Center for Clinical and Translational Science (CCaTS)
Clinical research and trials unit.
CCaTS' Clinical Research and Trials Unit (CRTU) offers investigators the support they need to conduct clinical research — specially trained nurses, registered dietitians, technical and support staff, and a large selection of procedural and laboratory services.
CRTU staff are uniquely trained to carry out research protocols with an emphasis on patient safety and research quality. Learn more about CRTU staff .
The CRTU has a physical facility at both campuses of Mayo Clinic Hospital — Rochester, plus mobile staff and a unique research vehicle.

Saint Marys CRTU
The Saint Marys CRTU is located on Domitilla 5 at Mayo Clinic Hospital, Saint Marys Campus, and supports both inpatient and outpatient clinical research studies.
Charlton CRTU
Located on Charlton 7 at Mayo Clinic Hospital, Methodist Campus, the Charlton CRTU focuses on outpatient studies.
Extended CRTU and Mobile CRTU
The Extended CRTU is a "virtual" unit that provides services anywhere on Mayo Clinic's campus in Rochester. Some clinical research protocols necessitate that samples and data be collected outside the Saint Marys or Charlton CRTUs, but still within the Rochester campus — for example, from someone arriving in the emergency department with heart attack symptoms, or from a patient undergoing neurosurgery.
The Mobile CRTU provides services to support research conducted off the Rochester campus in community settings across southeastern Minnesota.
Related Resources
- CRTU contacts
- Support services
- About CRTU staff
- Staff resources
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- See us on facebook
- See us on youtube
- See us on twitter
Every Subject and Every Sample Matters
The Clinical and Translational Research Unit (CTRU) provides clinical and translational research support to accelerate novel bedside diagnostics and treatments—and CTRU clinical and laboratory services are available to all Stanford University faculty, residents, research fellows, and collaborators.
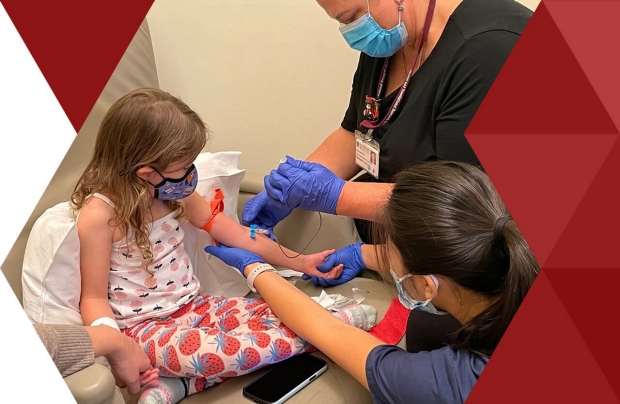
Stanford University School of Medicine’s Clinical and Translational Research Unit (CTRU) is the institute’s research-focused, ambulatory care, and laboratory services group.
Our mission is to accelerate translational research with a patient-focused goal of better health and science.
The CTRU collaborates and engages with academia and industry sponsors to advance research and patient care while maintaining a positive, competent, and compassionate research unit to ensure everyone is treated with dignity and care.
The CTRU provides research support that accelerates development and validation of novel therapeutics, bedside diagnostics, and other medical applications by improving the way clinical and translational research is conducted.
On average, the center supports over 350 clinical research studies annually for more than 200 faculty members. The studies stretch across multiple medical disciplines, and many are first-in-human trials, with novel therapies that have been discovered and developed at Stanford.
The CTRU supports research services in multiple locations, with the primary research clinic on Welch Road which is near Stanford Medicine, Health Care, and Children’s Health. The phlebotomy and lab services are also collocated to maximize same-day clinical trial services, while more advanced cellular isolations and other novel methodologies for specimen processing are located offsite.
Additional Resources
Study registration.
Interested in starting a study? Learn more about the tools and resources available to help get your study registered and launched with the CTRU, including accessing our online portal and learning about our overall process.
Get Started
Supported by NIH-NCATS-CTSA
The CTRU is supported by the Stanford CTSA Award from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health. Please cite the NIH-NCATS-CTSA grant # 5UL1TR003142.
See Guidelines
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Colon Rectal Surg
- v.27(2); 2014 Jun

Establishing a Successful Clinical Research Program
Daniele scoglio.
1 Department of Surgery, University of Washington Medical Center, Seattle, Washington
Alessandro Fichera
Clinical research (CR) is a natural corollary to clinical surgery. It gives an investigator the opportunity to critically review their results and develop new strategies. This article covers the critical factors and the important components of a successful CR program. The first and most important step is to build a dedicated research team to overcome time constraints and enable a surgical practice to make CR a priority. With the research team in place, the next step is to create a program on the basis of an original idea and new clinical hypotheses. This often comes from personal experience supported by a review of the available evidence. Randomized controlled (clinical) trials are the most stringent way of determining whether a cause–effect relationship exists between the intervention and the outcome. In the proper setting, translational research may offer additional avenues allowing clinical application of basic science discoveries.
CME Objectives : After reading this article, the reader should be able to understand the principals of research team building, to appreciate the different levels of scientific evidence, and to generate hypotheses.
Practicing surgeons are usually very driven individuals that invariably end up building a busy clinical practice. Clinical research (CR) is, in our opinion, a natural corollary to clinical surgery. In addition to academic advancement and visibility, CR gives investigators the opportunity to critically review their results and outcomes and potentially develop new strategies. It is our responsibility to encourage residents and fellows to consider getting involved in CR very early in their career.
