
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems

Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Chemistry Worksheets and Handouts (PDF for Printing)

This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Here is a list of worksheets. This site also has articles explaining these topics in detail.
- Label Parts of the Atom [ Google Apps worksheet ][ worksheet PDF ][ worksheet PNG ][ answers PNG ]
- Acid formulas [ PDF ][ Answers ]
- Balancing equations Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ] Worksheet #3 [ PDF ][ Answers ] Worksheet #4 [ PDF ][ Answers ]
- Chemical and Physical Changes [ PDF ][ Answers ]
- Chemistry scavenger hunt [ PDF clues ][ Answers ]
- Element names crossword [ PDF ][ Answers ]
- Element symbols – Symbols that make words [ PDF worksheet ][ Answers ]
- Element symbols – Countries of the world [ PDF ][ Answers ]
- More element symbol worksheets
- Homogeneous or Heterogeneous Mixtures [ PDF ][ Answers ]
- Intensive and Extensive Properties [ Worksheet ][ Answer Key ]
- Intrinsic and Extrinsic Properties [ PDF ][ Answers ]
- Ionic and Covalent Compounds (Names and Identification) [ PDF Worksheet ][ Answer Key ]
- Ionic Compound Names and Formulas [ PDF Worksheet ][ Answer Key ]
- Metric to English Unit Conversions [ PDF Worksheet ][ Answer Key ]
- Mixtures [ PDF ][ Answers ]
- Periodic table scavenger hunt [ PDF clues ][ Answers ]
- Reading a meniscus [ PDF ][ Answers ]
- Reading periodic table element information Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ]
- Scientific Notation [ PDF ][ Answers ]
- Significant digits Rules [ PDF ][ Answers ] Addition and subtraction [ PDF ][ Answers ] Multiplication and division [ PDF ][ Answers ]
- Types of Chemical Reactions [ Worksheet ][ Answers ]
In addition to these chemistry worksheets, there is a collection of word search puzzles .
Chemistry Handouts
These chemistry handouts illustrate chemistry concepts and offer examples.
- Amino acid side chains [ PDF ]
- Antimatter examples [ PNG ]
- Atom facts [ PNG ]
- Chemical properties [ JPG ]
- Colligative properties [ JPG ]
- Electron configurations [ PDF ]
- Element electronegativities [ PDF ]
- 118 Element Flash Cards [ PDF ]
- Element list [ PDF ]
- Endothermic reactions [ PNG ]
- Error calculations [ JPG ]
- Exothermic reactions [ JPG ]
- Heterogeneous mixtures [ JPG ]
- Hydrocarbon prefixes [ JPG ]
- Ionic compound properties [ PNG ]
- Genetic codons [ PDF ]
- Lewis structures [ JPG ]
- Litmus test [ PNG ]
- Magnetic vs non-magnetic metals [ JPG ]
- Mole ratio [ JPG ]
- Organic vs inorganic [ JPG ]
- Oxidation numbers [ JPG ]
- Periodic table Bingo game [ PDF ]
- pH indicators [ PNG ]
- Physical change [ JPG ]
- Physical properties [JPG ]
- Noble metals [ JPG ]
- Reactants and products [ JPG ]
- RNA vs DNA [ JPG ]
- States of matter [ JPG ]
- Visible spectrum [ JPG ]
Periodic Tables
There’s a printable periodic table for just about any purpose, but some of the most popular are listed here.

- 118 element vibrant periodic table [ PNG ]
- Actinides [ JPG ]
- Blank periodic table [ PDF ]
- Element charges [ JPG ]
- Element density [ PDF ]
- Element electrical conductivity [ PDF ]
- Element state of matter [ PDF ]
- Muted color 118 element periodic table [ PDF ]
- Native elements [ JPG ]
- Valence [ JPG ]

Biology Worksheets and Handouts
Is biology more your thing? We’ve got similar resources for the life sciences, including biology, biochemistry, cell biology, and anatomy.
Chemistry Worksheets Terms of Use
You are welcome to print these resources for personal or classroom use. They may be used as handouts or posters. They may not be posted elsewhere online, sold, or used on products for sale.
This page doesn’t include all of the assets on the Science Notes site. If there’s a table or worksheet you need but don’t see, just let us know!
Related Posts
Browse Course Material
Course info, instructors.
- Dr. Kimberly Berkowski
- Prof. Sarah O’Connor
Departments
As taught in.
- Organic Chemistry
Learning Resource Types
Organic chemistry i, assignments.

You are leaving MIT OpenCourseWare
Module 2: Atoms, Molecules, and Ions
Assignment: atoms, molecules, and ions.
This assignment can be found in Google Docs: Chemistry for Majors Assignment: Atoms, Molecules, and Ions
To make your own copy to edit:
- If you want a Google Doc : in the file menu of the open document, click “Make a copy.” This will give you your own Google Doc to work from.
- If you want a PDF or Word file : in the file menu of the open document, click “Download” and select the file type you would like to have (note: depending on the file type you select, the formatting could get jumbled).
Instructions
As you work these problems, consider and explain:
- What type of question is it?
- How do you know what type of question it is?
- What information are you looking for?
- What information do they give?
- How will you go about solving this?
- Show how to solve the problem.
- Be able to answer for a different reaction, number, set of conditions, etc.
- NH 4 and NH 4 Cl
- ZnO 2 and ZnCl 2
- H 2 O and HCl
- NO and NO 2
- CH 4 and CO 2
- A sample of chemical X is found to contain 5.0 grams of oxygen, 10.0 grams of carbon, and 20.0 grams of nitrogen. The law of definite proportion would predict that a 70 gram sample of chemical X should contain how many grams of carbon?
- All atoms of the same element are identical.
- Compounds are combinations of different atoms.
- A chemical reaction changes the way atoms are grouped together.
- Atoms are indestructible.
- Who was the first scientist to show that atoms emit any negative particles?
- The Rutherford experiment proved the Thomson “plum-pudding” model of the atom to be essentially correct.
- The Rutherford experiment was useful in determining the nuclear charge on the atom.
- Millikan’s oil-drop experiment showed that the charge on any particle was a simple multiple of the charge on the electron.
- The electric discharge tube proved that electrons have a negative charge.
- All of the above experiments gave the results described.
- An atom is mostly empty space.
- Almost all of the mass of the atom is concentrated in the nucleus.
- The protons and neutrons in the nucleus are very tightly packed.
- The number of protons and neutrons is always the same in the neutral atom.
- All of the above statements (A–D) are true.
- [latex]{}_{14}^{16}\text{C}[/latex]
- [latex]{}_{17}^{37}\text{Cl}[/latex]
- [latex]{}_{15}^{32}\text{P}[/latex]
- [latex]{}_{19}^{39}\text{K}[/latex]
- [latex]{}_{14}^{8}\text{N}[/latex]
- The element rhenium (Re) exists as two stable isotopes and 18 unstable isotopes. What does Rhenium-185 have in its nucleus?
- 20 protons and 20 neutrons
- 21 protons and 19 neutrons
- 22 neutrons and 18 protons
- 20 protons and 22 neutrons
- 21 protons and 20 neutrons
- How many protons, neutrons, and electrons does [latex]{}_{20}^{40}\text{Ca}^{2+}[/latex] have?
- How many protons, neutrons, and electrons are in [latex]{}_{19}^{39}\text{K}^+[/latex]
- There is twice as much mass of hydrogen as oxygen in each molecule.
- There are two hydrogen atoms and one oxygen atom per water molecule.
- There is twice as much mass of oxygen as hydrogen in each molecule.
- There are two oxygen atoms and one hydrogen atom per water molecule.
- None of these.
- K, alkali metal
- Ba, alkaline earth metal
- Ne, noble gas
- Ni, transition metal
- Calcium, Ca
- good conductors of heat
- often lustrous
- tend to gain electrons in chemical reactions
- cobalt(II) chloride
- magnesium oxide
- aluminum(III) oxide
- diphosphorus pentoxide
- All of the above names are correct
- iodine trichloride, ICl 3
- phosophorus pentoxide, P 2 O 5
- ammonia, NH 3
- sulfur hexafluoride, SF 6
- All of the above pairs are correct.
- How many oxygen atoms are there in one formula unit of Ca 2+ ?
- What is the correct name for FeO?
- What is the correct name for Ca 2+ ?
- What is the correct name for V 3+ ?
- What is the subscript of barium in the formula of barium sulfate?
- What is the formula for calcium bisulfate?
- Pb(NO 3 ) 2 , lead(II) nitrate
- NH 4 ClO 4 , ammonium perchlorate
- PO 4 3− , phosphate ion
- Mg(OH) 2 , magnesium hydroxide
- NO 3− , nitrite ion
- Authored by : Jessica Garber. Provided by : Tidewater Community College. License : CC BY: Attribution

