An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection


Biology of Viruses and Viral Diseases
Viruses exact an enormous toll on the human population and are the single most important cause of infectious disease morbidity and mortality worldwide. Viral diseases in humans were first noted in ancient times and have since shaped our history. Scientific approaches to the study of viruses and viral disease began in the 19th century and led to the identification of specific disease entities caused by viruses. Careful clinical observations enabled the identification of many viral illnesses and allowed several viral diseases to be differentiated (e.g., smallpox vs. chickenpox and measles vs. rubella). Progress in an understanding of disease at the level of cells and tissues, exemplified by the pioneering work of Virchow, allowed the pathology of many viral diseases to be defined. Finally, the work of Pasteur ushered in the systematic use of laboratory animals for studies of the pathogenesis of infectious diseases, including those caused by viruses.
The first viruses were identified as the 19th century ended. Ivanovsky and Beijerinck identified tobacco mosaic virus, and Loeffler and Frosch discovered foot-and-mouth disease virus. These observations were quickly followed by the discovery of yellow fever virus and the seminal research on the pathogenesis of yellow fever by Walter Reed and the U. S. Army Yellow Fever Commission. 1 By the end of the 1930s, tumor viruses, bacteriophages, influenza virus, mumps virus, and many arthropod-borne viruses had been identified. This process of discovery has continued with growing momentum to the present, with recently identified skin cancer–associated Merkel cell polyomavirus, 2 novel Old World arenaviruses causing fatal disease, 3 , 4 bat-related respiratory coronavirus 5 and reoviruses, 6 , 7 and novel swine- and avian-origin influenza viruses 8 , 9 counted among the most recent entries in the catalog of human disease-causing viruses.
In the 1940s, Delbruck, Luria, and others 10 , 11 used bacteriophages as models to establish many basic principles of microbial genetics and molecular biology and identified key steps in viral replication. The pioneering experiments of Avery, MacLeod, and McCarty 12 on the transformation of pneumococci established DNA as the genetic material and set the stage for corroborating experiments by Hershey and Chase using bacteriophages. 13 In the late 1940s, Enders and colleagues 14 cultivated poliovirus in tissue culture. This accomplishment led to the development of both formalin-inactivated (Salk) 15 and live-attenuated (Sabin) 16 vaccines for polio and ushered in the modern era of experimental and clinical virology.
In recent years, x-ray crystallography has allowed visualization of virus structures at an atomic level of resolution. Nucleotide sequences of entire genomes of most human viruses are known, and functional domains of many viral structural and enzymatic proteins have been defined. This information is being applied to the development of new strategies to diagnose viral illnesses and design effective antiviral therapies. Techniques to detect viral genomes, such as the polymerase chain reaction (PCR) and its derivatives, have proven superior to conventional serologic assays and culture techniques for the diagnosis of many viral diseases. Nucleic acid–based strategies are now used routinely in the diagnosis of infections caused by enteroviruses, hepatitis B virus (HBV), hepatitis C virus (HCV), herpesviruses, human immunodeficiency virus (HIV), and, with increasing frequency, respiratory and enteric viral pathogens. Furthermore, rapid developments in mass spectrometry and nucleotide sequencing technology are permitting the application of these tools to highly sensitive and specific virus detection in clinical specimens.
Perhaps an even more exciting development is the means to introduce new genetic material into viral genomes. Strategies now exist whereby specific mutations or even entire genes can be inserted into the genomes of many viruses. Such approaches can be exploited in the rational design of vaccines and the development of viral vectors for use in gene delivery. Furthermore, these powerful new techniques are leading to breakthroughs in foundational problems in viral pathogenesis, such as the nature of virus–cell interactions that produce disease, immunoprotective and immunopathologic host responses to infection, and viral and host determinants of contagion. Improved understanding of these aspects of viral infection will facilitate new approaches to the prevention, diagnosis, and treatment of viral diseases.
Virus Structure and Classification
The first classification of viruses as a group distinct from other microorganisms was based on the capacity to pass through filters of a small pore size (filterable agents). Initial subclassifications were based primarily on pathologic properties such as specific organ tropism (e.g., hepatitis viruses) or common epidemiologic features such as transmission by arthropod vectors (e.g., arboviruses). Current classification systems are based on the following: (1) the type and structure of the viral nucleic acid and the strategy used in its replication; (2) the type of symmetry of the virus capsid (helical vs. icosahedral); and (3) the presence or absence of a lipid envelope ( Table 134-1 ).
TABLE 134-1
Classification of Viruses
(+), message sense; (−), complement of message sense; DS, double-stranded; H, helical; I, icosahedral; S, spherical; SS, single-stranded.
Virus particles—virions—can be functionally conceived as a delivery system that surrounds a payload. The delivery system consists of structural components used by the virus to survive in the environment and bind to host cells. The payload contains the viral genome and often includes enzymes required for the initial steps in viral replication. In almost all cases, the delivery system must be removed from the virion to allow viral replication to commence.
In addition to mediating attachment to host cells, the delivery system also plays a crucial role in determining the mode of transmission between hosts. Viruses containing lipid envelopes are sensitive to desiccation in the environment and, for the most part, are transmitted by the respiratory, parenteral, and sexual routes. Nonenveloped viruses are stable to harsh environmental conditions and are often transmitted by the fecal-oral route.
Viral genomes exist in a variety of forms and sizes and consist of RNA or DNA (see Table 134-1 ). Animal virus genomes range in size from 3 kb, encoding only three or four proteins in small viruses such as the hepadnaviruses, to more than 300 kb, encoding several hundred proteins in large viruses such as the poxviruses. Viral genomes are single- or double-stranded and circular or linear. RNA genomes are composed of a single molecule of nucleic acid or multiple discrete segments, which can vary in number from as few as two in the arenaviruses up to 12 in some members of the Reoviridae. Viral nucleic acid is packaged in a protein coat, or capsid, that consists of multiple protein subunits. The combination of the viral nucleic acid and the surrounding protein capsid is often referred to as the nucleocapsid ( Fig. 134-1 ).

Schematic diagrams illustrating the structure of a nonenveloped icosahedral virus (A) and an enveloped helical virus (B). Nucleocapsid: combination of a viral nucleic acid and surrounding protein capsid.
Structural details of many viruses have now been defined at an atomic level of resolution ( Fig. 134-2 ). General features of virus structure can be gained from examination of electron micrographs of negatively stained virions and thin-section electron micrographs of virus-infected tissues and cultured cells. These techniques allow rapid identification of viral size, shape, symmetry, and surface features, presence or absence of an envelope, and intracellular site of viral assembly. Cryoelectron microscopy and computer image processing techniques are used to determine the three-dimensional structures of spherical viruses at a level of resolution far superior to that of negatively stained electron micrographs. A major advantage of cryoelectron microscopy is that it allows structural studies of viruses to be performed under conditions that do not alter native virion structure. Moreover, recent advances in cryoelectron microscopy have extended the achievable resolution of particle-associated proteins to near-atomic levels, sufficient to recognize characteristic features of secondary structural elements. 17 Image reconstructions of cryoelectron micrographs, sometimes in combination with x-ray crystallography, can also be used to investigate structural aspects of various virus functions, including receptor binding 18 , 19 , 20 and interaction with antibodies. 21 , 22 Identification of key motifs, such as receptor binding sites or immunodominant domains, provides the framework for understanding the structural basis of virus–cell interactions. Electron tomography with image reconstruction has been applied to architectural studies of viruses and intracellular foci of virus replication, rendering exquisite three-dimensional representations of particle organization and revealing the structure and subcellular origins of virus manufacturing centers. 23 , 24

Structural studies of poliovirus.
A, Negative-stained electron micrograph. B, Three-dimensional image reconstruction of cryoelectron micrographs. C, Structure determined by x-ray crystallography.
A number of general principles have emerged from studies of virus structure. In almost all cases, the capsid is composed of a repeating series of structurally similar subunits, each of which in turn is composed of only a few different proteins. The parsimonious use of structural proteins in a repetitive motif minimizes the amount of genetic information required to encode the viral capsid and leads to structural arrangements with symmetrical features. All but the most complex viruses exhibit either helical or icosahedral symmetry (see Table 134-1 ). Viruses with helical symmetry contain repeating protein subunits bound at regular intervals along a spiral formed by the viral nucleic acid. Interestingly, all known animal viruses that show this type of symmetry have RNA genomes. Viruses with icosahedral symmetry display twofold, threefold, and fivefold axes of rotational symmetry, and viral nucleic acid is intimately associated with specific capsid proteins in an ordered packing arrangement.
The use of repeating subunits with symmetrical protein-protein interactions facilitates the assembly of the viral capsid. In most cases, viral assembly appears to be a spontaneous process that occurs under the appropriate physiologic conditions and often can be reproduced when recombinant viral proteins are expressed in the absence of viral replication. 25 , 26 For many viruses, assembly of the capsid proceeds through a series of intermediates, each of which nucleates the addition of subsequent components in the assembly sequence.
One of the most poorly understood aspects of viral assembly is the process that ensures that the viral nucleic acid is correctly packaged into the capsid. In the case of viruses with helical symmetry, there may be an initiation site on the nucleic acid to which the initial capsid protein subunit binds, triggering the addition of subsequent subunits. The genomes of most DNA-containing viruses are inserted into preassembled capsid intermediates (procapsids) through adenosine triphosphate–driven mechanisms. 27 In preparations of many icosahedral viruses, empty capsids (i.e., capsids lacking nucleic acid) are frequently observed, indicating that assembly may proceed to completion without a requirement for the viral genome.
In some viruses, the nucleocapsid is surrounded by a lipid envelope acquired as the virus particle buds from the host cell cytoplasmic, nuclear, or endoplasmic reticular membrane (see Fig. 134-1 ). Inserted into this lipid bilayer are virus-encoded proteins (e.g., the hemagglutinin [HA] and neuraminidase proteins of influenza virus and gp41 and gp120 of HIV), which are exposed on the surface of the virus particle. These viral proteins usually contain a glycosylated hydrophilic external portion and internal hydrophobic domains that span the lipid membrane and anchor the protein into the viral envelope. In some cases, another viral protein, often termed a matrix protein, associates with the internal (cytoplasmic) surface of the lipid envelope, where it can interact with the cytoplasmic domains of the envelope glycoproteins. Matrix proteins may play roles in stabilizing the interaction between viral glycoproteins and the lipid envelope, directing the viral genome to intracellular sites of viral assembly, or facilitating viral budding. Matrix proteins can also influence a diverse set of cellular functions, such as inhibition of host cell transcription 28 , 29 and evasion of the cellular innate antiviral response. 30
Virus–Cell Interactions
Viruses require an intact cell to replicate and can direct the synthesis of hundreds to thousands of progeny viruses during a single cycle of infection. In contrast to other microorganisms, viruses do not replicate by binary fission. Instead, the infecting particle must disassemble in order to direct synthesis of viral progeny.
The interaction between a virus and its host cell begins with attachment of the virus particle to specific receptors on the cell surface. Viral proteins that mediate the attachment function (viral attachment proteins) include the following: single-capsid components that extend from the virion surface, such as the attachment proteins of adenovirus, 31 reovirus, 32 and rotavirus 33 , 34 ; surface glycoproteins of enveloped viruses, such as influenza virus 35 , 36 ( Fig. 134-3 ) and HIV 37 , 38 ; viral capsid proteins that form binding pockets that engage cellular receptors, such as the canyon formed by the capsid proteins of poliovirus 39 and rhinovirus 40 ; and viral capsid proteins that contain extended loops capable of binding receptors, such as foot-and-mouth disease virus. 41 Studies of the attachment of several diverse virus groups, including adenoviruses, coronaviruses, herpesviruses, lentiviruses, and reoviruses, indicate that multiple interactions between virus and cell occur during the attachment step. These observations indicate that a specific sequence of binding events between virus and cell optimizes specificity and contributes significant stability to the association. 42

The folded structure of the influenza virus hemagglutinin (HA) and its rearrangement when exposed to low pH.
A, The HA monomer. HA1 is blue, and HA2 is multicolored. The receptor-binding pocket resides in the virion-distal portion of HA1. The viral membrane would be at the bottom of this figure. B, Conformational change in HA induced by exposure to low pH. Note the dramatic structural rearrangement in HA2, in which amino acid residues 40-105 become a continuous alpha helix. Dashed lines indicate regions of undetermined structure. This model of HA in its fusion conformation is a composite of the HA1 domain structure and the low-pH HA2 structure.
One of the most dynamic areas of virology concerns the identification of virus receptors on host cells. This interest stems in part from the critical importance of the attachment step as a determinant of target cell selection by many viruses. Several virus receptors have now been identified ( Table 134-2 ), and three important principles have emerged from studies of these receptors. First, viruses have adapted to use cell surface molecules designed to facilitate a variety of normal cellular functions. Virus receptors may be highly specialized proteins with limited tissue distribution, such as complement receptors, growth factor receptors, or neurotransmitter receptors, or more ubiquitous components of cellular membranes, such as integrins and other intercellular adhesion molecules, glycosaminoglycans, or sialic acid–containing oligosaccharides. Second, many viruses use more than a single receptor to mediate multistep attachment and internalization. For example, adenovirus binds coxsackievirus and adenovirus receptor (CAR) 43 and the integrins α v β 3 or α v β 5 44 ; herpes simplex virus (HSV) binds heparan sulfate 45 , 46 , 47 and herpesvirus entry mediator (HVEM/HveA), 48 nectin 1 (PRR1/HveC), 49 or nectin 2 (PRR2/HveB) 50 ; HIV binds CD4 51 , 52 and chemokine receptors CXCR4 53 , 54 or CCR5 55 , 56 , 57 ; and reovirus binds sialylated glycans 58 , 59 and JAM-A. 60 , 61 Third, in many cases, receptor expression is not the sole determinant of viral tropism for particular cells and tissues in the host. Therefore, although receptor binding is the first step in the interaction between virus and cell, subsequent events in the viral replication cycle must also be supported for productive viral infection to occur.
TABLE 134-2
Receptors and Entry Mediators Used by Selected Human Viruses
Several viruses bind receptors expressed at regions of cell-cell contact. 62 Junctional adhesion molecule-A (JAM-A), which serves as a receptor for reovirus 60 and feline calicivirus, 63 and CAR, which serves as a receptor for some coxsackieviruses and adenoviruses, 43 are expressed at tight junctions 64 , 65 and adherens junctions. 66 , 67 Junctional regions are sites of enhanced membrane recycling, endocytic uptake, and intracellular signaling. 68 Therefore, it is possible that viruses have selected junction-associated proteins as receptors to usurp the physiologic functions of these molecules. In this regard, interactions of coxsackievirus with decay-accelerating factor elicit a tyrosine kinase–based signaling cascade that mediates subsequent interactions of the virus with CAR in tight junctions. 69 Structures of viral proteins or whole viral particles in complex with sialic acid have been determined for some viruses, including the influenza virus hemagglutinin (HA) 36 , 70 (see Fig. 134-3 ), polyomavirus, 71 , 72 , 73 , 74 foot-and-mouth disease virus, 75 reovirus attachment protein σ1, 58 , 59 and the VP8 domain of rotavirus capsid protein VP4. 34 Sialic acid binding in each of these cases occurs in a shallow groove at the surface of the viral protein. However, the architectures of the binding sites differ. Structures of complexes of viral proteins or viral particles and cell surface protein receptors have also been determined. These include adenovirus fiber knob and CAR, 76 Epstein-Barr virus (EBV) gp42 and major histocompatibility complex (MHC) class II protein, 77 HSV glycoprotein D and HVEM/HveA, 78 HIV gp120 and CD4, 38 measles virus HA and CD46 79 and SLAM (signaling lymphocyte-activation molecule), 80 reovirus σ1 and JAM-A, 61 and rhinovirus and ICAM-1 (intercellular adhesion molecule 1). 81 In several of these cases, the viral attachment proteins engage precisely the same domains used by their cognate receptors to bind natural ligands.
Penetration and Disassembly
Once attachment has occurred, the virus must penetrate the cell membrane, and the capsid must undergo a series of disassembly steps (uncoating) that prepare the virus for the next phases in viral replication. Enveloped viruses such as the paramyxoviruses and retroviruses enter cells by fusion of the viral envelope with the cell membrane ( Fig. 134-4 ). Attachment of these viruses to the cell surface induces changes in viral envelope proteins required for membrane fusion. For example, the binding of CD4 and certain chemokine receptors by HIV envelope glycoprotein gp120 induces a series of conformational changes in gp120 that lead to the exposure of transmembrane protein gp41. 82 , 83 Fusion of viral and cellular membranes proceeds through subsequent interactions of the hydrophobic gp41 fusion peptide with the cell membrane. 84 , 85 , 86 , 87

Mechanisms of viral entry into cells.
Nonenveloped (A) and enveloped (B) virus internalization by receptor-mediated endocytosis.
