Permission Letter for Research: 4 Templates
When a determined, focused, and talented student idolizes a particular person in your institution for his or her work then what you need to do to carry on your research under him or she is written the perfect permission letter.
It should be such that conveys your request in the most polite way. If you think you want some help in writing it then make sure to refer to some of the sample letters which are written by me.
Template: 1
(Name of the sender)
(Designation of the respective person)
(Name of the address)
Subject- letter of permission for research
Respected madam/sir,
This letter is to inform (mention the name of the person) that I (mention your name) student of (mention the name of the college or name of the institution) would like to say to you that I want to do my research under your guidance for the topic (mention any project name or title which is suitable for you).
I am a very hard-working student currently pursuing my (mention your pursuing degree) from (mention the name of the college or the name of the institution).
I would like to do my research paper under your guidance, I have seen your work prior also and I am very much interested in doing my research under your guidance. This is a letter request to you forgive me permission for this research.
I would like to request you please grant my permission so that I can learn more from you and I believe with your help I will grow more and will definitely enhance myself more. I would like to assure you that I will work hard and will work with all my dedication. Please grant my permission. I will be highly obliged to you.
For any further information or queries, you can reach me at (mention phone number) or through an email (mention email address).
Thanking you
Download Template : ( pdf, docs, ODT, RTF, txt, HTML, Epub, Etc )
Template: 2
[Name of the sender]
[Designation of the respective person]
[Name of the address]
Subject- Letter of Permission for Research
This letter is to bring to your notice that I [mention your name] student of [mention the name of the college or name of the institution] would like to start my research under your guidance for the topic [mention any project name or title which is suitable for you].
I am currently pursuing my [mention your pursuing degree] from [mention the name of the college or the name of the institution] and I am a very persevering student. It is my earnest wish and hopes that I complete my research paper under your supervision and guidance.
I had the opportunity to be introduced to your work previously and since then, I had been very much keen on conducting my research under your oversight. So with great hopes, I write this letter of request to you for giving me permission for this research.
I would be highly obliged if you kindly grant me permission so that I can get the chance to learn from you. Your immense knowledge will help me in enhancing my research work. I would like to promise you that I will put in all my hard work and dedication towards this research. Please oblige me by granting me permission.
For any further queries or information, you can reach me on my number [mention phone number] or through an email too [mention email address].
Template: 3
I beg to state that I [mention your name] student of [mention the name of the college or name of the institution] would like to start my research on the topic of [mention any project name or title which is suitable for you] under your care and guidance.
Currently, I am pursuing my [mention your pursuing degree] from [mention the name of the college or the name of the institution].
I have always been a consistent student all throughout my academic career. I assure you that I will keep rendering my same dedication towards my research paper too.
You have always been my inspiration since my high school days. I have read all your research papers and all other relevant works. I would earnestly request you to give me the opportunity to be under your supervision and guidance so that I can get to learn a lot many things from you.
I will surely reach the summit with your guidance. I would also like to give you my full assurance as nowhere I will let you down.
I will remain ever grateful if you kindly grant me permission for completing my research work under your keen guidance. Please do accord me the acceptance of your guidance and supervision and oblige.

Similar Posts:
- Farewell Manager Letter: 75 Templates
- How To Write a Cover Letter With No Experience: 82 Templates
- How to Write Approval Letter: 54+Template
- How to Write an Authorization Letter: 35+ Templates
- How to Write a Support Letter: 35+ Templates
- Hostel Joining Permission Letter: 4 Free Templates
- Orphanage Visit Permission Letter: 4 Free Templates
- How to Write a Permission Letter: 10 Free Templates
- Examination Permission Letter: 4 Templates
- How to Write a Request Letter: 10+ Free Templates
“Business, marketing, and blogging – these three words describe me the best. I am the founder of Burban Branding and Media, and a self-taught marketer with 10 years of experience. My passion lies in helping startups enhance their business through marketing, HR, leadership, and finance. I am on a mission to assist businesses in achieving their goals.”
Leave a Comment
Letters in English
Sample Letters, Letter Templates & Formats
Home » Letters » Permission Letters » Letter Seeking Permission to Conduct Research – Sample Letter Requesting Permission to do Research
Letter Seeking Permission to Conduct Research – Sample Letter Requesting Permission to do Research
To, The Supervisor, __________ (University Name), __________ (University’s Address)
Date: __/__/____ (Date)
Subject: Permission to conduct research
Respected Sir / Madam,
Respected, I am ____________ (Name) studying in ___________ (Department) in your esteemed ____________ university/ affiliated college (University/College’s Name).
Respectfully, I would bring into your kind concern that I want to conduct a research for ___________ (Research- Course Name) which involves _____________ (Name of the research). For the same I will be requiring _____________ (Required Material) and ____________ (Covered fields) fields will be covered in my research.
I am writing this letter to seek your permission and allowance for the above-said research. This research will be conducted under the guidance of Mr./Mrs./Ms. ____________ (Name of the Professor).
I shall be highly obliged if you look into this matter and approve my request.
Thanking you, Yours ____________ (Truly/ obediently), _________ (Your Name), _________ (Department), _________ (Contact Number)
Incoming Search Terms:
- research request letter examples
- how to write letter for requesting permission for research
- Letter to Municipal Corporation about Mosquitoes in Your Area – Sample Complaint Letter
- Request Letter Seeking Permission for Leave – Sample Permission Letter for Leave
Leave a Reply Cancel reply
You must be logged in to post a comment.
Privacy Overview
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)

Dissertation Format and Submission
- Format Guidelines
- Dissertation Submission
- Getting Survey Permissions
- Help Videos
Instrument Permission documents
Instrument Permissions FAQ
Download a pdf of this faq , download the template permission letter, permissions to use and reproduce instruments in a thesis/dissertation frequently asked questions, why might i need permission to use an instrument in my thesis/dissertation.
- Determine whether you need permission
- Identify the copyright holder
- Ask for permission
- Keep a record
- What if I can't locate the copyright holder?
If you want to use surveys, questionnaires, interview questions, tests, measures, or other instruments created by other people, you are required to locate and follow usage permissions. The instrument may be protected by copyright and/or licensing restrictions.
Copyright Protection
Copyright provides authors of original creative work with limited control over the reproduction and distribution of that work. Under United States law, all original expressions that are “fixed in a tangible medium” are automatically protected by copyright at the time of their creation. In other words, it is not necessary to formally state a declaration of copyright, to use the © symbol, or to register with the United States Copyright Office.
Therefore, you must assume that any material you find is copyrighted, unless you have evidence otherwise. This is the case whether you find the instrument openly on the web, in a library database, or reproduced in a journal article. It is your legal and ethical responsibility to obtain permission to use, modify, and/or reproduce the instrument.
If you use and/or reproduce material in your thesis/dissertation beyond the limits outlined by the “fair use” doctrine, which allows for limited use of a work, without first gaining the copyright holder’s permission, you may be infringing copyright.
Licensing/Terms of Use
Some instruments are explicitly distributed under a license agreement or terms of use. Unlike copyright, which applies automatically, users must agree to these terms in order to use the instrument. In exchange for abiding by the terms, the copyright holder grants the licensee specific and limited rights, such as the right to use the instrument in scholarly research, or to reproduce the instrument in a publication.
When you ask a copyright holder for permission to use or reproduce an instrument, you are in effect asking for a license to do those things.
How do I know if I need permission to use instruments in my thesis/dissertation research? (Adapted from Hathcock & Crews )
Follow the four-step process below:
1. Determine whether you need permission
There are different levels of permissions for using an instrument:
a) No permission required
i. The copyright holder has explicitly licensed the use of instrument for any purpose, without requiring you to obtain permission.
ii. If you are only using a limited portion of the instrument, your use may be covered under the Fair Use Doctrine. See more here: https://uhcl.libguides.com/copyright/fairuse .
iii. If the instrument was developed by the federal government or under a government grant it may be in the public domain, and permission is therefore not required.
iv. If the document was created before 1977, it may be in the public domain, and permission is therefore not required. See the Stanford Public Domain Flowchart at https://fairuse.stanford.edu/wp-content/uploads/2014/06/publicdomainflowchart.png .
b) Non-commercial/educational use: The copyright holder has licensed the instrument only for non-commercial research or educational purposes, without requiring you to obtain the permission of the copyright holder. Any other usage requires permission.