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.15: Assignment—Matter and Measurement
- Last updated
- Save as PDF
- Page ID 232976
To download a copy of the assignment, please click on the link Sample Questions .
As you work these matter and measurement problems, consider and explain:
- What type of question is it?
- How do you know what type of question it is?
- What information are you looking for?
- What information do they give?
- How will you go about solving this?
- Show how to solve the problem.
- Be able to answer for a different reaction, number, set of conditions, etc.
Sample Questions
Consider the following choices when answering questions 1–2:
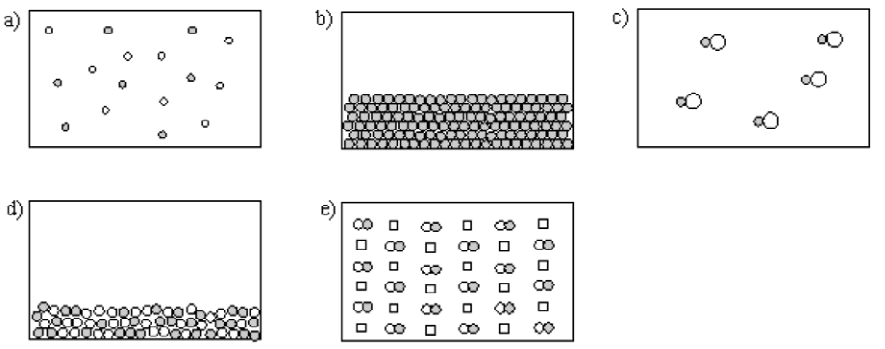
- Which best represents a gaseous compound?
- Which best represents a homogeneous mixture of an element and a compound?
- homogeneous mixture
- heterogeneous mixture
- pure mixture
- distilled mixture
- An example of a pure substance is ________.
- ________ are substances with constant composition that can be broken down into elements by chemical processes.
- The state of matter for an object that has a definite volume but not a definite shape is ________.
- Explain if the boiling of water is a physical or a chemical change and why.
- What is 409 Kelvin in Fahrenheit and in Celsius?
- How many grams are in 8.1 kilograms?
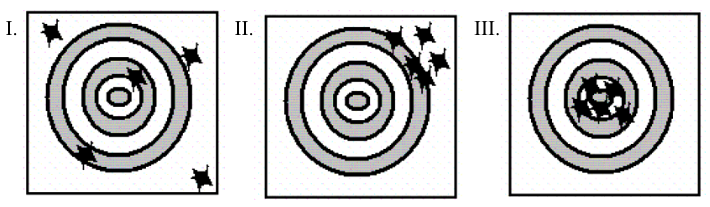
- Figure I only
- Figure II only
- Figure III only
- Figure I and Figure III
- Figure II and Figure III
The balance is
- Both accurate and precise.
- Accurate but imprecise.
- Precise but inaccurate.
- Both inaccurate and imprecise.
- Accuracy and precision are impossible to determine with the available information.
- A scientist obtains the number 0.045006700 on a calculator. If this number actually has four (4) significant figures, how should it be written?
- Express the number 0.000333 in scientific notation.
- Express 165,000 in exponential notation.
- You are asked to determine the perimeter of the cover of your textbook. You measure the length as 39.36 cm and the width as 24.83 cm. How many significant figures should you report for the perimeter?
- Consider the numbers 23.68 and 4.12. The sum of these numbers has ________ significant figures, and the product of these numbers has ________ significant figures.
- How many significant figures are in 0.00110?
- Convert 0.3980 m to mm.
- The distance of 21 km equals how many meters?
- Convert 59.4 mi to km. (1 m = 1.094 yard, 1 mi = 1760 yd)
- For spring break you and some friends plan a road trip to a sunny destination that is 2105 miles away. If you drive a car that gets 33 miles per gallon and gas costs $3.199/gal, about how much will it cost to get to your destination?
- Convert 7.9 kg to lb. (1 kg = 2.205 lb)
- A wavelength of red light is measured at 655 mm. What is this measurement in cm?
- A 20 mL sample of glycerol has a mass of 25.2 grams. What is the mass of a 57-mL sample of glycerol?
[reveal-answer q=”781529″]Show Sample Answers[/reveal-answer] [hidden-answer a=”781529″]
- elements, compounds, pure water, carbon dioxide etc
- liquid state
- physical change because the gaseous water is chemically the same as the liquid
- 8.1 × 10 3
- 3.33 × 10 -4
- 1.65 × 10 5
- 2.1 × 10 4 m
- 9.56 × 10 1 km
- 6.55 × 10 -5 cm
[/hidden-answer]
- Authored by : Jessica Garber. Provided by : Tidewater Community College. License : CC BY: Attribution
Library Subject Guides
- Subject Guides
- Assignment Research
Chemistry: Assignment Research
- Books and ebooks
- Dictionaries, Encyclopedias, Handbooks
- Journal Articles and Databases
- Journal Title Abbreviations
- Data and Properties
- Exam Papers (via AKO | LEARN)
- Past Tests (via AKO | LEARN)
- Products and Prices
- Safety Data Sheets
- Structure Drawing Tools and Nomenclature
- Information Competencies for Chemistry Undergraduates (Wikibook)
- Stages in the Research Process
- Citation Styles and EndNote
- Writing Guides
- Web Lectures
- Stay Current
- For Academics
- Library Navigator
Introduction
This guide to basic assignment research outlines a simple but effective approach to finding information for your assignment. It is based on the resources described elsewhere in this subject guide and on the UC Library web site. Depending on your topic and your level of study, you may need to rearrange or review these steps where necessary
Check the rest of this subject guide carefully for additional subject resources and, where available, appropriate topic guides
1. Define your topic
Make sure you understand the topic. Identify the main concepts or keywords in your question to help you develop a search strategy.
2. Gather background information

Use dictionaries and encyclopedias to find definitions and background information. Articles from specialised subject encyclopedias are authoritative and often substantial
Read more on
- Dictionaries, Encyclopedias and Handbooks for Chemistry
3. Think about what information you need

- How much information do you need? Lecturers often give guidelines on the number of sources you should use
- Do you need current information or is older material relevant? Sometimes you might need both, as you might have to give both the historic background and the current situation of a topic
- Do you need primary sources that give an original account of research, or secondary sources that are interpretations of someone else's work?
If you do not understand what you have to do for an assignment, ask your lecturer, your tutor or someone at the Academic Skills Centre
4. Find books
Search the library catalogue
- Check for books on High Demand .
- Use Title and Keyword anywhere searches to find additional material.
- When you find a useful title, click its subject headings to find books on similar subjects.

Read more on:
- Finding Chemistry Books and Ebooks
5. Find journal articles

6. Find information on the Internet

- a government department (.gov or .govt.nz)
- an academic (.edu or .ac.nz or published in a reputable journal)
- a business (what are they selling?)
- or a random non-expert?
Use Google Scholar to find reliable journal articles, or the Advanced Search features of Google to restrict your search to results from more reputable sources.
- Web searching
7. Evaluate your sources

- Critically Analyzing Information Sources (Cornell University)
- How to spot fake news .
8. Cite your sources
- Citing your sources
9. Write your assignment