Other viruses enter cells by some form of receptor-mediated endocytic uptake (see Fig. 134-4 ). For several viruses, virus–receptor complexes induce formation of clathrin-coated pits that invaginate from the cell membrane to form coated vesicles. 88 These vesicles are rapidly uncoated and fuse with early endosomes, which sort internalized proteins for recycling to the cell surface or other cellular compartments, such as late endosomes or lysosomes. For other viruses, virus–receptor complexes are taken into cells by caveolae in lipid rafts. 88 Enveloped viruses such as dengue virus, 89 influenza virus, 90 and Semliki Forest virus 91 exploit the acidic environment of the endocytic compartment to induce conformational changes in surface glycoproteins required for membrane fusion. High-resolution structures of the influenza virus HA at acidic pH illustrate a dramatic conformational alteration leading to the fusion-active state (see Fig. 134-3 ). 90
Endocytic uptake and acidification are also required for entry of some nonenveloped viruses such as adenovirus, 92 , 93 parvovirus, 94 and reovirus. 95 , 96 In these cases, acidic pH may facilitate disassembly of the viral capsid to enable subsequent penetration of endosomal membranes. In addition to acidic pH, endocytic cathepsin proteases are required for disassembly of several viruses, including Ebola virus, 97 Hendra virus, 98 reovirus, 99 and severe acute respiratory syndrome (SARS) coronavirus. 100
In contrast to enveloped viruses, nonenveloped viruses cross cell membranes using mechanisms that do not involve membrane fusion. This group of viruses includes several human pathogens, with adenoviruses, picornaviruses, and rotaviruses serving as prominent examples. Despite differences in genome and capsid composition, each of these viruses must penetrate cell membranes to deliver the genetic payload to the interior of the cell. Capsid rearrangements triggered by receptor binding, 101 , 102 acidic pH, 92 , 93 or proteolysis 103 , 104 serve essential functions in membrane penetration by some nonenveloped viruses. Although a precise understanding of the biochemical mechanisms that underlie viral membrane penetration is incomplete, small capsid proteins of several nonenveloped viruses, such as adenovirus, 105 poliovirus, 106 and reovirus, 107 are required for membrane penetration, perhaps by forming pores in host cell membranes.
Genome Replication
Once a virus has entered a target cell, it must replicate its genome and proteins. Replication strategies used by single-stranded RNA-containing viruses depend on whether the genome can be used as messenger (m)RNA. Translation-competent genomes, which include those of the coronaviruses, flaviviruses, picornaviruses, and togaviruses, are termed plus (+) sense and are translated by cellular ribosomes immediately following entry of the genome into the cytoplasm. For most viruses containing (+) sense RNA genomes, translation results in the synthesis of a large polyprotein that is cleaved into several smaller proteins through the action of viral and sometimes host proteases. One of these proteins is an RNA-dependent RNA polymerase (RdRp), which replicates the viral RNA. Genome replication of (+) sense RNA-containing viruses requires synthesis of a minus (–) sense RNA intermediate, which serves as template for production of (+) sense genomic RNA.
A different strategy is used by viruses containing (−) sense RNA genomes. The genomes of these viruses, which include the filoviruses, orthomyxoviruses, paramyxoviruses, and rhabdoviruses, cannot serve directly as mRNA. Therefore, viral particles must contain a co-packaged RdRp to transcribe (+) sense mRNAs using the (−) sense genomic RNA as template. Genome replication of (−) sense RNA-containing viruses requires synthesis of a (+) sense RNA intermediate, which serves as a template for production of (−) sense genomic RNA. Mechanisms that determine whether (+) sense RNAs are used as templates for translation or genome replication are not well understood.
RNA-containing viruses belonging to the family Reoviridae have segmented double-stranded (ds) RNA genomes. The innermost protein shell of these viruses (termed a single-shelled particle or core ) contains an RdRp that catalyzes the synthesis of (+) sense mRNA using as a template the (−) sense strand of each dsRNA segment. The mRNAs of these viruses are capped at their 5′-termini by virus-encoded enzymes and then extruded into the cytoplasm through channels in the single-shelled particle. 108 The (+) sense mRNAs also serve as a template for replication of dsRNA gene segments. Viral genome replication is thus completely conservative; neither strand of parental dsRNA is present in newly formed genomic segments.
The retroviruses are RNA-containing viruses that replicate using a DNA intermediate. The viral genomic RNA is (+) sense and single stranded; however, it does not serve as mRNA following viral entry. Instead, the retrovirus RNA genome is a template for synthesis of a double-stranded DNA copy, termed the provirus. Synthesis of the provirus is mediated by a virus-encoded RNA-dependent DNA polymerase or reverse transcriptase, so named because of the reversal of genetic information from RNA to DNA. The provirus translocates to the nucleus and integrates into host DNA. Expression of this integrated DNA is regulated for the most part by cellular transcriptional machinery. However, the human retroviruses HIV and human T-cell leukemia virus (HTLV) encode proteins that augment transcription of viral genes. Intracellular signaling pathways are capable of activating retroviral gene expression and play important roles in inducing high levels of viral replication in response to certain stimuli. 109 Transcription of the provirus yields mRNAs that encode viral proteins and genome-length RNAs that are packaged into progeny virions. Such a replication strategy results in persistent infection in the host because the viral genome is maintained in the host cell genome and replicated with each cell division.
With the exception of the poxviruses, viruses containing DNA genomes replicate in the nucleus and for the most part use cellular enzymes for transcription and replication of their genomes. Transcription of most DNA-containing viruses is tightly regulated and results in the synthesis of early and late mRNA transcripts. The early transcripts encode regulatory proteins and proteins required for DNA replication, whereas the late transcripts encode structural proteins. Several DNA-containing viruses, such as adenovirus and human papillomavirus (HPV), induce cells to express host proteins required for viral DNA replication by stimulating cell-cycle progression. For example, the HPV E7 protein binds the retinoblastoma gene product pRB and liberates transcription factor E2F, which induces the cell cycle. 110 , 111 To prevent programmed cell death in response to E7-mediated unscheduled cell cycle progression, the HPV E6 protein mediates the ubiquitylation and degradation of tumor suppressor protein p53. 112 , 113 , 114
Some DNA-containing viruses, such as the herpesviruses, can establish latent infections in the host. Unlike the retroviruses, genomes of the herpesviruses do not integrate into host chromosomes but instead exist as plasmid-like episomes. Mechanisms that govern establishment of latency and subsequent reactivation of replication are not well understood. However, microRNAs encoded by cytomegalovirus (CMV) and perhaps other herpesviruses may promote persistence by targeting viral and cellular mRNAs that control viral gene expression and replication and innate immune responses to viral infection. 115 , 116
A fascinating aspect of virus–cell interactions is the replication microenvironments established in infected cells. Viral replication is a sophisticated interplay of transcription, translation, nucleic acid amplification, and particle assembly. Furthermore, infection must proceed under sensitive pathogen surveillance systems trained on virus-associated molecular patterns (e.g., unmethylated CpG dinucleotides in DNA viral genomes) and replicative intermediates (e.g., dsRNA generated during RNA virus replication) that may impose impassable blocks to infection. 117 Partitioning of the viral replication machinery from the surrounding intracellular milieu satisfies a spatial requirement to concentrate viral proteins and nucleic acid for efficient genome amplification and encapsidation while simultaneously shielding viral products from cellular sensors that provoke antiviral innate immune responses. Hence, as a rule, viral replication is a localized process, occurring within morphologically discrete cytoplasmic or nuclear structures variously termed viral inclusions (or inclusion bodies ), virosomes, viral factories, or viroplasm. These entities are novel, metabolically active organelles formed by contributions from both virus and cell. Many highly recognizable features of viral cytopathic effect observed using light microscopy, such as dense nuclear inclusions or refractile cytoplasmic densities, represent locally concentrated regions of viral nucleic acid and protein.
Membrane-associated replicase complexes appropriated by (+) sense RNA viruses are perhaps the most conspicuous examples of compartmentalized viral replication. In cells infected by these viruses, intracellular membranes originating from the endoplasmic reticulum (ER; e.g., picornaviruses 118 , 119 ), ER-Golgi intermediate compartment and trans -Golgi network (e.g., flaviviruses 120 ), endolysosomal vesicles (e.g., alphaviruses 121 ), and autophagic vacuoles (e.g., poliovirus 122 ) are reduplicated and reorganized by viral proteins into platforms that anchor viral replication complexes consisting of the RdRp and other RNA-modifying enzymes necessary for RNA synthesis. Curiously, dsRNA viruses are thought to generate nonmembranous intracytoplasmic replication factories, even though their life cycles pass through a (+) polarity RNA intermediate. However, in an interesting functional parallel with (+) sense RNA viruses, the assembly pathway of rotavirus, a dsRNA virus, involves budding of immature particles into the ER, where a lipid envelope is transiently acquired and subsequently replaced by the outermost protein shell. 123 Perhaps additional roles for cellular membranes in non–membrane-bound viral replication complexes await discovery.
The tight relationship of RNA virus replication to cellular membranes is less predictable for DNA viruses. For example, in distinction to the supporting role of autophagy in the replication of some RNA viruses, autophagosomes (stress-induced, double-membraned vesicles that remove noxious cytoplasmic materials to lysosomes for degradation) defend against infection by HSV-1, which encodes a protein that inhibits induction of autophagy and accentuates viral virulence. 124 , 125 The replication and assembly complexes of many DNA viruses, including adenoviruses, herpesviruses, papillomaviruses, polyomaviruses, and parvoviruses, are associated with promyelocytic leukemia (PML) nuclear bodies, 126 , 127 which have been ascribed functions in diverse nuclear processes encompassing gene regulation, tumor suppression, apoptosis, and removal of aggregated or foreign proteins. 128 It appears that DNA viruses exploit PML bodies in a variety of ways, which include consolidation and disposal of misfolded viral proteins, sequestration of host-cell stress response factors that block infection, and segregation of interfering cellular DNA repair proteins from sites of viral replication. 129
The life cycles of all viruses that replicate in eukaryotic cells are physically and functionally intertwined with the cytoskeleton. Many viruses with nuclear replication programs, such as adenovirus, HSV, and influenza virus, are transported by motor proteins along microtubules toward the nucleus, resulting ultimately in release of the viral genome into the nucleoplasm through nuclear pores. 130 The microtubule network is also conscripted as an egress pathway by a number of enveloped viruses (e.g., HIV, HSV, vaccinia virus) for conveyance of immature particles to cytolemmal sites of virion budding. 131 Furthermore, microtubules and actin filaments may serve as anchorage points for nucleoprotein complexes that coordinate genome expression or replication with cytoplasmic replication programs, exemplified by parainfluenza virus (PIV), 132 reovirus, 133 and vaccinia virus. 134 Because the cytoskeleton is a decentralized organelle linking cellular structural elements to the metabolic and transport machineries, it is not surprising that viruses capitalize on this highly integrative system, which provides a stable platform for replication and enables purposeful movement of virions or subviral components within cells to facilitate the requisite partitioning of viral assembly and disassembly.
Cell Killing
Viral infection can compromise numerous cellular processes, such as nucleic acid and protein synthesis, maintenance of cytoskeletal architecture, and preservation of membrane integrity. 135 Many viruses are also capable of inducing the genetically programmed mechanism of cell death that leads to apoptosis of host cells. 136 , 137 Apoptotic cell death is characterized by cell shrinkage, membrane blebbing, condensation of nuclear chromatin, and activation of an endogenous endonuclease, which results in cleavage of cellular DNA into oligonucleosome-length DNA fragments. 138 These changes occur according to predetermined developmental programs or in response to certain environmental stimuli. In some cases, apoptosis may serve as an antiviral defense mechanism to limit viral replication by destruction of virus-infected cells or reduction of potentially harmful inflammatory responses elicited by viral infection. 139 In other cases, apoptosis may result from viral induction of cellular factors required for efficient viral replication. 136 , 137 Generally, RNA-containing viruses, including influenza virus, measles virus, poliovirus, reovirus, and Sindbis virus, induce apoptosis of host cells, whereas DNA-containing viruses, including adenovirus, CMV, EBV, HPV, and the poxviruses, encode proteins that block apoptosis. For some viruses, the duration of the viral infectious cycle may determine whether apoptosis is induced or inhibited. Viruses capable of completing an infectious cycle before induction of apoptosis would not require a means to inhibit this cellular response to viral infection. Interestingly, several viruses that cause encephalitis are capable of inducing apoptosis of infected neurons ( Fig. 134-5 ). 140 , 141 , 142

Reovirus-induced apoptosis in the murine central nervous system.
Consecutive sections of the hippocampus prepared from a newborn mouse 10 days following intracranial inoculation with reovirus strain type 3 Dearing. Cells were stained with (A) hematoxylin and eosin, (B) reovirus antigen, and (C) the activated form of apoptosis protease caspase-3. Cells that stain positive for reovirus antigen or activated caspase 3 contain a dark precipitate in the cytoplasm, including neuronal processes. Scale bars, 100 µm.
Antiviral Drugs
(Also see Chapters 43 to 47Chapter 43Chapter 44Chapter 45Chapter 46Chapter 47.)
Knowledge of viral replication strategies has provided insights into critical steps in the viral life cycle that can serve as potential targets for antiviral therapy. For example, drugs can be designed to interfere with virus binding to target cells or prevent penetration and disassembly once receptor engagement has occurred. Steps involved in the replication of the viral genome are also obvious targets for antiviral therapy. A number of antiviral agents inhibit viral polymerases, including those active against herpesviruses (e.g., acyclovir), HIV (e.g., zidovudine), and HBV (e.g., entecavir). Drugs that inhibit viral proteases have been developed; several are used to treat HCV 143 , 144 and HIV 145 infection. These drugs block the proteolytic processing of viral precursor polyproteins and serve as potent inhibitors of replication. Other viral enzymes also serve as targets for antiviral therapy. The influenza virus neuraminidase is required for the release of progeny influenza virus particles from infected cells. Oseltamivir and zanamivir bind the neuraminidase catalytic site and efficiently inhibit the enzyme. 146 These drugs have been used in the prophylaxis and treatment of influenza virus infection. 147
Better understanding of viral replication strategies and mechanisms of virus-induced cell killing is paving the way for the rational design of novel antiviral therapeutics. One of the most exciting approaches to the development of antiviral agents is the use of high-resolution x-ray crystallography and molecular modeling to optimize interactions between these inhibitory molecules and their target viral proteins. Such structure-based drug design has led to the development of synthetic peptides (e.g., enfuvirtide) that inhibit HIV entry by blocking gp41-mediated membrane fusion. 148 Other vulnerable steps in HIV replication are targets of drugs approved for patient treatment, including entry inhibitors that interfere with gp120 binding to CCR5 149 and agents that prevent proviral integration into cellular DNA through inhibition of viral integrase activity 150 (see Chapter 130). Several inhibitors of the HCV protease and polymerase are also in clinical development 151 (see Chapter 46).
Despite promising advances in rational antiviral drug design, current therapeutic approaches to some viral infections rely heavily on compounds with less specific mechanisms of action. One such agent, interferon (IFN)-α, efficiently inhibits a broad spectrum of viruses and is secreted by diverse cell types as part of the host innate immune response. Recombinant IFN-α is presently used to treat HBV and HCV infections. Ribavirin, a synthetic guanosine analogue, inhibits the replication of many RNA- and DNA-containing viruses through complex mechanisms involving inhibition of viral RNA synthesis and disturbances in intracellular pools of guanosine triphosphate. 152 , 153 This drug is routinely used to treat HCV infection and sometimes administered in aerosolized form to treat respiratory syncytial virus (RSV) lower respiratory tract infection in hospitalized children and in severely ill and immunocompromised patients. Ribavirin therapy reduces the mortality associated with certain viral hemorrhagic fevers, such as that caused by Lassa virus. 154 Broader-spectrum therapies exemplified by IFN-α and ribavirin remain part of the first-line defense against emerging pathogens and other susceptible viruses for which biochemical and structural information is insufficient to design high-potency agent-specific drugs.
Virus–Host Interaction
One of the most formidable challenges in virology is to apply knowledge gained from studies of virus–cell interactions in tissue culture systems to an understanding of how viruses interact with host organisms to cause disease. Virus–host interactions are often described in terms of pathogenesis and virulence. Pathogenesis is the process whereby a virus interacts with its host in a discrete series of stages to produce disease ( Table 134-3 ). Virulence is the capacity of a virus to produce disease in a susceptible host. Virulence is often measured in terms of the quantity of virus required to cause illness or death in a predefined fraction of experimental animals infected with the virus. Virulence is dependent on viral and host factors and must be measured using carefully defined conditions (e.g., virus strain, dose, and route of inoculation; host species, age, and immune status). In many cases, it has been possible to identify roles played by individual viral and host proteins at specific stages in viral pathogenesis and to define the importance of these proteins in viral virulence.
TABLE 134-3
Stages in Virus–Host Interaction
The first step in the process of virus–host interaction is the exposure of a susceptible host to viable virus under conditions that promote infection ( Fig. 134-6 ). Infectious virus may be present in respiratory droplets or aerosols, in fecally contaminated food or water, or in a body fluid or tissue (e.g., blood, saliva, urine, semen, or a transplanted organ) to which the susceptible host is exposed. In some cases, the virus is inoculated directly into the host through the bite of an animal vector or through the use of a contaminated needle. Infection can also be transmitted from mother to infant through virus that has infected the placenta or birth canal or by virus in breast milk. In some cases, acute viral infections result from the reactivation of endogenous latent virus (e.g., reactivation of HSV giving rise to herpes labialis) rather than de novo exposure to exogenous virus.

Entry and spread of viruses in human hosts.
