportion of children are infected, even a very low rate of severe illness might translate to a high absolute number of cases. Moreover, in low/middle-income countries (LMICs), the impact of COVID-19 in children may be greater due to comorbidities that impact immunity, including diarrhoea, dengue fever, tuberculosis, malnutrition, stunting and anaemia. 33 Similary, in high-income countries, children from deprived and ethnic minority groups are more frequently infected with SARS-CoV-2, which might be due to a greater likelihood of living with unvaccinated adults or in multigenerational and overcrowded households. 43 44 These children have also been reported to have more severe COVID-19 and to more frequently suffer from PIMS-TS. 45–47
Protection against PIMS-TS
The risk of PIMS-TS is low, affecting less than 0.1% of SARS-CoV-2-infected children. Although up to 70% of children with PIMS-TS are admitted to intensive care units, 48 49 almost all patients recover without sequelae. 11–20 48 50 51 Between 79% and 100% of abnormal cardiac findings are reported to resolve within 14–30 days after hospital discharge. 48 52 53 Six months after discharge, 96% of children have a normal echocardiography, and renal, haematological, otolaryngological and neurological abnormalities have largely resolved. 45 However, the long-term consequences of PIMS-TS remain uncertain and the death rate from PIMS-TS is estimated to be 1%–2%. 48 49 There is no evidence to date on whether vaccination protects against PIMS-TS: although by protecting against SARS-CoV-2 infection it may well also protect against post-infectious sequelae; data are needed to confirm this. Since the pathogenesis of PIMS-TS remains unclear, there is also a theoretical risk that antibodies induced by COVID-19 vaccination could cause PIMS-TS, though there is no evidence of this to date.
Protection against long COVID-19
While vaccination prevents infection with SARS-CoV-2 to a degree and thus, presumably, persistent symptoms following the infection, more data are needed to determine accurately the incidence of long COVID-19 in children. 21 Studies to date report a prevalence ranging from 1.2% to 66%. 54–64 However, most of these studies have substantial limitations, including a lack of a clear case definition, the absence of a control group without infection, inclusion of children without laboratory-confirmed SARS-CoV-2 infection, follow-up at arbitrary time points and high non-responder bias. 54–63 65–68 Of the five studies to date that have included controls, 55 59 61 65 two did not find a difference in the prevalence of persistent symptoms between infected and uninfected children. 61 65 This highlights the difficulty of separating COVID-19-related symptoms from those attributable to other factors associated with the pandemic, such as lockdowns and school closures. The three that did find a difference had significant limitations, including potential selection bias due to a high non-responder rate, that could lead to an overestimate of the risk of long COVID-19. 55 59
Prevention of community transmission
Another advantage of vaccinating children is helping decrease transmission and thus reducing severe cases in adults and the risk of new virus variants emerging. As well as reducing disease, COVID-19 vaccines also reduce infection. Initial studies reported that vaccinated individuals who become infected are less likely to transmit the virus due to decreased viral load and duration of virus shedding 69 70 and as a consequence, transmission from vaccinated individual to household contacts is significantly lower 71 (by 50% in one study 69 ). However, more recent studies done since the Delta variant became dominant show similar viral loads in vaccinated and unvaccinated individuals. 72–75
Children, including young children, can transmit SARS-CoV-2. 76 Nonetheless, even though transmission in schools can contribute to the circulation of SARS-CoV-2, 77 the rate of transmission in educational settings is low and index cases are often adults. 78–81 The risk of infection in schools correlates strongly with local community infection rates, which can be reduced by vaccinating older age groups. Nevertheless, the risk of transmission in different age groups and settings might change with the emergence of new virus variants of concern. For the Delta variant, it has been suggested that infected fully vaccinated individuals are as likely to transmit SARS-CoV-2 as infected unvaccinated individuals, although for shorter duration. 82 83 However, recent data from Australia reported a low risk of transmission in educational settings with protection measurements in place, even with the Delta variant (the transmission rate from adults to children was 8%, from children to adults 1.3% and from children to other children 1.8%). 84
Earlier in the pandemic, it was reported that index cases in households were more likely to be a parent or adolescent than a young child. 6 85–87 However, one study suggests that children and adolescents are more likely to infect others. 88 Another study reported that household transmission was more common from children aged 0–3 years than from children aged 14–17 years. 89 However, this might change with the Delta or other new variants. In a population with low numbers of vaccinated adults, infected children transmitted the Delta variant to 70% of households (in 57% of households all members became infected). 84 Nevertheless, once a large proportion of the adult population is vaccinated, preventing transmission to them from unvaccinated children becomes less important. There is a stronger argument for vaccinating children and adolescents who live with immunosuppressed or other high-risk household members, not only for the protection of the latter but also to benefit the mental health of the former. Also, in LMICs children under 12 years of age form a larger proportion of the population and might therefore have a larger role in tranmission.
Another consideration is that, once SARS-CoV-2 becomes endemic, primary SARS-CoV-2 infection in early childhood, when COVID-19 is mild, with subsequent boosting from ongoing exposure at older ages, may bring about population immunity, as seen with common circulating coronaviruses, more effectively than mass immunisation. 90
Avoidance of indirect (population-level) harms
Vaccinating children and adolescents might help reduce the indirect harms caused by quarantine, lockdowns, repeat testing, school exclusion and closures, and other policies aimed at reducing community transmission, although the extent to which mass vaccination is necessary to achieve this remains unclear. Also, if the purpose of lockdowns and school closures is to protect adults, the incremental benefit of vaccinating children will be minimal once most adults are protected through vaccination. The possibility that vaccination might become a requirement for children for international travel is another consideration.
Potential risks of vaccinating children
Risk of adverse effects.