See our writing guides page for books that have useful hints for writing on technical subjects.
Visit the Academic Skills Centre for workshops and/or personal help.
- << Previous: Websites
- Next: Information Competencies for Chemistry Undergraduates (Wikibook) >>
- Last Updated: Mar 8, 2024 10:37 AM
- URL: https://canterbury.libguides.com/chem
Home / Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
Stereochemistry and Chirality
By James Ashenhurst
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
Last updated: February 20th, 2024 |
Assigning R and S Configurations With the Cahn-Ingold-Prelog (CIP) Rules
- Enantiomers are stereoisomers that are non-superimposable mirror images of each other (by the way, molecules that are superimposable mirror images of each other are considered to be identical molecules).
- Enantiomers rotate plane-polarized light in equal and opposite directions, but there is no straightforward way to trace back the absolute configuration of a molecule to the direction of optical rotation
- A tetrahedral atom with four different substituents (a chiral center) can have two different configurations. A naming scheme developed by Cahn, Ingold and Prelog (CIP) is used for assigning the terms R or S to each chiral center. (When all the (R,S) designations of a molecule are specified, this is referred to as its “ absolute configuration”.)
- In the CIP protocol, each atom attached to the chiral center is ranked in order of atomic number (highest = #1, lowest = #4). (We go further down the chain in the event of ties. )
- With the #4 substituent in the back: if #1, #2, and #3 trace a clockwise path, the chiral center is assigned (R) . If they trace a counterclockwise path the chiral center is (S) .
- When #4 is in the front or on the side, some useful tips and tricks can be used to avoid having to rotate the whole molecule ( See also: How To Draw The Enantiomer of A Chiral Molecule ).
- For breaking ties, it’s useful to keep track of which carbons you’re working on with the “dot method”.
Table of Contents
- Chiral Centers And The Problem Of Naming
- The Cahn-Ingold-Prelog System For Naming Chiral Centers
- Oh No! What Do We Do When #4 Is Not In The Back?
- Assigning R/S When #4 Is In The Front: A Short Cut
- Assigning R/S When #4 Is In The Plane Of The Page
- Breaking Ties With The “Dot Technique”
- Conclusion: Assigning R and S With CIP
(Advanced) References and Further Reading
This post was co-authored by Matt Pierce of Organic Chemistry Solutions . Ask Matt about scheduling an online tutoring session here .
1. Chiral Centers And The Problem Of Naming
Previously on MOC we’ve described enantiomers : molecules that are non-superimposable mirror images of each other. Perhaps the most memorable example is these “enantiocats”.
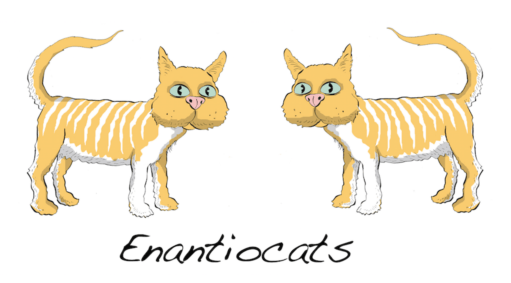
Each of these cats is said to be “chiral”: they lack a plane of symmetry.
What causes molecules to have chirality?
The most common source of chirality is a “chiral centre”: typically a tetrahedral carbon attached to four different “groups”, or “substituents”. For each chiral centre there are two (and only two!) different ways of arranging the 4 different substituents, which gives rise to two different configurations. [If you don’t believe there are only two, see Single Swap Rule ].
The purpose of this post is to introduce and describe the nomenclature we use to describe these configurations: the (R)/(S) notation, or Cahn-Ingold Prelog Rules.
Let’s look at a simple example.
Both of these molecules are 1-bromo-1-chloroethane. But they are not exactly the same molecule, in the same way that your left shoe is not exactly the same as your right. They are non-superimposable mirror images of each other. How do we communicate this difference?
One way would be to describe their physical properties. For example, although these two molecules have the same boiling point, melting point, and share many other physical properties, they rotate plane-polarized light in equal and opposite directions, a property called optical rotation ( See Optical Rotation and Optical Activity ) We could use (+)-1-bromo-1-chloroethane to refer to the isomer that rotates polarized light to the right (clockwise, or “dextrorotatory”) and use (-)-1-bromo-1-chloroethane to refer to the isomer that rotates polarized light to the left (counterclockwise, or “levorotatory”).
However this nomenclature suffers from a serious problem. There is no simple correlation between the arrangement of substituents around a chiral centre and the direction in which polarized light is rotated . Another solution is needed.
2. The Cahn Ingold Prelog (CIP) System For Naming Chiral Centers
A solution to this quandary was proposed by Robert Cahn, Chris Ingold, and Vladimir Prelog in 1966. The resulting “CIP” protocol works as follows:
- Prioritize the four groups around a chiral center according to atomic number . The highest atomic number is assigned priority #1, and the lowest atomic number is assigned priority #4.
- Orient the chiral centre such that the #4 priority substituent is pointing away from the viewer. For our purposes, it’s enough for it merely to be attached to a “dashed” bond.
- If the path traced from 1-2-3 is clockwise , the chiral center is assigned ( R ) (from Latin, rectus )
- If the path traced is counter clockwise , the chiral center is assigned ( S ) (from the Latin sinister)
- Now we have a better way to describe molecules [A] and [B] shown above. Molecule [A] is named ( R )-1-bromo-1-chloroethane, and molecule [B] is named ( S )-1-bromo-1-chloroethane:
We should reiterate that the designations (R) and (S) bear no relationship to whether a molecule rotates plane-polarized light clockwise (+) or counterclockwise (-). For example the most common naturally occurring configuration of the amino acid alanine is (S), but its optical rotation (in aqueous acid solution) is (+).
3. What About When #4 Is Not In The Back?
That seems simple enough! “Is that it?”, you might ask.
Uh, no. As it happens, there’s a few bumps in the road toward determining (R)/(S) once we get beyond the simple example above.
These “trickier cases” fall into three main categories.
- What if the #4 substituent is not helpfully pointing away from the viewer , as it was in our example above? What if it’s in the “front” (i.e. attached to a “wedged” bond) or, heaven forbid, in the plane of the page?
- Assigning priorities in complex situations . What do we do in situations where a chiral centre has two or more identical atoms attached? In other words, how do we break ties?
- What do we do if the molecule is drawn a peculiar way , such as in Fischer or Newman projections ?
We’re not going to be able to fully address all of these issues in this post. But we can certainly deal with #1 and make some headway with #2. For #3, see How To Determine R/S On A Fischer Projection and How to Determine R/S on a Newman Projection
4. Determining R/S When The #4 Substituent Is In Front (i.e. on a “Wedge”): A Short Cut
Let’s first consider the molecule below. The name of this molecule is ( R )-1-fluoroethanol. It is listed below with priorities assigned based on atomic number. In this case F>O>C>H. So F is #1 and H is #4. The tricky part here is that the #4 priority is pointing out of the page (on a “wedge”).
How do we determine (R)/(S) in this case? There are two ways to do it.
Many instructors will tell you to “simply” rotate the molecule in your head so that the #4 priority is on a dash. Then you can assign R or S in the traditional way. This “simple” advice is not always an easy task for beginners.
Thankfully, it is technically unnecessary to perform such a mental rotation.
Here’s a way around this. When the #4 priority is on a wedge you can just reverse the rules. So now we have two sets of rules:
If the #4 priority is on a dash :
- Clockwise = R
- Counterclockwise = S
If the #4 priority is on a wedge , reverse the typical rules:
- Clockwise = S
- Counterclockwise = R
R and S can easily be assigned to either picture of the molecule. I still encourage you to use a model kit and learn how to do so, however. Organic chemistry is much easier to understand, and much more beautiful, if you can master how to visualize a tetrahedral carbon atom.
See also, How To Draw The Enantiomer of A Chiral Molecule
5. Determining R/S When The #4 Group Is In The Plane Of The Page?
What if the #4 priority is in the plane of the paper, for example on a line? In this case it’s impossible to assign R and S in the traditional way. You’d have a 50:50 shot of getting it correct: not good odds. Again, if you can redraw the molecule in your head, then it’s better to just do that. If you can’t do this reliably then you need to learn the “single swap” concept.
Here’s how it works. Swapping any two groups on a chiral centre will flip the configuration of the chiral centre from R to S (and vice versa). [ We previously talked about the “single swap rule” here ]
Knowing this, we can do a nifty trick.
- Take the #4 substituent (in the plane of the page) and swap it with the substituent in the back [If the configuration is R, this will switch it to S. If the configuration is S, this will flip it to R. We’ll need to account for this in step #3].
- Redraw the chiral centre, and determine R/S on the new chiral centre which now has group #4 in the back.
- Whatever result you got, flip it to its opposite to account for the fact that you did a single swap in step #1.
Here’s an example. Note that here I first switched #4 and #3, but the main point is to switch two groups so that #4 is out of the plane of the paper.
This method always works, assuming you’ve determined the four priorities accurately. (It also works for cases when #4 is on a wedge).
However, sometimes we’re not in the position of dealing with 4 different atoms attached to a chiral carbon. For instance, it’s possible to have chiral carbons which are attached to 4 carbons . So how do we break the ties in these cases?
6. Determining CIP Priorities: Breaking “Ties” With The “Dot Technique”
The quick answer is to use the “dot technique”. Here’s how it works. Let’s do it for 4-ethyl-4-methyloctane, above.
- Go outward from the chiral centre to each of the surrounding 4 atoms and assign priorities (based on atomic number) to each of these atoms. [Sometimes it’s helpful to draw dots on each of these atoms.]
3. Compare each list, atom by atom. In our example, since C>H, (C,H,H) takes priority over (H,H,H) so the CH 3 group is assigned priority #4.
4. If there is still a tie, move the dots to the highest ranking atom in the list (i.e. the atom with highest atomic number). The dots are helpful because they help you to keep track of where you are, which can be important in complex examples.
5. In this case, we keep moving along the chain. By the way, if you ever reach the end of the chain without determining a difference, that means that the groups are identical and it isn’t a chiral centre after all.
6. By this point we have enough information to assign (R)/(S). Since priority #4 is in the front, we can also break out our “opposite rule” for good measure:
7. Conclusion: The Cahn-Ingold-Prelog Rules For Assigning R and S Configurations
In the next post we’ll go into some trickier examples with determining R/S, including how to deal with double bonds, rings, and isotopes. In a future post, we’ll get into determining R/S in the Fischer and Newman projections.
Thanks to Matt Pierce for making major contributions to this article.
Ask Matt about scheduling an online tutoring session here .
Related Articles
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) – The Method of Dots
- How To Draw The Enantiomer Of A Chiral Molecule
- How To Determine R and S Configurations On A Fischer Projection
- Assigning R/S To Newman Projections (And Converting Newman To Line Diagrams)
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- On Cats, Part 4: Enantiocats
- On Cats, Part 6: Stereocenters
- Stereochemistry Practice Problems and Quizzes (MOC Membership)
- How To Draw A Bond Rotation
- Specification of Molecular Chirality R. S. Cahn, Sir Christopher Ingold, V. Prelog Angew. Chem. Int. Ed. 1966, 5 (4), 385-415 DOI: 10.1002/anie.196603851 This is not the first paper on the topic by the authors (see Refs. 4 and 5), but it is a major publication and an attempt to consolidate all the information on chirality in a single place. This paper discusses the various types of chirality possible in chemistry (not just at tetrahedral carbons!) and how to assign chirality unambiguously.
- Basic Principles of the CIP‐System and Proposals for a Revision Dr. Vladlmir Prelog and Prof. Dr. Günter Helmchen Angew. Chem. Int. Ed. 1982 , 21 (8), 567-583 DOI: 10.1002/anie.198205671 An update to Ref. #1, which addresses a lot of edge cases that may come up in complex stereochemical assignments.
- CHIRALITY IN CHEMISTRY Vladimir Prelog Nobel Lecture, 1975 Prelog’s Nobel Lecture. Nobel Lectures are fascinating to read as they give insight into the life of scientists and the path to discovery, which is rarely linear.
- “Absolutely” simple stereochemistry Philip S. Beauchamp Journal of Chemical Education 1984, 61 (8), 666 DOI : 10.1021/ed061p666 This paper describes a simple method for determining stereochemistry of tetrahedral carbons using the hands, suitable for undergraduate students of organic chemistry.
- A simple hand method for Cahn-Ingold-Prelog assignment of R and S configuration to chiral carbons Martin P. Aalund and James A. Pincock Journal of Chemical Education 1986, 63 (7), 600 DOI : 10.1021/ed063p600 A follow-up to the previous paper (Ref #4), but sadly it is incomplete!
- A Web-Based Stereochemistry Tool to Improve Students’ Ability to Draw Newman Projections and Chair Conformations and Assign R/S Labels Nimesh Mistry, Ravi Singh, and Jamie Ridley Journal of Chemical Education 2020, 97 (4), 1157-1161 DOI : 10.1021/acs.jchemed.9b00688 This paper discusses a web-based tool that helps students with visualization of chiral compounds and assignment of stereochemistry as per the Cahn-Ingold-Prelog (CIL) rules. See ref. 34 in the paper for the link.
00 General Chemistry Review
- Lewis Structures
- Ionic and Covalent Bonding
- Chemical Kinetics
- Chemical Equilibria
- Valence Electrons of the First Row Elements
- How Concepts Build Up In Org 1 ("The Pyramid")
01 Bonding, Structure, and Resonance
- How Do We Know Methane (CH4) Is Tetrahedral?
- Hybrid Orbitals and Hybridization
- How To Determine Hybridization: A Shortcut
- Orbital Hybridization And Bond Strengths
- Sigma bonds come in six varieties: Pi bonds come in one
- A Key Skill: How to Calculate Formal Charge
- The Four Intermolecular Forces and How They Affect Boiling Points
- 3 Trends That Affect Boiling Points
- How To Use Electronegativity To Determine Electron Density (and why NOT to trust formal charge)
- Introduction to Resonance
- How To Use Curved Arrows To Interchange Resonance Forms
- Evaluating Resonance Forms (1) - The Rule of Least Charges
- How To Find The Best Resonance Structure By Applying Electronegativity
- Evaluating Resonance Structures With Negative Charges
- Evaluating Resonance Structures With Positive Charge
- Exploring Resonance: Pi-Donation
- Exploring Resonance: Pi-acceptors
- In Summary: Evaluating Resonance Structures
- Drawing Resonance Structures: 3 Common Mistakes To Avoid
- How to apply electronegativity and resonance to understand reactivity
- Bond Hybridization Practice
- Structure and Bonding Practice Quizzes
- Resonance Structures Practice
02 Acid Base Reactions
- Introduction to Acid-Base Reactions