In this article, we will cover what the authors believe are critical factors and important components of a successful CR program, based in part on the available literature, but predominantly on personal experience.
Success in CR does not come easy in the current economic environment, in striking contrast to the academic world of the 1970s and 1980s. What we consider individual and institutional factors are equally important. Investigators must be extremely motivated, especially in this time of increased demand for productivity and attention to relative value unit generation. An investigator must be organized to allocate time and resources, and to seek adequate training and mentorship to ask meaningful, realistic, and controversial questions. It is critical to understand the volume, complexity, and type of referrals available to the investigator and to start to focus early on very specific questions. Building a manageable and sustainable database can then be used to answer these questions. Investigators should also explore the available resources (cooperative group participation and membership, clinical trials, industry sponsored trials, available databases) and try to become an active participant early in their career.
At the institutional level, several components ought to be present and readily available for a young investigator to be successful. Institutional commitment for protected time, administrative support, and resources are critical factors that young surgeons should be looking for when starting a career that involves a CR component. Without time, resources, and support, it is virtually impossible to develop a successful CR program. Finally, an environment that fosters interdepartmental and intradepartmental collaboration will facilitate mentorship, a critical factor in the development of a junior investigator.
The Clinical Research Team
Depending on the available institutional resources, research infrastructure and a dedicated team may already be in place. Without proper support it is unreasonable to expect any CR to be successful. Lack of time is one of the most common reasons that surgeons give for not participating in clinical trials. The development of a research team can help overcome this barrier and enable a surgical practice to make CR a priority. The composition of a research team varies, but the goal for all is the same: alleviate the burdens of clinical trial participation. Members of the team should include CR associates (CRAs), CR nurses, non-nursing CRAs, data managers, regulatory staff, and administrative support staff. A research coordinator, who is usually broadly experienced with both CR and with the practice's operation, often oversees the team. Matching individuals with suitable tasks ensures that the program's resources are being used appropriately and that staff enjoy and remain challenged by their responsibilities. Depending on a program's organizational structure, the responsibilities assigned to each may differ greatly from one research program to another, both within and outside the United States. Tasks assigned to the staff include screening for potential study candidates, determining eligibility, coordinating the patient calendar, preparing documents for submission to Institutional Review Boards, filing amendments, submitting safety data, conducting patient education, obtaining informed consent, and assessing potential adverse events. No single individual could expect to fulfill all of these tasks. When resources permit, it is also helpful to have a manager or coordinator who supervises the program by overseeing quality assurance, staffing, budgeting, and site audits. 1 This person will work closely with the primary investigator to ensure that all investigator responsibilities are being met. Poor delegation leads to inefficient use of the program's resources and may cause staff to become dissatisfied with their duties. The CR manager can help ensure that tasks are being delegated appropriately.
Effective management requires shared commitment to excellence, mutual respect for each team member's role, and effective communication. Once staff members are trained and data management systems are implemented, this infrastructure has to be maintained and refreshed continually. Ultimately, a leader of an effective research program must acknowledge the value of each of its members while promoting a culture of teamwork and commitment to delivering high-quality care to patients.
Basic Steps to Develop a Research Program
With a research team in place, the next step is to develop a program. Taking an entrepreneurial approach is a successful mechanism when developing a CR program. Maintaining a sustainable program requires fiscal planning, much like a business. Researchers are often frustrated that per-patient reimbursement does not always cover the actual costs of conducting a trial and that reimbursement is usually given after patient enrollment. With National Cancer Institute (NCI) cooperative group trials, inadequate federal funding is a known and accepted fact that unfortunately deters smaller community-based practices from participation in cooperative group trials. A recent study determined that the average cost for each patient on a clinical trial is approximately $6,000 versus a per-patient reimbursement of only $2,000. 2 It is important to be aware of alternative funding mechanisms or institutional support that may be available to supplement program needs. Some physicians and their staff receive salary support from the institution to participate in CR, a great employment option for individuals dedicated to conducting trials. Physicians who are not salaried can build a similar mechanism into their practice. For example, because research requires additional time that is not reimbursed by insurance and does not typically generate revenue, it may be possible to add physician reimbursement as a cost covered by the study budget. Reimbursing physicians for their time is a reasonable study cost and helps create a research culture within the institution and institutions clearly benefit from offering a broad menu of clinical trials. If the program cannot be adequately funded by federally funded trials, adding industry trials may be an alternative. Though investigators are generally pleased by the higher reimbursement rates provided by industry, some complain that industry trials are less stimulating and provide fewer opportunities for publication. Always be selective before choosing trials and consider the question being investigated as well as patient demographics. It is important to be cognizant that if investigators open a trial that cannot accrue, they tax the program budget by wasting time and resources. Since NCI per-patient reimbursement alone is often insufficient, many additional options are available through NCI and other federal sources. 3 Joining a community clinical oncology program (CCOP) is a great option for community sites dedicated to research. CCOPs benefit from having access to numerous phase I, II, and III trials and have significant autonomy. CCOPs also manage their own budgets and receive some funding before patient enrollment, unlike standard cooperative group partnerships. Becoming a CCOP requires a previous record of success. If a program is still in initial stages, consider becoming an affiliate member of a cooperative group instead. This enables a researcher to partner with a member institution and participate in all trials offered through the institution's cooperative group affiliation. In this mechanism, reimbursement is provided after patients are enrolled and is initially given to the member institution, which is then responsible for channeling funds to partner institutions. Joining the NCI Clinical Trials Support Unit is also an option worth pursuing for programs at all levels. Also, NCI has many investigator-initiated funding opportunities, including training grants and administrative supplements, all of which are listed on the NCI Web site. In addition to federal options, enhancing knowledge of funding opportunities offered through philanthropic organizations can be beneficial. From professional societies to advocacy organizations, most offer varying levels of grants, some exceeding several million dollars in annual funding. Many of the grants can be used to supplement the research one is already pursuing, such as ASCO's (American Society of Clinical Oncology) community oncology research grants. 4 Be clear about the requirements associated with grants funded by nonprofit organizations. Most researchers find these grants helpful, but some are not applicable because of conflicts of interest or inability to meet associated requirements. Also, do not automatically dismiss grants for small amounts of funding; instead, consider realistic ways to incorporate these mechanisms into your program. Smaller grants can be useful to fund feasibility studies or pilot projects. Using supplemental funding mechanisms can greatly enhance a CR program. As federal funding availability decreases, it has become more and more competitive also to be funded through alternative mechanisms.