Some major steps in viral spread and invasion of target organs are shown. Neural spread is not illustrated. GI, gastrointestinal; HIV, human immunodeficiency virus; HPV, human papillomavirus.
Exposure of respiratory mucosa to virus by direct inoculation or inhalation is an important route of viral entry into the host. A simple cough can generate up to 10,000 small, potentially infectious aerosol particles, and a sneeze can produce nearly 2 million. The distribution of these particles depends on a variety of environmental factors, the most important of which are temperature, humidity, and air currents. In addition to these factors, particle size is an important determinant of particle distribution. In general, smaller particles remain airborne longer than larger ones. Particle size also contributes to particle fate after inhalation. Larger particles (>6 µm) are generally trapped in the nasal turbinates, whereas smaller particles may ultimately travel to the alveolar spaces of the lower respiratory tract.
Fecal-oral transmission represents an additional important route of viral entry into the host. Food, water, or hands contaminated by infected fecal material can facilitate the entry of a virus via the mouth into the gastrointestinal tract, the environment of which requires viruses that infect by this route to have certain physical properties. Viruses capable of enteric transmission must be acid stable and resistant to bile salts. Because conditions in the stomach and intestine are destructive to lipids contained in viral envelopes, most viruses that spread by the fecal-oral route are nonenveloped. Interestingly, many viruses that enter the host via the gastrointestinal tract require proteolysis of certain capsid components to infect intestinal cells productively. Treatment of mice with inhibitors of intestinal proteases blocks infection by reovirus 155 and rotavirus, 156 which demonstrates the critical importance of proteolysis in the initiation of enteric infection by these viruses. The host microbiota is essential for infection by some viruses. 157 , 158
To produce systemic disease, a virus must cross the mucosal barrier that separates the luminal compartments of the respiratory, gastrointestinal, and genitourinary tracts from the host's parenchymal tissues. Studies with reovirus illustrate one strategy used by viruses to cross mucosal surfaces to invade the host after entry into the gastrointestinal tract. 159 , 160 After oral inoculation of mice, reovirus adheres to the surface of intestinal microfold cells (M cells) that overlie collections of intestinal lymphoid tissue (Peyer's patches). In electron micrographs, reovirus virions can be followed sequentially as they are transported within vesicles from the luminal to the subluminal surface of M cells. Virions subsequently appear within Peyer's patches and then spread to regional lymph nodes and extraintestinal lymphoid organs such as the spleen. A similar pathway of spread has been described for poliovirus 161 and HIV, 162 suggesting that M cells represent an important portal for viral invasion of the host after entry into the gastrointestinal tract.
Once a virus has entered the host, it can replicate locally or spread from the site of entry to distant organs to produce systemic disease (see Fig. 134-6 ). Classic examples of localized infections in which viral entry and replication occur at the same anatomic site include respiratory infections caused by influenza virus, RSV, and rhinovirus; enteric infections produced by norovirus and rotavirus; and dermatologic infections caused by HPV (warts) and paravaccinia virus (milker's nodules). Other viruses spread to distant sites in the host after primary replication at sites of entry. For example, poliovirus spreads from the gastrointestinal tract to the central nervous system (CNS) to produce meningitis, encephalitis, or poliomyelitis. Measles virus and varicella-zoster virus (VZV) enter the host through the respiratory tract and then spread to lymph nodes, skin, and viscera. Pathobiologic definitions of viruses based on spread potential have begun to blur amid accumulating evidence that model agents of localized infection may disseminate to distant sites. For example, rotavirus, an important cause of pediatric acute gastroenteritis, replicates vigorously in villous tip epithelial cells of the small intestine but is also frequently associated with viral antigen and RNA in blood, the clinical significance of which is unclear. 163 Influenza virus is another case in point; viral RNA in blood is detected at a substantial frequency in hematopoietic cell transplant recipients and correlates with more severe disease and increased mortality. 164
Release of some viruses occurs preferentially from the apical or basolateral surface of polarized cells, such as epithelial cells. In the case of enveloped viruses, polarized release is frequently determined by preferential sorting of envelope glycoproteins to sites of viral budding. Specific amino-acid sequences in these viral proteins direct their transport to a particular aspect of the cell surface. 165 , 166 Polarized release of virus at apical surfaces may facilitate local spread of infection, whereas release at basolateral surfaces may facilitate systemic invasion by providing virus access to subepithelial lymphoid, neural, or vascular tissues.
Many viruses use the bloodstream to spread in the host from sites of primary replication to distant target tissues (see Fig. 134-6 ). In some cases, viruses may enter the bloodstream directly, such as during a blood transfusion or via an arthropod bite. More commonly, viruses enter the bloodstream after replication at some primary site. Important sites of primary replication preceding hematogenous spread of viruses include Peyer's patches and mesenteric lymph nodes for enteric viruses, bronchoalveolar cells for respiratory viruses, and subcutaneous tissue and skeletal muscle for alphaviruses and flaviviruses. In the case of reovirus, infection of endothelial cells leads to hematogenous dissemination in the host. 167 , 168
Pioneering studies by Fenner with mousepox (ectromelia) virus suggest that an initial low-titer viremia (primary viremia) serves to seed virus to a variety of intermediate organs, where a period of further replication leads to a high-titer viremia (secondary viremia) that disseminates virus to the ultimate target organs ( Fig. 134-7 ). 169 It is often difficult to identify primary and secondary viremias in naturally occurring viral infections. However, replication of many viruses in reticuloendothelial organs (e.g., liver, spleen, lymph nodes, bone marrow), muscle, fat, and even vascular endothelial cells can play an important role in maintaining viremia. 168

Pathogenesis of mousepox virus infection.
Successive waves of viremia are shown to seed the spleen and liver and then the skin.
Viruses that reach the bloodstream may travel free in plasma (e.g., enteroviruses and togaviruses) or in association with specific blood cells. 170 A number of viruses are spread hematogenously by macrophages (e.g., CMV, HIV, measles virus) or lymphocytes (e.g., CMV, EBV, HIV, HTLV, measles virus). Although many viruses have the capacity to agglutinate erythrocytes in vitro (a process called hemagglutination), only in exceptional cases (e.g., Colorado tick fever virus) are erythrocytes used to transport virus in the bloodstream.
The maintenance of viremia depends on the interplay among factors that promote virus production and those that favor viral clearance. A number of variables that affect the efficiency of virus removal from plasma have been identified. In general, the larger the viral particle, the more efficiently it is cleared. Viruses that induce high titers of neutralizing antibodies are more efficiently cleared than those that do not induce humoral immune responses. Finally, phagocytosis of virus by cells in the host reticuloendothelial system can contribute to viral clearance.
A major pathway used by viruses to spread from sites of primary replication to the nervous system is through nerves. Numerous diverse viruses, including Borna disease virus, coronavirus, HSV, poliovirus, rabies virus, reovirus, and Venezuelan equine encephalitis virus (VEE), are capable of neural spread. Several of these viruses accumulate at the neuromuscular junction after primary replication in skeletal muscle. 171 , 172 HSV appears to enter nerve cells via receptors that are located primarily at synaptic endings rather than on the nerve cell body. 173 Spread to the CNS by HSV, 174 rabies virus, 171 , 172 and reovirus 175 , 176 can be interrupted by scission of the appropriate nerves or by chemical agents that inhibit axonal transport. Neural spread of some of these viruses occurs by the microtubule-based system of fast axonal transport. 177
Viruses are not limited to a single route of spread. VZV, for example, enters the host by the respiratory route and then spreads from respiratory epithelium to the reticuloendothelial system and skin via the bloodstream. Infection of the skin produces the characteristic exanthem of chickenpox. The virus subsequently enters distal terminals of sensory neurons and travels to dorsal root ganglia, where it establishes latent infection. Reactivation of VZV from latency results in transport of the virus in sensory nerves to skin, where it gives rise to vesicular lesions in a dermatomal distribution characteristic of zoster or shingles .
Poliovirus is also capable of spreading by hematogenous and neural routes. Poliovirus is generally thought to spread from the gastrointestinal tract to the CNS via the bloodstream, although it has been suggested that the virus may spread via autonomic nerves in the intestine to the brainstem and spinal cord. 178 , 179 This hypothesis is supported by experiments using transgenic mice expressing the human poliovirus receptor. 180 When these mice are inoculated with poliovirus intramuscularly in the hind limb, virus does not reach the CNS if the sciatic nerve ipsilateral to the site of inoculation is transected. 181 Once poliovirus reaches the CNS, axonal transport is the major route of viral dissemination. Similar mechanisms of spread may be used by other enteroviruses.
The capability of a virus to infect a distinct group of cells in the host is referred to as tropism. For many viruses, tropism is determined by the availability of virus receptors on the surface of a host cell. This concept was first appreciated in studies of poliovirus when it was recognized that the capacity of the virus to infect specific tissues paralleled its capacity to bind homogenates of the susceptible tissues in vitro. 182 The importance of receptor expression as a determinant of poliovirus tropism was conclusively demonstrated by showing that cells not susceptible for poliovirus replication could be made susceptible by recombinant expression of the poliovirus receptor. 183 In addition to the availability of virus receptors, tropism can also be determined by postattachment steps in viral replication, such as the regulation of viral gene expression. For example, some viruses contain genetic elements, termed enhancers, that act to stimulate transcription of viral genes. 184 , 185 Some enhancers are active in virtually all types of cells, whereas others show exquisite tissue specificity. The promoter-enhancer region of John Cunningham (JC) polyomavirus is active in cultured human glial cells but not in HeLa cervical epithelial cells. 186 Cell-specific expression of the JC virus genome correlates well with the capacity of this virus in immunocompromised persons to produce progressive multifocal leukoencephalopathy, a disease in which JC virus infection is limited to oligodendroglia in the CNS.
Specific steps in virus–host interaction, such as the route of entry and pathway of spread, also can strongly influence viral tropism. For example, encephalitis viruses such as VEE are transmitted to humans by insect bites. These viruses undergo local primary replication and then spread to the CNS by hematogenous and neural routes. 187 After oral inoculation, VEE is incapable of primary replication and spread to the CNS, illustrating that tropism can be determined by the site of entry into the host. Influenza virus buds exclusively from the apical surface of respiratory epithelial cells, 188 which may limit its capacity to spread within the host and infect cells at distant sites.
A wide variety of host factors can influence viral tropism. These include age, nutritional status, and immune responsiveness, as well as certain genetic polymorphisms that affect susceptibility to viral infection. Age-related susceptibility to infection is observed for many viruses, including reovirus, 189 , 190 RSV, 191 , 192 , 193 and rotavirus. 194 , 195 The increased susceptibility in young children to these viruses may in part be due to immaturity of the immune response but also may be related to intrinsic age-specific factors that enhance host susceptibility to infection. Nutritional status is a critical determinant of the tropism and virulence of many viruses. For example, persons with vitamin A deficiency have enhanced susceptibility to measles virus infection. 196 , 197 Similarly, the outcome of most viral infections is strongly linked to the immune competence of the host.
The genetic basis of host susceptibility to viral infections is complex. Studies with inbred strains of mice indicate that genetic variation can alter susceptibility to viral disease by a variety of mechanisms. 198 These can involve differences in immune responses, variability in the ability to produce antiviral mediators such as IFN, and differential expression of functional virus receptors. Polymorphisms in the expression of chemokine receptor CCR5, which serves as a co-receptor for HIV, 55 , 56 , 57 are associated with alterations in susceptibility to HIV infection. 199 , 200
Persistent Infections
Many viruses are capable of establishing persistent infections, of which two types are recognized: chronic and latent. Chronic viral infections are characterized by continuous shedding of virus for prolonged periods of time. Congenital infections with rubella virus and CMV and persistent infections with HBV and HCV are examples of chronic viral infections. Latent viral infections are characterized by maintenance of the viral genome in host cells in the absence of viral replication. Herpesviruses and retroviruses can establish latent infections. The distinction between chronic and latent infections is not readily apparent for some viruses, such as HIV, which can establish both chronic and latent infections in the host. 201 , 202 , 203 Viruses capable of establishing persistent infections must have a means of evading the host immune response and a mechanism of attenuating their virulence. Lentiviruses such as equine infectious anemia virus 204 and HIV 205 , 206 , 207 are capable of extensive antigenic variation resulting in escape from neutralizing antibody responses by the host.
Several viruses encode proteins that directly attenuate the host immune response (e.g., the adenovirus E3/19K protein 208 and CMV US11 gene product 209 block cell surface expression of MHC class I proteins, resulting in diminished presentation of viral antigens to cytotoxic T lymphocytes [CTLs]). The poxviruses encode a variety of immunomodulatory molecules including CrmA, which blocks T-cell–mediated apoptosis of virus-infected cells. 210 In some cases (e.g., the CNS), preferential sites for persistent viral infections are not readily accessible by the immune system, 211 which may favor establishment of persistence.
Viruses and Cancer
Several viruses produce disease by promoting malignant transformation of host cells. Work by Peyton Rous with an avian retrovirus was the first to demonstrate that viral infections can cause cancer. 212 Rous sarcoma virus encodes an oncogene, v -src, which is a homologue of a cellular proto-oncogene, c -src. 213 , 214 Cells infected with Rous sarcoma virus become transformed. 215 , 216 , 217 , 218 , 219 Several viruses are associated with malignancies in humans. EBV is associated with many neoplasms, including Burkitt's lymphoma, Hodgkin's disease, large B-cell lymphoma, leiomyosarcoma, and nasopharyngeal carcinoma. HBV and HCV are associated with hepatocellular carcinoma. HPV is associated with cervical cancer and a variety of anogenital and esophageal neoplasms. Kaposi sarcoma–associated herpesvirus is associated with Kaposi sarcoma and primary effusion lymphoma in persons with HIV infection.
Often, the linkage of a virus to a particular neoplasm can be attributed to transforming properties of the virus itself. For example, EBV encodes several latency-associated proteins that are responsible for immortalization of B cells; these proteins likely play crucial roles in the pathogenesis of EBV-associated malignancies. 220 Similarly, HPV encodes the E6 and E7 proteins that block apoptosis 112 , 113 , 114 and induce cell cycle progression, 110 , 111 respectively. It is hypothesized that unregulated expression of these proteins induced by the aberrant integration of the HPV genome into host DNA is responsible for malignant transformation. 221 The tumorigenicity of polyomaviruses, which are oncogenic in rodent species, is mediated by a family of viral proteins known as tumor (T) antigens. Reminiscent of the HPV E6 and E7 proteins, T antigens induce cell cycling and block the ensuing cellular apoptotic response to unscheduled cell division. 222 The normally episomal polyomavirus genome becomes integrated into cellular DNA during neoplastic transformation of nonpermissive cells unable to support the entire viral replication program, which would otherwise culminate in cell death. Discovery of a human polyomavirus clonally integrated into cells of an aggressive form of skin cancer, Merkel cell carcinoma, 2 substantiates the long-standing suspicion that polyomaviruses can also promote neoplasia in humans.
In other cases, mechanisms of malignancy triggered by viral infection are less clear. HCV is an RNA-containing virus that lacks reverse transcriptase and a means of viral genome integration. However, chronic infection with HCV is strongly associated with hepatocellular cancer. 223 It is possible that increased cell turnover and inflammatory mediators elicited by chronic HCV infection increase the risk of genetic damage, which results in malignant transformation. Some HCV proteins may also play a contributory role in neoplasia. For example, the HCV core protein can protect cells against apoptosis induced by a variety of stimuli, including tumor necrosis factor-α (TNF-α). 224
Viral Virulence Determinants
Viral surface proteins involved in attachment and entry influence the virulence of diverse groups of viruses. For example, polymorphisms in the attachment proteins of influenza virus, 225 , 226 polyomavirus, 227 reovirus, 228 rotavirus, 229 and VEE 230 are strongly linked to virulence and can be accurately termed virulence determinants. Viral attachment proteins can serve this function by altering the affinity of virus–receptor interactions or modulating the kinetics of viral disassembly. Importantly, sequences in viral genomes that do not encode protein can also influence viral virulence. Mutations that contribute to the attenuated virulence of the Sabin strains of poliovirus are located in the 5′ nontranslated region of the viral genome. 231 These mutations attenuate poliovirus virulence by altering the efficiency of viral protein synthesis.
A number of viruses encode proteins that enhance virulence by modulation of host immune responses. Illustrative examples include the influenza A NS1 protein, which interferes with activation of cellular innate immune responses to viral infection, 232 and translation products of the adenovirus E3 transcriptional unit, which serve to prevent cytotoxic T-cell recognition of virally infected cells and block immunologically activated signaling pathways that lead to infected-cell death. 208 , 233 In many cases, these proteins are dispensable for viral replication in cultured cells. In this way, immunomodulatory viral virulence determinants resemble classic bacterial virulence factors such as various types of secreted toxins.