As with any vaccine, there are potential rare adverse effects of COVID-19 vaccines. The development of myocarditis or pericarditis after mRNA vaccines has been a recent concern, 91 92 particularly in male adolescents (studies reporting 6.3–6.7 cases per 100 000 second vaccine doses in males aged 12–17 years, 91 93 and 15.1 cases per 100 000 second vaccine doses in males aged 16–19 years 94 ). Another study reported an incidence of 10.7 cases per 100 000 persons in males aged 16–29 years. 95 Of these patients, approximately 6% required intensive care admission. 96 However, most recovered without sequelae (86% had resolution of symptoms after mean duration of 35 days). 97 98 Importantly, even in this age group, recent reports suggest the risk of myocarditis associated with COVID-19 is higher (see above).
The risk of thrombosis after viral vector vaccines observed rarely in adults also needs to be considered. The thrombotic risk in children or adolescents is less 99 and no cases have been reported to date in this age group. However, since the pathogenesis underlying thrombosis associated with COVID-19 vaccines is thought to differ from that for clots from other causes, such as stasis and the contraceptive pill, further data from children are necessary. As thrombotic events have either not been observed or appear to be very rare in Asia, Africa and Latin America, some countries are considering these vaccines as an option. The theoretical risk of COVID-19 vaccines triggering PIMS-TS has been raised but there are no reports of this to date. 100
Long-term safety
The lack of long-term safety data is another consideration. Longer term follow-up of myocarditis cases is needed to exclude any possibility of myocardial fibrosis and associated dysfunction or arrhythmia risk. Two studies showed a high prevalence of late gadolinium enhancement in MRIs in patients suffering from post-vaccine myocarditis. 97 101 Further studies are needed to establish whether this resolves or evolves into fibrosis. As discussed above, information on this risk is also needed for myocarditis resulting from SARS-CoV-2 infection.
Although the majority of adverse vaccine effects occur early after vaccination, any unforeseen adverse effects could undermine vaccine confidence and reduce vaccination rates against other diseases. 102
Vaccine supply
The currently limited global COVID-19 vaccine supply is another factor to consider. To date, many LMICs have only been able to vaccinate less than 5% of their population despite the COVAX programme. At this time, available supplies might be better prioritised for vaccinating adults with a higher risk of severe COVID-19 and death, including healthcare workers. 103 Another consideration is the higher immunogenicity of mRNA vaccines in children, meaning that one dose or a reduced dose might be sufficient to protect this age group. 25 On the other hand, the infrastructure to upscale the production of COVID-19 vaccines already exists and strategies for boosting global supply have been outlined. 104
Since the risks of intensive care admission or death in children are so low, the cost–benefit ratio of COVID-19 vaccination in children is higher. However, the emergence of new variants might change this if these variants cause more frequent or more severe disease in children. 105 The cost of vaccination also needs to be balanced against the reduction in community transmission that might be achieved through vaccinating children, which would enable a faster return to pre-pandemic economic stability with associated benefits to children.
Other immunisation programmes
Routine immunisation programmes for children and adolescents have been disrupted by the pandemic. 106 107 Implementing a universal COVID-19 vaccine programme for these age groups runs the risk of causing further delays by using up existing delivery resources and personnel. This in turn may harm children by resulting in more cases of vaccine-preventable infections and diseases such as cervical cancer, meningitis, measles and pertussis. However, if COVID-19 vaccination is combined with the administration of other routine vaccines, this problem might be reduced.
Concluding remarks
In summary, the case for vaccinating all healthy children against COVID-19 is more difficult than for adults as the balance of risks and benefits is more nuanced. If COVID-19 remains a generally mild disease in children and in vaccinated adults, it may not be necessary to vaccinate all children. 90 108 In addition, it is important to consider different age groups separately; the balance of risk and benefit of vaccination is likely to differ between infants, young children and adolescents. Children under 5 years of age are likely to need separate consideration to those 5–11 years of age. Continued monitoring of disease severity across all age groups is crucial. If a variant of concern emerges with increased severity in children (as is, for example, the case for Middle East respiratory syndrome-related coronavirus), this would alter the risk–benefit equation. 90 In LMICs, where the burden of COVID-19 is higher in the paediatric population as a result of comorbidities, there may be a lower threshold for vaccinating children. A one-dose schedule (as now recommended in the UK and Norway) 109 110 or a reduced-dose vaccine might be an option for this age group; this might also reduce the risk of myocarditis with the second dose of mRNA vaccines. Although mass COVID-19 vaccination of all ages, including children under 12 years of age, may become the general approach globally in the future, it seems wise at present to weigh up the risks and benefits with caution and to proceed with care.
Ethics statements
Patient consent for publication.
Not required.
- Götzinger F ,
- Santiago-García B ,
- Noguera-Julián A , et al
- Zimmermann P ,
- Castagnoli R ,
- Licari A , et al
- Ludvigsson JF
- Buettcher M ,
- Bernhard-Stirnemann S
- Harwood R ,
- ↵ World Health Organization. COVID-19 detailed surveillance data dashboard . Available: https://covid19.who.int [Accessed 27 July 2021 ].