Sample Permission for Educational Use:
Test content may be reproduced and used for non-commercial research and educational purposes without seeking written permission. Distribution must be controlled, meaning only to the participants engaged in the research or enrolled in the educational activity. Any other type of reproduction or distribution of test content is not authorized without written permission from the author and publisher. Always include a credit line that contains the source citation and copyright owner when writing about or using any test.
Source: Marta Soto, “How Permissions Work in PsycTests,” APA Databases & Electronic Resources Blog. American Psychological Association. http://blog.apapubs.org/2016/12/21/how-permissions-work-in-psyctests/ .
Even if you are not required to obtain permission to use the instrument, consider contacting the author for ideas on how to administer and analyze the test. Authors often welcome further use of their work, and may request you send them a copy of your final work.
c) Permission required: Instruments that require you to obtain the permission of the copyright holder, regardless of whether the use is for educational or commercial purposes. This may be because the copyright holder
- has important directions for how the test must be administered and analyzed
- wants to make sure the most current version is being used
- charges users a fee in order to administer the test
If you cannot locate the permissions, you are required to identify the copyright holder and contact them to ask about permission to use the instrument.
2. Identify the copyright holder (Adapted from Crews )
The next step is to identify who owns the copyright. The copyright holder is usually the creator of the work. If the copyright owner is an individual, you will need to do the usual Internet and telephone searches to find the person. Be ready to introduce yourself and to explain carefully what you are seeking.
Some authors transfer copyright to another entity, such as a journal publisher or an organization. In these cases, you must obtain permission from that entity to use or reproduce the instrument. You can often identify the owner by locating a © copyright notice, but as mentioned above, not all copyrighted works have a notice.
Check the following sources to locate instruments, their copyright holders, and their permission statements:
- Mental Measurements Yearbook: https://uhcl.idm.oclc.org/login?url=https://search.ebscohost.com/login.aspx?authtype=ip,uid&profile=ehost&defaultdb=mmt
- PsycTESTS: https://uhcl.idm.oclc.org/login?url=https://search.ebscohost.com/login.aspx?authtype=ip,uid&profile=ehost&defaultdb=pst
- Neumann Library Tests & Measures help: https://uhcl.libguides.com/PSYC/tests
- Library assistance e-mail: [email protected]
You may need to contact the author or publisher directly to find out who owns the copyright. Publishers often have websites that prescribe a method for contacting the copyright owner, so search the publisher website for a permissions department or contact person. Be sure to confirm the exact name and address of the addressee, and call/e-mail the person or publishing house to confirm the copyright ownership.
- The copyright owner may prefer or require that permission requests be made using a certain medium (i.e. fax, mail, web form, etc.). If you do not follow instructions, you may not get a reply.
- Telephone calls may be the quickest method for getting a response from the owner, but they should be followed up with a letter or e-mail in order to document the exact scope of the permission. E-mail permissions are legally acceptable in most cases, but getting a genuine signature is usually best.
- The request should be sent to the individual copyright holder (when applicable) or permissions department of the publisher in question. Be sure to include your return address, telephone and fax numbers, e-mail address, and the date at the top of your letter or message. If you send the permission request by mail, include a self-addressed, stamped return envelope.
- Make the process easy for the copyright owner. The less effort the owner has to put forth, the more likely you will get the permission you need. If you are using conventional mail, include a second copy of your request for the owner’s records.
- State clearly who you are, your institutional affiliation (e.g., University of Houston-Clear Lake), and the general nature of your thesis/dissertation research.
Do not send permissions letters to all possible rightsholders simultaneously. Taking the time to find the person who most likely holds the copyright will better yield success. If you do not have much information about who actually owns the copyright, be honest with your contacts, and they may be able to help you find the right person.
3. Ask for permission (Adapted from Crews )
Once you have identified the copyright holder, you must determine the scope of your permission request. Some copyright owners furnish their own permission form that you may download from their website.
If the copyright owner does not provide a permission agreement form, you may write your own letter ( click here to download a template ). Requests should be made in writing; e-mail is fine for this purpose. A most effective letter will include detailed information concerning your request for permission to use the work. Include the following information:
- Who: Introduce yourself. Tell who you are, your degree program, and a brief overview of your research.
- Why: Tell why you are contacting that person or entity for permission.
- What: Be as specific as possible when you cite and describe the instrument you wish to use. Include whether you plan to use the entire instrument, or if you plan on modifying or adapting any of the questions.
- How: Tell how you plan to use the instrument. Specify the parameters of your research study, and include any important information about the way you will administer the instrument and/or analyze the results.
- When: Expected length of the project and time to complete the thesis/dissertation.
Important : Obtaining permission to use an instrument is not the same as obtaining permission to reproduce the instrument in your appendix. If you intend on providing a copy of the instrument in an appendix, ask for separate permissions to do that.
Click here to download a template letter . Feel free to modify and adapt this template for your purposes.
4. Keep a record
After securing permission to use and/or reproduce the instrument, save a copy of the correspondence and the agreement. Documentation allows you to demonstrate to others that you have the legal right to use the owner's work. In the unlikely event that your use of the work is ever challenged, you will need to demonstrate your good faith efforts. That challenge could arise far in the future, so keep a permanent file of the records. Moreover, you might need to contact that same copyright owner again for a later use of the work, and your notes from the past will make the task easier.
Upload a copy of your permission letter in Vireo with your thesis/dissertation, or include it as an appendix in the document itself.
What if I can't locate the copyright holder? (Adapted from Hathcock & Crews & Pantalony )
In some cases, you may never get a response from the copyright holder or you may never even be able to identify who they are or how to contact them. It can be difficult to know how to proceed when you reach a dead end. Unfortunately, no matter how diligently you have tried to get permission, these efforts cannot completely eliminate the risk of infringement should you proceed to use the work.
Assuming you have diligently investigated your alternatives, do not want to change your project, and remain in need of the elusive copyright permission, the remaining alternative is to explore a risk-benefit analysis. You need to balance the benefits of using that particular material in your given project against the risks that a copyright owner may see your project, identify the materials, and assert the owner’s legal claims against you. Numerous factual circumstances may be important in this evaluation. The “benefit” may depend upon the importance of your project and the importance of using that particular material. The “risks” may depend upon whether your project will be published or available on the Internet for widespread access—as theses and dissertations will. You ought to investigate whether the work is registered with the U.S. Copyright Office and weigh the thoroughness of your search for the copyright owner and your quest for appropriate permission.
Undertaking this analysis can be sensitive and must be advanced with caution and with careful documentation. You may be acting to reduce the risk of liability, but you have not eliminated liability. A copyright owner may still hold rights to the material. Members of the University of Houston-Clear Lake community should consult with their chair or the Neumann Library to discuss their options.
Portions of this FAQ are used and adapted from:
Crews, Kenneth and Rina Elster Pantalony. “Special Cases.” Columbia University Copyright Advisory Services. https://copyright.columbia.edu/basics/special-cases.html . Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
Crews, Kenneth. “Asking for Permission.” Columbia University Advisory Services. https://copyright.columbia.edu/basics/permissions-and-licensing.html . Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
Hathcock, April. “Getting Permission.” NYU Libraries Copyright Library Guide, https://guides.nyu.edu/c.php?g=276785&p=1845968 . Licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
- << Previous: Dissertation Submission
- Next: Help Videos >>
- Last Updated: Feb 12, 2024 11:35 AM
- URL: https://uhcl.libguides.com/dissertation

- Creative Letter
- Letter for research proposal
In this article, we will discuss the format and structure of a formal letter for a research proposal. A research proposal is a document that outlines the objectives, methodology, and potential outcomes of a research project. It is typically submitted to funding agencies, academic institutions, or potential collaborators for consideration and approval.