Host Responses to Infection
The immune response to viral infection involves complex interactions among leukocytes, nonhematopoietic cells, signaling proteins, soluble proinflammatory mediators, antigen-presenting molecules, and antibodies. These cells and molecules collaborate in a highly regulated fashion to limit viral replication and dissemination through recognition of broadly conserved molecular signatures, followed by virus-specific adaptive responses that further control infection and establish antigen-selective immunologic memory. The innate antiviral response is a local, transient, antigen-independent perimeter defense strategically focused at the site of virus incursion into an organ or tissue. Mediated by ancient families of membrane-associated and cytosolic molecules known as pattern recognition receptors (PRRs), the innate immune system detects pathogen-associated molecular patterns (PAMPs), which are fundamental structural components of microbial products including nucleic acids, carbohydrates, and lipids. 234 Viral PAMPs in the form of single-stranded (ss)RNA, dsRNA, and DNA evoke the innate immune response through two groups of PRRs: the transmembrane Toll-like receptors (TLRs) and the cytosolic nucleic acid sensors. The latter include retinoic acid inducible gene-I (RIG-I)-like receptors, nucleotide-binding domain and leucine-rich-repeat containing proteins (NLRs) such as NLRP, and DNA sensors. 235 Nucleic acid binding by PRRs activates signaling pathways leading to the production and extracellular release of IFN-α, IFN-β, and proinflammatory cytokines such as interleukin (IL)-1β and IL-18. IFN-α and IFN-β engage the cell surface IFN-α/β receptor and thereby mediate expression of hundreds of gene products that corporately suppress viral replication and establish an intracellular antiviral state in neighboring uninfected cells. Well-described IFN-inducible gene products include the latent enzymes dsRNA-dependent protein kinase (PKR) and 2′,5′-oligoadenylate synthetase (OAS), both of which are activated by dsRNA. 236 PKR inhibits the initiation of protein synthesis through phosphorylation of translation initiation factor eIF2α. The 2′,5′-oligoandenylates generated by OAS bind and activate endoribonuclease RNAse L, which degrades viral mRNA. In addition to mediating an intracellular antiviral state, IFN-α/β also stimulates the antigen-independent destruction of virus-infected cells by a specialized population of lymphocytes known as natural killer (NK) cells. 237 Importantly, IFNs bridge innate and adaptive antiviral immune responses through multiple modes of action, which include enhancing viral antigen presentation by class I MHC proteins, 238 promoting the proliferation of MHC class I–restricted CD8 + CTLs, 239 and facilitating the functional maturation of dendritic cells. 240 Proinflammatory mediators IL-1β and IL-18 pleiotropically stimulate and amplify the innate immune response through induction of other inflammatory mediators, immune cell activation, and migration of inflammatory cells into sites of infection. 241 These molecules perform essential functions in host antiviral defense. 242
The adaptive immune response confers systemic and enduring pathogen-selective immunity through expansion and functional differentiation of viral antigen-specific T and B lymphocytes. Having both regulatory and effector roles, T lymphocytes are centrally positioned in the scheme of adaptive immunity. The primary cell type involved in the resolution of acute viral infection is the CD8 + CTL, which induces lethal proapoptotic signaling in virus-infected cells upon recognition of endogenously produced viral protein fragments presented by cell surface MHC class I molecules. Less frequently, CD4 + T cells, which recognize MHC class II–associated viral oligopeptides processed from exogenously acquired proteins, also demonstrate cytotoxicity against viral antigen-presenting cells. 243 The usual function of CD4 + T lymphocytes is to orchestrate and balance cell-mediated (CTL) and humoral (B lymphocyte) responses to infection. Classes of CD4 + helper T-cell subsets—Th1, Th2, Th17, Treg (regulatory T), and Tfh (follicular helper T)—have been defined based on characteristic patterns of cytokine secretion and effector activities. 244 , 245 Th1 and Th2 lymphocytes are usually associated with the development of cell-mediated and humoral responses, respectively, to viral infection. Th17 and Treg CD4 + subsets are important for control of immune responses and prevention of autoimmunity, but their precise roles in viral disease and antiviral immunity are not clear. For certain persistent viral infections, such as those caused by HIV and HSV, Treg cells might exacerbate disease through suppression of CTLs or, paradoxically, ameliorate illness by attenuating immune-mediated cell and tissue injury. 246 Tfh cells promote differentiation of antigen-specific memory B lymphocytes and plasma cells within germinal centers. 247 Therefore, Tfh cells likely occupy a central place in the humoral response to viral infection and vaccination. Although Tfh cell functions are not unique to antiviral responses, chronic viral infections including HBV and HIV appear to stimulate proliferation of these cells. 248 , 249 The Tfh phenotype may interconvert with other T-helper lineage profiles and thus represent a differentiation intermediate rather than a unique CD4 + T lymphocyte subset. 245
The primacy of cell-mediated immune responses in combating viral infections is revealed by the extreme vulnerability of individuals to chronic and life-threatening viral diseases when cellular immunity is dysfunctional. Those with acquired immunodeficiency syndrome (AIDS) exemplify the catastrophic consequences of collapsing cell-mediated immunity; progressive multifocal leukoencephalopathy caused by JC polyomavirus, along with severe mucocutaneous and disseminated CMV, HSV, and VZV infections, are frequent complications of vanishing CD4 + T cells. Similarly, iatrogenic cellular immunodeficiency associated with hematopoietic stem cell and solid-organ transplantation or antineoplastic treatment regimens predisposes to severe, potentially fatal infections with herpesviruses and respiratory viral pathogens such as adenovirus, PIV, and RSV, 250 all of which normally produce self-limited illness in immunocompetent hosts. Prevention and management of serious viral respiratory infections are significant challenges in myelosuppression units because of the communicability of respiratory viruses and paucity of effective drugs to combat these ubiquitous agents. Individuals with significantly impaired cell-mediated immunity are also at increased risk for enhanced viral replication and systemic disease following immunization with live, attenuated viral vaccines (e.g., measles-mumps-rubella [MMR] and VZV vaccines). Hence, live viral vaccines are generally contraindicated for immunocompromised persons (see Chapter 321). TNF-α inhibitor therapy, increasingly employed to manage a variety of rheumatologic and inflammatory diseases, enhances the risk of HBV reactivation with potentially life-threatening consequences. 251 Preventive and interventional HBV treatment strategies are necessary to circumvent complications of uncontrolled viral replication in these patients.
In contrast to cell-mediated immune mechanisms, humoral responses are usually not a determinative factor in the resolution of primary viral infections. (One notable exception is a syndrome of chronic enteroviral meningitis in the setting of agammaglobulinemia. 252 ) However, for most human viral pathogens, the presence of antibody is associated with protection against initial infection in vaccinees or reinfection in hosts with a history of natural infection. 253 Longitudinal studies indicate that levels of protective serum antibodies (induced by natural infection or immunization) to common viruses, including EBV, measles, mumps, and rubella, are remarkably stable, with calculated antibody half-lives ranging from several decades to thousands of years. 254 The protective role of antibodies on secondary exposure is frequently explained as interruption of viremic spread where a hematogenous phase is involved, such as occurs with measles, mumps, and rubella viruses, poliovirus, VZV, and most arboviruses. Nevertheless, most human viruses, excluding insect-transmitted agents, enter their hosts by transgression of a mucosal barrier, frequently undergoing primary replication in mucosal epithelium or adjacent lymphoid tissues. Neutralizing IgA exuded onto mucosal epithelial surfaces may protect against primary infection at this portal of viral entry. A classic example is gut mucosal immunity induced by orally administered Sabin poliovirus vaccine containing live-attenuated virus. Secretory IgA against poliovirus blocks infection at the site of primary replication and consequently interrupts the chain of viral transmission, although fully virulent revertant viruses arise at regular frequency in vaccine recipients, who may develop disease and also transmit revertant strains to nonimmune individuals. 255 Clinical and experimental studies of immunity to HIV have led to the recognition that resident immune responses at exposed mucosal surfaces are likely critical components of host resistance to primary HIV infection, and achievement of potent mucosal immunity has emerged as an important consideration for the design of candidate HIV vaccines. 256 Despite the appearance of serum neutralizing antibodies to HIV several weeks after infection, viral eradication is thwarted by selection of neutralization-resistant variant strains from a mutant pool, which is perpetually replenished because of extreme plasticity within neutralization determinants on the viral envelope glycoproteins. 257 Identification of epitopes bound by broadly neutralizing antiviral antibodies has provided potential new targets for structure-based vaccine design. 258
Protection against viral infection by serum immunoglobulins is often correlated with antibody-mediated neutralization of viral infectivity in cultured cells. Antibodies interrupt the viral life cycle at early steps, which may include cross-linking virion particles into noninfectious aggregates, steric hindrance of receptor engagement, and interference with viral disassembly. 259 It is presumed that virus neutralization in cell culture by human serum is reflective of antibody activity in the intact host, but the mechanistic basis of infection blockade and disease prevention by antibodies in vivo is difficult to define precisely. For example, exclusively in vivo functions of the humoral antiviral response include Fc-mediated virion phagocytosis 260 , 261 and antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC responses require effectors from both the innate and adaptive systems, NK cells and antibodies, respectively. 262 The basis of ADCC is FcγRIIIa receptor-dependent recognition by NK cells of virus-specific IgG bound to antigens expressed on the surface of infected cells, leading to release of perforin and granzymes from NK cells that eventuate in target cell apoptosis. Neutrophils, lymphocytes, and macrophages also possess Fc receptors and may participate in ADCC.
Key References
The complete reference list is available online at Expert Consult.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.1.3: Virus Infections and Hosts
- Last updated
- Save as PDF
- Page ID 97116

Learning Objectives
By the end of this section, you will be able to do the following:
- List the steps of replication and explain what occurs at each step
- Describe the lytic and lysogenic cycles of virus replication
- Explain the transmission of plant and animal viruses
- Discuss some of the diseases caused by plant and animal viruses
- Discuss the economic impact of plant and animal viruses
Viruses are obligate, intracellular parasites. A virus must first recognize and attach to a specific living cell prior to entering it. After penetration, the invading virus must copy its genome and manufacture its own proteins. Finally, the progeny virions must escape the host cell so that they can infect other cells. Viruses can infect only certain species of hosts and only certain cells within that host. Specific host cells that a virus must occupy and use to replicate are called permissive . In most cases, the molecular basis for this specificity is due to a particular surface molecule known as the viral receptor on the host cell surface. A specific viral receptor is required for the virus to attach. In addition, differences in metabolism and host-cell immune responses (based on differential gene expression) are a likely factor in determining which cells a virus may target for replication.
Steps of Virus Infections
A virus must use its host-cell processes to replicate. The viral replication cycle can produce dramatic biochemical and structural changes in the host cell, which may cause cell damage. These changes, called cytopathic effects , can change cell functions or even destroy the cell. Some infected cells, such as those infected by the common cold virus known as rhinovirus, die through lysis (bursting) or apoptosis (programmed cell death or “cell suicide”), releasing all progeny virions at once. The symptoms of viral diseases result both from such cell damage caused by the virus and from the immune response to the virus, which attempts to control and eliminate the virus from the body.
Many animal viruses, such as HIV (human immunodeficiency virus) , leave the infected cells of the immune system by a process known as budding , where virions leave the cell individually. During the budding process, the cell does not undergo lysis and is not immediately killed. However, the damage to the cells that the virus infects may make it impossible for the cells to function normally, even though the cells remain alive for a period of time. Most productive viral infections follow similar steps in the virus replication cycle: attachment, penetration, uncoating, replication, assembly, and release (Figure 21.8).
A virus attaches to a specific receptor site on the host cell membrane through attachment proteins in the capsid or via glycoproteins embedded in the viral envelope. The specificity of this interaction determines the host—and the cells within the host—that can be infected by a particular virus. This can be illustrated by thinking of several keys and several locks, where each key will fit only one specific lock.
Link to Learning
This video explains how influenza attacks the body.
Viruses may enter a host cell either with or without the viral capsid. The nucleic acid of bacteriophages enters the host cell “naked,” leaving the capsid outside the cell. Plant and animal viruses can enter through endocytosis (as you may recall, the cell membrane surrounds and engulfs the entire virus). Some enveloped viruses enter the cell when the viral envelope fuses directly with the cell membrane. Once inside the cell, the viral capsid degrades, and then the viral nucleic acid is released and becomes available for replication and transcription.
Replication and Assembly
The replication mechanism depends on the viral genome. DNA viruses usually use host-cell proteins and enzymes to replicate the viral DNA and to transcribe viral mRNA, which is then used to direct viral protein synthesis. RNA viruses usually use the RNA core as a template for synthesis of viral genomic RNA and mRNA. The viral mRNA directs the host cell to synthesize viral enzymes and capsid proteins, and assemble new virions.
Of course, there are exceptions to this pattern. If a host cell does not provide the enzymes necessary for viral replication, viral genes supply the information to direct synthesis of the missing proteins. Retroviruses, such as HIV (group VI of the Baltimore classification scheme), have an RNA genome that must be reverse transcribed into DNA, which then is incorporated into the host cell genome. To convert RNA into DNA, retroviruses must contain genes that encode the virus-specific enzyme reverse transcriptase that transcribes an RNA template to DNA. Reverse transcription never occurs in uninfected host cells—the enzyme reverse transcriptase is only derived from the expression of viral genes within the infected host cells. The fact that HIV produces some of its own enzymes not found in the host has allowed researchers to develop drugs that inhibit these enzymes without affecting the host’s metabolism.
This approach has led to the development of a variety of drugs used to treat HIV and has been effective at reducing the number of infectious virions (copies of viral RNA) in the blood to non-detectable levels in many HIV-infected individuals.
The last stage of viral replication is the release of the new virions produced in the host organism, where they are able to infect adjacent cells and repeat the replication cycle. As you’ve learned, some viruses are released when the host cell dies, and other viruses can leave infected cells by budding through the membrane without directly killing the cell.
Visual Connection
Influenza virus is packaged in a viral envelope that fuses with the plasma membrane. This way, the virus can exit the host cell without killing it. What advantage does the virus gain by keeping the host cell alive?
Watch a video on viruses, identifying structures, modes of transmission, replication, and more.
Different Hosts and Their Viruses
As you’ve learned, viruses often infect very specific hosts, as well as specific cells within the host. This feature of a virus makes it specific to one or a few species of life on Earth. On the other hand, so many different types of viruses exist on Earth that nearly every living organism has its own set of viruses trying to infect its cells. Even prokaryotes, the smallest and simplest of cells, may be attacked by specific types of viruses. In the following section, we will look at some of the features of viral infection of prokaryotic cells. As we have learned, viruses that infect bacteria are called bacteriophages (Figure 21.9). Archaea have their own similar viruses.
Bacteriophages
Most bacteriophages are dsDNA viruses, which use host enzymes for DNA replication and RNA transcription. Phage particles must bind to specific surface receptors and actively insert the genome into the host cell. (The complex tail structures seen in many bacteriophages are actively involved in getting the viral genome across the prokaryotic cell wall.) When infection of a cell by a bacteriophage results in the production of new virions, the infection is said to be productive . If the virions are released by bursting the cell, the virus replicates by means of a lytic cycle (Figure 21.10). An example of a lytic bacteriophage is T4, which infects Escherichia coli found in the human intestinal tract. Sometimes, however, a virus can remain within the cell without being released. For example, when a temperate bacteriophage infects a bacterial cell, it replicates by means of a lysogenic cycle (Figure 21.10), and the viral genome is incorporated into the genome of the host cell. When the phage DNA is incorporated into the host-cell genome, it is called a prophage . An example of a lysogenic bacteriophage is the λ (lambda) virus, which also infects the E . coli bacterium. Viruses that infect plant or animal cells may sometimes undergo infections where they are not producing virions for long periods. An example is the animal herpesviruses , including herpes simplex viruses, the cause of oral and genital herpes in humans. In a process called latency , these viruses can exist in nervous tissue for long periods of time without producing new virions, only to leave latency periodically and cause lesions in the skin where the virus replicates. Even though there are similarities between lysogeny and latency, the term lysogenic cycle is usually reserved to describe bacteriophages. Latency will be described in more detail in the next section.
Which of the following statements is false?
- In the lytic cycle, new phages are produced and released into the environment.
- In the lysogenic cycle, phage DNA is incorporated into the host genome.
- An environmental stressor can cause the phage to initiate the lysogenic cycle.
- Cell lysis only occurs in the lytic cycle.
Plant Viruses
Most plant viruses, like the tobacco mosaic virus, have single-stranded (+) RNA genomes. However, there are also plant viruses in most other virus categories. Unlike bacteriophages, plant viruses do not have active mechanisms for delivering the viral genome across the protective cell wall. For a plant virus to enter a new host plant, some type of mechanical damage must occur. This damage is often caused by weather, insects, animals, fire, or human activities like farming or landscaping. Movement from cell to cell within a plant can be facilitated by viral modification of plasmodesmata (cytoplasmic threads that pass from one plant cell to the next). Additionally, plant offspring may inherit viral diseases from parent plants. Plant viruses can be transmitted by a variety of vectors, through contact with an infected plant’s sap, by living organisms such as insects and nematodes, and through pollen. The transfer of a virus from one plant to another is known as horizontal transmission , whereas the inheritance of a virus from a parent is called vertical transmission .
Symptoms of viral diseases vary according to the virus and its host (Table 21.4). One common symptom is hyperplasia , the abnormal proliferation of cells that causes the appearance of plant tumors known as galls . Other viruses induce hypoplasia , or decreased cell growth, in the leaves of plants, causing thin, yellow areas to appear. Still other viruses affect the plant by directly killing plant cells, a process known as cell necrosis . Other symptoms of plant viruses include malformed leaves; black streaks on the stems of the plants; altered growth of stems, leaves, or fruits; and ring spots, which are circular or linear areas of discoloration found in a leaf.