- Stokes EK ,
- Zambrano LD ,
- Anderson KN , et al
- Verdoni L ,
- Gervasoni A , et al
- Whittaker E
- Toubiana J ,
- Poirault C ,
- Corsia A , et al
- Riphagen S ,
- Gonzalez-Martinez C , et al
- Moraleda C ,
- Serna-Pascual M ,
- Soriano-Arandes A
- Cheung EW ,
- Zachariah P ,
- Gorelik M , et al
- Whittaker E ,
- Bamford A ,
- Kenny J , et al
- Godfred-Cato S , et al
- Pittet LF ,
- European Medicines Agency
- National Institute for Health Research
- National Advisory Committee on Immunization
- Frenck RW ,
- Kitchin N , et al
- Zhou H , et al
- Alatovic J , et al
- Centers for Disease Control and Prevention
- Du H , et al
- Bhuiyan MU ,
- Hassan MZ , et al
- Delahoy MJ ,
- Whitaker M , et al
- Siegel DA ,
- Cool AJ , et al
- Havers FP ,
- Whitaker M ,
- Self JL , et al
- O'Halloran A , et al
- Singer ME ,
- Boehmer TK ,
- Kompaniyets L ,
- Lavery AM , et al
- Gierada M ,
- Fralick M , et al
- American Academy of Pediatrics
- Fernandes DM ,
- Oliveira CR ,
- Guerguis S , et al
- Anderson EJ ,
- Campbell JD ,
- Creech CB , et al
- Ogunyemi D ,
- Mantilla R ,
- Markus A , et al
- Vahidy FS ,
- Nicolas JC ,
- Meeks JR , et al
- Abdel-Mannan O ,
- Grant K , et al
- Kanthimathinathan HK , et al
- Brighouse J , et al
- Feldstein LR ,
- Tenforde MW ,
- Friedman KG , et al
- Oster ME , et al
- Shingleton J ,
- Bennett E , et al
- Riollano-Cruz M ,
- Akkoyun E ,
- Briceno-Brito E , et al
- Minocha PK ,
- Phoon CKL ,
- Verma S , et al
- Ramcharan T ,
- Lai CY , et al
- Osmanov IM ,
- Spiridonova E ,
- Navaratnam AMD
- Molteni E ,
- Canas LS , et al
- Buonsenso D ,
- Munblit D ,
- De Rose C , et al
- Crawford N ,
- McNab S , et al
- Stephenson T ,
- Shafran R ,
- De Stavola B , et al
- Olsson-Åkefeldt S ,
- Hertting O , et al
- Puhan MA , et al
- Blomberg B ,
- Mohn KG-I ,
- Brokstad KA , et al
- Pucuka Z , et al
- Ayoubkhani D ,
- Pawelek P ,
- Blankenburg J ,
- Wekenborg MK ,
- Espuny Pujol F ,
- Brackel CLH ,
- Buddingh EP , et al
- Ashkenazi-Hoffnung L ,
- Shmueli E ,
- Harris RJ ,
- Zaidi A , et al
- Levine-Tiefenbrun M ,
- Shah AS V ,
- Gribben C ,
- Riemersma KK ,
- Grogan BE ,
- Kita-Yarbro A
- Musser JM ,
- Christensen PA ,
- Johnson H , et al
- Xiang Ong SW ,
- Yousaf AR ,
- Chang K , et al
- Litvinova M ,
- Liang Y , et al
- Ismail SA ,
- Lopez Bernal J , et al
- National Centre for Immunisation Research and Surveillance NCIRS
- National Institute for Public Health and the Environment, Netherlands
- Ladhani SN ,
- Baawuah F ,
- Beckmann J , et al
- Public Health England
- McLean HQ ,
- Grijalva CG ,
- Hanson KE , et al
- Siebach MK ,
- Piedimonte G ,
- Maltezou HC ,
- Magaziotou I ,
- Dedoukou X , et al
- Liu M-J , et al
- Daneman N ,
- Schwartz KL , et al
- Lavine JS ,
- Bjornstad ON ,
- Gargano JW ,
- Wallace M ,
- Hadler SC , et al
- Israelian Ministry of Health
- Bozkurt B ,
- Mevorach D ,
- Cedar N , et al
- Witberg G ,
- Hoss S , et al
- Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention
- Steele JM ,
- Fonseca B , et al
- Goddard K , et al
- Ignjatovic V ,
- Blumenthal JA ,
- Sperotto F ,
- Chamberlain S , et al
- Our World in Data
- Castillo JC ,
- Athey S , et al
- ↵ American Academy of Pediatrics . Available: https://protect-au.mimecast.com/s/kTiQCp8AxKsn7BoR2HPuc2Q?domain=downloads.aap.org [Accessed 10 Aug 2021 ].
- Santoli JM ,
- Lindley MC ,
- DeSilva MB , et al
- Centers for Disease Control and Prevention, Messonnier N
- Coleman PG ,
- Woolhouse ME
- UK joint Committee on vaccination and immunisation
- Norwegian Institute of Public Health
- Mizumoto K ,
- Milani GP ,
- Bottino I ,
- Rocchi A , et al
- Mytton OT ,
- Bonell C , et al
- Zhang Z-B , et al
- Szablewski CM ,
- Brown MM , et al
- Russell S ,
- Medicines and Healthcare products Regulatory Agency
Twitter @Dr_Petzi, @PittetLaure, @adamhfinn, @ajpollard1, @nigeltwitt
Contributors PZ drafted the initial manuscript. All authors critically revised the manuscript and approved the final manuscript as submitted.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer The views expressed in this article do not necessarily represent the views of the DHSC, JCVI, NIHR or WHO.
Competing interests AJP is chair of UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI), but does not participate in policy decisions on COVID-19 vaccine. He is a member of the WHO’s SAGE. AJP is chief investigator on clinical trials of Oxford University’s COVID-19 vaccine funded by NIHR. Oxford University has entered a joint COVID-19 vaccine development partnership with AstraZeneca. AF is an investigator in trials and studies of COVID-19 vaccines manufactured by Pfizer-BioNTech, AstraZeneca, Janssen, Valneva and Sanofi but receives no personal remuneration or benefits for this work. He is a member of the UK Joint Committee on Vaccination and Immunisation and chairs the WHO Euro Regional Technical Advisory Group of Experts (ETAGE) on immunisation.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Persuasive messaging to increase COVID-19 vaccine uptake intentions
Affiliations.
- 1 Yale Institute for Global Health, New Haven, CT, USA; Department of Internal Medicine, Section of Infectious Diseases, Yale School of Medicine, New Haven, CT, USA.
- 2 Institution for Social and Policy Studies, Yale University, New Haven, CT, USA; Center for the Study of American Politics, Yale University, New Haven, CT, USA.