Writing a well-crafted research proposal letter is crucial for conveying the significance and feasibility of your research project. It should effectively communicate your research goals and convince the recipient of the letter to support or collaborate on your research. In the following sections, we will provide examples of research proposal letters and offer suggestions for writing an impactful letter.
Letter Example 1: Request for Research Funding
Letter example 2: collaboration request, suggestions for writing a research proposal letter, conclusions, q: how long should a research proposal letter be, q: should i include my qualifications and research experience in the letter, q: how should i address the recipient in a research proposal letter, q: is it necessary to include a detailed budget breakdown in a research proposal letter, examples of research proposal letters.
Dear [Recipient's Name],
I am writing to submit a research proposal titled [Title of Research Project]. The objective of this research is to [briefly explain the purpose and significance of the research].
The proposed research methodology includes [describe the methods and techniques you plan to use]. By conducting this research, we aim to [state the expected outcomes and potential contributions to the field].
We kindly request funding support of [amount] to cover the research expenses, including [mention specific costs such as equipment, materials, or participant compensation]. This research has the potential to [explain the potential impact and benefits of the research].
We believe that our research aligns with the goals and priorities of [funding agency or organization]. We would be grateful for your consideration and support. Please find attached a detailed research proposal for your review.
Thank you for your time and consideration. We look forward to the opportunity to discuss this research proposal further.
[Your Name]
I hope this letter finds you well. I am writing to propose a research collaboration between [your institution/organization] and [recipient's institution/organization].
The objective of this collaboration is to combine our expertise in [specific areas] and conduct a comprehensive research study on [topic]. Our combined resources and knowledge will allow us to address key research questions and achieve significant breakthroughs in this field.
We propose a research timeline of [duration] and suggest dividing the research responsibilities as follows: [outline the division of tasks and responsibilities].
We believe that this collaboration will not only enhance our research capabilities but also foster knowledge exchange and contribute to the advancement of [field of study]. We kindly request your consideration and support for this collaboration.
We look forward to discussing this opportunity further and exploring the potential synergies between our institutions. Thank you for your attention.
- Clearly state the objective and significance of your research.
- Describe the research methodology and expected outcomes concisely.
- Tailor your letter to the goals and priorities of the recipient.
- Provide a detailed budget breakdown if requesting funding.
- Highlight any previous research achievements or relevant experience.
- Express enthusiasm for potential collaboration opportunities.
- Use a professional and polite tone throughout the letter.
- Proofread for grammar and spelling errors before submitting.
Writing a research proposal letter requires careful consideration of the recipient's needs and interests. By following the proper format and structure and clearly conveying your research objectives, methodology, and potential outcomes, you increase the chances of getting your proposal accepted or securing a collaboration opportunity.
A: A research proposal letter should be concise and to the point. It is recommended to keep it within one to two pages, focusing on the key aspects of your research project.
A: Yes, it is beneficial to highlight your qualifications and research experience in the letter. This helps establish your credibility and expertise in the field, increasing the chances of your proposal being considered favorably.
A: It is best to address the recipient formally using their appropriate title and last name (e.g., Dr. Smith, Professor Johnson). If you are unsure about the preferred form of address, you can do some research or simply use a generic salutation such as "Dear Sir/Madam."
A: If you are requesting funding for your research, it is advisable to include a detailed budget breakdown. This demonstrates your careful planning and provides transparency regarding how the funding will be utilized for the research project.
- Letter for Mom in Spanish
- Letter for rejection
Related Posts
Letter for Trip to Parents
Letter for working from home
Letter for Court Appearance
Letter for Request of Approval
Letter for Court Template
Letter for travel permission
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This website uses its own and third-party cookies to guarantee you the best experience on our website. Cookies
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
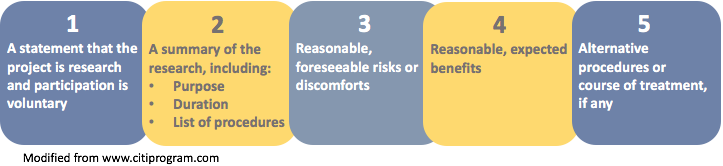
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
- Brief protocol for exempt research including data management and security questionnaire
Informed Consent Guidance
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 05/8/2023.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 05/08/2023
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools.
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects.
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent ages 7-11
- Child assent ages 12-14
- Parent permission
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
- Privacy Policy
Buy Me a Coffee

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.

Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.14(2); 2018 Jun

How to obtain informed consent for research
1 University of Messina, “G. Martino” Hospital, Messina, Italy
Amelia Licari
2 University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [1]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [2]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [3].
Short abstract
The process of obtaining informed consent for clinical trials is tightly regulated; complications arise in circumstances when consent may be waived, or when needed from vulnerable populations http://ow.ly/rEMe30j5MVq
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [ 1 ]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [ 2 ]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [ 3 ].
The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the subject must be given sufficient time to consider participation. However, informed consent is not merely a form that is signed, but is a process in which the subject has an understanding of the research and its risks, and it is tightly described in ethical codes and regulations for human subject research [ 2 ].
Educational aims
- To provide a comprehensive overview of issues in obtaining informed consent in clinical research.
- To describe the process of obtaining informed consent in clinical trials.
- To highlight the circumstances under which informed consent can be waived.
- To review the setting of obtaining informed consent from “vulnerable populations”.
The informed consent process
The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [ 4 ]. Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation. Conditions posing practical challenges in obtaining informed consent from the real subject may include situations of medical emergency or obtaining consent from “vulnerable” subjects and/or children [ 5 ].
Research-related information must be presented to enable people to voluntarily decide whether or not to participate as a research subject. For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject’s participation; a detailed description of study treatment or intervention and of any experimental procedures (including, in the case of randomised clinical trials (RCTs), also blinding and randomisation); a statement that participation in research is voluntary; probable risks and benefits associated with research participation; details of the nature of the illness and possible outcome if the condition is left untreated; availability, risks and benefits of alternative treatments; information about procedures adopted for ensuring data protection/confidentiality/privacy, including duration of storage of personal data; details about the handling of any incidental findings of the research; description of any planned genetic tests; details of insurance coverage in case of injury; reference contacts for any further answers to pertinent questions about the research and the subject’s rights and in case of any research-related injury to the subject; and any other information that seems necessary for an informed decision to be taken by the subject. Of particular importance, a statement offering the subject the opportunity to withdraw at any time from the research without consequences must be provided during the information disclosure [ 2 ]. Specific information should be provided in case of research projects involving children, incapacitated adults not able to give informed consent, illiterate populations, etc. (as will be described later in this article).
The information about the research should be given by a physician or by other individuals ( i.e. researchers) with appropriate scientific training and qualifications [ 6 ]. Furthermore, the location where the informed consent is being discussed, and the subject’s physical, emotional and psychological capability, must be taken into consideration when taking consent from a human subject.
Informed consent: when is it not necessary?
After institutional review board (IRB) or independent ethics committee approval is achieved, obtaining informed consent from each human subject prior to his/her participation in clinical trial is mandatory [ 5 ]. However, when specific circumstances occur, the informed consent can be waived, and “research without consent” is possible, which allows enrolment of patients without their consent, under strict regulation [ 7 ]. In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [ 8 ].
The first condition, of “impracticability”, occurs when obtaining informed consent is burdened by high impact in terms of time and economic resources or could compromise the study’s validity [ 8 ]. The second condition means that, although physicians are requested to ensure that the patient has understood the aim of the research and the risks and/or benefits associated with study participation, the researchers are also advised to respect the patient’s decision-making capacity, not interfering with his/her decisions and acting always in the patient’s best interest [ 9 ]. The third condition leads to justification of waiving consent when the clinical relevance and public health importance are potentially high [ 8 ].