Plant viruses can seriously disrupt crop growth and development, significantly affecting our food supply. They are responsible for poor crop quality and quantity globally, and can bring about huge economic losses annually. Others viruses may damage plants used in landscaping. Some viruses that infect agricultural food plants include the name of the plant they infect, such as tomato spotted wilt virus, bean common mosaic virus, and cucumber mosaic virus. In plants used for landscaping, two of the most common viruses are peony ring spot and rose mosaic virus. There are far too many plant viruses to discuss each in detail, but symptoms of bean common mosaic virus result in lowered bean production and stunted, unproductive plants. In the ornamental rose, the rose mosaic disease causes wavy yellow lines and colored splotches on the leaves of the plant.
Animal Viruses
Animal viruses, unlike the viruses of plants and bacteria, do not have to penetrate a cell wall to gain access to the host cell. The virus may even induce the host cell to cooperate in the infection process. Non-enveloped or “naked” animal viruses may enter cells in two different ways. As a protein in the viral capsid binds to its receptor on the host cell, the virus may be taken inside the cell via a vesicle during the normal cell process of receptor-mediated endocytosis . An alternative method of cell penetration used by non-enveloped viruses is for capsid proteins to undergo shape changes after binding to the receptor, creating channels in the host cell membrane. The viral genome is then “injected” into the host cell through these channels in a manner analogous to that used by many bacteriophages.
Enveloped viruses also have two ways of entering cells after binding to their receptors: receptor-mediated endocytosis, or fusion . Many enveloped viruses enter the cell by receptor-mediated endocytosis in a fashion similar to that seen in some non-enveloped viruses. On the other hand, fusion only occurs with enveloped virions. These viruses, which include HIV among others, use special fusion proteins in their envelopes to cause the envelope to fuse with the plasma membrane of the cell, thus releasing the genome and capsid of the virus into the cell cytoplasm.
After making their proteins and copying their genomes, animal viruses complete the assembly of new virions and exit the cell. As we have already discussed using the example the influenza virus, enveloped animal viruses may bud from the cell membrane as they assemble themselves, taking a piece of the cell’s plasma membrane in the process. On the other hand, non-enveloped viral progeny, such as rhinoviruses, accumulate in infected cells until there is a signal for lysis or apoptosis, and all virions are released together.
As you will learn in the next module, animal viruses are associated with a variety of human diseases. Some of them follow the classic pattern of acute disease , where symptoms get increasingly worse for a short period followed by the elimination of the virus from the body by the immune system and eventual recovery from the infection. Examples of acute viral diseases are the common cold and influenza. Other viruses cause long-term chronic infections , such as the virus causing hepatitis C, whereas others, like herpes simplex virus, only cause intermittent symptoms. Still other viruses, such as human herpesviruses 6 and 7, which in some cases can cause the minor childhood disease roseola, often successfully cause productive infections without causing any symptoms at all in the host, and thus we say these patients have an asymptomatic infection .
In hepatitis C infections, the virus grows and reproduces in liver cells, causing low levels of liver damage. The damage is so low that infected individuals are often unaware that they are infected, and many infections are detected only by routine blood work on patients with risk factors such as intravenous drug use. On the other hand, since many of the symptoms of viral diseases are caused by immune responses, a lack of symptoms is an indication of a weak immune response to the virus. This allows the virus to escape elimination by the immune system and persist in individuals for years, all the while producing low levels of progeny virions in what is known as a chronic viral disease. Chronic infection of the liver by this virus leads to a much greater chance of developing liver cancer, sometimes as much as 30 years after the initial infection.
As already discussed, herpes simplex virus can remain in a state of latency in nervous tissue for months, even years. As the virus “hides” in the tissue and makes few if any viral proteins, there is nothing for the immune response to act against, and immunity to the virus slowly declines. Under certain conditions, including various types of physical and psychological stress, the latent herpes simplex virus may be reactivated and undergo a lytic replication cycle in the skin, causing the lesions associated with the disease. Once virions are produced in the skin and viral proteins are synthesized, the immune response is again stimulated and resolves the skin lesions in a few days or weeks by destroying viruses in the skin. As a result of this type of replicative cycle, appearances of cold sores and genital herpes outbreaks only occur intermittently, even though the viruses remain in the nervous tissue for life. Latent infections are common with other herpesviruses as well, including the varicella-zoster virus that causes chickenpox. After having a chickenpox infection in childhood, the varicella-zoster virus can remain latent for many years and reactivate in adults to cause the painful condition known as “shingles” (Figure 21.11).
Some animal-infecting viruses, including the hepatitis C virus discussed above, are known as oncogenic viruses : They have the ability to cause cancer. These viruses interfere with the normal regulation of the host cell cycle either by introducing genes that stimulate unregulated cell growth (oncogenes) or by interfering with the expression of genes that inhibit cell growth. Oncogenic viruses can be either DNA or RNA viruses. Cancers known to be associated with viral infections include cervical cancer, caused by human papillomavirus (HPV) (Figure 21.12), liver cancer caused by hepatitis B virus, T-cell leukemia, and several types of lymphoma.
This video shows the life cycle of a virus.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
Bringing the World's Best Biology to You
Virus Structures
Part 1: virus structures: general principles.
- Duration: 49:50
00:00:02.08 Hello, I'm Stephen Harrison of Harvard Medical School, 00:00:05.25 Children's Hospital Boston, and the Howard Hughes 00:00:08.05 Medical Institute. This is the first of three lectures on virus 00:00:14.13 structures. This first lecture will be about general features 00:00:18.28 of the molecular organization of virus particles. The 00:00:22.15 second two will be about specific properties of virus 00:00:26.07 particles relevant to the molecular mechanism of infecting 00:00:30.22 a cell. Viruses are carriers of genetic information from one 00:00:37.04 cell to another; in that sense, they're effectively 00:00:41.16 extracellular organelles. The infectious virus particle, 00:00:46.18 sometimes called a "virion," is a molecular machine that 00:00:50.01 packages viral genomes, escapes from the infected cell, 00:00:54.22 survives transfer from one cell to another, and attaches, 00:00:59.01 penetrates, and initiates replication in the new host cell. 00:01:03.21 It's thus not just a passive package, but rather an active 00:01:12.15 payload deliverer. Now most people know viruses as 00:01:17.17 pathogens because the virus bears the genetic 00:01:21.12 information needed to usurp the cellular biosynthetic 00:01:25.24 machinery and replicate itself. The selective advantage 00:01:31.10 for evolution of the virus may be a selective disadvantage 00:01:34.21 for the host, and as a result, hosts evolve defense 00:01:39.25 mechanisms, the immune system in the case of humans 00:01:45.08 and other higher vertebrates. Now, viruses come in two 00:01:52.17 major flavors: enveloped viruses, in which the infectious 00:01:57.22 virus particle is surrounded by a lipid bilayer membrane 00:02:02.20 derived from host cell membrane; and non-enveloped 00:02:06.27 viruses, rather unimaginatively, that have no lipid bilayer 00:02:11.12 membrane, and the protective coat is just protein. These 00:02:15.01 two structural modes correspond to different modes of exit 00:02:21.09 and entry into cells, different mechanisms of assembly, 00:02:25.13 and different mechanisms of infection, as we'll see in the 00:02:31.18 course of this lecture and the next two. Now, just as 00:02:36.17 quick examples, on the left is an example of a non- 00:02:44.12 enveloped virus particle, a rotovirus particle. This image is 00:02:49.17 based on reconstructions from many electron 00:02:55.00 micrographs, and we'll go into some of the details of that 00:02:59.12 in the third lecture. And on the other side is an example of 00:03:05.25 an enveloped virus particle, also studied by electron 00:03:11.11 microscopy, and in the cross section of the image that 00:03:16.07 you see at the bottom, you can clearly see evidence of 00:03:19.22 the lipid bilayer with α-helical segments of the protein on 00:03:25.25 the outside traversing it. Just to remind you of sizes and 00:03:33.07 distances, both the rotovirus particle and the Sindbis virus 00:03:37.24 particle, as the right-hand one was called, have outer 00:03:42.23 shells that are about 700 angstroms in diameter, or 70 00:03:46.15 nanometers. That's about a millionth the size of a tennis 00:03:50.11 ball. Recall that chemical bonds that are between one 00:03:54.23 and two angstroms in length, and that's why chemists use 00:04:01.06 angstroms rather than nanometers; it's the natural unit of a 00:04:04.26 chemical bond. So when I say 700 angstroms, you can 00:04:08.04 think of that as 500-700 atoms across. Of course it's a 00:04:13.11 volume, and so the molecular mass of these particles is 00:04:17.08 some tens of millions of daltons. So bear in mind during 00:04:23.10 this lecture the following three questions. We'll talk about 00:04:28.15 more than just these three, but the main point of the 00:04:34.00 lecture will be to try to introduce you to the following 00:04:37.26 issues. First, why most non-enveloped viruses and a 00:04:41.29 number of smaller enveloped viruses have highly 00:04:45.05 symmetric structures. Second, what do the building 00:04:49.05 blocks of these particles look like? Turns out that the 00:04:52.14 same kinds of building blocks have been used over and 00:04:54.27 over again in the evolution of different viruses, even 00:04:58.02 viruses with very different replication strategies. And 00:05:01.08 finally, what the outer proteins of some enveloped viruses 00:05:04.27 look like. So let's begin with symmetry. What does 00:05:09.20 symmetry mean? Symmetry, as suggested by the image 00:05:14.00 on the left, means that there's some operation, in the case 00:05:18.12 of physical objects some physical operation like a rotation, 00:05:22.07 that brings the object into self-coincidence. In this case, if 00:05:26.16 you rotated this figure by 120° about the axis represented 00:05:31.29 by that triangle, and you closed your eyes while you did it, 00:05:36.02 you wouldn't realize that you had done the rotation. That's 00:05:39.22 called a threefold axis, and as you can imagine, symmetry 00:05:42.27 of more complicated objects can have other symmetry 00:05:46.14 axes, and so the icosahedron (we'll come back to that in 00:05:50.09 a minute) represented on the right has fivefold axes, 00:05:53.23 threefold axes, and twofold axes of symmetry. Some 00:05:59.06 viruses have helical symmetry. Helical symmetry is 00:06:05.11 represented by a screw axis, and so tobacco mosaic 00:06:09.12 virus, which was studied historically as one of the very 00:06:13.01 first viruses for which detailed biochemistry and detailed 00:06:17.13 structure became available, is a helical array in which the 00:06:22.23 nucleic acid, the RNA, is wound into a groove on the 00:06:27.17 protein subunit and winds up with the protein, which forms 00:06:35.22 this helical array. There are a number of other helical 00:06:39.23 organizations in virus particles: Vesicular stomatitis virus is 00:06:44.16 a much more complicated enveloped virus with an outer 00:06:47.18 glycoprotein, that's what this "G" is, on the right. But as 00:06:52.13 you can imagine, helical symmetry yields elongated 00:06:57.25 particles that get unwieldy. And so far more common is 00:07:04.01 the isometric, that is, roughly spherical characteristics, of 00:07:09.19 virus particles with icosahedral symmetry. The 00:07:13.06 icosahedron, one of the Platonic solids, the fanciest one, 00:07:16.28 so to speak, with 20 triangular faces, is simply a 00:07:20.18 representation of icosahedral symmetry. An object needn't 00:07:25.17 have icosahedral shape in order to have icosahedral 00:07:29.26 symmetry. And likewise, I could destroy the symmetry of 00:07:33.13 this object by painting an asymmetric object on each 00:07:37.17 face, rather than an object with threefold symmetry. The 00:07:44.09 icosahedral symmetry is represented or characterized by, 00:07:53.09 as I said, twofold axes, fivefold axes, and threefold axes. 00:07:58.27 And if you place a single asymmetric subunit into a space 00:08:07.24 governed by icosahedral symmetry and then operate on it 00:08:11.28 with the symmetry axes, you get 59 others, that is, there 00:08:16.21 are 60 locations in all that are related to each other by 00:08:23.27 these various symmetry axes, by these various symmetry 00:08:27.00 operations. And so a particle with icosahedral symmetry 00:08:33.27 will have 60 subunits. What I've flashed in here is a sort 00:08:39.24 of schematic representation of what might be a protein 00:08:43.22 subunit, to suggest that a small particle with 60 protein 00:08:49.28 subunits appropriately interfaced with each other can form 00:08:55.02 an icosahedral structure. Now this is indeed a schematic 00:09:00.21 representation of an actual virus particle. Parvovirus is 00:09:05.14 one of the very smallest and simplest of the viruses, have 00:09:10.04 a single kind of protein subunit that forms a small shell, 00:09:18.25 and 60 of them decorate or assemble into that shell, as 00:09:24.02 suggested here. And so, if we take a slightly closer look 00:09:30.14 at that protein subunit in a traditional ribbon diagram 00:09:35.19 representing the fold of the polypeptide chain, you see 00:09:39.26 that it's based on a quite simple, compact domain 00:09:44.21 represented here in red, with large loops emanating from 00:09:49.23 it. That compact domain has this sort of fold, it's called a 00:09:55.03 "jelly roll β-barrel," or sometimes a "cupin fold," and this 00:09:59.04 particular representation comes from the structure of 00:10:03.10 canine parvovirus, a virus of dogs, as you can imagine. 00:10:08.09 But the parvovirus family includes viruses, such as adeno- 00:10:14.26 associated viruses, now being used as vectors for efforts 00:10:21.04 at gene therapy. So in this case, the simple jelly roll β- 00:10:28.16 barrel structure has been elaborated with loops in order to 00:10:33.09 make a particle of adequate size, as shown here. And 00:10:39.00 that particle can package about a 5 kilobase single- 00:10:45.22 stranded DNA genome. Since the molecular mass of the 00:10:51.14 coat protein is about 50 kilodaltons, there's just enough 00:10:55.23 volume inside to package that genome, of which about a 00:11:00.19 third is actually given over to encoding for the coat 00:11:04.11 protein. So that's a relatively expensive way of spending 00:11:08.29 your genetic information. You've got to dedicate a full 00:11:12.04 one-third just to specifying the cardboard box, if you wish, 00:11:18.18 with which you're going to deliver the payload that you 00:11:22.24 actually wish to deliver. FedEx wouldn't like a system in 00:11:27.26 which a third of the weight were in the box. So what 00:11:38.05 about trying to package larger genomes with larger 00:11:41.24 coding capacity? One example, although still by no 00:11:46.29 means as efficient as the viruses we'll start to talk about, 00:11:55.03 are the so-called picornaviruses. These are small positive- 00:11:59.04 strand RNA viruses, of which poliovirus and the human 00:12:03.10 common cold virus (human rhinoviruses) are good 00:12:06.27 examples. In this case, there are three different protein 00:12:11.26 subunits, each with one of these β jelly roll designs, very, 00:12:17.11 very similar to that red β jelly roll in the canine parvovirus, 00:12:22.21 that assemble as shown into an icosahedral structure. 