I Have an Idea. How Do I Formulate a Hypothesis and Pursue the Answer?
Over the last several years, medical and health professionals have begun using evidence-based medicine (EBM) in their practice integrating best available research and their clinical expertise with the specific patient clinical scenario. In the early 1970s, Cochrane 5 criticized the lack of reliable evidence behind a plethora of health care interventions commonly accepted at the time. Rigorous evaluation of these interventions highlighted the need for an increase in evidence in medicine, planting the seed for EBM. David Sackett of McMaster University used the term “critical appraisal” to describe extracting evidence from systematically examined medical literature in the early 1980s. 6
The actual term EBM was coined by Dr. Gordon Guyatt of McMaster University in 1990. An initial group of physicians from McMaster University joined forces with specialists from a variety of institutions to create the Evidence-Based Working Group. This group became responsible for adopting the idea of EBM and presented it in the pivotal report announcing it as a new medical paradigm: “Evidence-Based Medicine: A New Approach to Teaching the Practice of Medicine.” 7
The availability of systematic reviews, medical databases, the Cochrane Library, and evidence-based journals, for example, focusing on articles of immediate clinical use, has significantly improved research and clinical decision making. For example, in 1997, when the National Library of Medicine announced it was offering free access to the first-line web-based medical databases MEDLINE and PubMed, usage jumped 10-fold to a total of 75 million searches annually. 8 Availability and accessibility of information have also increased with the advent of second-line databases such as the Cochrane Library, UpToDate, and Best Evidence along with EBM-related journals such as the ACP Journal Club and Evidence-Based Medicine . Furthermore, with electronic peer review and electronic publication, new evidence is rapidly available and disseminated.
The greatest difficulty for some junior investigator is how to generate a hypothesis. It is our personal conviction that the seed of a new clinical hypothesis comes from personal experience corroborated and supported by review of the available evidence.
Specific hypothesis-generating questions could arise from clinical findings, differential diagnoses, manifestations, harm, etiology, therapy, prevention, diagnostic tests, and prognosis ( Table 1 ). However, the last two questions that need to be answered before starting a study are: why is this work needed and who would benefit from it?
In CR, studies can be classified into descriptive and analytic studies. Descriptive studies are observational studies and describe general disease characteristics. They include cross-sectional studies, surveillance studies, case reports, and case series. 9 Analytic studies test a hypothesis about a casual relation between exposure and outcome. 10 They can be observational, such as case–control and cohort studies, or controlled, such as the randomized controlled trial (RCT) ( Table 2 ).
In observational studies, the physician in conjunction with the patient recommends the approach/treatment indicated, as it happens in routine clinical practice, and it could be considered more clinically relevant.
Even though a RCT carries the highest level of evidence, it may not be the most appropriate design for all clinical questions due to technical or ethical issues. 10 11 12 In these situations, observational studies are the second best option to evaluate the efficacy and safety of a specific intervention. These studies often provide initial evidence useful in generating a hypothesis that can be tested further with analytic studies. 10
Cohort studies, case–control studies, and case series are all different types of observational studies. Cohort and case–control studies differ from case series in that they make use of a comparison group. Case series belong to a group of descriptive studies that do not test the hypothesis of treatment efficacy. A case series follows a group of patients who have a similar diagnosis or are undergoing a certain procedure over a specific period of time.
Treatment safety and diagnostic accuracy are the principal outcomes that can be assessed fairly and reliably in a case series. In the assessment of either outcome, no control group is necessary and long-term follow-up can be obtained readily, especially with a retrospective design.
The external validity is also acceptable in case series that includes a diverse range of patients. By including patients with different characteristics and cointerventions, the study sample is more likely to be representative of the population of interest. In a RCT, however, relatively stringent inclusion criteria and selection of only those patients who wish to participate decrease the extent to which the results can be applied to common clinical practice.