The formal literature identifies different types of RCTs and classifies them into three macro-areas: 1) RCTs based on infeasibility of informed consent; 2) RCTs that omit informed consent only for control groups; and 3) RCTs that omit informed consent entirely.
RCTs based on infeasibility of informed consent
Emergency clinical studies, involving critically ill subjects, represent an exception to the requirement of informed consent. The investigated life-saving therapy and the medical intervention may be required immediately, not permitting the researchers to wait and respect all procedures of obtaining informed consent. Within this context, the researchers will be able to proceed with patient recruitment, also without the subject’s consent to treatment, when, prior to the study, the IRB has ascertained the presence of mandatory conditions ( table 1 ) [ 10 ].
Table 1
Conditions to be met in emergency clinical study
Cluster randomised studies include cluster-cluster and individual-cluster research [ 11 ]. In cluster-cluster designs ( e.g. studies on infectious disease prevention), the intervention involves the entire target community, so that single subjects cannot refuse it [ 12 ]. Conversely, in individual-cluster designs ( e.g. studies on primary care), although the intervention involves all the selected community, the right to refuse treatment is allowed. Under this circumstance, the omission of informed consent is justified only when the treatment refusal undermines the validity of the research study and/or procedures [ 13 ].
RCTs that omit informed consent only for control groups
In Zelen’s single-consent model ( e.g. RCTs in infectious or oncological diseases), randomisation occurs prior to any consent, and informed consent is sought only from individuals assigned to experimental treatment [ 14 ]. In the control group, the physicians do not make substantial changes in routine patient care, so informed consent is not required for patient enrolment [ 8 ].
In order to improve study recruitment, Zelen developed the double-consent design. Specifically, informed consent is requested for subjects to be involved in the study but not for the randomisation, preventing psychological distress [ 14 ].
In follow-up studies, the nested consent model ( e.g. for single cohort studies) or cohort multiple RCTs model ( e.g. for multiple cohort studies) is applied. In these variants, patients give their consent for prospective follow-up; however, they remain blinded to any randomised experimental interventions [ 15 ].
In trials using the model of “consent to postponed information”, the informed consent process is carried out after the study is completed [ 16 ].
All these RCT types aim to avoid unnecessary stress in patients who will not receive the new promising experimental treatment. Moreover, these clinical study designs do not affect the standard therapeutic approach or infringe the rights of the patients in the control group; therefore, the clinical trial can proceed without obtaining informed consent [ 8 ].
RCTs that omit informed consent entirely
Based on the fact that patients are assigned to standard care interventions, no informed consent is sought either in low-risk pragmatic RCTs [ 17 ] or in prompted optional randomisation trials [ 18 , 19 ]. However, in a low-risk pragmatic RCT, patients do not have the possibility to choose one of the two standard treatments, whereas in a prompted optional randomisation trial, both the researchers and the enrolled patients can choose one type of treatment over another, despite the randomisation results [ 6 ].
Special needs: vulnerable patients
A “vulnerable population” is defined as a disadvantaged community subgroup unable to make informed choices, protect themselves from inherent or intended risks, or keep their own interests safeguarded [ 20 ]. In the health domain, “vulnerable populations” refers to physical vulnerability ( e.g. pregnant women, fetuses, children, orphans, students, employees, prisoners, the military, and those who are chronically or terminally ill), psychological vulnerability (cognitively and intellectually impaired individuals) and social vulnerability (those who are homeless, from ethnic minorities, are immigrants or refugees) [ 20 ].
Due to a compromised free will and inability to make conscious decisions, several ethical dilemmas (related to communications, privacy and treatment) often arise when research involves these populations. Guaranteeing protection of rights, safety, data privacy and confidentiality of vulnerable subjects are prerogatives of good clinical practice, and law dispositions are regulated and strictly monitored by the applicable authorities [ 21 ].
Physical vulnerability
For a long time, pregnant women were excluded from clinical research because of their “vulnerability”. Although pregnant women are able to make informed and conscious choices, they have been considered “vulnerable” due to the potential risks to the fetus, who is also considered as a “patient” [ 22 ]. More recently, with the consideration of pregnant women as “scientifically complex” rather than “vulnerable” subjects, it has been permitted to involve this category in research trials [ 23 ]. The “scientific complexity” reflects both ethical and physiological complexity. The ethical aspects are secondary to the need to find a balance between interests of the fetus and the mother. The physiological aspects are strictly related to the pregnancy status [ 24 ].
Research studies involving pregnant women and fetuses have to satisfy specific federal regulations ( table 2 ). The following appropriate precautions should be taken in research studies involving pregnant women: no pregnant woman may be involved as a subject in a human clinical research project unless the purpose of the research is to meet the health needs of the mother and the fetus will be placed at risk only to the minimum extent necessary to meet such needs, or the risk to the fetus is minimal [ 25 ].
Table 2
Conditions to be met in research studies involving pregnant women and fetuses
Researchers can enrol pregnant women only when the mother and/or the father are legally competent. In fact, the consent to participate in research may be either self-directed (only the mother’s consent is required) or made with the guidance of the woman’s partner. However, the father’s consent need not be obtained when: 1) the research activity is directed to the health needs of the mother; 2) the father’s identity is doubtful; 3) the father is absent; or 4) a pregnancy from rape has occurred [ 26 ]. The consent signature requirements from the mother and father are summarised in table 3 . Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient’s health (mother and/or fetus) will be in danger.
Table 3
Consent signature requirements for pregnant women and children
# : consent requirements are the same whether the risk is “no more than minimal” or “more than minimal”.
Medical students and employees, who take part in numerous aspects of patient care in primary, secondary and tertiary care settings, are often invited to participate in human studies as volunteers. Frequently, the requesting researcher is their supervisor or instructor, who may push them to participate in the study, which can negatively influence their decision and also violate the consent legitimacy. Therefore, in order to protect these subjects against “coercion” or “undue influence”, when an investigator wishes to recruit medical students or employees, they must first obtain IRB approval for inclusion in the study of these vulnerable subgroups [ 27 ].
Prisoners, defined as any individual involuntarily confined or detained in a penal institution, are considered as “vulnerable” because they may be coerced into study participation, and also, due to both cognitive and psychiatric disorders, they can show an impaired ability to provide voluntary informed consent [ 28 ]. To protect this population, the Office for Human Research Protections has stipulated federal regulations according to which the only studies that may involve prisoners are those with independent and valid reasons for involving them ( table 4 ) [ 25 ].
Table 4
Studies that may involve prisoners
Due to the context of war in which they work, as well as the critical care setting in which they are treated, military subjects often receive medical care and/or participate in biomedical research under an “implied consent” condition. Moreover, the superior–subordinate relationship contributes to favour coercion or undue influence, making this population vulnerable [ 29 ]. To curb this phenomenon and to ensure that participation is truly voluntary, the US Dept of Defense agencies have adopted requirements similar to those that govern medical research that applies to the civilian population. Accordingly, the medical research recruitment session happens in the absence of superiors, and the informed consent is obtained prior to participating in a medical research study. The presence of an ombudsman guarantees and verifies that the participation is voluntary and that the information provided during recruitment is complete, accurate and clear. A payment as an incentive is acceptable but it must not be used to legitimise a coercive interference. Additional protection is provided to students at service academies, especially those aged <18 years. However, when emergency research is conducted or the research study advances the development of a medical product needed by the armed forces, informed consent will not be required [ 29 ].
Psychological vulnerability
Mental disability may compromise the self-determination and decision-making capacities [ 30 ]. Researchers interested in enrolling individuals with cognitive disorders are invited to apply different strategies to promote a better understanding of information-gathering processes. Simplifying the questions and content, adopting supportive technologies, using a more simple language, and spending more time for the information process have been suggested as useful and valid measures. When all these strategies prove to be insufficient, the investigators are required to obtain consent from a legally authorised representative [ 30 ].