00:12:27.06 And so one-sixtieth of this structure has three jelly rolls, a 00:12:32.06 red one, a blue one, and a green one, designated in that 00:12:37.10 order, VP3, VP1, and VP2, for the colors as I named 00:12:42.03 them, forming the sort of assembly that you see here. 00:12:48.03 Now, those three subunits, as I said, look strikingly like 00:12:54.22 that same β jelly roll we saw in the parvovirus subunit, but 00:12:59.29 the loops are a little less extensive, because in this case, 00:13:03.05 with three subunits, the size of the particle doesn't need 00:13:09.22 to be additionally augmented by taking up space with 00:13:13.20 those loops, and one can still package adequate 00:13:16.14 amounts of RNA. One other feature of the architecture of 00:13:21.13 this particle that's noteworthy, and we're going to see in 00:13:27.01 various forms as we look at even more complicated 00:13:30.24 viruses particles, is the nature of the interaction among 00:13:38.19 the subunits, which not only involves interfaces between 00:13:46.09 pre-folded, rigid domains of subunits, but an elaborate 00:13:53.07 inner scaffold and a little bit of an outer scaffold made by 00:13:58.17 parts of this protein's subunit that fold up only when then 00:14:04.17 particle assembles. And so on the right, you can see 00:14:06.29 some hint of this in a blow-up of VP1, VP2, and VP3, 00:14:13.02 where you can see that, in addition to the jelly roll 00:14:18.15 domains, there are extended arms, they happen to be N- 00:14:24.06 terminal and extend inward in the particle, that fold 00:14:28.10 together when the particle assembles. Now these viruses 00:14:34.15 manage to package a nine kilobase single-stranded RNA 00:14:38.07 genome, but still use about a third of the genome to 00:14:42.16 encode the coat. The same size of package can be 00:14:50.23 achieved with a single kind of subunit, if that subunit can 00:14:55.13 have multiple conformers. Why does it need to have 00:14:58.22 multiple conformers? I told you that icosahedral symmetry 00:15:05.25 requires that there be 60 and only 60 identical structures 00:15:15.11 that form an icosahedrally symmetric shell, so that if you 00:15:19.21 want to use 180 protein subunits, as in the 00:15:23.14 picornaviruses, either they have to come in three chemically distinct 00:15:29.07 kinds, three colors, if you wish, or they need to have three 00:15:34.18 distinct conformers. In this example, from a simple plant 00:15:39.08 virus called tomato bushy stunt virus, shows that indeed 00:15:44.29 one can make a very similar package with the jelly roll β- 00:15:49.17 barrels packed essentially in the same orientation and the 00:15:54.13 same packing style, so to speak, as in the picornaviruses, 00:16:01.11 but where there's only one kind of subunit, and blue, red, 00:16:05.20 and green correspond to three different conformations of 00:16:09.21 that subunit. Those conformers can be achieved by 00:16:14.11 alternate hinges between rigid domains, as shown here, 00:16:19.18 between the two different major conformations. The red 00:16:25.10 and the blue in the previous slide are actually extremely 00:16:29.10 similar to each other and would be represented by what 00:16:33.08 you see here on the right. And a second conformation not 00:16:38.18 only with a somewhat different hinge but also with an N- 00:16:43.00 terminal arm folded up in an ordered way, whereas it's 00:16:47.04 disordered and hangs into the center of the virus particle 00:16:50.15 on the other conformation. So then again one sees here 00:16:56.29 that there is an elaborate inner scaffold that dictates the 00:17:01.27 assembly formed by parts of the protein subunit that are 00:17:08.17 not rigidly folded, are not ordered, until the assembly 00:17:13.24 comes together. We can have a look at that scaffold, it's 00:17:18.00 instructive, in this blow-up of the particle by just focusing 00:17:23.24 on those 60 of the 180 subunits that have an ordered 00:17:28.11 arm. And if we now focus in on that array of protein 00:17:36.10 subunits, 60 of the 180, and look over here at where 00:17:41.23 three of them interact at a threefold axis of the 00:17:45.27 icosahedral symmetry, you see that there's an inner 00:17:49.10 scaffold formed by the N-terminal arms of the protein 00:17:52.22 subunit that dictates the size and characteristic of the 00:17:59.25 whole assembly. These arm-like extensions, which fold 00:18:05.02 together to form an inner scaffold, also form flexible links 00:18:08.19 to the RNA. This is a good example of the undemanding 00:18:14.07 packaging of a genome, as I like to call it. If the package 00:18:20.04 required either a specific nucleotide sequence for a lot of 00:18:25.14 the RNA, or a defined structure in three dimensions, let's 00:18:30.22 say, for the RNA, then the RNA could not evolve to 00:18:34.19 encode the other functions that matter: an RNA- 00:18:39.21 dependent RNA polymerase, for example. And so 00:18:44.03 packaging of nucleic acids in viruses like these involve 00:18:49.25 both a short packaging sequence or packaging signal 00:18:57.20 that is recognized by a few copies of the protein subunit 00:19:03.14 and that can act as an assembly origin; and then a large 00:19:07.21 of nonspecific, charge-neutralizing interactions to 00:19:11.07 condense the RNA into the center of the particle. And so 00:19:15.19 in the case of tomato bushy stunt virus, at the tip of the 00:19:19.00 arm is a very positively charged polypeptide segment that 00:19:25.14 condenses the RNA and neutralizes the strong negative 00:19:30.02 charge on the phosphates. The specific recognition 00:19:36.15 interactions in the case of bushy stunt virus, we don't 00:19:40.05 have a picture of, but we do have a picture of how that 00:19:44.05 same part of the arm recognizes a specific packaging 00:19:49.24 sequence in the case of a related plant virus called alfalfa 00:19:54.03 mosaic virus. And in that case, a positively charged, 25- 00:19:59.13 residue-or-so, N-terminal segment that is not well ordered 00:20:07.15 on the protein subunit as it folds on its own, co-folds with 00:20:14.05 a short packaging sequence represented here by a 00:20:20.07 standard two-dimensional sequence representation of two 00:20:24.02 stem-loops. And the three-dimensional structure 00:20:28.28 represented here leads to a specific recognition, because 00:20:34.28 the stem-loops form a defined three-dimensional 00:20:38.29 interaction stabilized by their folding together with the N- 00:20:48.04 terminal arm of a small number of subunits. Probably one 00:20:52.22 dimer is responsible for recognizing this pair of stem-loops, 00:20:57.18 and the full packaging sequence might have three such 00:21:00.26 pairs and three dimers, but the protein shell is composed 00:21:07.12 of a much larger number, and the all remaining protein 00:21:09.28 subunits will have nonspecific, positively charged, 00:21:16.12 charge-neutralizing interactions with the RNA. Now, let's 00:21:22.14 go on and talk about still larger and more complicated 00:21:25.11 virus particles. Here's a representation, a surface 00:21:28.20 representation, of a papillomavirus. Papillomaviruses 00:21:34.09 cause warts and, in some cases, cancer in humans and 00:21:41.12 many other animals. The recently introduced vaccine 00:21:46.22 against human papillomavirus 16, 18, and one or two 00:21:50.28 other types, is a vaccine that prevents transmission of the 00:21:56.03 virus, which causes cervical cancer. So this surface 00:22:01.05 representation shows you that these viruses, which 00:22:05.00 package a double-strand DNA genome, are based on an 00:22:13.07 assembly of pentameric building blocks. In this case, the 00:22:20.11 pentameric building blocks are positioned not only at 00:22:26.22 positions of fivefold symmetry in this icosahedral shell, but 00:22:31.29 also at a general nonsymmetrical position so that this 00:22:37.09 pentamer is actually surrounded by six other pentamers. A 00:22:42.02 fivefold peg in a sixfold hole, so to speak. This sort of 00:22:48.24 assembly can nonetheless be stabilized by the same sorts 00:22:53.09 of principles that we've seen in the simpler viruses, 00:22:57.04 namely, the tying together of rigid or relatively rigid building 00:23:02.20 blocks by flexible, and hence potentially multidirectional, 00:23:11.00 arms. So here the pentameric assembly of the protein L1 00:23:17.22 that forms this structure is represented here, and as you 00:23:22.27 see, there are loops coming out of it with dotted lines 00:23:26.22 here, that form the interactions between the pentamers 00:23:33.08 shown here. And of course, since this is a fivefold peg in 00:23:36.19 a sixfold hole, its arms have to be directed in different 00:23:41.18 ways, but the pentamer itself is a rigid, fivefold-symmetric 00:23:47.01 object, just like its chemically identical mate here on a 00:23:53.20 fivefold position. Now, this subunit, the L1 subunit, is also 00:24:01.27 based on the same sort of β jelly roll building block that 00:24:06.15 we saw in the positive strand RNA viruses that we were 00:24:11.05 just talking about, and it's elaborated by various loops that 00:24:17.19 vary from virus type to virus type, just one of the reasons 00:24:24.08 that these viruses come in a great variety of serotypes, of 00:24:31.25 immunologically distinct types, because these loops, 00:24:36.19 which are on the outside of the virus particle (that is, this 00:24:39.24 is the part of the pentamer that faces outward, and this is 00:24:46.11 the part that would face inward, this would be the inside 00:24:49.13 of the virus, this the outside of the virus)... these loops are 00:24:53.25 free to vary evolutionarily because they're not so critical 00:24:59.07 for the formation of the stable assembly or for forming the 00:25:05.04 rigid pentamer, and hence can respond, if you wish, to 00:25:10.05 the pressures of their coevolution with the human immune 00:25:16.17 response, or the immune response of the particular animal 00:25:19.28 that they infect. Now, a similar principle, if you wish, 00:25:29.14 namely, reuse of the same kind of building block but in 00:25:35.01 environments that don't have a simple symmetry, is 00:25:43.02 exemplified by the adenoviruses. These are even much 00:25:46.27 larger structures, and I will try to make a few points by 00:25:53.06 talking about the adenovirus structure. The particle has a 00:25:58.28 strikingly icosahedral shape with fibers coming out of the 00:26:04.13 fivefold positions that are responsible for cell attachment. 00:26:08.20 The main part of the coat is represented by a protein 00:26:12.29 called "hexon" because it forms these sorts of 00:26:16.14 hexagonally packed arrays, but in fact the hexon is not a 00:26:19.20 hexamer, it's a trimer. It's a trimer, however, with two of 00:26:24.05 these β jelly roll domains, rather similar in their overall 00:26:28.00 shape, next to each other, so it has a kind of hexagonal 00:26:31.14 outline. As a result, the face of the icosahedron does 00:26:39.13 have threefold symmetry, and the whole structure has 00:26:42.20 threefold symmetry, but the hexon itself actually is only a 00:26:49.15 threefold, and not a sixfold, symmetric entity. Now one 00:26:54.25 quite interesting aspect of the structure here is that there 00:27:01.26 is a bacteriophage called PRD1 (and indeed several 00:27:05.13 other bacteriophages now known) that has essentially 00:27:09.19 exactly the same design. Adenoviruses are viruses of 00:27:13.11 humans and vertebrates and actually a large number of 00:27:17.06 other animal species, so with this structure, one can make 00:27:25.08 the point that even viruses of bacteria have strong 00:27:32.23 resemblances in their design to those of humans and 00:27:41.20 plants, for that matter. Indeed you saw similarity between 00:27:45.19 the plant viruses, like tomato bushy stunt virus, and the 00:27:51.08 picornaviruses, such as polio and the human common 00:27:54.02 cold virus. This doesn't mean, in my own view, that these 00:27:59.10 viruses are so ancient, if you wish, in their design, in their 00:28:05.12 structure, that they antedated the divergence of bacteria 00:28:10.17 and animals, or animals and plants. Rather, we know that 00:28:15.11 viruses can jump species. They can jump from insects... 00:28:19.20 indeed, there are viruses that infect both insects and 00:28:22.28 people, and there viruses that infect both insects and 00:28:26.29 plants. And so the transfer of genetic information that I 00:28:31.17 alluded to at the very beginning of the talk, the notion that 00:28:35.06 a virus particle is package that gets genetic material from 00:28:41.09 one kind of cell to another, may well be true not just for 00:28:45.13 the cells within you or between you and another individual 00:28:50.23 of the same species, but across species. We know that 00:28:55.05 flu jumps from swine to people, as we all learned from the 00:28:59.09 2009 pandemic, or from birds to people. But also, 00:29:05.09 ultimately, through eons of time, from one kingdom to 00:29:10.24 another. At any rate, it does means that the structures 00:29:15.20 we're talking about show a striking similarity and a striking 00:29:19.11 unity, whatever the evolutionary details. In the case of the 00:29:24.04 adenoviruses, the subunit on the fivefold axis is a different 00:29:31.27 protein subunit from the hexon. It's got one β jelly roll 00:29:37.10 domain instead of two, so that again there's a kind of 00:29:42.21 duplication and elaboration as this structure develops into 00:29:48.29 a much larger shall to package, in this case, a 35 00:29:54.01 kilobase-pair, double-strand DNA genome, much, much 00:29:58.04 larger genome. And indeed there are viruses based on 00:30:02.09 very similar kinds of protein subunits, the same double jelly 00:30:07.08 roll structure with a separate, related but genetically and 00:30:13.02 chemically distinct, single jelly roll pentamer on the fivefold 00:30:17.20 axes. There are even much larger viruses based on this 00:30:23.25 kind of subunit. Now, an interesting point in relating the 00:30:31.02 adenovirus structure to the bacteriophage that I 00:30:36.21 mentioned is based on or has a similar kind of major outer 00:30:44.00 shell subunit, are the mechanisms by which the virus 00:30:53.16 forms a defined and specific structure. As you can 00:30:56.27 imagine, in this sort of structure, how in the course of 00:31:00.26 assembly is the relationship between one fivefold position 00:31:06.03 and another fivefold position determined? How is the size 00:31:09.22 of this structure determined, rather than allowing, let us 00:31:14.19 say, multiple hexons to start forming much bigger and 00:31:18.11 bigger triangles? In the case of the phage, the answer is 00:31:26.15 particularly simple. One stripped off the outer shell of 00:31:31.15 hexon-like subunits and penton-like subunits, and 00:31:34.27 discovered that, from the x-ray crystal structure of this 00:31:38.22 particle, that there is an extended protein called a "tape 00:31:44.11 measure" protein by the investigators who discovered 00:31:47.14 this, that in effect stretches from a fivefold position here to 00:31:54.17 a twofold position here and then meets another one, 00:31:57.28 twofold symmetric to the next fivefold, and that 00:32:01.15 organization, think of the scaffolds that we talked about 00:32:06.28 before, governs the fixed size of the particle. In this case, 00:32:12.27 the scaffold protein is not an arm of the same protein 00:32:17.15 subunit, it's a separate protein, but the same principle 00:32:21.25 applies, and likewise, in the adenovirus particle, there are 00:32:26.12 several different so-called "glue" or "cement" proteins 00:32:30.19 that form, in effect, a scaffold that knits together the 00:32:34.18 structure in a way that leaves no ambiguity for the size 00:32:44.07 and characteristic of the final particle. In all of these 00:32:50.24 structures, the papillomaviruses, the adenoviruses, the 00:32:55.15 picornaviruses, the plant viruses such as tomato bushy 00:33:02.21 stunt, we see a simple construction principle at work that 00:33:09.16 is a little bit like an assembly line, like a factory assembly 00:33:13.23 line. There is in all cases a fixed assembly unit, happens 00:33:19.03 to be a dimer in the case of the coat protein of TBSV. 00:33:24.05 You saw that it was a pentamer in the case of the L1 00:33:26.21 protein of the papillomaviruses. The same of the 00:33:30.14 polyomaviruses like SV40. And you saw that the 00:33:35.04 adenovirus hexon, the trimeric adenovirus hexon, is 00:33:38.24 likewise a mass-produced assembly unit. But in order to 00:33:44.18 determine how that mass-produced assembly unit fits into 00:33:49.16 a defined structure of larger size, how the positioning of 00:33:57.18 that subunit doesn't simply lead to errors in the building of 00:34:03.09 a larger or smaller particle, there's a framework or scaffold 00:34:07.07 just as in the construction of a building, let's say, that 00:34:10.10 ensures accurate placement of these mass-produced 00:34:15.15 assembly units. And we've also seen that, interestingly 00:34:19.07 enough, there's a recurring architectural motif that has 00:34:23.17 appeared in the evolution of these structures (and it's a 00:34:26.09 complicated one, so it probably evolved only once) over 00:34:30.21 and over again. Now you might well ask, is this the only 00:34:34.22 architectural motif? Why are all viruses based on a so- 00:34:41.12 similar building block, and the answer is, that isn't the 00:34:44.00 case. There's at least one other, and that sometimes is 00:34:47.09 called the "HK97" fold, after the bacteriophage HK97 in 00:34:55.05 which it was discovered. You can see that this protein 00:34:57.13 subunit looks quite different, it's got some α-helices, it's a 00:35:00.20 somewhat irregular-looking structure, and it's found in the 00:35:07.12 bacteriophage P22 and a large number of other double- 00:35:11.01 strand DNA bacteriophage, where it forms a shell with a 00:35:22.10 number of these subunits forming both hexamers and 00:35:25.15 pentamers, so that there are 60 hexamers and 12 00:35:30.14 pentamers (there are always 12 pentamers in any 00:35:33.12 icosahedral structure), as suggested here. These viruses 00:35:39.03 assemble with an inner scaffold, but the scaffold in this 00:35:43.16 case is discarded by proteolytic digestion in some cases. 