When designed and properly conducted, a case series can be a sensible alternative to studies with higher levels of evidence, with the additional advantage of time and cost savings. 13
A RCT is a study in which subjects are randomly assigned to one of two groups: one (the experimental group) receiving the intervention that is being tested, and the other (the comparison group or control) receiving an alternative (conventional) treatment. The two groups are then followed-up to see if there are any differences in outcome. The results and subsequent analysis of the trial are used to assess the effectiveness of the intervention, which is the extent to which a treatment, procedure, or service does patients more good than harm. RCTs are the most stringent way of determining whether a cause–effect relationship exists between the intervention and the outcome. 14 In the hierarchy of evidence, RCT (Level 1) are followed by cohort studies (Level 2), case–control studies (Level 3), case series (Level 4), and expert opinion (Level 5). 10
RCT is based on a good hypothesis formulated a priori. Having chosen a subject to research and a specific hypothesis to be tested, preparation should be thorough and is best documented in the form of a protocol that will outline the proposed methodology. An appropriate rationale for the study will follow with a relevant literature review, which is focused on any existing evidence relating to the condition or interventions to be studied. The subject to be addressed should be of clinical, technical, or translational significance to afford relevance to the study, and the hypothesis to be evaluated must contain outcomes that can be accurately measured. The subsequent study design (population sampling, randomization, applying the intervention, outcome measures, analysis, etc.) will need to be defined to permit a true evaluation of the hypothesis being tested. In practice, this will be the best compromise between what is ideal and what is practical. Writing a thorough and comprehensive protocol in the planning stages of the research project is essential. Peer review of a written protocol allows others to criticize the methodology constructively at a stage when appropriate modification is possible. Seeking advice from experienced researchers, particularly involving a local research and development support unit, or some other similar advisory center, can be very beneficial. It is far better to identify and correct errors in the protocol at the design phase than to try to adjust for them in the analysis phase. Articles rarely get rejected for publication because of inappropriate analysis, which is remediable, but rather because of design flaws. There are several steps in performing an RCT, all of which need to be considered while developing a protocol. The first is to choose an appropriate (representative) sample of the population from which to recruit. Having measured relevant baseline variables, the next task is to randomize subjects into one of two (or more) groups, and subsequently to perform the intervention as appropriate to the assignment of the subject.
Choosing the right population is crucial because poor sampling will undermine the generalizability of the study or, even worse, reduce the validity if sampling bias is introduced. 15 The task begins with deciding what kind of subjects to study and how to go about recruiting them. The target population is that population to which it is intended to apply the results. It is important to set inclusion and exclusion criteria defining target populations that are appropriate to the research hypothesis. These criteria are also typically set to make the researchers' task realistic, for within the target population there must be an accessible/appropriate sample to recruit. For the findings of the study to be generalizable to the population as a whole, the sample must be representative of the population from which it is drawn. The best design is consecutive sampling from the accessible population.
If the inclusion criteria are broad, it will be easy to recruit study subjects and the findings will be generalizable to a comparatively large population. Exclusion criteria need to be defined and will include such subjects who have conditions which may contraindicate the intervention to be tested, subjects who will have difficulty complying with the required regimens, those who cannot provide informed consent, etc. Then having determined an appropriate sample to recruit, it is necessary to estimate the size of the sample required to allow the study to detect a clinically important difference between the groups being compared. After deciding on the population to be studied and the sample size required, it will now be possible to plan the appropriate amount of time (and money) required to collect the data necessary.
It will be important at the analysis stage to show that these potential confounding variables are equally distributed between the two groups; indeed, it is usual practice when reporting an RCT to demonstrate the integrity of the randomization process by showing that there is no significant difference between baseline variables (following CONSORT guidelines). 16 Randomization should equally distribute any confounding variables between the two groups, although it is important to be aware that differences in confounding variables may arise through chance. It is also essential that treatment allocations are concealed from the investigator until recruitment is irrevocable, so that bias (intentional or otherwise) cannot be introduced at the stage of assigning subjects to their groups. 17
Ideally, neither the study subjects nor anybody performing subsequent measurements and data collection should be aware of the study group assignment. Effective randomization will eliminate confounding by variables that exist at the time of accrual. Without effective blinding, if subject assignment is known by the investigator, bias can be introduced because extra attention may be given to the intervention group (intended or otherwise). 17
Once the intervention has been applied, the groups will be followed up and various outcome measures will be performed to evaluate the effect or otherwise of that intervention. The outcome measures to be assessed should be appropriate to the research question, and must be ones that can be measured accurately and precisely. Even if it has not been possible to blind the administration of the intervention, it should be possible to design the study so that outcome measurement is performed by someone who is blinded to the original treatment assignment. Probably, the most important prerequisite for conducting an RCT is one's commitment. Commitment relates to not only being involved with respect to one's role but more importantly being committed to the question rather than finding the answer. This commitment is compounded by the fact that, in every respect, conducting a surgical trial takes more time and effort compared with running a clinical practice. A meaningful trial may take anywhere from 5 to 10 years to complete. During this time, the surgical world has moved forward, techniques have been modified, case reports may have suggested complications with a particular procedure, and so on. 18 Successful surgical “trialists” are just as passionate about the research as any aspect of their clinical practice. They likely spent additional time learning how to perform research in the fields of clinical epidemiology, methodology, or public health in addition to their clinical fellowships.
A critical aspect of CR is quality control. Essentially, quality control issues occur in clinical procedures, measuring outcomes, and handling data. Ideally, any outcome measurement taken on a patient should be precise and reproducible; it should not depend on the observer who took the measurement. 15
However, it is often necessary to use multiple observers, especially in multicenter trials. Inevitably, there will be a principal investigator; this person will be responsible for assuring the quality of data measurement through motivation, appropriate delegation of responsibility, and supervision. An investigators' meeting before the study starts and regular visits to the team members or centers by the principal investigator during data collection allow for communication, supervision, early detection of problems, and feedback are good for motivation.