Social vulnerability
Similarly to other vulnerable populations, research involving the homeless, ethnic minorities, immigrants and refugees is regulated by laws and specific procedures. Cultural and language differences, “undocumented” migrant status, and the precarious legal positions of these subjects raise several ethical issues, such as whether the participation is truly voluntary, or there are unrealistic expectations, or any benefits for their “status”.
Obtaining informed consent in these groups is extremely complex. A friendly procedure has been identified as the best way to adequately involve these vulnerable groups. A health centre or community building could represent an accessible location. The reimbursement of travel expenses for applicants can be a valid solution to obtain a representative sample for the clinical research. Clear and simple language, emphasising confidentiality, with the help of professional interpreters, can tempt migrants to sign the consent form. Lastly, the possibility of receiving something back in return for their contribution may enable successful enrolment of migrants in research [ 31 ].
Special needs: children
Because of their young age as well as their limited emotional and intellectual abilities, children are considered to be legally incompetent to give valid informed consent; thus, to enrol a child in a research study, the permission by at least one parent or legal representative is mandatory ( table 3 ). For subjects aged <18 years, biological or adoptive parents or legal guardians (persons having both legal capacity and responsibility) can give consent on behalf of their child, exercising free power of choice without any form of coercion. While married mothers and fathers both have parental responsibility, unmarried parents can exert parental responsibility only if they are named individually on the child’s birth certificate. Also, divorced parents maintain parental responsibility, but it is necessary to know to whom the child’s custody has been assigned [ 32 ]. However, on this matter, the European laws and regulations are not harmonised and several discrepancies are present in each country [ 33 ].
Despite potential benefits for the research subjects, the failure of parents to give consent (or their refusal to give consent) is not a rare circumstance [ 34 ]. It can be the case that researchers are dealing with underage parents, so that, although underage parents are responsible for representing their children, as minors themselves they are not considered to be sufficiently mature; therefore, they will be not able to give valid consent. Literacy and socioeconomic levels have been identified as the most common reasons for parental non-response [ 34 ]. Clarity and adequate explanation of research information materials should be part of effective planning to overcome language and social barriers.
In clinical studies in which the adopted methodology constitutes “less than minimal risks” for children, passive parental consent represents a possible way to more easily obtain informed parental consent [ 34 ]. Furthermore, parents can be informed with regard to a possible study involving their children, and, at the time of data collection, only the child’s assent is required. In fact, although the child’s decision-making capacity and understanding of the research project in which he/she will be involved may be limited, the Medical Research Council have shown that, when study details are provided and communicated in a clear and adequate manner, the child can be able to reach a decision and participate consciously in the research [ 35 ]. “Assent” is the term coined to express the child’s willingness to participate in clinical trials despite their young age. The “assent” should include and respect the following key points: 1) helping the child to acquire disease awareness; 2) explaining the potential impact of the experimental treatment; 3) evaluating the child’s ability to understand and adapt to new situations or challenges; and 4) positively influencing the patient’s willingness to participate in clinical trials [ 36 ]. Although the “assent” is not mandatory for research offering a direct benefit for the child, it arises from the need to respect paediatric research subjects [ 37 ]. The evaluation of the capacity to provide the “assent” is based on developmental stage, intellectual abilities and life or disease experience. Usually, the cut-off age of 7 years is used for the beginning of logical thought processes and rational decision making [ 38 ]. However, “assent” for children aged <7 years can be also required once the ability to read and write has been verified [ 32 ]. Figures 1 and and2 2 summarise the parental and assent permission requirements, respectively.

Flow chart of parental permission requirements.

Flow chart of child assent requirements.
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial. However, when it is impracticable to obtain consent, and the research does not infringe the principle of self-determination and also provides significant clinical relevance, the researcher is legally authorised to proceed without informed consent. Furthermore, in order to preserve the self-determination and decision-making rights, specific law dispositions are applied when vulnerable populations are enrolled in clinical trials.
Self-evaluation questions
- a) Diagnosis
- b) Risks and benefits of treatment
- c) Alternatives to treatment
- d) Family’s wishes
- a) When a minor is considered as emancipated
- b) When a patient is found to be incompetent
- c) When immediate treatment is necessary to prevent death or permanent impairment
- d) When the subject is aged >18 years
- a) Minor is married or divorced
- b) Minor on active duty in the US armed forces
- c) Minor is considered self-sufficient by a court
- d) Minor having a son
Suggested answers
- All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent.
- Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
- When specific circumstances occur, informed consent can be waived: if it is impracticable to obtain consent, if the research does not infringe the principle of self-determination, and if the research provides significant clinical relevance.
- Participation of vulnerable patients in clinical trials is regulated by specific law dispositions.
Conflict of interest: None declared.
3 Must-Have Templates for Requesting Permission Easily
When drafting a letter of request for permission, it’s essential to maintain a clear, respectful tone, and ensure the letter is structured to convey your request effectively. Here are three unique detailed templates for different contexts:
Template 1: Request for Permission to Use Facility
[Your Name] [Your Address] [City, State, Zip Code] [Email Address] [Phone Number] [Date]
[Recipient’s Name] [Recipient’s Title] [Company/Organization Name] [Address] [City, State, Zip Code]
Dear [Recipient’s Name],
I am writing on behalf of [Your Organization’s Name], a [Brief Description of Your Organization] that serves [Brief Description of Target Audience or Purpose]. We are planning to host a [Type of Event] on [Date] and are currently seeking an ideal venue that aligns with our event’s needs and values.
Given the reputation of your [Facility/Institution] for [Mention any known attributes or qualities, e.g., spaciousness, accessibility, etc.], we believe it would be the perfect setting for our event. We are particularly impressed by [Specific Feature or Offering of the Venue] and feel that hosting our event at your venue would significantly enhance the experience for our attendees.
We kindly request permission to use your [Specific Facility or Area, e.g., auditorium, conference room, etc.] for this purpose. We anticipate [Number of Attendees] and our event would include [Brief Description of Activities, e.g., speeches, workshops, etc.]. We are committed to adhering to all facility usage policies and will ensure the venue is maintained in its original condition.
We are open to discussing any terms or conditions that you may have for the use of your facility, including rental fees, insurance requirements, and any other details necessary. Our team is ready to coordinate closely with your staff to ensure a successful and seamless event.
Thank you for considering our request. We are hopeful for the opportunity to bring our event to your esteemed venue and believe it would be mutually beneficial. Please let us know if you require any further information or would like to arrange a meeting to discuss this request in detail.
[Your Name] [Your Position] [Your Organization’s Name] [Contact Information]
Template 2: Request for Permission to Conduct Research
[Your Name] [Your Position/Title] [Institution or Company Name] [Department or School Name] [Address] [City, State, Zip Code] [Email Address] [Phone Number] [Date]
[Recipient’s Name] [Recipient’s Title] [Organization/Institution Name] [Department Name] [Address] [City, State, Zip Code]
I am [Your Name], a [Your Position, e.g., graduate student, researcher, etc.] at [Your Institution or Company Name], currently working on a project that explores [Brief Description of Research Topic]. My research aims to [Brief Description of Research Aim], which I believe will contribute valuable insights to the field of [Field of Study].
To further this research, I am seeking your permission to [Specific Request, e.g., access specific data, conduct surveys/interviews, use certain facilities, etc.] at [Organization/Institution Name]. [Explain why their organization/institution is crucial for your research, and mention any specific departments, data sets, populations, etc., you wish to access].
I assure you that all research activities will be conducted with the utmost respect for [Organization/Institution Name]’s policies and ethical guidelines. Additionally, I am committed to ensuring the confidentiality and anonymity of all participants and data involved. [If applicable, mention any institutional review board (IRB) approval or ethical clearance you have obtained].
I am more than willing to provide a detailed research proposal, outlining my methodology, ethical considerations, and how the results will be disseminated. Your support would be invaluable to my research, and I am hopeful for a positive response.
Thank you for considering my request. I am looking forward to the possibility of conducting my research with the support of your esteemed [organization/institution]. Please feel free to contact me at [Your Contact Information] for any further information or clarification.