- Acid Base Reactions In Organic Chemistry
- The Stronger The Acid, The Weaker The Conjugate Base
- Walkthrough of Acid-Base Reactions (3) - Acidity Trends
- Five Key Factors That Influence Acidity
- Acid-Base Reactions: Introducing Ka and pKa
- How to Use a pKa Table
- The pKa Table Is Your Friend
- A Handy Rule of Thumb for Acid-Base Reactions
- Acid Base Reactions Are Fast
- pKa Values Span 60 Orders Of Magnitude
- How Protonation and Deprotonation Affect Reactivity
- Acid Base Practice Problems
03 Alkanes and Nomenclature
- Meet the (Most Important) Functional Groups
- Condensed Formulas: Deciphering What the Brackets Mean
- Hidden Hydrogens, Hidden Lone Pairs, Hidden Counterions
- Don't Be Futyl, Learn The Butyls
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Branching, and Its Affect On Melting and Boiling Points
- The Many, Many Ways of Drawing Butane
- Wedge And Dash Convention For Tetrahedral Carbon
- Common Mistakes in Organic Chemistry: Pentavalent Carbon
- Table of Functional Group Priorities for Nomenclature
- Summary Sheet - Alkane Nomenclature
- Organic Chemistry IUPAC Nomenclature Demystified With A Simple Puzzle Piece Approach
- Boiling Point Quizzes
- Organic Chemistry Nomenclature Quizzes
04 Conformations and Cycloalkanes
- Staggered vs Eclipsed Conformations of Ethane
- Conformational Isomers of Propane
- Newman Projection of Butane (and Gauche Conformation)
- Introduction to Cycloalkanes (1)
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Calculation of Ring Strain In Cycloalkanes
- Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
- Cyclohexane Conformations
- Cyclohexane Chair Conformation: An Aerial Tour
- How To Draw The Cyclohexane Chair Conformation
- The Cyclohexane Chair Flip
- The Cyclohexane Chair Flip - Energy Diagram
- Substituted Cyclohexanes - Axial vs Equatorial
- Ranking The Bulkiness Of Substituents On Cyclohexanes: "A-Values"
- Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
- Fused Rings - Cis-Decalin and Trans-Decalin
- Naming Bicyclic Compounds - Fused, Bridged, and Spiro
- Bredt's Rule (And Summary of Cycloalkanes)
- Newman Projection Practice
- Cycloalkanes Practice Problems
05 A Primer On Organic Reactions
- The Most Important Question To Ask When Learning a New Reaction
- Learning New Reactions: How Do The Electrons Move?
- The Third Most Important Question to Ask When Learning A New Reaction
- 7 Factors that stabilize negative charge in organic chemistry
- 7 Factors That Stabilize Positive Charge in Organic Chemistry
- Nucleophiles and Electrophiles
- Curved Arrows (for reactions)
- Curved Arrows (2): Initial Tails and Final Heads
- Nucleophilicity vs. Basicity
- The Three Classes of Nucleophiles
- What Makes A Good Nucleophile?
- What makes a good leaving group?
- 3 Factors That Stabilize Carbocations
- Equilibrium and Energy Relationships
- What's a Transition State?
- Hammond's Postulate
- Learning Organic Chemistry Reactions: A Checklist (PDF)
- Introduction to Free Radical Substitution Reactions
- Introduction to Oxidative Cleavage Reactions
06 Free Radical Reactions
- Bond Dissociation Energies = Homolytic Cleavage
- Free Radical Reactions
- 3 Factors That Stabilize Free Radicals
- What Factors Destabilize Free Radicals?
- Bond Strengths And Radical Stability
- Free Radical Initiation: Why Is "Light" Or "Heat" Required?
- Initiation, Propagation, Termination
- Monochlorination Products Of Propane, Pentane, And Other Alkanes
- Selectivity In Free Radical Reactions
- Selectivity in Free Radical Reactions: Bromination vs. Chlorination
- Halogenation At Tiffany's
- Allylic Bromination
- Bonus Topic: Allylic Rearrangements
- In Summary: Free Radicals
- Synthesis (2) - Reactions of Alkanes
- Free Radicals Practice Quizzes
07 Stereochemistry and Chirality
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- The Meso Trap
- Optical Rotation, Optical Activity, and Specific Rotation
- Optical Purity and Enantiomeric Excess
- What's a Racemic Mixture?
- Chiral Allenes And Chiral Axes
- Stereochemistry Practice Problems and Quizzes
08 Substitution Reactions
- Introduction to Nucleophilic Substitution Reactions
- Walkthrough of Substitution Reactions (1) - Introduction
- Two Types of Nucleophilic Substitution Reactions
- The SN2 Mechanism
- Why the SN2 Reaction Is Powerful
- The SN1 Mechanism
- The Conjugate Acid Is A Better Leaving Group
- Comparing the SN1 and SN2 Reactions
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- Common Blind Spot: Intramolecular Reactions
- The Conjugate Base is Always a Stronger Nucleophile
- Substitution Practice - SN1
- Substitution Practice - SN2
09 Elimination Reactions
- Elimination Reactions (1): Introduction And The Key Pattern
- Elimination Reactions (2): The Zaitsev Rule
- Elimination Reactions Are Favored By Heat
- Two Elimination Reaction Patterns
- The E1 Reaction
- The E2 Mechanism
- E1 vs E2: Comparing the E1 and E2 Reactions
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- Bulky Bases in Elimination Reactions
- Comparing the E1 vs SN1 Reactions
- Elimination (E1) Reactions With Rearrangements
- E1cB - Elimination (Unimolecular) Conjugate Base
- Elimination (E1) Practice Problems And Solutions
- Elimination (E2) Practice Problems and Solutions
10 Rearrangements
- Introduction to Rearrangement Reactions
- Rearrangement Reactions (1) - Hydride Shifts
- Carbocation Rearrangement Reactions (2) - Alkyl Shifts
- Pinacol Rearrangement
- The SN1, E1, and Alkene Addition Reactions All Pass Through A Carbocation Intermediate
11 SN1/SN2/E1/E2 Decision
- Identifying Where Substitution and Elimination Reactions Happen
- Deciding SN1/SN2/E1/E2 (1) - The Substrate
- Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
- SN1 vs E1 and SN2 vs E2 : The Temperature
- Deciding SN1/SN2/E1/E2 - The Solvent
- Wrapup: The Quick N' Dirty Guide To SN1/SN2/E1/E2
- Alkyl Halide Reaction Map And Summary
- SN1 SN2 E1 E2 Practice Problems
12 Alkene Reactions
- E and Z Notation For Alkenes (+ Cis/Trans)
- Alkene Stability
- Alkene Addition Reactions: "Regioselectivity" and "Stereoselectivity" (Syn/Anti)
- Stereoselective and Stereospecific Reactions
- Hydrohalogenation of Alkenes and Markovnikov's Rule
- Hydration of Alkenes With Aqueous Acid
- Rearrangements in Alkene Addition Reactions
- Halogenation of Alkenes and Halohydrin Formation
- Oxymercuration Demercuration of Alkenes
- Hydroboration Oxidation of Alkenes
- m-CPBA (meta-chloroperoxybenzoic acid)
- OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes
- Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
- Cyclopropanation of Alkenes
- A Fourth Alkene Addition Pattern - Free Radical Addition
- Alkene Reactions: Ozonolysis
- Summary: Three Key Families Of Alkene Reaction Mechanisms
- Synthesis (4) - Alkene Reaction Map, Including Alkyl Halide Reactions
- Alkene Reactions Practice Problems
13 Alkyne Reactions
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- Partial Reduction of Alkynes With Lindlar's Catalyst
- Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
- Alkyne Hydroboration With "R2BH"
- Hydration and Oxymercuration of Alkynes
- Hydrohalogenation of Alkynes
- Alkyne Halogenation: Bromination, Chlorination, and Iodination of Alkynes
- Alkyne Reactions - The "Concerted" Pathway
- Alkenes To Alkynes Via Halogenation And Elimination Reactions
- Alkynes Are A Blank Canvas
- Synthesis (5) - Reactions of Alkynes
- Alkyne Reactions Practice Problems With Answers
14 Alcohols, Epoxides and Ethers
- Alcohols - Nomenclature and Properties
- Alcohols Can Act As Acids Or Bases (And Why It Matters)
- Alcohols - Acidity and Basicity
- The Williamson Ether Synthesis
- Ethers From Alkenes, Tertiary Alkyl Halides and Alkoxymercuration
- Alcohols To Ethers via Acid Catalysis
- Cleavage Of Ethers With Acid
- Epoxides - The Outlier Of The Ether Family
- Opening of Epoxides With Acid
- Epoxide Ring Opening With Base
- Making Alkyl Halides From Alcohols
- Tosylates And Mesylates
- PBr3 and SOCl2
- Elimination Reactions of Alcohols
- Elimination of Alcohols To Alkenes With POCl3
- Alcohol Oxidation: "Strong" and "Weak" Oxidants
- Demystifying The Mechanisms of Alcohol Oxidations
- Protecting Groups For Alcohols
- Thiols And Thioethers
- Calculating the oxidation state of a carbon
- Oxidation and Reduction in Organic Chemistry
- Oxidation Ladders
- SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi
- Alcohol Reactions Roadmap (PDF)
- Alcohol Reaction Practice Problems
- Epoxide Reaction Quizzes
- Oxidation and Reduction Practice Quizzes
15 Organometallics
- What's An Organometallic?
- Formation of Grignard and Organolithium Reagents
- Organometallics Are Strong Bases
- Reactions of Grignard Reagents
- Protecting Groups In Grignard Reactions
- Synthesis Problems Involving Grignard Reagents
- Grignard Reactions And Synthesis (2)
- Organocuprates (Gilman Reagents): How They're Made
- Gilman Reagents (Organocuprates): What They're Used For
- The Heck, Suzuki, and Olefin Metathesis Reactions (And Why They Don't Belong In Most Introductory Organic Chemistry Courses)
- Reaction Map: Reactions of Organometallics
- Grignard Practice Problems
16 Spectroscopy
- Degrees of Unsaturation (or IHD, Index of Hydrogen Deficiency)
- Conjugation And Color (+ How Bleach Works)
- Introduction To UV-Vis Spectroscopy
- UV-Vis Spectroscopy: Absorbance of Carbonyls
- UV-Vis Spectroscopy: Practice Questions
- Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model
- Infrared Spectroscopy: A Quick Primer On Interpreting Spectra
- IR Spectroscopy: 4 Practice Problems
- 1H NMR: How Many Signals?
- Homotopic, Enantiotopic, Diastereotopic
- Diastereotopic Protons in 1H NMR Spectroscopy: Examples
- C13 NMR - How Many Signals
- Liquid Gold: Pheromones In Doe Urine
- Natural Product Isolation (1) - Extraction
- Natural Product Isolation (2) - Purification Techniques, An Overview
- Structure Determination Case Study: Deer Tarsal Gland Pheromone
17 Dienes and MO Theory
- What To Expect In Organic Chemistry 2
- Are these molecules conjugated?
- Conjugation And Resonance In Organic Chemistry
- Bonding And Antibonding Pi Orbitals
- Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion
- Pi Molecular Orbitals of Butadiene
- Reactions of Dienes: 1,2 and 1,4 Addition
- Thermodynamic and Kinetic Products
- More On 1,2 and 1,4 Additions To Dienes
- s-cis and s-trans
- The Diels-Alder Reaction
- Cyclic Dienes and Dienophiles in the Diels-Alder Reaction
- Stereochemistry of the Diels-Alder Reaction
- Exo vs Endo Products In The Diels Alder: How To Tell Them Apart
- HOMO and LUMO In the Diels Alder Reaction
- Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
- Diels-Alder Reaction: Kinetic and Thermodynamic Control
- The Retro Diels-Alder Reaction
- The Intramolecular Diels Alder Reaction
- Regiochemistry In The Diels-Alder Reaction
- The Cope and Claisen Rearrangements
- Electrocyclic Reactions
- Electrocyclic Ring Opening And Closure (2) - Six (or Eight) Pi Electrons
- Diels Alder Practice Problems
- Molecular Orbital Theory Practice
18 Aromaticity
- Introduction To Aromaticity
- Rules For Aromaticity
- Huckel's Rule: What Does 4n+2 Mean?
- Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems
- Antiaromatic Compounds and Antiaromaticity
- The Pi Molecular Orbitals of Benzene
- The Pi Molecular Orbitals of Cyclobutadiene
- Frost Circles
- Aromaticity Practice Quizzes
19 Reactions of Aromatic Molecules
- Electrophilic Aromatic Substitution: Introduction
- Activating and Deactivating Groups In Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution - The Mechanism
- Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution
- Understanding Ortho, Para, and Meta Directors
- Why are halogens ortho- para- directors?
- Disubstituted Benzenes: The Strongest Electron-Donor "Wins"
- Electrophilic Aromatic Substitutions (1) - Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) - Nitration and Sulfonation
- EAS Reactions (3) - Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Nucleophilic Aromatic Substitution (NAS)
- Nucleophilic Aromatic Substitution (2) - The Benzyne Mechanism
- Reactions on the "Benzylic" Carbon: Bromination And Oxidation
- The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions
- More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger
- Aromatic Synthesis (1) - "Order Of Operations"
- Synthesis of Benzene Derivatives (2) - Polarity Reversal
- Aromatic Synthesis (3) - Sulfonyl Blocking Groups
- Birch Reduction
- Synthesis (7): Reaction Map of Benzene and Related Aromatic Compounds
- Aromatic Reactions and Synthesis Practice
- Electrophilic Aromatic Substitution Practice Problems
20 Aldehydes and Ketones
- What's The Alpha Carbon In Carbonyl Compounds?
- Nucleophilic Addition To Carbonyls
- Aldehydes and Ketones: 14 Reactions With The Same Mechanism
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones
- Grignard Reagents For Addition To Aldehydes and Ketones
- Wittig Reaction
- Hydrates, Hemiacetals, and Acetals
- Imines - Properties, Formation, Reactions, and Mechanisms
- All About Enamines
- Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles (Part 2)
- Aldehydes Ketones Reaction Practice
21 Carboxylic Acid Derivatives
- Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)
- Addition-Elimination Mechanisms With Neutral Nucleophiles (Including Acid Catalysis)
- Basic Hydrolysis of Esters - Saponification
- Transesterification
- Proton Transfer
- Fischer Esterification - Carboxylic Acid to Ester Under Acidic Conditions
- Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
- LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes
- Di-isobutyl Aluminum Hydride (DIBAL) For The Partial Reduction of Esters and Nitriles
- Amide Hydrolysis
- Thionyl Chloride (SOCl2)
- Diazomethane (CH2N2)
- Carbonyl Chemistry: Learn Six Mechanisms For the Price Of One
- Making Music With Mechanisms (PADPED)
- Carboxylic Acid Derivatives Practice Questions
22 Enols and Enolates
- Keto-Enol Tautomerism
- Enolates - Formation, Stability, and Simple Reactions
- Kinetic Versus Thermodynamic Enolates
- Aldol Addition and Condensation Reactions
- Reactions of Enols - Acid-Catalyzed Aldol, Halogenation, and Mannich Reactions
- Claisen Condensation and Dieckmann Condensation
- Decarboxylation
- The Malonic Ester and Acetoacetic Ester Synthesis
- The Michael Addition Reaction and Conjugate Addition
- The Robinson Annulation
- Haloform Reaction
- The Hell–Volhard–Zelinsky Reaction
- Enols and Enolates Practice Quizzes
- The Amide Functional Group: Properties, Synthesis, and Nomenclature
- Basicity of Amines And pKaH
- 5 Key Basicity Trends of Amines
- The Mesomeric Effect And Aromatic Amines
- Nucleophilicity of Amines
- Alkylation of Amines (Sucks!)
- Reductive Amination
- The Gabriel Synthesis
- Some Reactions of Azides
- The Hofmann Elimination
- The Hofmann and Curtius Rearrangements
- The Cope Elimination
- Protecting Groups for Amines - Carbamates
- The Strecker Synthesis of Amino Acids
- Introduction to Peptide Synthesis
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions
- Amine Practice Questions
24 Carbohydrates
- D and L Notation For Sugars
- Pyranoses and Furanoses: Ring-Chain Tautomerism In Sugars
- What is Mutarotation?
- Reducing Sugars
- The Big Damn Post Of Carbohydrate-Related Chemistry Definitions
- The Haworth Projection
- Converting a Fischer Projection To A Haworth (And Vice Versa)
- Reactions of Sugars: Glycosylation and Protection
- The Ruff Degradation and Kiliani-Fischer Synthesis
- Isoelectric Points of Amino Acids (and How To Calculate Them)
- Carbohydrates Practice
- Amino Acid Quizzes