- 3 Institution for Social and Policy Studies, Yale University, New Haven, CT, USA; Center for the Study of American Politics, Yale University, New Haven, CT, USA; Department of Political Science, Yale University, New Haven, CT, USA.
- 4 Yale Institute for Global Health, New Haven, CT, USA; Department of Internal Medicine, Section of Infectious Diseases, Yale School of Medicine, New Haven, CT, USA; Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, USA; Yale School of Nursing, West Haven, CT, USA.
- 5 Institution for Social and Policy Studies, Yale University, New Haven, CT, USA; Center for the Study of American Politics, Yale University, New Haven, CT, USA; Department of Political Science, Yale University, New Haven, CT, USA. Electronic address: [email protected].
- PMID: 34774363
- PMCID: PMC8531257
- DOI: 10.1016/j.vaccine.2021.10.039
Widespread vaccination remains the best option for controlling the spread of COVID-19 and ending the pandemic. Despite the considerable disruption the virus has caused to people's lives, many people are still hesitant to receive a vaccine. Without high rates of uptake, however, the pandemic is likely to be prolonged. Here we use two survey experiments to study how persuasive messaging affects COVID-19 vaccine uptake intentions. In the first experiment, we test a large number of treatment messages. One subgroup of messages draws on the idea that mass vaccination is a collective action problem and highlighting the prosocial benefit of vaccination or the reputational costs that one might incur if one chooses not to vaccinate. Another subgroup of messages built on contemporary concerns about the pandemic, like issues of restricting personal freedom or economic security. We find that persuasive messaging that invokes prosocial vaccination and social image concerns is effective at increasing intended uptake and also the willingness to persuade others and judgments of non-vaccinators. We replicate this result on a nationally representative sample of Americans and observe that prosocial messaging is robust across subgroups, including those who are most hesitant about vaccines generally. The experiments demonstrate how persuasive messaging can induce individuals to be more likely to vaccinate and also create spillover effects to persuade others to do so as well. The first experiment in this study was registered at clinicaltrials.gov and can be found under the ID number NCT04460703 . This study was registered at Open Science Framework (OSF) at: https://osf.io/qu8nb/?view_only=82f06ecad77f4e54b02e8581a65047d7.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- COVID-19 Vaccines*
- United States
- Vaccination
- COVID-19 Vaccines
Associated data
- ClinicalTrials.gov/NCT04460703
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Examining persuasive message type to encourage staying at home during the COVID-19 pandemic and social lockdown: A randomized controlled study in Japan
- • We examined persuasive message types in terms of a narrator encouraging self-restraint.
- • Messages from a governor, an expert, a physician, a patient, and a resident were compared.
- • The message from a physician increased intention to stay at home the most.
- • The physician’s message conveyed the crisis of collapse of the medical system.
Behavioral change is the only prevention against the COVID-19 pandemic until vaccines become available. This is the first study to examine the most persuasive message type in terms of narrator difference in encouraging people to stay at home during the COVID-19 pandemic and social lockdown.
Participants (n = 1,980) were randomly assigned to five intervention messages (from a governor, a public health expert, a physician, a patient, and a resident of an outbreak area) and a control message. Intention to stay at home before and after reading messages was assessed. A one-way ANOVA with Tukey’s or Games–Howell test was conducted.
Compared with other messages, the message from a physician significantly increased participants’ intention to stay at home in areas with high numbers of people infected (versus a governor, p = .002; an expert, p = .023; a resident, p = .004).
The message from a physician―which conveyed the crisis of overwhelmed hospitals and consequent risk of people being unable to receive treatment―increased the intent to stay at home the most.
Practice implications
Health professionals and media operatives may be able to encourage people to stay at home by disseminating the physicians’ messages through media and the internet.
1. Introduction
The outbreak of the coronavirus disease 2019 (COVID-19) has emerged as the largest global pandemic ever experienced [ 1 ]. Experts have proposed that social lockdown will lead to improvements such as controlling the increase in the number of infected individuals and preventing a huge burden on the healthcare system [ [2] , [3] , [4] ]. Governments of many countries across the world have declared local and national social lockdown [ 4 , 5 ]. In April 2020, the Japanese government declared a state of emergency, which allows prefectural governors to request residents to refrain from unnecessary and nonurgent outings from home [ 6 ]. However, despite such governor declarations, people in various countries have resisted and disregarded calls to stay at home [ [7] , [8] , [9] ]. Because social lockdown is the only existing weapon for prevention of the pandemic until vaccines becomes available to treat COVID-19, behavioral change in individuals regarding staying at home is crucial [ 3 , 4 ]. Many news articles about COVID-19 are published daily by the mass media and over the internet. Such articles convey messages from governors, public health experts, physicians, COVID-19 patients, and residents of outbreak areas, encouraging people to stay at home. This is the first study to examine which narrator’s message is most persuasive in encouraging people to do so during the COVID-19 pandemic and social lockdown.
2.1. Participants and design
Participants were recruited from people registered in a survey company database in Japan. The eligibility criterion was men and women aged 18–69 years. Exclusion criteria were individuals who answered screening questions by stating: that they cannot go out because of illness or disability; that they have been diagnosed with a mental illness; or/and that they or their family members have been infected with COVID-19. A total of 1,980 participants completed the survey from May 9–11, 2020, when the state of emergency covered all prefectures in Japan. Participants were included according to the population composition ratio in Japan nationwide by gender, age, and residential area. Participants were randomly assigned either to a group that received an intervention message (i.e., from a governor, a public health expert, a physician, a patient, and a resident of the outbreak area) or to one that received a control message. The study was registered as a University Hospital Medical Information Network Clinical Trials Registry (number: UMIN000040286) on May 1, 2020. The methods of the present study adhered to CONSORT guidelines. The protocol was approved by the ethical review committee at the Graduate School of Medicine, University of Tokyo (number: 2020032NI). All participants gave written informed consent in accordance with the Declaration of Helsinki.