Yours sincerely,
[Your Name] [Your Position/Title] [Institution or Company Name] [Contact Information]
Template 3: Request for Permission for Photography or Filming
I am reaching out to request permission to conduct a [photography/filming] session at your [Location/Venue], specifically in [Specific Location/Area, if applicable]. I am a [Your Occupation/Role, e.g., freelance photographer, filmmaker, etc.], and I am currently working on a project titled “[Project Name],” which focuses on [Brief Description of Project].
Your [Location/Venue] presents the perfect backdrop for this project due to its [Mention Specific Features, e.g., unique architecture, beautiful landscapes, historical significance, etc.]. We believe that capturing our visuals in such a setting would greatly enhance the aesthetic and narrative depth of our project.
The photography/filming is scheduled to take place on [Date] from [Start Time] to [End Time]. Our team will consist of [Number of People in Team], and we plan to use [Brief Description of Equipment]. We will ensure that our activities do not disrupt the normal operations of your [Location/Venue] and will comply with all requested guidelines and restrictions.
We are also prepared to discuss any permit fees or other conditions you may require for granting this permission. Our team is committed to respecting the property and will take all necessary steps to leave the location in its original state post-production.
Thank you for considering our request. We are excited about the possibility of using your beautiful location for our project and are hopeful for a positive response. Please let me know if there are any forms I need to complete or if there are any further details or discussions you would require.
Warm regards,
[Your Name] [Your Occupation/Role] [Contact Information]
Each template can be adjusted based on your specific needs and the details of your request. It’s important to personalize the letter with the recipient’s name and details relevant to your request to make it as effective as possible
Related Articles
Permission letter for child to travel with friend: how to draft it right, sample letter of permission to travel for a wedding: free & effective, sample letter of request asking permission to use a school venue: free & effective, sample letter of permission to use property: free & effective, sample letter to request to attend a conference: free & effective, permission letter to leave early from school: the simple way, leave a comment cancel reply.
Your email address will not be published. Required fields are marked *

Permission Letter for Research
A permission letter for research is written in respect of a request letter for conducting a research program in a certain field of the interest. The letter grants one to carry on his/her research program after observing the benefits of research for a wide perspective. An authority of the concerned department issues such letter to allow an organization or a company to go further in their proposal. The letter also deals with the details of the research program in order to help the company.
Here in the example stated below, an authority writes this letter to permit a research group for conducting their research on the flora and fauna in a certain area.
Sample Permission Letter for Research
Jonathan Browne
Research student,
Winstonville College,
Redgrove Road,
October 8, 2014
Subject: Permission for conducting research
Dear Mr. Browne,
I am pleased to inform that I give you permission in respect of your research request of studying the flora and fauna around the Livingstone Lake under the principal investigator Mr. Lawrence Dawkings of Winstonville College. Your initiative is appreciable and I am ready to support this research at my best.
As I also cherish the same thought, the Livingstone property is the great resource of the flora and fauna and if your group explores this thing scientifically, it would be a great thing. Please visit website www.lakelivingstone.org that tells you about the rules and regulation of the property and gives you some important information that may help in your study.
We wish you all the best in your research.
Thanking you,
Yours truly,
Riley Jones
Given Below are a few Permission Letter samples for a clearer Idea.
Police Permission Letter for Loudspeaker in English If you have a wedding in your family or there is a party in your house and you are planning to have a DJ arrangement or loudspeaker, it is important that you get permission from police.
Permission Letter for Industrial Visit A permission letter for industrial visit, as its name says, is written for seeking the permission of an industrial visit as a part of the educational system.
Permission Letter For A Project A permission letter for a project is written to seek permission from an authority for conducting a project.
Field Trip Permission Letter A field trip permission letter is written to permit an individual going for a field trip and the letter
Permission Giving Letter A permission giving letter is written to allow an individual for doing a certain task so the letter
Top Search:
- letter of permission for research
- letter of permission to conduct research
View all contributions by Marisa
Related Posts
Leave a Comment
Next post: 7 Ideas for Writing a Permission Letter for Research
Previous post: Tips on How to Write a Project Permission Letter
- Acceptance Letters
- Acknowledgement Letters
- Advice Letter
- Agreement Letter
- Announcement Letter
- Apology Letter
- Appeal Letter
- Application Letter
- Appointment Letter
- Appraisal Letter
- Appreciation Letter
- Approval Letter
- Authorization Letter
- Birthday Letter
- Breakup Letter
- Business Letter
- Cancellation Letter
- Certificate Format
- Certification Letter
- Charity Letter
- Claim Letter
- Collection Letter
- Complaint Letter
- Compliment Letter
- Condolence Letter
- Confirmation Letter
- Congratulations Letter
- Consent Letter
- Cover Letter
- Credit Letter
- Criticism Letter
- Delegation Letters
- Dismissal Letter
- Dispute Letter
- Donation Letter
- Employee Letter
- Encouragement Letters
- Endorsement Letter
- Evaluation Letter
- Farewell Letter
- Feedback Letter
- Follow Up Letter
- Friendly Letter
- Friendship Letter
- Fundraising Letter
- Get Well Letters
- Goodbye Letter
- Grievance Letter
- Inquiry Letter
- Internship Letter
- Interview Letter
- Introduction Letter
- Invitation Letter
- Leave Letter
- Love Letter
- Marketing Letter
- Memo Formats
- Miscellaneous Letter
- Notification Letters
- Order Letter
- Permission Letter
- Promotion Letter
- Proposal Letter
- Recommendation Letter
- Reference Letter
- Request Letter
- Resignation Letter
- Retirement Letter
- Romantic Letter
- Sales Letter
- Scholarship Letter
- Sorry Letter
- Sponsorship Letter
- Suggestion Letter
- Sympathy Letter
- Termination Letter
- Thank You Letter
- Transfer Letter
- Transmittal Letter
- Uncategorized
- Verification Letter
- Warning Letter
- Welcome Letter
- Withdrawal Letters
Recent Posts
- Letter Format Due to Late Fee by School
- Letter of Contract Agreement for Teachers Template
- Simple Letter of Consent for New Project Format
- Letter of Consent for Child to Travel with Grandparents
- Sample Letter Format to Judge Asking for Leniency by Wife
Popular Letters
- transfer request letter due to family problem
- goodbye letter to co-workers
- personal thank you letter appreciation
- self introduction letter
- apology letter to customer
- transfer request letter due to parent\s illness
- apology letter to teacher for not attending class
- application letter for teacher job for fresher
- Resignation Letter 2 Week Notice
- letter of interest for a job
Sample Donation Letter
School Donation Request Letter Template Sponsorship Thank You Letter Donation Letter for a Sick Person Donation Letter for Flood Victims Donation Request Letter for Cancer Patients Donation Thank You Letter How to Write a Donation Letter
Letter Writing Tips
Tips to Write a Wedding Welcome Letter Tips for Writing an Employee Warning Letter Tips for Writing a Transmittal Letter Tips for Writing a Employee Transfer Letter How to Write a Thank You Letter How to Write a Contract Termination Letter Tips for Writing an Effective Sponsorship Letter
Word & Excel Templates
Printable Word and Excel Templates
Request Letter for Research Project Collaboration/acceptance
- Download 1009
- File Size 23 KB
- File Count 1
- Create Date June 7, 2023
- Last Updated June 7, 2023

- Library Guides
- Survey & Instrument Permissions
Copyright: Survey & Instrument Permissions
- Requesting Permissions to Use and Reproduce Instruments in a Thesis/Dissertation [PDF] Download a PDF copy of the below FAQ
- Sample Permission Letter Template Download a template letter for requesting permissions
Permissions to Use and Reproduce Instruments in a Thesis/Dissertation Frequently Asked Questions
Why might i need permission to use an instrument in my thesis/dissertation.
- How do I know if I need permission to use instruments in my thesis/dissertation research?