25 Fun and Miscellaneous
- A Gallery of Some Interesting Molecules From Nature
- Screw Organic Chemistry, I'm Just Going To Write About Cats
- On Cats, Part 1: Conformations and Configurations
- On Cats, Part 2: Cat Line Diagrams
- Organic Chemistry Is Shit
- The Organic Chemistry Behind "The Pill"
- Maybe they should call them, "Formal Wins" ?
- Why Do Organic Chemists Use Kilocalories?
- The Principle of Least Effort
- Organic Chemistry GIFS - Resonance Forms
- Reproducibility In Organic Chemistry
- What Holds The Nucleus Together?
- How Reactions Are Like Music
- Organic Chemistry and the New MCAT
26 Organic Chemistry Tips and Tricks
- Common Mistakes: Formal Charges Can Mislead
- Partial Charges Give Clues About Electron Flow
- Draw The Ugly Version First
- Organic Chemistry Study Tips: Learn the Trends
- The 8 Types of Arrows In Organic Chemistry, Explained
- Top 10 Skills To Master Before An Organic Chemistry 2 Final
- Common Mistakes with Carbonyls: Carboxylic Acids... Are Acids!
- Planning Organic Synthesis With "Reaction Maps"
- Alkene Addition Pattern #1: The "Carbocation Pathway"
- Alkene Addition Pattern #2: The "Three-Membered Ring" Pathway
- Alkene Addition Pattern #3: The "Concerted" Pathway
- Number Your Carbons!
- The 4 Major Classes of Reactions in Org 1
- How (and why) electrons flow
- Grossman's Rule
- Three Exam Tips
- A 3-Step Method For Thinking Through Synthesis Problems
- Putting It Together
- Putting Diels-Alder Products in Perspective
- The Ups and Downs of Cyclohexanes
- The Most Annoying Exceptions in Org 1 (Part 1)
- The Most Annoying Exceptions in Org 1 (Part 2)
- The Marriage May Be Bad, But the Divorce Still Costs Money
- 9 Nomenclature Conventions To Know
- Nucleophile attacks Electrophile
27 Case Studies of Successful O-Chem Students
- Success Stories: How Corina Got The The "Hard" Professor - And Got An A+ Anyway
- How Helena Aced Organic Chemistry
- From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around
- How Serge Aced Organic Chemistry
- Success Stories: How Zach Aced Organic Chemistry 1
- Success Stories: How Kari Went From C– to B+
- How Esther Bounced Back From a "C" To Get A's In Organic Chemistry 1 And 2
- How Tyrell Got The Highest Grade In Her Organic Chemistry Course
- This Is Why Students Use Flashcards
- Success Stories: How Stu Aced Organic Chemistry
- How John Pulled Up His Organic Chemistry Exam Grades
- Success Stories: How Nathan Aced Organic Chemistry (Without It Taking Over His Life)
- How Chris Aced Org 1 and Org 2
- Interview: How Jay Got an A+ In Organic Chemistry
- How to Do Well in Organic Chemistry: One Student's Advice
- "America's Top TA" Shares His Secrets For Teaching O-Chem
- "Organic Chemistry Is Like..." - A Few Metaphors
- How To Do Well In Organic Chemistry: Advice From A Tutor
- Guest post: "I went from being afraid of tests to actually looking forward to them".
Comment section
22 thoughts on “ introduction to assigning (r) and (s): the cahn-ingold-prelog rules ”.
In a chiral molecule, two groups are attached to it with the normal line bond ,the third is shown through a wedge and hydrogen is not shown..can I conclude that the hydrogen is a dash ?
Yes! The dashed hydrogen is implied!
Thanks. Move the dots. Could not find this before.
Glad you found it useful James!
- Pingback: Terpene University: Part 8 - Limonene Effects and Benefits - Omega Equipment & Supply Blog
During my studies for 11th grade and 12th grade, we had a brilliant Organic Chemistry teacher who taught the concepts beautifully. In addition, I had a passion (more of a “study crush”) on Chemistry in general and Organic Chemistry in particular. To such an extent that this topic of R and S enantiomers is still ingrained in memory. Though I am in a completely different area now of Machine Learning and Analytics in the Healthcare space in Industry, primarily a Software Engg job. Out of sheer curiosity, I googled “Chirality Detection Machine Learning” and voila !! such cool, intereesting papers I came across where they combine Bayesian Learning and Convolutional Neural Networks (Advanced ML Theory) to detect chirality in Nanoparticles. So application of ML in cutting edge Physics. Amazing stuff :!
Most people don’t learn chirality until 2nd year university in north america, so you are ahead of the curve
- Pingback: 23 Juli: Hari Kelahiran Vladimir Prelog – Departemen Keilmiahan HMD Kimia FMIPA UI
I just only want to know the CIP system of Nomenclature
Man this website proved to be a boon for me in quarantine…keep it up🔥🔥 The best content of organic chem I could get in such an incredible way
Thank you so much!! :) This was a great refresher on chirality and you explained it in such a straightforward manner. Appreciate it!
What to do if the compound is not denoted using the dash and wedge but simple bond line notation or expanded notation ?
Can you show an example? There has to be some kind of indicator. If all four bonds from the chiral center are shown as simple line notation there is no way to tell if it is R or S. It’s ambiguous.
Thank you so much, you are a true life saver???
I have a lot of trouble rotating molecules in my head, so these tips feel like magic to me!!! Thank you soooo much :DDDD Btw I also go to McGill!
The molecule used to explain the dot technique is labelled as 3-ethyl-3-methyloctane, however shouldn’t the molecule be named as 4-ethyl-4-methyloctane? The branches are on the fourth carbon…
Shoot. You are right. Thanks for the catch. Fixed!
Thank You so much :)
Thanks!! You saved my org chem exam
I was having trouble with this when 4 was in the plane of the page. This technique is so easy. Thanks
Kindly take my work into consideration in your website.
Abstract:- “The Keval’s Method” is developed for the determination of absolute configuration of a chiral carbon in a Fisher Projection and Wedge-Dash Projection just by simple calculations. This method is easily applicable over both Fisher as well as Wedge-Dash Projection. Various methods for determining absolute configuration have been developed and published till now, some of them used fingers and hands and other used exchanging elements. “Keval’s Method” is the first method in which a chiral carbon is taken to be an origin and the branches to axes, also it is purely calculation based method where absolute configuration is found based on the nature of calculated answer without using fingers and hands and also without exchanging elements.
Your’ Thankfully Keval Chetanbhai Purohit 5th-Computer Engineering, Vishwakarma Government Engineering College, Mo- 7226953531
Thank you very much, I now understand the R/S, its not easy to rotate a compound in your mind……
Leave a Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me via e-mail if anyone answers my comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Last modified: Jun 30, 2023
Access Online Assignments
Chapter 1: matter—solids, liquids, and gases.
- 1.1 Molecules Matter
- 1.2 Molecules in Motion
- 1.3 The Ups and Downs of Thermometers
- 1.4 Moving Molecules in a Solid
- 1.5 Air, It's Really There
Chapter 2: Changes of State
- 2.1 Heat, Temperature, and Conduction
- 2.2 Changing State—Evaporation
- 2.3 Changing State—Condensation
- 2.4 Changing State—Freezing
- 2.5 Changing State—Melting
Chapter 3: Density
- 3.1 What is Density?
- 3.2 Finding Volume—The Water Displacement Method
- 3.3 Density of Water
- 3.4 Density—Sink and Float for Solids
- 3.5 Density—Sink and Float for Liquids
- 3.6 Density—Temperature and Density
Chapter 4: The Periodic Table & Bonding
- 4.1 Protons, Neutrons, and Electrons
- 4.2 The Periodic Table
- 4.3 The Periodic Table & Energy Level Models
- 4.4 Energy Levels, Electrons, and Covalent Bonding
- 4.5 Energy Levels, Electrons, and Ionic Bonding
Chapter 5: The Water Molecule and Dissolving
- 5.1 Water is a Polar Molecule
- 5.2 Surface Tension
- 5.3 Why Does Water Dissolve Salt?
- 5.4 Why Does Water Dissolve Sugar?
- 5.5 Using Dissolving to Identify an Unknown
- 5.6 Does Temperature Affect Dissolving?
- 5.7 Can Liquids Dissolve in Water?
- 5.8 Can Gases Dissolve in Water?
- 5.9 Temperature Changes in Dissolving
Chapter 6: Chemical Change
- 6.1 What is a Chemical Reaction?
- 6.2 Controlling the Amount of Products in a Chemical Reaction
- 6.3 Forming a Precipitate
- 6.4 Temperature and Rate of a Chemical Reaction
- 6.5 A Catalyst and the Rate of Reaction
- 6.6 Using Chemical Change to Identify an Unknown
- 6.7 Energy Changes in Chemical Reactions
- 6.8 pH and Color Change
- 6.9 Neutralizing Acids and Bases
- 6.10 Carbon Dioxide Can Make a Solution Acidic
What Are Online Assignments?
Middle School Chemistry’s Interactive Chemistry Lesson Modules are a set of digital resources designed to complement your in-class, inquiry-based chemistry lessons. Originally developed during the pandemic as remote learning tools, these Google Forms modules have been repurposed for the post-pandemic era, providing an engaging and flexible way to support and assess student learning.
You can use Online Assignments for
- In-class reinforcement: Use the modules during class time to support hands-on activities, providing students with additional context and visuals to enhance understanding.
- Homework assignments: Assign modules as homework to reinforce concepts covered in class and encourage independent learning.
- Review and assessment: Utilize the modules as a review tool before exams, enabling students to self-assess their understanding and identify areas for improvement.
- Flipped classroom: Implement a flipped classroom approach, where students engage with the module content before class and use class time for active learning and discussion.
How to Use Online Assignments
How to copy, edit, and assign.
Youtube ID: hrNceDbTksQ
- Click on a remote learning assignment to copy it to your Google Drive
- Do not edit the copy link when you click on it
- Create a copy in your Google Drive and make any edits you wish
- Click on the “eyeball” symbol at the top right to preview the form and view the videos, which only appear as images in your editable copy
- Assign your copy to your students
- Students will receive an email with their responses after they submit
- Grade student work by clicking on “Responses” at the top of the editable form in your Google Drive
Online Assignments Walkthrough
Check out this short walkthrough to see how you can use these assignments with your students.
Youtube ID: DDy3GakQHXw
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
Chemistry Education Research and Practice
Examining the role of assignment design and peer review on student responses and revisions to an organic chemistry writing-to-learn assignment.