Data will subsequently need to be transcribed onto a computer database from these forms. The database should also be set up so that it is similar in format to the forms, allowing for easy transcription of information. The database can be preprepared to accept only variables within given permissible ranges and that are consistent with previous entries, along with alerts to the user for missing values. Ideally, data should be entered in duplicate, with the database only accepting data that are concordant with the first entry; this, however, is time consuming, and it may be adequate to check randomly selected forms with a printout of the corresponding datasheet to ensure transcription error is minimal, acting appropriately if an unacceptably high number of mistakes are discovered.
In conclusion, a well-designed, methodologically sound RCT evaluating an intervention provides strong evidence of a cause–effect relationship if one exists; it is therefore powerful in changing practice to improve patient outcome, this being the ultimate goal of research on therapeutic effectiveness.
More and more emphasis has been placed on translation science (or translational research) as a very appealing clinical application of basic science discoveries. The dominant view of translation science overly emphasizes the translation of the results of “basic,” “bench,” or discovery research into clinical application through the conduct of clinical trials. We believe that translation is much more than the conduct of clinical trials to test discoveries. It begins with translating the questions that arise out of the need for knowledge in the “real world” into discovery research; translating the findings of discovery research into clinical or policy application through clinical or policy research; translating the findings of clinical or policy research into action at the clinical or policy level. Integrating these three translation gaps into a model of evidence-based health could lead to improvement of health outcomes through translating knowledge into action. 19
Surgeons are the health care providers with access to tissue samples, the key link between the bench and the bedside. For that reason we, as surgeons, are often approached by bench researchers with questions and hypotheses that need clinical confirmation. For junior investigators, in our opinion, this is a very appealing and rewarding way to get involved in research, establishing relationships with potential mentors in the basic science world to obtain academic visibility. Furthermore, correlative science and translational components are often part of multidisciplinary federally funded Research Translation Core (RTC). Tissue samples are often archived in these large studies and are available to study participants with the appropriate questions, expertise and support, offering great opportunities for clinical investigators with an interest in translational research.
The available resources at the institutional, regional, and national level may seem overwhelming and it is easy for junior investigators to get confused, discouraged, and slowly withdraw from research. To be successful in CR, it is critical to be at the right place (adequate institutional support, resources, and protected time) at the right time (stable department, availability of knowledgeable mentors willing to collaborate and support) and ask original questions. While it is appropriate initially to follow in the footsteps of a mentor, it is very important to branch out and develop personal research directions.
There are several common mistakes that are made by young investigators. Lack of focus and specificity seems to be a very common one. Reporting large retrospective clinical reviews, while worthwhile several decades ago, does not serve any purpose in today's environment and is unlikely to be published in a quality journal. As clinical practice tends to super specialize, so should CR. Focusing early on a specific question does not limit the scope of research, but rather makes it more appealing. There are questions that may require several generations of investigators to be answered. As the surgeon develops into a more experienced researcher, several different research avenues may be present. It is even more important at this stage to remain focused on a specific topic. The most successful clinical, translational, and basic researchers are the ones who have persisted and remain faithful to their main research path.
No funds, grants, or support was received to complete the study.
Conflict of Interest The authors declare no conflict of interest.


Research Staffing Support
Conducting clinical research requires the support of a team of individuals with specialized experience. Within the NINDS Clinical Trials Unit (CTU), two offices provide Research Staffing Support.
The Research Staffing Support Office (RSSO) provides centralized research staffing support in 3 main categories: Protocol Navigation (PN), Patient Care Coordination (PCC), and clinical research staff hiring support for PNs, PCCs, and Research Coordinators (RCs) primarily through the SOAR contract mechanism.
The Research Coordinator Office (RCO) provides centralized research staffing support for Research Coordinators (RN and non-RN), as well as hiring support for these positions.
Protocol Navigation: PNs provide PI support in the writing of clinical research protocols, consents and other supporting documents, regulatory submissions and approvals of those documents, as well as maintenance of the site regulatory binder for PIs as assigned. PNs serve as regulatory experts to advise PIs and study teams on current applicable regulations that govern their protocols.
Patient Care Coordination: PCCs provide PI support in the services required to coordinate research participant visits. This includes managing all aspects of participant scheduling, such as new participant visits, tracking participant visit windows to ensure visits are scheduled per protocol, travel, lodging, clinic visits, procedure appointments, participant communications, and generally liaising between the study team and the research participants to ensure study appointments run smoothly.
Research Coordination: RCs (Including RNs serving as RCs) provide guidance and management services for research studies involving human subjects. RCs have a wide variety of research responsibilities, highlights of which are listed here:
- Provide PI feedback in protocol development discussions regarding feasibility of protocol implementation and execution with a specific focus on clinical issues, available resources, study coordination, participant safety, and data quality.
- Assist Investigators in community outreach, recruitment, and screening of research participants.
- Coordination and management of most daily activities of the study and ensure that study activities follow established protocol, Standard Operating Procedures (SOP), and utilizes approved forms, templates, and practices.
- Integral in the creation and maintenance of MOPs, Source Document Forms, and Case Report Forms.
- Assist researchers with study testing and other duties associated with the completion of study visits and ensure protocol and human subject safety regulation compliance.
- Document and/or ensure documentation of research participant condition, adverse events, concomitant medication use, protocol compliance, response to study drug, and other re-quired data points.
Clinical Research Staff Hiring Support:
Identifying and selecting qualified candidates for clinical research support staff can be daunting and can have a significant effect on clinical protocol execution. The CTU can assist study teams with the screening and interviewing of potential candidates for clinical research staff support positions, i.e., Protocol Navigator, Research Coordinator, and Patient Care Coordinator positions within the NINDS Division of Intramural Research.