- How do I ask for permission to use and reproduce an instrument in my thesis/dissertation?
- What if I can't locate the copyright holder?
If you want to use surveys, questionnaires, interview questions, tests, measures, or other instruments created by other people, you are required to locate and follow usage permissions. The instrument may be protected by copyright and/or licensing restrictions.
Copyright Protection
Copyright provides authors of original creative work with limited control over the reproduction and distribution of that work. Under United States law, all original expressions that are “fixed in a tangible medium” are automatically protected by copyright at the time of their creation. In other words, it is not necessary to formally state a declaration of copyright, to use the © symbol, or to register with the United States Copyright Office.
Therefore, you must assume that any material you find is copyrighted, unless you have evidence otherwise. This is the case whether you find the instrument openly on the web, in a library database, or reproduced in a journal article. It is your legal and ethical responsibility to obtain permission to use, modify, and/or reproduce the instrument.
If you use and/or reproduce material in your thesis/dissertation beyond the limits outlined by the “fair use” doctrine, which allows for limited use of a work, without first gaining the copyright holder’s permission, you may be infringing copyright.
Licensing/Terms of Use
Some instruments are explicitly distributed under a license agreement or terms of use. Unlike copyright, which applies automatically, users must agree to these terms in order to use the instrument. In exchange for abiding by the terms, the copyright holder grants the licensee specific and limited rights, such as the right to use the instrument in scholarly research, or to reproduce the instrument in a publication.
When you ask a copyright holder for permission to use or reproduce an instrument, you are in effect asking for a license to do those things.
How do I know if I need permission to use instruments in my thesis/dissertation research? (Adapted from Hathcock & Crews )
Follow the four-step process below:
1. Determine whether you need permission
There are different levels of permissions for using an instrument:
a) No permission required
i. The copyright holder has explicitly licensed the use of instrument for any purpose, without requiring you to obtain permission.
ii. If you are only using a limited portion of the instrument, your use may be covered under the Fair Use Doctrine. See more the University of Minnesota’s Thinking Through Fair Use tool at https://www.lib.umn.edu/copyright/fairthoughts .
iii. If the instrument was developed by the federal government or under a government grant it may be in the public domain, and permission is therefore not required.
iv. If the document was created before 1977, it may be in the public domain, and permission is therefore not required. See the Stanford Public Domain Flowchart at https://fairuse.stanford.edu/wp-content/uploads/2014/06/publicdomainflowchart.png .
b) Non-commercial/educational use: The copyright holder has licensed the instrument only for non-commercial research or educational purposes, without requiring you to obtain the permission of the copyright holder. Any other usage requires permission.
Sample Permission for Educational Use:
Test content may be reproduced and used for non-commercial research and educational purposes without seeking written permission. Distribution must be controlled, meaning only to the participants engaged in the research or enrolled in the educational activity. Any other type of reproduction or distribution of test content is not authorized without written permission from the author and publisher. Always include a credit line that contains the source citation and copyright owner when writing about or using any test.
Source: Marta Soto, “How Permissions Work in PsycTests,” APA Databases & Electronic Resources Blog. American Psychological Association. http://blog.apapubs.org/2016/12/21/how-permissions-work-in-psyctests/ .
Even if you are not required to obtain permission to use the instrument, consider contacting the author for ideas on how to administer and analyze the test. Authors often welcome further use of their work, and may request you send them a copy of your final work.
c) Permission required: Instruments that require you to obtain the permission of the copyright holder, regardless of whether the use is for educational or commercial purposes. This may be because the copyright holder
- has important directions for how the test must be administered and analyzed
- wants to make sure the most current version is being used
- charges users a fee in order to administer the test
If you cannot locate the permissions, you are required to identify the copyright holder and contact them to ask about permission to use the instrument.
2. Identify the copyright holder (Adapted from Crews )
The next step is to identify who owns the copyright. The copyright holder is usually the creator of the work. If the copyright owner is an individual, you will need to do the usual Internet and telephone searches to find the person. Be ready to introduce yourself and to explain carefully what you are seeking.
Some authors transfer copyright to another entity, such as a journal publisher or an organization. In these cases, you must obtain permission from that entity to use or reproduce the instrument. You can often identify the owner by locating a © copyright notice, but as mentioned above, not all copyrighted works have a notice.
Check the following sources to locate instruments, their copyright holders, and their permission statements:
- Goldman, Bert A., and American Psychological Association. Directory of Unpublished Experimental Mental Measures., 1974. (print): https://okstate-stillwater.primo.exlibrisgroup.com/permalink/01OKSTATESTILL_OKSTAT/16c9pn6/alma998132075502681
- EBSCO Publishing, and Buros Institute of Mental Measurements. Mental Measurements Yearbook. Ipswich, MA: EBSCO Publishing, 1990. Available at https://library.okstate.edu/databases/m/mental-measurements-yearbook
- PsycTESTS: https://library.okstate.edu/databases/p/psyctests
- OSU Library guide to Finding Tests & Measurements: https://info.library.okstate.edu/tests
You may need to contact the author or publisher directly to find out who owns the copyright. Publishers often have websites that prescribe a method for contacting the copyright owner, so search the publisher website for a permissions department or contact person. Be sure to confirm the exact name and address of the addressee, and call/e-mail the person or publishing house to confirm the copyright ownership.
- The copyright owner may prefer or require that permission requests be made using a certain medium (i.e. fax, mail, web form, etc.). If you do not follow instructions, you may not get a reply.
- Telephone calls may be the quickest method for getting a response from the owner, but they should be followed up with a letter or e-mail in order to document the exact scope of the permission. E-mail permissions are legally acceptable in most cases, but getting a genuine signature is usually best.
- The request should be sent to the individual copyright holder (when applicable) or permissions department of the publisher in question. Be sure to include your return address, telephone and fax numbers, e-mail address, and the date at the top of your letter or message. If you send the permission request by mail, include a self-addressed, stamped return envelope.
- Make the process easy for the copyright owner. The less effort the owner has to put forth, the more likely you will get the permission you need. If you are using conventional mail, include a second copy of your request for the owner’s records.
- State clearly who you are, your institutional affiliation (e.g., Oklahoma State University), and the general nature of your thesis/dissertation research.
Do not send permissions letters to all possible rightsholders simultaneously. Taking the time to find the person who most likely holds the copyright will better yield success. If you do not have much information about who actually owns the copyright, be honest with your contacts, and they may be able to help you find the right person.
How do I ask for permission to use and reproduce an instrument in my thesis/dissertation? (Adapted from Crews )
Once you have identified the copyright holder, you must determine the scope of your permission request. Some copyright owners furnish their own permission form that you may download from their website.
If the copyright owner does not provide a permission agreement form, you may write your own letter ( click here to download a template ). Requests should be made in writing; e-mail is fine for this purpose. A most effective letter will include detailed information concerning your request for permission to use the work. Include the following information:
- Who: Introduce yourself. Tell who you are, your degree program, and a brief overview of your research.
- Why: Tell why you are contacting that person or entity for permission.
- What: Be as specific as possible when you cite and describe the instrument you wish to use. Include whether you plan to use the entire instrument, or if you plan on modifying or adapting any of the questions.
- How: Tell how you plan to use the instrument. Specify the parameters of your research study, and include any important information about the way you will administer the instrument and/or analyze the results.
- When: Expected length of the project and time to complete the thesis/dissertation.
Important : Obtaining permission to use an instrument is not the same as obtaining permission to reproduce the instrument in your appendix. If you intend on providing a copy of the instrument in an appendix, ask for separate permissions to do that.
Click here to download a template letter . Feel free to modify and adapt this template for your purposes.
Keep a record
After securing permission to use and/or reproduce the instrument, save a copy of the correspondence and the agreement. Documentation allows you to demonstrate to others that you have the legal right to use the owner's work. In the unlikely event that your use of the work is ever challenged, you will need to demonstrate your good faith efforts. That challenge could arise far in the future, so keep a permanent file of the records. Moreover, you might need to contact that same copyright owner again for a later use of the work, and your notes from the past will make the task easier.