* Corresponding authors
a Department of Chemistry & Biochemistry, University of Wisconsin – Milwaukee, Milwaukee, WI 53211, USA
b Department of Chemistry, University of Michigan, Ann Arbor, Michigan 48109, USA E-mail: [email protected]
Research on student learning in organic chemistry indicates that students tend to focus on surface level features of molecules with less consideration of implicit properties when engaging in mechanistic reasoning. Writing-to-learn (WTL) is one approach for supporting students’ mechanistic reasoning. A variation of WTL incorporates peer review and revision to provide opportunities for students to interact with and learn from their peers, as well as revisit and reflect on their own knowledge and reasoning. However, research indicates that the rhetorical features included in WTL assignments may influence the language students use in their responses. This study utilizes machine learning to characterize the mechanistic features present in second-semester undergraduate organic chemistry students’ responses to two versions of a WTL assignment with different rhetorical features. Furthermore, we examine the role of peer review on the mechanistic reasoning captured in students’ revised drafts. Our analysis indicates that students include both surface level and implicit features of mechanistic reasoning in their drafts and in the feedback to their peers, with slight differences depending on the rhetorical features present in the assignment. However, students’ revisions appeared to be primarily connected to the peer review process via the presence of surface features in the drafts students read (as opposed to the feedback received). These findings indicate that further scaffolding focused on how to utilize information gained from the peer review process ( i.e. , both feedback received and drafts read) and emphasizing implicit properties could help support the utility of WTL for developing students’ mechanistic reasoning in organic chemistry.
Article information
Download citation, permissions.
F. M. Watts, S. A. Finkenstaedt-Quinn and G. V. Shultz, Chem. Educ. Res. Pract. , 2024, Advance Article , DOI: 10.1039/D4RP00024B
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
- Book A Demo
Transform Your Chemistry Students
Aktiv Chemistry reimagines chemistry courses with an approach that fosters student engagement both during and after class – in-person or online.
Adopted by faculty at 700+ campuses