CLINICAL RESEARCH UNIT

The Clinical Research Unit (CRU) is a VA Puget Sound facility that provides expert clinical services for VA research.
SIBCR supports the CRU by serving on the advisory board and by supporting a portion of the personnel costs. The CRU is located at the VA Puget Sound Seattle campus, on the fifth floor of the Mental Health Research Building (building 101).
- Reception area
- 3 Exam rooms
- 2 Single-bed procedure/observation rooms
- 2 Double-bed procedure/observation rooms
- 1 Interview/conference room
- 2 Observation/Nursing Stations
- 1 Physiology (exercise/training) room
- 1 Lab/sample processing room
- 1 Phlebotomy room
- 4 Bathrooms
- Omnicell pharmacy unit
- ParEx supply and distribution system
- EKG Machines
- Refrigerated Centrifuge
- Hologic bone densitometer (DEXA)
- -80-degree freezer storage
PROCEDURES
- Lumbar puncture
- Insulin tolerance tests
- ACTH stimulation
- Glucose Tolerance
- IV infusions
- Regulatory assistance
- Blood draws/sampling/shipping
- Medical History
The CRU is staffed by Magdalena (Meg) Wojtowicz, BSN, RN and Lauren Paulsen, Research Coordinator, both of whom have extensive experience in supporting clinical research studies. The CRU’s advisory board is composed of investigator members and representatives from VA R&D and SIBCR with Jose Garcia, MD, serving as Director.
INFORMATION
Documents: CRU Intake Form CRU Budget Justification Example CRU Equipment Example CRU Facilities Resources Example CRU Support Letter Outline
Web Links: VA Puget Sound Research Webpage IRBNet VA R&D SharePoint (VA internal access only) Clinical Research Unit Core SharePoint (VA internal access only)
Hours of Operation Monday – Friday 6:30 AM to 3 PM
For more information, please contact: Meg Wojtowicz, 206-277-4681
CTSU Members

Linking Practice to Progress

Integration Collaboration Research

Today’s Clinical Trials for Tomorrow’s Cancer Treatments

Connecting Investigators to NCI Cancer Research
The Cancer Trials Support Unit (CTSU) is a service of the National Cancer Institute (NCI) designed to facilitate access to NCI-funded clinical trials for qualified clinical sites and to support the management and conduct of those clinical trials. CTSU Membership provides access to a wide range of information and support services for qualified investigators and research staff. The CTSU Registration Page provides additional details regarding member access. For those who are not CTSU Members this website provides a listing of active protocols that the CTSU supports along with links to resources for additional information on NCI-funded clinical trials.
The NCI launched the CTSU in 1999 to streamline and harmonize support services for phase three Cooperative Group cancer clinical trials funded by the NCI. Since that time the scope of the CTSU has expanded to include support of multiple NCI-funded networks and clinical trials of all phases and types including cancer treatment, prevention and control, advanced imaging and correlative science studies. The CTSU collaborates with the NCI and its funded organizations to develop and support operational processes and informatics solutions leading to cost-effective solutions that reduce administrative burden on the clinical sites.
Under guidance of the NCI, the CTSU provides centralized services to support the following goals and objectives:
- Facilitate investigator and research staff participation in selected NCI multi-center programs and their clinical trials.
- Increase investigator and patient awareness and enrollment to cancer clinical trials.
- Provide standardized, integrated, and comprehensive support services to selected NCI multi-center programs.
- Identify best practices and streamline or eliminate redundant processes and procedures.
- Improve operational efficiency, enhance productivity and deliver products offering measurable business value to selected NCI multi-center programs.
NCI cancer research networks supported by the CTSU include:
- NCI National Clinical Trials Network (NCTN) - is a clinical trials research network that provides an infrastructure for NCI treatment, screening, and diagnosis trials. The infrastructure allows investigators to begin clinical trials quicker, reach conclusions faster, and offer patients studies that incorporate precision medicine at over 3,000 clinical sites.
- NCI Experimental Therapeutics Clinical Trials Network (ETCTN) - is a clinical trials network that evaluates innovative cancer treatments using a coordinated, collaborative, and inclusive team-based approach to early phase experimental therapeutic clinical trials.
- NCI Community Oncology Research Program (NCORP) - is a community-based program that brings cancer clinical trials, as well as cancer care delivery research, to individuals in their own communities, thereby generating a broadly applicable evidence base that contributes to improved patient outcomes and a reduction in cancer disparities.
Additional NCI and NIH services that work in conjunction with the CTSU include:
- NCI Central Institutional Review Board (CIRB) - reduces administrative burden of local institutional review boards and investigators by partnering with local institutions to provide human subject protections by conducting IRB reviews of NCI-sponsored trials.
- NCI Clinical Trials Search - NCI's website helps you find NCI-supported clinical trials that are taking place across the United States, Canada, and internationally. The list includes all NCI network trials.
- NIH ClinicalTrials.gov - is a registry and results database that provides easy access to information on clinical studies of human participants conducted around the world.
Coronavirus (COVID-19): Latest Updates | Visitation Policies Visitation Policies Visitation Policies Visitation Policies Visitation Policies | COVID-19 Testing | Vaccine Information Vaccine Information Vaccine Information
About UR CTSI
Office of clinical research.