2.2. Intervention and control messages
We searched news articles about COVID-19 using Yahoo! JAPAN News ( https://news.yahoo.co.jp ), the largest Japanese news portal site. We also searched videos posted by residents of outbreak areas such as New York using YouTube ( https://www.youtube.com/user/YouTubeJapan ). By referring to these articles and videos, we created five intervention messages from a governor, a public health expert, a physician, a patient, and a resident of an outbreak area. The content of each message encouraged readers to stay at home. We included threat and coping messages in each intervention message based on protection motivation theory (PMT) [ 10 , 11 ]. Appendix A shows the five intervention messages used in this study, translated into English for this report. For a control message we obtained textual information about bruxism from the website of the Ministry of Health, Labour and Welfare ( https://www.e-healthnet.mhlw.go.jp/ ).
2.3. Measures
The primary outcome was intention to stay at home. The secondary outcomes were PMT constructs (i.e., perceived severity, vulnerability, response efficacy, and self-efficacy). Participants responded to two or three questions for each measure (see Appendix B ). These measures were adapted and modified from previous studies [ [12] , [13] , [14] , [15] ]. All primary and secondary outcomes were measured before and after the participants read intervention or control messages, and mean scores were calculated. Higher scores indicated greater intention and perception. All participants were asked for their sociodemographic information before they read intervention or control messages.
2.4. Sample size
Based on the effect size in a previous randomized controlled study [ 16 ], we estimated a small effect size (Cohen’s d = .20) in the current study. We conducted a power analysis at an alpha error rate of .05 (two-tailed) and a beta error rate of .20. The power analysis indicated that 330 participants were required in each of the intervention and control groups.
2.5. Statistical analysis
A one-way analysis of variance (ANOVA) was conducted with the absolute change in mean values for each measure before and after intervention as the dependent variable and the group assignment as the independent variable. For multiple comparisons, Tukey’s test was conducted on significant main effects where appropriate. The Games–Howell test was performed when the assumption of homogeneity of variances was not satisfied. Additionally, we conducted subgroup analyses including only participants who lived in 13 “specified warning prefectures,” where the number of infected individuals showed a marked increase [ 17 ]. A p value of <.05 was considered significant in all statistical tests. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM, Armonk, NY, USA).
Table 1 shows the participants’ characteristics. Table 2 , Table 3 present a comparison among the five intervention groups using one-way ANOVA and multiple comparisons when including all prefectures and only participants who lived in the specified warning prefectures, respectively. More significant differences between intervention messages were found in the specified warning prefectures compared with all prefectures. In Table 3 , the Games–Howell test indicates that the message from a physician increased participants’ intention to stay at home significantly more than other narrators’ messages (versus a governor, p = .002; an expert, p = .023; a resident, p = .004). Multiple comparisons demonstrated that the message from a physician increased participants’ perceived severity (versus a governor, p = .015), response efficacy (versus a resident, p = .014), and self-efficacy (versus a governor, p = .022; a patient, p = .009) significantly more than other narrators’ messages.
Participants’ sociodemographic information.
Comparison of amount of change before and after intervention among groups when including all prefectures (N = 1,980).
Comparison of amount of change before and after intervention among groups when including only the “specified warning prefectures” (N = 1,274).
4. Discussion and conclusion
4.1. discussion.
As Appendix A shows, the message from a physician specifically communicated the critical situation of hospitals being overwhelmed and the consequent risk of people being unable to receive treatment. Depiction of the crisis of overwhelmed hospitals may have evoked heightened sensation that elicited sensory, affective, and arousal responses in recipients. Social lockdown presumably evoked psychological reactance in many individuals [ 18 ]. Psychological reactance is considered one of the factors that impedes individuals’ staying at home during a pandemic [ 18 ]. Studies of psychological reactance have indicated that heightened sensation is the feature of a message that reduces psychological reactance [ 19 , 20 ]. Additionally, in Japan recommendations by physicians have a strong influence on individuals’ decision making owing to the remnants of paternalism in the patient–physician relationship [ 21 ]. These may constitute the reasons for the message from a physician generating the greatest impact on recipients’ protection motivation.
Public health professionals, governors, media professionals, and other influencers should use messages from physicians and disseminate relevant articles through the media and social networking services to encourage people to stay at home. It is important that health professionals and media have a network and collaborate with one another [ 22 ]. To build relationships and provide reliable resources, health professionals are expected to hold press conferences and study meetings with journalists. Through such networking, journalists can acquire accurate information in dealing with the pandemic, such as using messages from physicians to encourage people to stay at home. Consequently, journalists should disseminate such messages. It is also important that governments, municipalities, medical associations, and other public institutions convey messages from physicians and that the media effectively spread those messages. Owing to the advances of Web 2.0 [ 23 ], health professionals’ grassroots communication with journalists and citizens via social media may provide opportunities for many people to access persuasive messages from physicians.
4.1.1. Limitations
First, the content of the intervention messages in this study may not represent voices of all governors, public health experts, physicians, patients, and residents of outbreak areas. Second, it is not clear from this study which sentences in the intervention message made the most impact on recipients and why. Third, this study assessed intention rather than actual behavior. Finally, it is unclear as to what extent the present findings are generalizable to populations other than the Japanese participants in this study.
4.2. Conclusion
In areas with high numbers of infected people, the message from a physician, which conveyed the crisis of hospitals being overwhelmed and the consequent risk of people being unable to receive treatment, increased the intention to stay at home to a greater extent than other messages from a governor, a public health expert, a patient with COVID-19, and a resident of an outbreak area.
4.3. Practice implications
Governors, health professionals, and media professionals may be able to encourage people to stay at home by disseminating the physicians’ messages through media such as television and newspapers as well as social networking services on the internet.