00:35:48.24 In this case, it's actually reused; it exits from the particle 00:35:53.08 and gets reused in the case of P22. And the particle then 00:36:00.24 changes some details of its organization as the scaffold 00:36:04.27 exits, as part of the process by which the double-strand 00:36:09.18 DNA is injected, actually pumped, if you wish, into the 00:36:14.20 particle at the next stage in assembly. So these are cases 00:36:19.02 in which the shell preassembles around a scaffold. The 00:36:23.20 scaffold is ejected, either chewed up or literally ejected 00:36:31.17 and reused, and a series of events involving motor 00:36:36.10 proteins are responsible for inserting DNA into these 00:36:42.22 structures. Now you could ask whether this is true only of 00:36:48.16 bacteriophage, answer: "no." You might anticipate that 00:36:52.09 the answer would be no from what I told you about 00:36:55.04 adenovirus and PRD1 for example. Here are two 00:37:01.07 bacteriophage protein subunits that have this sort of 00:37:05.04 structure, but the herpesviruses, of which the herpes 00:37:09.05 simplex 1, the cold sore virus, is one example, are based 00:37:15.24 on a much more elaborately looped, elaborately 00:37:21.13 decorated, version of the same fundamental fold. The 00:37:26.04 structure that we have at the moment is from electron 00:37:28.25 microscopy and not yet at the same resolution that the x- 00:37:33.04 ray structures of these subunits have yielded, but you can 00:37:37.29 probably see in this relatively low resolution representation 00:37:43.03 of the herpesvirus that this part, for example, there's a 00:37:47.20 long α-helix, corresponds to the much simpler, 00:37:53.03 undecorated fold you see here. And then these are loopy 00:37:56.04 structures that stick out and make the protein subunit 00:37:59.10 much larger and have to do with other interactions that 00:38:03.11 the protein subunit of the herpes particle makes. The 00:38:07.12 herpesvirus particle is more complicated, it's both larger 00:38:09.27 and more complicated than the phage particles, and so 00:38:13.25 there are other interactions of those surface loops that are 00:38:18.18 important. Herpesviruses like the phage have very tightly 00:38:25.20 coiled DNA that is inside, that's pumped into them in this 00:38:31.23 reconstruction from electron cryomicroscopy, you can 00:38:34.23 actually see the coiling of the DNA. The DNA is actually 00:38:39.20 coiled this way, that is, circumferentially about the axis of 00:38:45.00 the particle, it's injected through one vertex, and as you 00:38:48.09 see, there's a specialized internal structure here, to which 00:38:53.19 then the tail of the phage that ultimately injects it back 00:38:58.04 into a new host cell, is attached. The cross section here 00:39:04.21 looks as if you have circumferential layers of density in the 00:39:12.12 other direction because, as you can see from this 00:39:15.05 diagram, DNA coiled about a vertical axis, if the order is 00:39:25.23 such that, from particle to particle, there isn't exactly a 00:39:29.16 piece of DNA here, but this one might be here or here, 00:39:34.10 then on average, you will get radial shells of density as 00:39:40.07 you see here, fitting tightly into the interior of the particle, I 00:39:46.03 like to say, with a gardener's analogy (it might not be 00:39:50.10 relevant for all people listening), like winding a hose into a 00:39:54.06 hose pot, or rope into a bucket. Now finally, let's talk a 00:40:01.26 little bit about enveloped viruses. Enveloped viruses 00:40:08.25 acquire their envelope, in general, their membrane (this is 00:40:12.04 not true of all enveloped viruses, but true of almost all of 00:40:19.08 them), by budding out of the cell, either out of the cell 00:40:22.22 surface, or into an intracellular compartment such as the 00:40:29.03 endoplasmic reticulum or the Golgi apparatus, and then 00:40:33.16 being transported out. And in that budding process, wrap 00:40:38.19 themselves, if you wish, in a membrane that's derived from 00:40:42.23 host cell lipids, although host cell proteins are in general 00:40:47.21 excluded. Some of the smaller enveloped viruses have 00:40:53.21 icosahedral symmetry, and their structure and assembly is 00:40:58.15 determined by regular interactions within an icosahedral 00:41:03.08 shell, just as the ones you've seen in the non-enveloped 00:41:06.26 viruses. But larger and less regular enveloped viruses are 00:41:12.08 also seen, such as HIV or influenza, in which the protein 00:41:21.12 interactions are less perfect, but that doesn't matter for 00:41:26.11 protecting the nucleic acid nearly so much, because the 00:41:30.03 lipid bilayer in effect is an impermeable barrier against 00:41:35.07 agents that might get in and degrade or damage or 00:41:41.00 cleave the nucleic acid. So the budding process that I 00:41:48.06 mentioned can either involve, as in the case of the so- 00:41:52.02 called alphaviruses, or which Sindbis virus is one of the 00:41:56.18 prototypes and well studied... and a recent human 00:42:02.01 outbreak of an alphavirus is the chikungunya virus, which 00:42:07.03 had a major outbreak in the French island of Réunion, 00:42:12.22 and led to considerable interest and publicity about the 00:42:16.21 properties of that virus. The alphaviruses have a core that 00:42:22.25 preassembles in the cytoplasm and then two species of 00:42:28.05 glycoprotein that are synthesized on the rough ER, 00:42:33.29 exported to the cell surface, and then the particle buds 00:42:39.03 out through a process by which the inward-directed C- 00:42:47.01 terminal tips of the glycoprotein, which stick through the 00:42:52.09 membrane in a single α-helical segment (you might 00:42:55.04 remember very early on, I showed you a cross section 00:42:58.05 that showed that), interact one-to-one with the 00:43:05.04 icosahedrally symmetric core that's assembled rather like 00:43:09.21 a non-enveloped virus in the cytoplasm, and buds out. In 00:43:16.07 other cases, such as influenza, there's no preassembled 00:43:21.03 inner particle, but rather, the assembly occurs at the 00:43:24.20 membrane, as you see here, where the inner structures 00:43:28.18 and the glycoproteins that incorporate in the membrane 00:43:33.20 come together as part of the elaborate budding event. 00:43:38.17 Separate cellular machinery, in some cases, is then 00:43:42.05 needed to the finish the pinching off, whereas these 00:43:44.27 viruses don't seem to need a separate pinching off 00:43:47.23 mechanism. In the case of HIV, these micrographs show 00:43:54.15 particularly dramatic examples of HIV budding. It's 00:43:58.27 directed in this case by the interaction of the N-terminal 00:44:04.21 domain of the inner protein, the so-called "Gag" gene 00:44:09.10 product, and that protein has a myristoyl group at its N 00:44:17.06 terminus and a very positively charged surface, and 00:44:22.10 interacts with the membrane to drive budding, as shown 00:44:25.20 here. In these micrographs, you can see that the HIV 00:44:33.01 particle is rather sparsely decorated with an envelope 00:44:38.25 glycoprotein that has the function of attaching the virus 00:44:44.17 particle to a new host cell and mediating viral entry. In the 00:44:51.14 case of the smaller icosahedrally symmetric enveloped 00:44:57.11 viruses, like dengue virus for example, the outer coat is 00:45:03.29 much more tightly packed, it forms a very regular array, in 00:45:08.14 case with 180 subunits of the protein whose structure is 00:45:14.21 shown up here, forming a perfect icosahedral array, and it 00:45:18.26 is an assembly of that array that drives particle budding. In 00:45:27.23 all cases of enveloped viruses, the entry process (and 00:45:33.21 that will be the topic of the next part of this set of lectures) 00:45:39.28 involves fusion of the viral membrane with a membrane of 00:45:44.17 the host cell. So just as the assembly process, the 00:45:48.24 maturation process, the exit process, involved budding 00:45:53.19 out and pinching off, so entry involves the reverse 00:45:58.15 process: attachment and fusion of the two membranes. 00:46:03.17 We'll talk about fusion in great detail in the next part of 00:46:07.02 this series, but just to give you a hint of what's to come, 00:46:10.20 an important of all of these viral envelope proteins is that, 00:46:16.17 under suitable circumstances, they can be triggered to 00:46:20.24 undergo a major conformational rearrangement. It's that 00:46:25.26 rearrangement that drives the fusion event, so that in the 00:46:31.14 case of the dengue virus particle, there is a 00:46:33.24 rearrangement from the dimeric structure shown here, a rather plate-like 00:46:42.14 organization of two somewhat elongated protein subunits, 00:46:48.27 into a trimer in which hydrophobic residues at the tip of 00:46:54.05 one of the domains, this yellow domain, so-called "domain 00:46:57.05 II," cluster together at one end of the trimer and interact 00:47:02.18 with the target cell membrane in order to begin the 00:47:06.03 process by which the two membranes are brought 00:47:09.03 together. In the case of dengue virus, this conformational 00:47:13.10 change is triggered by proton-binding, a signal that the 00:47:17.26 virus has arrived in the low pH compartment of an 00:47:21.19 endosome. In other cases, other signals are read out, so 00:47:27.00 to speak, by the fusion mechanism. We can look at this in 00:47:36.00 one more slide, where the interaction with the target cell 00:47:40.13 membrane is shown, and there is a zipping-up process of 00:47:44.08 the C-terminal part of the subunit that actually is part of 00:47:49.28 the pinching together of the two membranes, and leading 00:47:54.10 to an elaborate bit of molecular machinery. Now not all 00:48:04.06 enveloped glycoproteins form such a regular array. In the 00:48:08.11 case of the influenza virus particle, the proteins on the 00:48:13.03 surface of the virus particle sticking out from the 00:48:17.15 membrane are rather spike-like. There are two of them, as 00:48:22.14 you probably know, or two species, the hemagglutinin 00:48:26.06 and neuraminidase, the "H" and "N" of H1N1 or H5N1, 00:48:32.18 that you read about when pandemics threaten. The 00:48:38.12 hemagglutinin is the protein that undergoes a low pH- 00:48:44.16 triggered conformational rearrangement to drive fusion. 00:48:50.05 We'll be hearing quite a lot about that in the next part. 00:48:54.17 The hemagglutinin shown here is a spike-like structure as 00:48:58.13 I mentioned, its molecular design doesn't look anything 00:49:02.10 like that of the envelope protein of dengue virus, it's a 00:49:08.14 stalk-like structure, and long α-helices project the 00:49:19.04 receptor-binding site at the top about 120 or 130 00:49:24.23 angstroms away from the membrane. We'll use that 00:49:30.13 structure to discuss fusion mechanisms in much more 00:49:33.22 detail in Part 2 of this series. See you then. 00:49:40.17
Part 2: Viral Membrane Fusion
- Duration: 32:55
00:00:03.24 Hello, I'm Stephen Harrison from the Harvard Medical 00:00:07.02 School, Children's Hospital Boston, and the Howard 00:00:09.18 Hughes Medical Institute. Welcome to Part 2 of this series 00:00:15.17 on virus structures. This part is about viral membrane 00:00:19.08 fusion, the process by which enveloped viruses get into 00:00:23.20 cells. As those of you who watched Part 1 will know, 00:00:29.23 enveloped viruses, those with lipid bilayer membranes, 00:00:33.10 acquire their membrane by budding out through the 00:00:37.13 surface or into an internal compartment of the host cell. 00:00:44.22 And likewise, they penetrate cells that they are about to 00:00:49.04 infect by fusion, a reverse of the budding process, by 00:00:53.13 fusion of viral and cellular membranes. Different viruses 00:00:58.18 have different triggers or sensors, if you wish, to initiate 00:01:05.15 the fusion process. Influenza virus, which enters through 00:01:10.14 endosomes, depends on the low pH of the endosome to 00:01:13.22 initiate fusion. Viruses such as HIV can fuse at the cell 00:01:18.21 surface, and they depend on the sensing the receptor, 00:01:24.18 which triggers conformational changes of its own and in, 00:01:30.15 in the case of HIV, a co-receptor as well. What is 00:01:33.17 membrane fusion? Membrane fusion is, in the simplest 00:01:38.05 sense, making one bilayer out of two. But it's a relatively 00:01:43.15 complicated process in practice, although it's 00:01:47.15 thermodynamically downhill, that is, the fused structure is 00:01:52.17 ultimately stabler than the two separate structures, but 00:01:57.16 there's a substantial kinetic barrier, and it's overcoming 00:02:01.02 that kinetic barrier that is the role of the viral fusion 00:02:06.15 proteins, or of cellular fusion proteins. So an intermediate 00:02:14.20 in the fusion process is generally accepted to be a 00:02:19.20 structure in which the apposed monolayers, the apposed 00:02:26.15 leaflets, of the two bilayers have merged, but not yet the 00:02:31.08 distal ones, and that's called a "hemifusion" structure, or 00:02:34.22 a "hemifusion stalk." And while there's some debates 00:02:38.17 about the detailed organization of the hemifusion 00:02:41.23 intermediate, it's clear from a number of studies that that is 00:02:47.17 an important step on route to fusion. Indeed, the barrier 00:02:52.10 between two bilayers and the hemifusion structure is one 00:02:58.26 of the major kinetic barriers in this process of fusion, and 00:03:04.04 there is probably a kinetic barrier between hemifusion and 00:03:08.04 the ultimate merging of the distal leaflets that lead to the 00:03:13.12 formation of a fusion pore. In the case of viral proteins, 00:03:19.25 there's a sequence of events that's reasonably 00:03:23.12 stereotypical, it turns out, even though the molecular 00:03:29.17 machinery driving this series of events may look very 00:03:34.27 different. That is, the fusion proteins of different viruses, 00:03:39.18 although from the point of view of their protein 00:03:41.17 architecture may be very different, the underlying process 00:03:47.04 that they catalyze (and there's a real sense in which this a 00:03:50.26 catalysis, since as I said it's thermodynamically downhill, 00:03:55.05 but with a high kinetic barrier)... the sequence of events 00:03:58.11 that they catalyze is reasonably stereotypical in all cases. 00:04:04.24 And so, before events begin, the fusion protein is in some 00:04:14.02 conformation, and this is a purely schematic 00:04:16.10 representation, and there are two bilayers: the bilayer of the membrane 00:04:24.02 in the virus, and the bilayer of the membrane of the cell to 00:04:29.07 which the virus is attached. Some event, proton-binding 00:04:37.08 or receptor-binding, induces or fixes a conformational 00:04:41.26 change in the fusion protein that leads to the formation of 00:04:48.13 an extended intermediate in which a hydrophobic 00:04:51.17 element, either an N-terminal peptide or a loop in the 00:04:56.02 middle of an extended part of the protein structure, 00:05:00.00 interacts with the target cell membrane. And that 00:05:03.17 extended intermediate, which is transient, then collapses 00:05:08.18 into a structure that is ultimately a stable structure for the 00:05:15.05 fusion protein, and drags the two membranes together. As 00:05:19.14 I suggested, there are probably kinetic barriers from the 00:05:22.23 point of view of the lipid bilayer itself, both between the 00:05:25.28 two bilayers state and the hemifusion state, and between 00:05:29.20 the hemifusion state and the final formation of a fusion 00:05:33.07 pore, and it is the role of fusion protein to lower that 00:05:38.06 kinetic barrier, as suggested by these dashed, red lines. 00:05:45.12 We'll talk almost entirely about the fusion protein of 00:05:50.19 influenza virus, the so-called hemagglutinin. It's a member 00:05:57.03 of a class of viral fusion proteins, all of which have the 00:06:00.17 following properties, and it's sometimes because they 00:06:04.04 were the earliest ones characterized in molecular 00:06:07.06 structural terms, have come to be called "Class I" viral 00:06:11.02 fusion proteins. These proteins are synthesized as a 00:06:15.00 precursor, which is cleaved, usually, en route to the cell 00:06:23.23 surface, by a protease in the late compartments of the 00:06:31.16 secretory pathway (furin, for example) into an N-terminal 00:06:38.03 element, which is usually a receptor-binding domain (some 00:06:43.07 viruses have proteins like this, but have a separate 00:06:46.27 receptor-binding protein) and a fusion modular, the C- 00:06:52.21 terminal half in general, which is anchored by a C-terminal 00:06:56.11 transmembrane segment in the viral membrane. Examples 00:07:00.09 of this sort of protein (they are all trimeric assemblies of 00:07:06.14 this sort of organization) are influenza, HIV, and the 00:07:13.00 filoviruses such as Ebola. In the case of influenza, where 00:07:19.28 the protein hemagglutinin sticks off of the surface of the 00:07:24.13 virus... along with another protein, which is an enzyme, 00:07:29.26 called neuraminidase, and we won't talk about that today. 00:07:33.07 The hemagglutinin is a trimeric structure, as I suggested, 00:07:38.06 with three functions. It binds the virus to its receptor, the 00:07:43.07 receptor is sialic acid on glycolipids or glycoproteins on 00:07:47.25 the surface of the target cell. It has structures on the 00:07:54.03 outside that can vary without compromising its two other 00:08:00.19 essential functions, so that the virus can evolve to 00:08:05.01 escape neutralization by the immune system of its hosts. 00:08:11.18 And finally, it is, as I've suggested, the protein that 00:08:15.02 catalyzes the membrane fusion process when suitably 00:08:19.02 triggered by proton-binding. So as I've said, it's 00:08:23.00 synthesized as a precursor. This diagram is overly 00:08:26.28 complicated, but all that matters for today is that, at the N 00:08:32.28 terminus of the so-called HA2... the precursor is called 00:08:38.22 HA0, and the two fragments are known as HA1 00:08:42.26 (hemagglutinin 1) and HA2. At the N terminus of HA2 is a 00:08:50.05 hydrophobic peptide exposed, if you wish (it's actually not 00:08:55.12 exposed in the structure, but made N-terminal rather 00:08:59.24 internal by the cleavage process), that interacts with the 00:09:06.19 target cell membrane and is known as the fusion peptide. 00:09:10.20 And then there is a transmembrane segment very near the 00:09:13.06 C terminus that anchors the protein in the viral membrane. 