The Office of Clinical Research (OCR) provides tools and services to help URMC faculty and staff with the administration of clinical trials. By streamlining the processes behind clinical research, we hope to empower our clinical research teams to do more high-impact clinical trials that can advance clinical discovery and offer patients and community members more options and opportunities. We also make it easier for researchers to comply with clinical trial rules and regulations to produce successful outcomes.
Director Ashlee Lang, MPH Phone: (585) 275-8370 [email protected]
The OCR has many services to assist with your clinical trials
Please email the [email protected] with any questions related to OCR services and support.
Ashlee Lang, MPH, Joins UR CTSI as Director of the Office of Clinical Research
Ashlee Lang, MPH, joined the UR CTSI as the new director of the Office of Clinical Research at the beginning of December, 2023. Lang has more than 15 years of experience in the clinical research field, with expertise in global clinical trials, regulatory compliance, clinical laboratory science, program management, operations, and more.
The OCR maintains a set of resources related to key aspects of clinical trials that departments involved in clinical trials can utilize. URMC research coordinators, administrators, and faculty can access and download tip sheets, memos, directions, and other important documentation to help them with various stages and aspects of clinical trials.
URMC Clinical Research Study Start-Up Manual
The purpose of the URMC Clinical Research Study Start-Up Manual is to review best practices concerning Study Start-Up within the University of Rochester (UR) and is to be used as an overall guideline for individuals within Study Teams to use as applicable.
Shared Information
Please note you must access these resources through Box.
Study Participants
Potential study participants can learn more about health research and clinical trials at URMC.
Office for Human Subject Protection
The Office for Human Subject Protection (OHSP) supports the administration of the University of Rochester’s Human Research Protection Program.
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Clinical Research Coordinator
- Obstetrics and Gynecology
- Columbia University Medical Center
- Opening on: Apr 13 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Temporary
- End Date if Temporary: 05/31/2025
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $62,400 - $65,000
Position Summary
The primary role of this position is to support clinical research involving Maternal Fetal Medicine Research. The employee will interact with pregnant women, their families and clinical staff as it relates to clinical research protocols and clinical trials, industry funded and grant funded, being implemented in the outpatient setting.
Responsibilities
- Handle completion of GCP, HIPAA and applicable regulatory training.
- Complete certification requirements for assigned protocols.
- Screen designated schedules or patient lists for eligible subjects.
- Approach and verify eligibility subjects; and enroll and consent eligible subjects.
- Complete research study visits as delineated in assigned protocol and manual of operations set forth by sponsor and supervisor.
- Complete telephone follow-up and telephone reminder calls for study participants, during these phone calls the person will need to administer study questionnaire as assigned.
- Schedule research visits and coordinate the collection of all research data points as assigned, whether through research visits, chart abstraction or telephone.
- Handle collection through venipuncture, processing, transporting and shipping of biological specimens as assigned and by steps delineated in the protocol or manual of operations.
- Complete study documents and files some examples might include case report forms, worksheets and medical record notes.
- Maintain confidentiality of documents and files such as HIPAA.
- Informing relevant clinical staff regarding subject protocol participation.
- Assist in other research related activities and projects as needed.
- Regular collaboration with the PI and other research staff; and lead CRC of project or work area.
- Perform other related duties and responsibilities as assigned/requested.
Minimum Qualifications
- Phlebotomy Certified (or other forms of certification in lieu of phlebotomy such as certified medical assistant, nursing degree, medical degree) - current or obtained within 3 months.
- Incumbent must be self-directed and able to make independent decision within the parameters of all federal, state, institutional and departmental guidelines.
- Experience in a patient care setting and clinical research experience.
- Complete proficiency in written and spoken English and Spanish.
Preferred Qualifications
- Master’s or another advanced degree may substitute in part for experience.
- Excellent interpersonal, written/oral communication, and organizational skills.
- Proficiency in Microsoft Office.
The Department of Obstetrics and Gynecology is dedicated to the goal of building a multicultural faculty and staff committed to teaching, working and serving in a diverse community, and strongly encourages applications from candidates of traditionally underrepresented backgrounds.
We are continuously seeking to recruit individuals who will enhance the diversity of our workplace and the effectiveness of our organization.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Program Coordinator II
Administrative assistant, chief of staff -sr director.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .

IMAGES
VIDEO
COMMENTS
Clinical Research Support Unit. NYU Grossman School of Medicine's Clinical Research Support Unit oversees the financial administration of clinical research studies. We provide support services for interventional and noninterventional studies funded from any source. In addition, we oversee the financial administration of studies supported by ...
Clinical Research Support. We connect researchers with the resources they need to conduct their clinical studies that will improve prevention and treatment methods. On This Page. Center for Biomedical Ethics. Cancer Institute Clinical Trials Office (SCI-CTO) Clinical and Translational Research Unit (CTRU) Clinical Research Quality (CRQ)
The Clinical Research Support Unit currently supports three prestigious research consortia: MAGIC-Mount Sinai Acute GVHD International Consortium: Composed of 10 major stem cell transplant centers in the United States and Europe who are collaborating to use this new scoring system to test new treatments for acute GVHD. Contact: Rachel Young The Myeloproliferative Neoplasms Research Consortium ...
Overview. McMaster's Faculty of Health Sciences' Clinical Research Support Unit (CRSU) will support the next generation of clinical researchers lead collaborative studies that drive change in clinical practice and mobilizing research insights beyond academia. Our mandate is to decrease barriers to conduct clinical research for early and mid-career researchers with or without formal ...
CCaTS' Clinical Research and Trials Unit (CRTU) offers investigators the support they need to conduct clinical research — specially trained nurses, registered dietitians, technical and support staff, and a large selection of procedural and laboratory services. CRTU staff are uniquely trained to ...