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant number 19K10615).
CRediT authorship contribution statement
Tsuyoshi Okuhara: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Funding acquisition. Hiroko Okada: Methodology, Investigation, Writing - review & editing. Takahiro Kiuchi: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgement
We thank Hugh McGonigle, from Edanz Group ( https://en-author-services.edanzgroup.com/ac ), for editing a draft of the manuscript.
Appendix A.
Intervention: the message from a governor.
The following is a message from the governor of your local area.
Please avoid leaving your house as much as possible.
Staying at home can save lives and prevent the spread of infection.
Intervention: The message from an expert
The following is a message from an infectious disease control expert.
Intervention: The message from a physician
The following is a message from an emergency medical care doctor.
Intervention: The message from a patient
The following is a message from a patient who is infected with the novel coronavirus.
Intervention: The message from a resident
The following is a message from an individual who lives in an area where an outbreak of novel coronavirus has occurred.
A control message
According to the traditional definition, grinding one’s teeth is when somebody makes a sound by strongly grinding the teeth together, usually unconsciously or while asleep. Nowadays, it is often referred to as ‘teeth grinding,’ a term which also covers various actions that we do while awake.
Whether you are sleeping or awake, the non-functional biting habit of grinding one’s teeth dynamically or statically, or clenching one’s teeth, can also be referred to as bruxism (sleep bruxism if it occurs at night). Bruxism can be categorized into the movements of: sliding the upper and lower teeth together like mortar and pestle (grinding); firmly and statically engaging the upper and lower teeth (clenching); and dynamically bringing the upper and lower teeth together with a tap (tapping).
Bruxism is difficult to diagnose, as it often has no noticeable symptoms. Stress and dentition are thought to be causes of bruxism, but it is currently unclear and future research is anticipated.
Splint therapy, which involves the use of a mouthpiece as an artificial plastic covering on one’s teeth, and cognitive behavioral therapy are being researched as treatments for bruxism.
Appendix B.
All questions above were on a scale of 1–6, ranging from “extremely unlikely” to “unlikely,” “a little unlikely,” “a little likely,” “likely,” and “extremely likely.”
- Share full article
Advertisement
Subscriber-only Newsletter
David Wallace-Wells
The new age of d.i.y. medicine.

By David Wallace-Wells
Opinion Writer
“Cavities are a communicable disease, and if you’re among the 90 percent of Americans who’s ever had one, you probably got them from your mother.”
So begins “The Rise and Impending Fall of the Dental Cavity,” a remarkably engrossing and, for me, genuinely eye-opening survey of the history and science of tooth decay, published last week by the pseudonymous Cremieux Recueil in a Substack newsletter. The bacterium Streptococcus mutans might not seem like the likeliest subject for a 7,600-word general-interest deep dive, but Recueil takes detours into the immaculate teeth of dinosaurs, the practice of Neolithic dentistry, the agricultural and industrial revolutions and their effect on our diets and the dental agony of America’s founding fathers.
His essay is a kind of masterpiece of an emergent form of internet argumentation — one that has roots in the blogosphere and the message-board culture of an earlier era but really flowered in the pandemic years: extremely long, exhaustively researched, often compiled by obsessive nonexperts and aimed at a contrarian lesson about public health, say, or educational achievement or the origins of the pandemic. For me, the archetypal example is probably the 10-part investigation , with nine “interludes,” into the causes of American obesity published in 2021 by a pair of anonymous researchers, calling themselves Slime Mold Time Mold, who proposed environmental contamination of our water table by the runoff of the mood stabilizer lithium as the driver of the country’s skyrocketing body mass index and have since undertaken the staging of a large-scale, self-supervised community trial of what they call the “potato diet.”
In this case, the lesson was about what is going on in the bacteria pools we call mouths and what we could do to clean them up. Probably, you remember admonitions from childhood that eating candy will rot your teeth, but that story turns out to be a bit simplistic: The problem isn’t that your teeth hate sugar but that Streptococcus mutans loves it. And when it consumes sugar, the byproduct is lactic acid, which is what really starts to eat away at your dental enamel. Not everyone has an oral microbiome dominated by Streptococcus mutans, but chances are if you do, it was passed to you by your parents, very early on — and if you eat any sugar, you’re very likely to suffer tooth decay.
In places like the United States — where drugs are advertised directly to consumers, pharmacies are lined with whitening toothpaste and yuppie dentists hawk Invisalign between fillings — you might have come to see oral health as primarily a cosmetic matter. (Perhaps, given the costs, even a scam.) But probably a quarter of Americans and more than a third of the world have untreated cavities or tooth decay, and there is an awful lot of science linking oral hygiene with overall health and well-being. The connections are both direct (untreated cavities can host infections, which can spread elsewhere in the body, causing cellulitis and osteomyelitis, among other infections, and other forms of oral bacteria have been linked to colon and colorectal cancers) and indirect (tooth loss is correlated with higher all-cause mortality, with studies of large-scale tooth loss finding large increases in all-cause mortality risk). That’s one reason, over the past few decades, there have been periodic efforts to develop a vaccine for tooth decay, focused on Streptococcus mutans.
But “Impending Fall” was not prompted by an F.D.A. approval of such a vaccine, a successful large-scale clinical trial or even news of such a trial getting underway. It wasn’t even occasioned by the publication of new academic research or a new book. Instead, it referred to the rollout of a new product called Lumina, conceived by the start-up Lantern Bioworks and sold to customers directly as a probiotic supplement — and an opportunity to participate in something more like a health-care version of a beta-test soft launch.
“Lumina does not have F.D.A. approval, the endorsement of major scientific organizations or any of the other trappings that sound medicine is supposed to have,” acknowledged Richard Hanania — a prominent anti-woke, pro-vaccine Substack provocateur whose views on race my colleague Jamelle Bouie called “rancid” and whose furious history of social justice was endorsed by Peter Thiel, Tyler Cowen and Vivek Ramaswamy, among others — reflecting on his decision to “let some genetically engineered bacteria colonize my mouth.” So why had he done this?