All-in-one platform
Comprehensive learning & assessment.
From in-class active learning, homework assignments, and now secure online quizzes and exams, Aktiv Chemistry’s all-in-one platform provides a comprehensive set of features for formative and summative assessments.
In-class or Synchronous Online
Take attendance, post polls & quizzes, enhance traditional worksheets, or promote think-pair-share activities. Learn More
Homework & Practice
Assign interactive problem sets with instant feedback and automatic grading that sync with institutional LMS gradebooks. Learn More

Timed Online Quizzes & Exams
Create summative assessments with question pools, algorithmic problems, and optional integrated proctoring. Learn More
SCAFFOLDED, VISUAL, AND INTERACTIVE
Experience chemistry like never before.
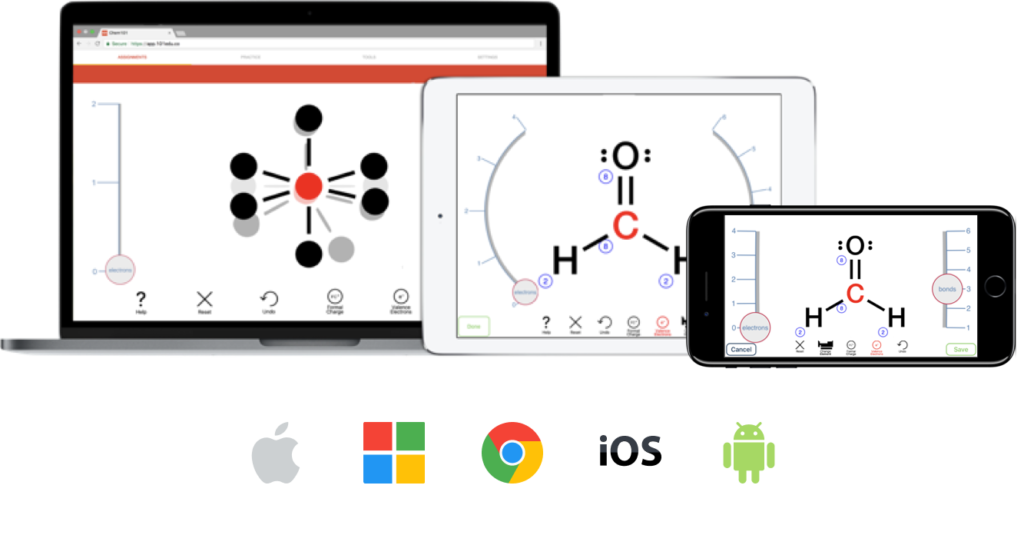
Students draw Lewis structures and visualize VSEPR with an intuitive, game-like interface that makes learning fun.
Students drag-and-drop tiles to set up scaffolded conversions with units, stoichiometry, densities, concentrations, and much more.
Allow students to focus on chemistry and not syntax errors by easily writing chemical nomenclature and equations with smart suggestions. Supports both inorganic and organic compounds.
Scaffolded and step- by-step learning for chemical, weak acid-base, and solubility equilibria problems.
Students draw skeletal and structural formulas with the groundbreaking and intuitive AktivGrid.
OVER 15,000 PROBLEMS AND ACTIVITIES
Learn more about our courses, general chemistry.
Support chemistry and STEM majors across the year-long sequence.
Intro to Chemistry
Support non-STEM majors or prepare students for General Chemistry.
GOB Chemistry
Support nursing or allied health majors in a 1-term or 2-term format.
Organic Chemistry
Designed to, support your course.
Use Any Device
Bring your own device. Any device. Students and instructors can access the Aktiv Learning app from any iPhone, iPad, or Android Device. Additionally, the platform is fully accessible on Web with any Mac, PC, or Chromebook.
Aktiv Chemistry’s mobile-first design ensures that students receive the same experience no matter where they are. Students take advantage of the Aktiv Learning mobile app to work and study on-the-go in places like riding the bus or train, or even on campus when they have downtime.
Gradebook & LMS Integration
Aktiv Chemistry’s gradebook can be synced with any popular campus LMS such as Canvas, Blackboard, Moodle, or D2L. The gradebook and associated columns can be customized to separate the various assignment types, to present individual assignments, or display summary columns for each type.
Every Aktiv Chemistry activity has a multitude of grading policies that can be customized depending on the assignment type. Settings include points per problem, participation credit, late submissions with penalties, variable attempts per problem, penalties for incorrect attempts, and more.
Algorithmic Problems & Question Pools
Aktiv Chemistry offers additional security with both Question Pools and Algorithmic Problems. These features randomize the content that is delivered to each student during homeworks, quizzes, or exams. Instructors can also randomize the order of questions on any assignment.
With Algorithmic Problems, built-in variables create thousands of question variants to be delivered that randomize numbers, words, or compounds within the problem statement. With Question Pools, instructors can group together a set of similar questions and deliver a subset of them at random to students.
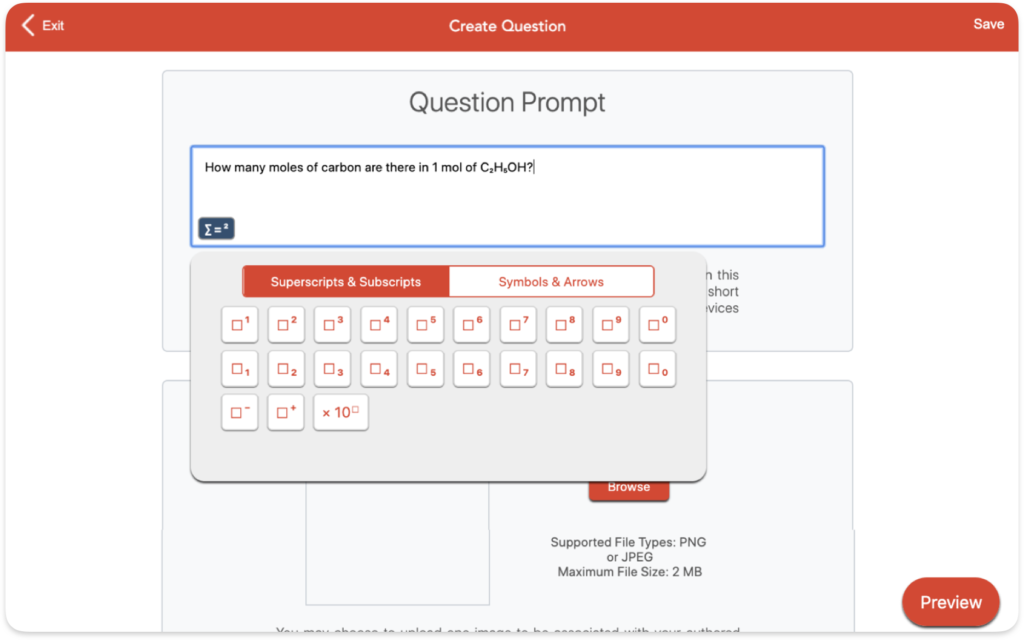
Question Authoring
Aktiv Chemistry’s authoring tool allows instructors to easily write, save, and assign your own questions with targeted instructional feedback. Input superscripts, subscripts, and special symbols with a breeze.
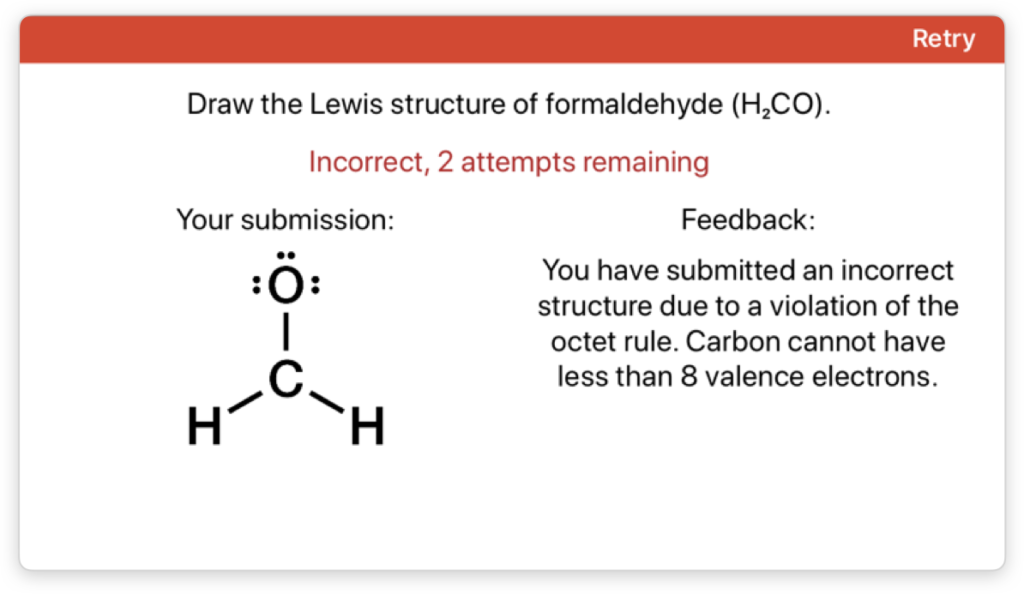
Targeted Student Feedback
Every Aktiv Chemistry question contains targeted instructional feedback that provides students with helpful hints when they submit incorrect answers. When students run out of attempts, they are presented with a step-by-step detailed solution that fully explains how to solve the problem.
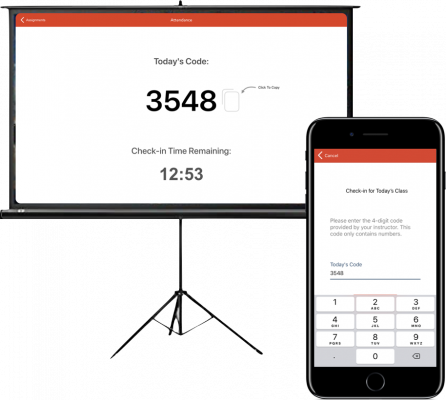
Aktiv Chemistry’s attendance tool allows instructors to easily take student attendance by randomizing a check in code for each class or lecture. Customize timing to have a strict or lenient lateness policy.
OpenStax Chemistry Integration
Aktiv Learning is proud to be a partner of OpenStax in order to deliver more affordable course materials for chemistry students.
General Chemistry students now have one button access directly to the OpenStax Chemistry textbooks when working on a Aktiv Chemistry problem. Access to learning content has never been easier.
- Gradebook & LMS Integration
- Algorithms & Question Pools
- Attendance
Success Stories
Professors from over 700 colleges and universities use Aktiv Chemistry to engage students inside and outside of the classroom. Learn how some of them have transformed their courses.