Clinical Research Support Center. 717 Delaware Street SE, Room 140 Minneapolis, MN 55414. A collaboration among: Research and Innovation Office Clinical and Translational Science Institute Fairview Health Services University of Minnesota Physicians. Contact [email protected] 612-625-4000.
The Tisch Cancer Institute (TCI) offers a wide range of clinical trials for patients with cancer. Our Clinical Research Support Unit (CRSU) offers Phase I, II, and III trials.. Phase One clinical trials test a potential drug's dosing, confirm effectiveness, and look for side effects.; Phase Two trials compare the new drug, already studied in the phase one trial, to a therapy that is known to ...
The Clinical Research Support Unit. Tel: 212-824-7391 [email protected]. View Calendar. Events. TCI Careers Forms and Information. Clinical Trial Flowcharts. PRMS Policies, Forms, and Meeting Schedules. PRMS Review Process. Share. Contact Us. 1 Gustave L. Levy Place New York, NY 10029-5674.
The CTRU provides research support that accelerates development and validation of novel therapeutics, bedside diagnostics, and other medical applications by improving the way clinical and translational research is conducted. On average, the center supports over 350 clinical research studies annually for more than 200 faculty members.
The NINDS Clinical Trials Unit (CTU) is comprised of a group of clinical research experts who collaborate to provide NINDS intramural investigators with support in developing and executing clinical research studies. CTU members have extensive experience and knowledge regarding clinical research regulations, IRB and scientific review procedures ...
The Aston Clinical Research Core and Clinical Research Unit (CRU) is a fee-for-service, institutional resource designed to support the safe and ethical conduct of research while enhancing the research experience for study participants.
Charges for Clinical Research Unit (CRU) ... Letters of Support. Search Clinical Trials. Join Our Mailing List. Ask a Navigator. Facebook Twitter Instagram. Johns Hopkins Institute for Clinical & Translational Research 750 E. Pratt Street, 16th Floor Baltimore, MD 21202; 410.361.7880;
Clinical research (CR) is a natural corollary to clinical surgery. It gives an investigator the opportunity to critically review their results and develop new strategies. ... Seeking advice from experienced researchers, particularly involving a local research and development support unit, or some other similar advisory center, can be very ...
The Clinical Research Unit (CRU) offers services to clinical research teams to launch and support clinical research studies. The CRU is located at Dartmouth Hitchcock Medical Center. Services are available to any clinical research team from: Dartmouth College; Dartmouth Hitchcock Medical Center and Clinics; Geisel School of Medicine at Dartmouth
Together the units provide a centralized mechanism to support cancer clinical research and assist HDFCCC members with conducting clinical studies in compliance with all federal, state and local regulatory requirements. ... UCSF Clinical Research Resource HUB; This unit is supported by a National Cancer Institute Cancer Center Support Grant ...
The TCI Clinical Research Support Unit with an Early Phase Trials Unit provides the infrastructure to conduct novel, investigator-initiated protocols developed by TCI investigators and supported by CRSU staff members. The TCI's catchment area includes the New York City Metropolitan area. TCI's cancer research programs are focused on the ...
The Industry Clinical Contracts Office and Fiscal Management groups within Clinical Research Support address the contract negotiation for industry trials and budget setup on study start-up for Fred Hutch. During the life of the study, activities include invoicing and booking of revenue, as well as the renegotiation of contracts, when necessary.
The NIA/IRP Clinical Research Unit (CRU) is a state-of-the-art clinical research center designed to study human aging and age-related diseases. CRU, located at MedStar Harbor Hospital in Baltimore, has a pharmacy equipped for compounding research drugs, an apheresis facility for PBMCs donation, a dedicated glucose clamp facility to study whole ...
Within the NINDS Clinical Trials Unit (CTU), two offices provide Research Staffing Support. The Research Staffing Support Office (RSSO) provides centralized research staffing support in 3 main categories: Protocol Navigation (PN), Patient Care Coordination (PCC), and clinical research staff hiring support for PNs, PCCs, and Research ...
The Clinical Research Unit (CRU) is a VA Puget Sound facility that provides expert clinical services for VA research. SIBCR supports the CRU by serving on the advisory board and by supporting a portion of the personnel costs. The CRU is located at the VA Puget Sound Seattle campus, on the fifth floor of the Mental Health Research Building ...
The CRU includes a number of resources for investigators to undertake clinical research, including nursing support, specimen processing, and research space. The Clinical Research Unit (CRU) is the hub for conducting clinical and translational research at the Icahn School of Medicine at Mount Sinai (ISMMS). Established in 1963 as a General ...
The Cancer Trials Support Unit (CTSU) is a service of the National Cancer Institute (NCI) designed to facilitate access to NCI-funded clinical trials for qualified clinical sites and to support the management and conduct of those clinical trials. ... (NCTN) - is a clinical trials research network that provides an infrastructure for NCI ...
Ashlee Lang, MPH, joined the UR CTSI as the new director of the Office of Clinical Research at the beginning of December, 2023. Lang has more than 15 years of experience in the clinical research field, with expertise in global clinical trials, regulatory compliance, clinical laboratory science, program management, operations, and more.
Position Summary. The primary role of this position is to support clinical research involving Maternal Fetal Medicine Research. The employee will interact with pregnant women, their families and clinical staff as it relates to clinical research protocols and clinical trials, industry funded and grant funded, being implemented in the outpatient setting.