He wasn’t motivated by the science or even the reputation of the company’s scientists. “The real reason I brushed my teeth with Lumina,” he writes, “was Scott Alexander told me to.”
Alexander, if you don’t know, is the nom de plume of one of the tech world’s most prominent public intellectuals — a Bay Area psychiatrist who used to publish a website of Rationalist musings and investigations called Slate Star Codex and now publishes one called Astral Codex Ten — including, recently, a 15,000-word summary of a 15-hour debate about the origins of the pandemic and a persuasive defense of the utilitarian-philanthropic movement effective altruism . Like Hanania, Alexander was supportive of vaccines and was cautious about Covid, though he has been critical of the F.D.A., which he believes could’ve brought us those vaccines much more quickly. In other words, though his worldview can skew right, he is not exactly the conventional liberal stereotype of the anti-science crusader but much closer to its sociological opposite. And in December he published a long FAQ-style interview with the founder of Lantern Bioworks, which amounted to a kind of endorsement, though Alexander was typically careful to avoid making claims about Lumina’s efficacy, given that it has not endured conventional clinical trials, and to disclose his conflicts of interests (his friends at the company and the consulting work his wife did for it).
To a lay reader like me, the idea appears promising: a $250 one-time treatment to crowd out the bacteria that are in my mouth now, producing lactic acid anytime I eat sugar, with an engineered variety that will not. (The process is a bit like gene drive proposals to outbreed disease-carrying mosquitoes with varieties that can’t harbor malaria or dengue or other diseases menacing to humans.) But it is nevertheless disorienting to find myself, reading about Lumina, in the position to decide, on my own, whether it’s worth it or safe to give a novel bacterium a permanent home in my microbiome. (“Once you use it, it’s in your mouth approximately forever,” Alexander writes.) And to thereby undertake what is essentially an unproven and untested treatment without any traditional reassuring oversight. (As Ruxandra Teslo puts it , “Most of the direct data comes from small studies in rats, and well … most humans are not rats.”)
And yet this position is an increasingly common one, at least in certain corners of the internet, especially since Covid upended an awful lot about not just our lives and our health but also the structure of our epistemic faith. Suddenly, groceries weren’t safe, and then they were; parks weren’t, either, and then they were; masks didn’t work, and then they did, and then maybe they didn’t again. Early on, especially, guidance seemed to shift almost by the week, which emboldened certain people to try to sort it all out for themselves, while others languished in sometimes fearful confusion. In 2020 there were those who placed their pandemic bets on ivermectin and hydroxychloroquine, infamously, but also those hawking vitamin D as a Covid fix and those who didn’t want to wait for the conclusion of clinical trials and instead assembled their own versions of the Covid vaccines, administering them in the form of nasal sprays. The pandemic’s rise of at-home test kits was not just about Covid-19 — which, in fact, became widely available only after many months of regulatory and messaging obstacles — but also about Lyme disease, hormone levels, S.T.I.s, menopause, vitamin D, DNA sequencing, thyroid function and many other health indicators. By 2021, a majority of states had passed laws restricting public health authorities from taking actions against future pandemics. In the aftermath of the Covid emergency, the country’s biggest pharmaceutical and perhaps biomedical story has been the spectacular rise of Ozempic, which technically hasn’t even gotten F.D.A. approval for weight loss (though its semaglutide cousin Wegovy has).
Off-label use is nothing new, of course; some estimates suggest up to one-third of prescriptions for common drugs in the United States may be written for purposes other than those originally intended. And skepticism about the American medical establishment didn’t begin with the pandemic, given decades of hostility toward the authorities like the F.D.A. and an even longer national infatuation with quick fixes, “secret knowledge” and quack cures. But in part because no one was happy with how the pandemic went and because everyone wanted to believe it would have been easy to handle it better, it did help cultivate what my colleague Michelle Goldberg has called “a coalition of the distrustful” — an anarchic sort of D.I.Y. health and wellness counterestablishment, one that mixes disdain for much conventional wisdom with great faith in the ability of smart people on the internet to do better. “Substackism,” Max Read recently called it, on his own Substack.
“The anti-woke wellness corner of Substack is just one portion of a large and loose network of influencers, podcasters, gurus, scientists, pseudoscientists, quacks, dietitians and scammers,” Read wrote this month. “What links all of these diverse content producers together is less a particular level (or absence) of scientific rigor or expertise (sometimes these guys are absolutely correct!) and more an outsider attitude — a mistrust of institutions.”
To most Americans, not just on the right, this all feels not just familiar but intuitive: that the apparent stumbles of our public health establishment through the past few years would produce distrustful backlash against elite authorities. And there were missteps and mistakes: early on about testing kits and aerosol spread, in the middle of the pandemic about natural immunity and breakthrough infections and in the post-emergency phase about the universal value of Paxlovid, for instance, which, one recent study suggests, may offer little to no clinical benefit for the fully vaccinated.
But it is also the case that those elites and that establishment produced in the same period what is not just the great success of the pandemic but probably the most impressive public-health achievement in a generation: the record-time design, production, clinical testing and delivery of an entirely new kind of vaccine, which, when rolled out less than a year after the country’s first publicly identified Covid case, saved the lives of several million Americans and more than 10 million people living abroad.
How should we balance those mistakes and those contributions? Or the pandemic wisdom of the establishment against those who promised that Covid would kill only a few thousand Americans or who believe the pandemic experience was an unequivocal indictment of the scientific establishment? And how far should we take the argument that the F.D.A. could often move faster?