Upload a copy of your permission letter in Vireo with your thesis/dissertation, or include it as an appendix in the document itself.
What if I can't locate the copyright holder? (Adapted from Hathcock & Crews & Pantalony )
In some cases, you may never get a response from the copyright holder or you may never even be able to identify who they are or how to contact them. It can be difficult to know how to proceed when you reach a dead end. Unfortunately, no matter how diligently you have tried to get permission, these efforts cannot completely eliminate the risk of infringement should you proceed to use the work.
Assuming you have diligently investigated your alternatives, do not want to change your project, and remain in need of the elusive copyright permission, the remaining alternative is to explore a risk-benefit analysis. You need to balance the benefits of using that particular material in your given project against the risks that a copyright owner may see your project, identify the materials, and assert the owner’s legal claims against you. Numerous factual circumstances may be important in this evaluation. The “benefit” may depend upon the importance of your project and the importance of using that particular material. The “risks” may depend upon whether your project will be published or available on the Internet for widespread access—as theses and dissertations will. You ought to investigate whether the work is registered with the U.S. Copyright Office and weigh the thoroughness of your search for the copyright owner and your quest for appropriate permission.
Undertaking this analysis can be sensitive and must be advanced with caution and with careful documentation. You may be acting to reduce the risk of liability, but you have not eliminated liability. A copyright owner may still hold rights to the material. Members of the Oklahoma State University community should consult with their chair, the OSU Library, and/or the OSU Office of Legal Counsel at https://regents.okstate.edu/office-legal-counsel to discuss their options.
Crews, Kenneth and Rina Elster Pantalony. “Special Cases.” Columbia University Copyright Advisory Services. https://copyright.columbia.edu/basics/special-cases.html . Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
Crews, Kenneth. “Asking for Permission.” Columbia University Advisory Services. https://copyright.columbia.edu/basics/permissions-and-licensing.html . Licensed under Creative Commons Attribution 4.0 International (CC BY 4.0).
Hathcock, April. “Getting Permission.” NYU Libraries Copyright Library Guide, https://guides.nyu.edu/c.php?g=276785&p=1845968 . Licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
This guide was originally compiled and published by Clarke Iakovakis at the University of Houston-Clear Lake at https://uhcl.libguides.com/ETD/permissions . The same author has recompiled, adapted, and redistributed the material on this page.
- Last Updated: Aug 1, 2023 10:44 AM
- URL: https://info.library.okstate.edu/copyrightbasics
Calculate the price
Minimum Price
Hire experienced tutors to satisfy your "write essay for me" requests.
Enjoy free originality reports, 24/7 support, and unlimited edits for 30 days after completion.
Is buying essays online safe?
Shopping through online platforms is a highly controversial issue. Naturally, you cannot be completely sure when placing an order through an unfamiliar site, with which you have never cooperated. That is why we recommend that people contact trusted companies that have hundreds of positive reviews.
As for buying essays through sites, then you need to be as careful as possible and carefully check every detail. Read company reviews on third-party sources or ask a question on the forum. Check out the guarantees given by the specialists and discuss cooperation with the company manager. Do not transfer money to someone else's account until they send you a document with an essay for review.
Good online platforms provide certificates and some personal data so that the client can have the necessary information about the service manual. Service employees should immediately calculate the cost of the order for you and in the process of work are not entitled to add a percentage to this amount, if you do not make additional edits and preferences.
1035 Natoma Street, San Francisco
This exquisite Edwardian single-family house has a 1344 Sqft main…
My experience here started with an essay on English lit. As of today, it is quite difficult for me to imagine my life without these awesome writers. Thanks. Always.
Finished Papers

IMAGES
VIDEO
COMMENTS
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group. It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally.
Template: 1. To, (Name of the sender) (Designation of the respective person) (Name of the address) Subject- letter of permission for research. Respected madam/sir, This letter is to inform (mention the name of the person) that I (mention your name) student of (mention the name of the college or name of the institution) would like to say to you ...
I am writing this letter to seek your permission and allowance for the above-said research. This research will be conducted under the guidance of Mr./Mrs./Ms. ____________ (Name of the Professor). I shall be highly obliged if you look into this matter and approve my request.
A permission request letter is one where an individual, organization, or group is requesting permission to perform an act or obtain information. A Permission request letter for data collection for research is one where someone, such as a college student, is requesting data to complete research work. For instance, if someone is attending ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
If the copyright owner does not provide a permission agreement form, you may write your own letter (click here to download a template). Requests should be made in writing; e-mail is fine for this purpose. A most effective letter will include detailed information concerning your request for permission to use the work.
Request to reproduce material in a [thesis/major research paper] I am a graduate student at Brock University [in X program - e.g. completing my PhD in Psychology] and am preparing my final [thesis/major research paper]. I would like your permission to include in my [thesis/major research paper] [an excerpt/excerpts] from your
Writing a research proposal letter requires careful consideration of the recipient's needs and interests. By following the proper format and structure and clearly conveying your research objectives, methodology, and potential outcomes, you increase the chances of getting your proposal accepted or securing a collaboration opportunity.
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS) Phone: (734) 936-0933. Fax: (734) 936-1852. [email protected]. Defines the term "informed consent process" and provides tips and other information to craft an appropriate informed consent document for a human subjects study and Univeristy of Michigan IRB review.
The same three-step wizard is used for submission of letters of intent and proposals. A user with the PI, SPO, or AOR role can initiate and submit a letter of intent in Research.gov. For a funding opportunity that requires AOR submission, the PI or SPO must share edit access including submission permission for the letter of intent with the AOR. 1
A written permission letter is a vital document that grants access to laboratory facilities, and it is frequently required of students and researchers for their projects, experiments, and research studies. This article aims to simplify the concept of permission letters, shedding light on their importance, the typical addressees, and the process of crafting an effective one. A permission letter ...
General Consent Form Templates. Social and Behavioral Research Projects (last updated 03/16/2023) Biomedical Research Projects (last updated 07/18/2022) Consent Form Templates for Specific Biomedical Procedures. MRI and fMRI. Blood Collection by Finger Stick. Blood Collection by Venipuncture. Oral Consent Template. Guidance for Protocols ...
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
The consent signature requirements from the mother and father are summarised in table 3. Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient's health (mother and/or fetus) will be in danger.
Permission Letter for Research - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free.
Template 2: Request for Permission to Conduct Research. I am [Your Name], a [Your Position, e.g., graduate student, researcher, etc.] at [Your Institution or Company Name], currently working on a project that explores [Brief Description of Research Topic]. My research aims to [Brief Description of Research Aim], which I believe will contribute ...
In the Permission Letters and Emails, you'll find templates designed to help you formally request or grant approval for various activities or actions. These templates cover a wide range of scenarios, from seeking permission for a field trip, conducting research, using copyrighted materials, to granting access to personal data and many more.
A permission letter for research is written in respect of a request letter for conducting a research program in a certain field of the interest. ... authority of the concerned department issues such letter to allow an organization or a company to go further in their proposal. The letter also deals with the details of the research program in ...
Re. Request for Research Project Acceptance. Hello Mr./Ms. [NAME HERE]. I am writing this letter to express that I am interested in conducting a research project under your kind guidance and supervision. I am a [mention your year/semester/session] student in [degree and program] at [university name]. After a lot of thought and thorough study of ...
Follow the four-step process below: 1. Determine whether you need permission. There are different levels of permissions for using an instrument: a) No permission required. i. The copyright holder has explicitly licensed the use of instrument for any purpose, without requiring you to obtain permission. ii.
Permission Letter For Research Proposal, Essay On Disadvantages Of Artificial Intelligence, Essay Of Lualhati Bautista, Examples Of Nursing Smart Goal About Proffessionalism, Truman Show Symbolism Essay, Scholarship Editing Services Usa, Realtor Resume Free Sample