¹ This quote was provided at a time where Aktiv Chemistry was named Chem101. We have replaced the use of Chem101 in any direct quotes with Aktiv Chemistry to minimize confusion.
Industry-Leading Support
Every course and teaching style is different. That’s why all instructors who adopt Aktiv Mathematics receive personalized support to help you and your students succeed.
10 MIN RESPONSE TIME
Every student and instructor has access to our support, which can be reached by e-mail, live chat, SMS text, or phone. Extended hours make sure everyone is taken care of even on late nights and weekends.
SUCCESS TEAM
In addition to our support line, every instructor is assigned an individual member of Aktiv Learning’s Success team who helps with training, group logistics, master courses, LMS integrations, and much more.
Speak to a Specialist
One of our Learning Specialists will give you a tour of the Aktiv Chemistry or Aktiv Mathematics learning platforms and provide a free instructor playground account with access to the content library.
I Want to Learn More About:
PERSPECTIVE article
This article is part of the research topic.
Organic Chemistry Education Research into Practice
Ungrading in organic chemistry: Students assessing themselves and reflecting on their learning Provisionally Accepted

- 1 Eckerd College, United States
The final, formatted version of the article will be published soon.
The focus on grades has diminished the focus on learning. One strategy that aims to return students' attention to what they are actually learning (and not just earning) is ungrading. Ungrading is thought of as any strategy in which instructors do not assign a number or letter grade to students' assignments and assessments. Instead, faculty may 1) provide thorough feedback and engage in dialogue with students about their work, and perhaps, 2) allow students to assign their own grade. Whichever style of ungrading they choose, the scholars that have been forging the path for ungrading come from a variety of fields and perspectives, including STEM instructors in more recent years. The focus on incorporating ungrading practices into the organic chemistry curriculum provided here is adapted from a variety of practitioners, and especially the foundational work of chemistry professor Clarissa Sorensen-Unruh. In addition to discussing the current ungrading practices in various fields, we will use this perspective article to share our own experience with and lessons learned from beginning to incorporate ungrading in the undergraduate organic chemistry curriculum, both as it relates to the implementation of the practice and our own perceptions of the student experience and learning outcomes. Ultimately, the goal is to allow students to see the significance of the process of learning and to engage in some metacognitive work that they can apply to different assignments, whether in our class or not. If we want students to focus on learning, perhaps they should do the grading themselves.
Keywords: Grading, Ungrading, Organic Chemistry, Chemistry education research, metacognition
Received: 29 Feb 2024; Accepted: 29 Mar 2024.
Copyright: © 2024 Ferguson and Bonner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Prof. Jalisa Ferguson, Eckerd College, St. Petersburg, United States
People also looked at
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Class 10 Chemistry Assignments
We have provided below free printable Class 10 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 10 Chemistry . These Assignments for Grade 10 Chemistry cover all important topics which can come in your standard 10 tests and examinations. Free printable Assignments for CBSE Class 10 Chemistry , school and class assignments, and practice test papers have been designed by our highly experienced class 10 faculty. You can free download CBSE NCERT printable Assignments for Chemistry Class 10 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Chemistry Class 10. Students can click on the links below and download all Pdf Assignments for Chemistry class 10 for free. All latest Kendriya Vidyalaya Class 10 Chemistry Assignments with Answers and test papers are given below.
Chemistry Class 10 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 10 Chemistry . Students and teachers can download and save all free Chemistry assignments in Pdf for grade 10th. Our expert faculty have covered Class 10 important questions and answers for Chemistry as per the latest syllabus for the current academic year. All test papers and question banks for Class 10 Chemistry and CBSE Assignments for Chemistry Class 10 will be really helpful for standard 10th students to prepare for the class tests and school examinations. Class 10th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 10 Chemistry Download in Pdf

Advantages of Class 10 Chemistry Assignments
- As we have the best and largest collection of Chemistry assignments for Grade 10, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Chemistry textbooks for Class 10 .
- All Chemistry assignments for Class 10 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 10 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Chemistry chapter wise worksheets and assignments for free in Pdf
- Class 10 Chemistry question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 10 Chemistry students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Chemistry Class 10 which you can download in Pdf
We provide here Standard 10 Chemistry chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Chemistry in Grade 10, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Chemistry Grade 10 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 10 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 10 Biology Assignments

Class 10 Mathematics Arithmetic Progression Assignments

Class 10 German Assignments
- Accessibility
- Main SQA Website
- Using the site
- > Subjects
- > Chemistry
- > National 5
- > Assignment
In this section
Select a subject Accounting Administration and IT Applications of Mathematics Apprenticeships Art and Design Baccalaureates Biology Business Management Care Chemistry Childcare & Development Classical Studies Computing Science Core Skills Dance Design and Manufacture Drama Economics Engineering Science English Environmental Science ESOL Fashion and Textiles French Gaelic Gaidhlig Geography German Graphic Communication Health and Food Technology History HN Human Biology Italian Latin Mandarin Mathematics Mathematics of Mechanics Media Modern Studies Music Music Technology National 1 & 2 Philosophy Photography Physical Education Physics Politics Practical Cake Craft Practical Cookery Practical Electronics Practical Metalworking Practical Woodworking Psychology RMPS Science NPA's Scots Language Skills for Work Sociology Spanish Statistics SVQ Urdu
- Question Paper
- Advanced Higher
- Presentations
- Course Reports
- Additional resources for sessions 2020-22
National 5 Chemistry - assignment
Assignment 2023 (all links open as pdf files), candidate 1 - particle size and rate of reactions.
- Candidate 1 Evidence
Candidate 2 - Combustion of Alcohol's
- Candidate 2 Evidence
Candidate 3 - How Acid Concentration affect reaction rates
- Candidate 3 Evidence
Candidate 4 - the voltage of metals in the electrochemical series
- Candidate 4 Evidence
- Candidates 1 to 4 Commentaries
Assignment 2022 (All links open to PDF files)
- Candidates 1 to 3 Commentaries
- Terms & Conditions
- Back To Top

IMAGES
VIDEO
COMMENTS
ZnO 2 and ZnCl 2. H 2 O and HCl. NO and NO 2. CH 4 and CO 2. A sample of chemical X is found to contain 5.0 grams of oxygen, 10.0 grams of carbon, and 20.0. grams of nitrogen. The law of definite proportion would predict that a 70 gram sample of chemical.
Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Module 20: Organic Chemistry: Assignment: Nuclear Chemistry: Module 21: Nuclear Chemistry: Discussions. The following discussion assignments will also be preloaded (into the discussion-board tool) in your learning management system if you import the course. They can be used as is, modified, or removed. You can preview them below:
The Aktiv Chemistry gradebook can be set up to display summary columns or individual columns depending on the assignment type as well. Industry-leading Support Any course using Aktiv Chemistry comes with our industry-leading support featuring average response times of 14 minutes and extended hours that include late nights and weekends.
Resources for Teaching High School Chemistry. ChemEd X, published under the ACS Division of Chemical Education, curated this list of resources and lessons that teachers can use over the coming weeks. COVID-19 is a monster situation to deal with, but you can still take control of your learning and keep up with classes.
Physical Chemistry. Menu. More Info Syllabus Calendar Readings Lecture Notes Lecture Videos Assignments Exams Assignments. Problem Set solutions Problem Set 1 (PDF) Problem ... assignment_turned_in Problem Sets with Solutions. Download Course. Over 2,500 courses & materials
Problem Set 8 ( PDF ) ( PDF )#. Assignments section contains problem sets alonf with their solutions.
Module 1: Essential Ideas. Why It Matters: Essential Ideas. Chemistry in Context. Videos: History of Chemistry and the Scientific Method. Phases and Classification of Matter. Physical and Chemical Properties. Measurements. Measurement Uncertainty, Accuracy, and Precision. Videos: Significant Figures.
This assignment can be found in Google Docs: Chemistry for Majors Assignment: Atoms, Molecules, and Ions. If you want a Google Doc: in the file menu of the open document, click "Make a copy.". This will give you your own Google Doc to work from. If you want a PDF or Word file: in the file menu of the open document, click "Download" and ...
17 lbs. 6.55 × 10 -5 cm. 72 g. [/hidden-answer] CC licensed content, Original. Authored by: Jessica Garber. Provided by: Tidewater Community College. License: CC BY: Attribution. 1.15: Assignment—Matter and Measurement is shared under a CC BY 4.0 license and was authored, remixed, and/or curated by LibreTexts.
To find these you will need to search the recommended Chemistry databases. If you cannot find the kind of information you want on these databases, ask a subject librarian - we can help you choose the right database and the right keywords to use. 6. Find information on the Internet.
A tetrahedral atom with four different substituents (a chiral center) can have two different configurations. A naming scheme developed by Cahn, Ingold and Prelog (CIP) is used for assigning the terms R or S to each chiral center. (When all the (R,S) designations of a molecule are specified, this is referred to as its "absolute configuration".)
What Are Online Assignments? Middle School Chemistry's Interactive Chemistry Lesson Modules are a set of digital resources designed to complement your in-class, inquiry-based chemistry lessons. Originally developed during the pandemic as remote learning tools, these Google Forms modules have been repurposed for the post-pandemic era ...
A 24/7 free Chemistry homework AI tutor that instantly provides personalized step-by-step guidance, explanations, and examples for any Chemistry homework problem. Improve your grades with our AI homework helper! ... Get all your Chemistry assignments done with helpful answers in 10 seconds or less. No more asking friends for Chemistry help.
This study utilizes machine learning to characterize the mechanistic features present in second-semester undergraduate organic chemistry students' responses to two versions of a WTL assignment with different rhetorical features. Furthermore, we examine the role of peer review on the mechanistic reasoning captured in students' revised drafts.
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 12 Chemistry. Students and teachers can download and save all free Chemistry assignments in Pdf for grade 12th. Our expert faculty have covered Class 12 important questions and answers for Chemistry as per the latest syllabus for the current academic year.
This document contains instructions for teachers and lecturers, marking instructions and instructions for candidates for the National 5 Chemistry assignment. It must be read in conjunction with the course specification. This assignment is worth 20 marks (scaled to 25). The marks contribute 20% of the overall marks for the course assessment.
Aktiv Chemistry's gradebook can be synced with any popular campus LMS such as Canvas, Blackboard, Moodle, or D2L. The gradebook and associated columns can be customized to separate the various assignment types, to present individual assignments, or display summary columns for each type.
Higher Chemistry Assignment Assessment task. This document provides information for teachers and lecturers about the coursework component of this course in terms of the skills, knowledge and understanding that are assessed. It must be read in conjunction with the course specification. Valid from session 2020-21 and until further notice.
Higher Chemistry - assignment Assignment 2023 (All links open to PDF files) Full project: Vitamin C Concentration in Orange Juice. Full Project Candidate Evidence; Full Project Commentary; Sections: Summary, analysis, and evaluation sections. Sections Candidate Evidence; Sections Commentary . Assignment 2022 (All links open to PDF files ...
The focus on grades has diminished the focus on learning. One strategy that aims to return students' attention to what they are actually learning (and not just earning) is ungrading. Ungrading is thought of as any strategy in which instructors do not assign a number or letter grade to students' assignments and assessments. Instead, faculty may 1) provide thorough feedback and engage in ...
All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Chemistry Class 10. Students can click on the links below and download all Pdf Assignments for Chemistry class 10 for free. All latest Kendriya Vidyalaya Class 10 Chemistry Assignments with Answers and test papers are given below.
National 5 Chemistry - assignment Assignment 2023 (All links open as PDF files) Candidate 1 - Particle size and rate of reactions . Candidate 1 Evidence; Candidate 2 - Combustion of Alcohol's. Candidate 2 Evidence; Candidate 3 - How Acid Concentration affect reaction rates . Candidate 3 Evidence; Candidate 4 - the voltage of metals in the ...