Not everyone who doubts the wisdom of the F.D.A. is calling for its abolition, of course, and not everyone who’s wondering about the wisdom of seasonal Covid booster shots for the young believes that it would be good if parents seeking shots for their kids were reported to children’s services. Not everyone who was skeptical of those shots at the outset believes they have already killed millions. And not everyone who thinks American schools were closed too long believes those closings were a bigger public policy failure than the Iraq war. But for many, the pandemic experience does seem to have been a kind of gateway drug, and it isn’t just among anti-vaxxers that Covid cynicism has given way to a new age of medical libertarianism. In certain corners, it has come to look almost like a second mainstream, at least as sure of itself as the first.

IMAGES
VIDEO
COMMENTS
The Impact of the Covid-19 Pandemic on the Australian Economy Disadvantages of Covid-19 Vaccine Essay Analysis of Media in Response to the Global COVID-19 Pandemic Pros and Cons of Vaccinations: Argumentative Essay Death by Shot: Argumentative Essay on Vaccines Essay on Vaccines: Outline Why You Should Get Vaccinated Persuasive Essay Parental ...
Discussion. Although vaccines demonstrate effectiveness against this disease, vaccine hesitancy reveals concerns towards short-term and long-term side effects or adverse reactions such as post-inoculation death. Mandatory vaccination is used to provide herd immunity, but is refutable due to infringement of human rights and autonomy.
Examples of Persuasive Essay About Covid-19 Vaccine. Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your ...
The subsequent paragraphs are going to provide detailed explanations of the reasons why it is necessary for the COVID-19 vaccine to be taken. Firstly, the COVID-19 vaccine is necessary because it helps build protection against the virus. COVID-19 is a terrifying and dangerous communicable disease. The virus affects the respiratory organ of the ...
Please use one of the following formats to cite this article in your essay, paper or report: APA. Moore, Sarah. (2022, January 17). The Importance of Global COVID-19 Vaccination.
Caroline Gutman for The New York Times. 2. Hearing pro-vaccine messages from doctors, friends and relatives. For many people who got vaccinated, messages from politicians, national experts and the ...
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still ...
Here, two experts to make the case for and against mandatory COVID-19 vaccines. Alberto Giubilini, Senior Research Fellow, Oxford Uehiro Centre for Practical Ethics, University of Oxford. COVID-19 ...
Here we use two survey experiments to study how persuasive messaging affects COVID-19 vaccine uptake intentions. In the first experiment, we test a large number of treatment messages. One subgroup of messages draws on the idea that mass vaccination is a collective action problem and highlighting the prosocial benefit of vaccination or the ...
Widespread vaccination is generally regarded as the primary exit route out of the COVID-19 crisis, and for good reason. Even as we continue our collective struggle against the delta variant, we consistently observe reduced infections, morbidity, and mortality in fully vaccinated people living in nations fortunate enough to have supply and access to these products.
Guest Essay. Vaccine Mandates Are Coming. Good. June 28, 2021. ... Covid-19 is an emergency, and we don't have that much time. ... The mRNA vaccines, made by Moderna and Pfizer-BioNTech, will ...
The vaccine debate—including the argument as to whether vaccines are safe, effective, or could cause conditions like autism —has received a lot of attention from the media in recent years. With so much conflicting information being publicized, it can be a challenge to discern what is true and what is not.
Frontline health care personnel (HCP) were among the first to receive the COVID-19 vaccines. Physicians, nurses, allied health professionals, and others with direct patient contact or who handle biological materials are at high risk of exposure and illness and have duties of care and protection to patients, coworkers, and communities. Yet well into 2021, some HCP remain vaccine hesitant, an ...
Coronavirus Vaccines: Argumentative Essay. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. Thousands of people around the globe are dying every day due to Covid-19.
The initial argument in support of the COVID-19 vaccine mandates for health care workers must start with the principle of justice and consistency and their related ethical concepts that support fair and equitable treatment of individuals. Although justice is typically viewed through a patient-centric lens, it is reasonable to expect that health ...
Whether all children under 12 years of age should be vaccinated against COVID-19 remains an ongoing debate. The relatively low risk posed by acute COVID-19 in children, and uncertainty about the relative harms from vaccination and disease mean that the balance of risk and benefit of vaccination in this age group is more complex. One of the key arguments for vaccinating healthy children is to ...
For some COVID-19 vaccines, two doses are required . It's important to get the second dose if the vaccine requires two doses. For vaccines that require two doses, the first dose presents antigens - proteins that stimulate the production of antibodies - to the immune system for the first time. Scientists call this priming the immune response.
The COVID-19 vaccination will help keep you from getting the virus. COVID-19 vaccines were evaluated in clinical trials and have been approved because those studies show that the vaccine significantly reduces the probability of contracting the virus. Based on what has been proved about vaccines for other diseases, the COVID-19 vaccine may help ...
Some countries and companies are making bilateral deals, going around COVAX, driving up prices and attempting to jump to the front of the queue. COVID-19 vaccines are now being administered in 50 countries around the world, nearly all of which are wealthy nations. Seventy-five percent of doses have been deployed in only ten countries.
Covid-19 Vaccine Argumentative Essay. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. COVID-19, also known as the coronavirus, affected the whole world in 2020, and it is still taking effect in many countries. The world was put into quarantine and ...
Without high rates of uptake, however, the pandemic is likely to be prolonged. Here we use two survey experiments to study how persuasive messaging affects COVID-19 vaccine uptake intentions. In the first experiment, we test a large number of treatment messages. One subgroup of messages draws on the idea that mass vaccination is a collective ...
Behavioral change is the only prevention against the COVID-19 pandemic until vaccines become available. This is the first study to examine the most persuasive message type in terms of narrator difference in encouraging people to stay at home during the COVID-19 pandemic and social lockdown.
The pandemic's rise of at-home test kits was not just about Covid-19 — which, in fact, became widely available only after many months of regulatory and messaging obstacles — but also about ...