00:09:18.27 So, the representation here shows you the overall 00:09:23.23 structure of the hemagglutinin. This particular 00:09:29.20 representation is based on x-ray crystallography and does 00:09:33.15 not show the transmembrane segment or the very short 00:09:38.05 segment of about 11 residues that extends into the interior 00:09:43.12 of the virus particle or, before budding, into the cytosol of 00:09:47.07 the cell. As you see, most of the HA1 part, which would 00:09:54.03 be, let us say, red (and the HA2 part would be green of 00:10:00.28 one of the subunits), most of the HA1 part folds into a 00:10:05.27 globular domain at the top of the molecule. It contains the 00:10:11.01 site for binding sialic acid. HA2 forms a stalk that projects 00:10:17.18 it outward from the surface of the virus. The sialic acid- 00:10:25.03 binding site here (there's one on each of the three 00:10:28.19 subunits) faces outward; it's the one very conserved 00:10:34.22 feature of an otherwise antigenically variable surface that 00:10:41.00 the molecule presents to the outside world. Here's a 00:10:44.26 slightly more readable representation, both of the 00:10:51.02 monomer on the left, and of the trimeric, spike-like 00:10:58.05 hemagglutinin on the right. Let's look at the monomer. As I 00:11:04.02 said, the HA1 part is largely out at the surface with its 00:11:08.28 sialic acid-binding site, the HA2 part forms the stalk of the 00:11:15.12 molecule. The N terminus of HA2, remember that's the 00:11:20.19 fusion peptide, is here, tucked in along the threefold axis 00:11:26.16 of the trimer. And so the fusion peptide is hidden and 00:11:32.23 can't interact with hydrophobic targets in the structure of 00:11:40.27 the protein as we see it here, but as you'll see, once 00:11:45.01 exposed to low pH, once protons bind, a major 00:11:49.23 underfolding occurs that allows this fusion peptide to 00:11:54.02 emerge and interact with a target membrane. So here's 00:11:57.29 the low pH-triggered conformational change, and one 00:12:01.02 way of describing it from the point of view of the 00:12:03.21 monomer, is that the HA2 part turns itself inside out. That 00:12:11.19 is, the part of the HA2 (and perhaps it's easier to see in 00:12:16.01 this representation with colored segments)... the part of 00:12:20.05 HA2 that's on the outside in the trimer, which is red and 00:12:25.15 then merging into blue, is on the inside after the 00:12:30.19 conformational change, and the part that's on the inside 00:12:34.09 (green and yellow) turns around and comes up the 00:12:38.01 outside. This structure is most simply described as a trimer 00:12:47.06 of hairpin conformations. There's a fair amount of twisting 00:12:51.27 and turning at the turnaround of the hairpin, but 00:12:54.23 fundamentally, you can think of this as three polypeptide 00:12:58.10 chains that begin up here (with the purple arrow which 00:13:03.09 represents the fusion peptide, it's not represented here 00:13:06.28 since it's based on a crystal structure), comes down, and 00:13:10.29 then turns around and comes right back up to the 00:13:13.29 transmembrane segment, which would follow the yellow 00:13:17.24 arrow. So the hemagglutinin then undergoes two 00:13:27.25 irreversible changes in the course of its maturation and 00:13:33.07 exposure to low pH, because indeed the conformational 00:13:37.28 change I just showed you is irreversible. If you then n 00:13:41.12 eutralize, you don't go backwards. And that's because of 00:13:45.01 the first irreversible change, which is the cleavage of a 00:13:47.28 peptide bond. That now means that the structure we see, 00:13:53.05 which is very stable if you keep it at pH 7 (soluble flu 00:13:56.27 hemagglutinin can hang around for months or years stably 00:14:00.23 in the laboratory), but if you expose it to low pH very, very 00:14:05.05 rapidly, it rearranges as shown and that rearrangement 00:14:08.19 doesn't go backwards, and it doesn't go backwards 00:14:11.13 because there's no way of reknitting that peptide bond, 00:14:15.11 since this structure is actually not the lowest free energy 00:14:19.19 state, it's just there's a very high barrier here that's 00:14:22.11 lowered when protons bind. And it is that second change 00:14:31.16 and the free energy recovered from that second 00:14:36.00 conformational change that is coupled to the process of 00:14:40.18 membrane fusion. And so the fusion mechanism can be 00:14:46.15 thought of as cleaving the precursor, or priming this fusion 00:14:53.15 machinery; localizing the virus to the cell by receptor 00:15:02.09 binding, ultimately by uptake into the endosome; and the 00:15:09.02 triggering of refolding, in the case of flu, by low pH, in the 00:15:13.08 case of other viruses, let us say, by a receptor or co- 00:15:16.11 receptor binding, that leads to this stereotypical sequence 00:15:20.06 of events: Exposure of the fusion peptide (that's that 00:15:24.23 extended intermediate), insertion of the fusion peptide into 00:15:28.16 the target membrane, and a folding back of the protein 00:15:33.01 that brings together the target and viral membranes. And it 00:15:36.05 is that folding back that overcomes the first of the kinetic 00:15:40.06 barriers. There is a substantial kinetic barrier to squeezing 00:15:44.15 two membranes any closer together than about 10 or 15 00:15:47.12 angstroms. That is why liposomes, let's say, in solution are 00:15:52.25 stable, although once fused, they are even more stable. 00:15:59.26 But a liposome preparation doesn't spontaneously fuse 00:16:04.08 because of that kinetic barrier to bringing two bilayers 00:16:08.22 close together. And it is that process that is at least one of 00:16:15.03 the crucial ways in which these proteins facilitate 00:16:19.10 membrane fusion, and they do so by recovering free 00:16:23.13 energy in this fold-back process because the primed state 00:16:29.13 is, in one way or another, metastable. So the fusion of 00:16:40.00 membranes by influenza virus can be through of, then, as 00:16:45.13 a triggering process (we don't show actually the sialic acid 00:16:49.17 attachment here)... but a triggering process that leads to 00:16:55.03 dissociation of the HA1 domains at the top. There 00:17:00.13 happens to be a disulfide bond down here that keeps 00:17:03.07 HA1 from actually floating away, but some experiments 00:17:07.11 done already 10 or 15 years ago (actually, more than that, 00:17:11.20 nearly 20 years ago, now that I think of it), showed that if 00:17:16.02 you knit the tops together, then this process can't occur, 00:17:19.27 so we know that this dissociation of the tops from the 00:17:25.01 stalk occurs, and that allows the stalk, the HA2 stalk, to 00:17:32.11 unfold and refold, so to speak. That is, allows the fusion 00:17:37.12 peptide to flip up, associate with the target bilayer, and 00:17:47.21 then, along with the rest of the protein, collapse together 00:17:52.17 to squeeze the two bilayers together, leading to 00:17:56.24 membrane fusion. I said that the description of the post- 00:18:09.08 fusion conformation of the flu hemagglutinin of HA2 00:18:13.26 corresponds to a trimer of hairpin-like structures, and it 00:18:19.28 turns out that for large numbers of these so-called Class I 00:18:24.24 viral fusion proteins, that simple analogy is true. Indeed, in 00:18:29.05 the case of HIV and SIV, the hairpin is particularly simple. 00:18:34.06 It's just a helix coming down, a loop turning around, and a 00:18:38.18 helix coming up, and so the membrane fusion process is 00:18:46.17 nicely represented in this animation from Gaël McGill 00:18:52.11 based on the structure of the post-fusion state of the HIV 00:18:57.04 and SIV conformational proteins, which you can see 00:19:00.10 going from the extended intermediate at the beginning, to 00:19:05.09 a fused state at the end. So we can then ask, in the case 00:19:14.03 of flu hemagglutinin, which makes a post-fusion structure 00:19:19.01 that's also a trimer of hairpins (although as it happens, as 00:19:21.26 you saw, the outer layer isn't a simple α-helix, the 00:19:25.21 structure is a bit more complicated, but it's still 00:19:28.12 fundamentally just coming one way and going back the 00:19:32.24 other way), how many trimers are needed to make such a 00:19:35.24 fusion structure, and indeed, how long does the process 00:19:38.29 take? And so in some experiments that our laboratory 00:19:44.12 undertook with the collaboration of Antoine van Oijen, 00:19:49.08 through the work of a graduate student named Dan 00:19:52.11 Floyd, we sought to use contemporary techniques in 00:19:57.27 single-molecule fluorescence microscopy to try to carry 00:20:03.16 out measurements of fusion, looking at individual virus 00:20:07.22 particles. Because it was clear that the only way we could 00:20:10.29 begin to answer the questions I was just raising about 00:20:14.15 timescale and about numbers of hemagglutinins needed 00:20:20.29 could only be answered in that way. And the experimental 00:20:25.05 setup that Dan Floyd devised is shown schematically 00:20:31.02 here. A lipid bilayer supported on a thin layer of a dextran 00:20:38.11 polymer is doped with a bit of ganglioside, lipids that have 00:20:48.26 sialic acid on their head group and therefore are receptors 00:20:55.15 for flu hemagglutinin. An influenza virus that has been 00:21:04.09 exposed to two different fluorescent dyes, that has taken 00:21:07.17 up two different fluorescent dyes, is allowed to bind to this 00:21:11.12 surface. The green dye is a hydrophobic dye that inserts 00:21:16.03 into the membrane. The red dye is a more soluble dye that 00:21:19.26 can be soaked into the virus particle, and then the 00:21:21.24 excess washed out, and the virus used in the experiment 00:21:25.11 before any of it leaks back out. And so those two dyes 00:21:29.06 report, on the one hand, mixture of lipids in the two 00:21:33.27 membranes, and hence the hemifusion step, and 00:21:37.08 formation of an aqueous channel between the virus and 00:21:45.06 the solute in the swollen dextran polymer layer, that shows 00:21:54.26 the formation of a full fusion pore. And finally, there's a 00:21:59.05 fluorescein pH sensor to tell us... in the bilayer, fluorescein 00:22:04.01 is bleached when the pH drops below about pH 6, and 00:22:07.27 that tells us when, in the experiment you're about to see, 00:22:12.28 the pH in the region of the virus particle fell below a 00:22:16.15 critical value. And so, here's the kind of measurement that 00:22:22.01 is done, and you'll see here the recording both of the 00:22:32.04 signal from the pH sensor; the signal from the green dye 00:22:37.06 that's in the bilayer, the hydrophobic dye; and the signal 00:22:42.24 from the red fluorophore that is inside the virus particle. 00:22:48.04 And what happens when the pH drops is that, with a 00:22:51.10 certain time delay, there is suddenly a rise and then a 00:22:55.22 rapid fall of the fluorescence from the hydrophobic 00:23:03.00 fluorophore. That's because there's enough of it in the 00:23:08.04 membrane that the signal is quenched. This represents 00:23:12.27 de-quenching as the two membranes begin to merge, as 00:23:16.23 the hemifusion event occurs, and then the fluorophore 00:23:21.24 diffuses away in the target membrane. Then with a further 00:23:25.08 time delay, there is mixing of the content of the virus with 00:23:31.02 the aqueous substrate in the dextran layer underneath the 00:23:37.24 bilayer, and one sees loss of fluorescence from the dye 00:23:44.09 that was inside the virus particle as it diffuses away. And 00:23:49.10 so if one does lots of these measurements, and they can 00:23:54.08 be done in parallel because in a suitable microscope, as 00:23:58.06 you see here, there are lots of particles in a field, then one 00:24:02.12 can get a histogram of the times to hemifusion, that is, 00:24:07.02 lipid mixing, and the times to pore formation. We can do 00:24:11.03 this as a function of a variety of parameters, including the 00:24:14.23 final pH of the buffer that was flowed into the little 00:24:23.11 chamber in the microscope, and other parameters of the 00:24:26.28 experiment. Analyzing this kind of experiment, in which... 00:24:33.03 and I guess I should go back to explain that, as you see, 00:24:36.22 hemifusion always involves a rise and then a fall, and 00:24:42.05 when you have a kinetic event that has a delay of that 00:24:45.19 kind, and so then if we're looking at the time to 00:24:48.16 hemifusion, there is a certain delay that has a distribution 00:24:55.03 from particle to particle that looks like this, then you know 00:24:58.25 that there are multiple kinetic steps, whereas if there's a 00:25:01.15 simple, single kinetic step, you would just see a single 00:25:04.23 exponential decay, as you indeed do if you, on a particle- 00:25:12.10 by-particle basis, plot the time between hemifusion and 00:25:17.09 fusion. So to fit the kinetics of the hemifusion event, we 00:25:31.13 chose a relatively simply kinetic scheme with two 00:25:34.10 parameters, in which there might be "N" sequential steps, 00:25:46.09 rate-limiting steps, each with a similar rate constant "k," or 00:25:50.19 "N" independent parallel steps. And they turn out under 00:25:54.14 suitable conditions to have essentially the same sort of 00:25:57.17 functional behavior. And as a result, if we fit these 00:26:02.13 histograms that I showed you in the previous slide, we 00:26:06.22 find that the best fit involves an N of 3 and a rate 00:26:13.17 constant appropriate for the times involved: These 00:26:17.04 experiments were done at room temperature of about 20 00:26:21.08 seconds or so, as a kind of mean time to hemifusion 00:26:25.15 under the conditions of this experiment. Whereas the 00:26:29.29 pore-forming event was a single kinetic step from the 00:26:36.24 hemifusion state to pore formation. Now what's the 00:26:40.28 interpretation of this sort of kinetic analysis? Well, as I 00:26:46.16 said, there were several possibilities. One might be that 00:26:49.27 there are N sequential steps, three. Another might be that 00:26:54.09 there are three parallel steps. By looking at the pH 00:26:57.29 dependence, as indicated here, we found that N was 00:27:03.06 essentially independent of the final pH. And it seemed to 00:27:08.22 us unlikely that one could have N distinct sequential 00:27:12.14 steps that would vary identically with pH, whereas the 00:27:17.02 same is much more likely to be true of N parallel steps. 00:27:22.12 And so we've interpreted N as representing the number of 00:27:26.21 hemagglutinin molecules, the number of hemagglutinin 00:27:28.29 trimers, needed to form a successful fusion pore. That 00:27:35.13 number, of course, might be two in some cases and four 00:27:38.13 in others, but on average over the large number of events 00:27:43.20 analyzed, the number comes out to just about three. In 00:27:48.25 other words, that the free energy recovered from three 00:27:54.08 hemagglutinin conformational changes appears to be 00:27:57.22 sufficient to drive the process that I was showing you in 00:28:01.26 the previous slide. So, which of the various steps in this 00:28:10.06 sort of scheme are we looking at is the rate-limiting step in 00:28:17.06 this sort of analysis? From the pH dependence, and I 00:28:23.15 won't go into the details, we believe that there's an initial 00:28:29.20 rapid equilibrium between a protonated and unprotonated 00:28:35.03 state, but that as soon as this extended intermediate 00:28:39.23 forms, then the process is essentially irreversible. And 00:28:45.21 indeed, as a result of looking at some variance of the 00:28:53.28 hemagglutinin, we're pretty convinced that it is this step 00:28:58.17 that in the measurements I just showed you we're looking 00:29:01.20 at. So there happens to be a very conserved interaction 00:29:07.22 just where the fusion peptide tucks into the trimeric stalk. 00:29:16.08 And mutations here, either in a completely conserved 00:29:22.13 aspartic acid or a completely conserved glycine residue 00:29:26.22 that stabilize the tucking in, mutations here accelerate 00:29:31.04 fusion. And so, we take that as evidence that it is this 00:29:39.06 step that we're looking at. Now, in practice, on the 00:29:45.05 surface of the virus, there are very tightly packed 00:29:50.24 hemagglutinin molecules. There are two of them 00:29:53.13 superposed on this electron micrograph. And so it is also 00:29:59.11 plausible that three of these humagglutinins clustered in 00:30:06.09 one region might well be the minimum needed to catalyze 00:30:13.29 this fusion process. Also, because of the tight packing, it's 00:30:19.12 very unlikely that in the surface of the virus the proteins 00:30:24.03 can move around very much, and so again, the process 00:30:27.26 is presumably carried out by a local set of interactions at 00:30:33.26 an attachment point between the viral membrane and the 00:30:36.29 cell surface. So, these sorts of measurements obviously 00:30:43.09 are just the beginning at trying to understand the details of 00:30:48.21 this sort of process, but here, from now just about 50 00:30:52.28 years ago is an electron micrograph of influenza virus in 00:31:00.15 what would now be called an endosome, to indicate to 00:31:04.11 you, to give you a bit of perspective, and to suggest to 00:31:06.21 you that, from this sort of information, at a stage when one 00:31:13.28 didn't even know what the molecules on the surface of 00:31:17.01 the virus might be, we're now at a stage... we're at the 00:31:20.15 level of dissecting the kinetics of the events and, hence, 00:31:25.19 trying to understand sensitive points for neutralization by 00:31:29.17 antibodies, for example. We can actually get at the 00:31:37.19 molecular details of the process that would lead to the 00:31:45.07 release of the nucleoproteins from inside the particle (you 00:31:51.27 can actually see some of them in cross section here 00:31:55.03 probably) into the cytoplasm through fusion of the lipid 00:31:59.07 bilayer of the virus with the lipid bilayer of the endosome. 00:32:04.26 I've mentioned Dan Floyd and Antoine van Oijen, I should 00:32:08.07 mention also Tijana Ivanovic and John Skehel as 00:32:12.20 collaborators in the measurements I've been showing you, 00:32:15.26 illustrating how one can use structure and biophysical 00:32:21.03 measurements to dissect the fusion mechanism. And I 00:32:25.29 should add further credit to Gaël McGill, whose animation 00:32:30.16 of the HIV fusion mechanism is particularly helpful in trying 00:32:35.02 to understand what we believe these fusion proteins are 00:32:39.00 doing. Thank you very much. 00:32:43.03
Part 3: Non-Enveloped Virus Entry
- Duration: 30:55
00:00:03.22 Hello, I'm Stephen Harrison of Harvard Medical School, 00:00:07.26 Children's Hospital, and the Howard Hughes Medical 00:00:10.09 Institute. Welcome to Part 3 of this series of lectures on 00:00:15.13 virus structure. In this part, we'll talk about the structure of 00:00:20.07 a non-enveloped virus particle and its implications for the 00:00:26.12 mechanism by which this sort of virus particle gets into 00:00:29.26 cells. As you'll recall, viruses come in two major flavors: 00:00:37.27 enveloped viruses with lipid bilayers, like influenza virus 00:00:42.01 that was the subject of Part 2; and non-enveloped 00:00:47.05 viruses, viruses that have a tightly fitting protein coat to 00:00:52.29 protect the nucleic acid, but no lipid bilayer, such as 00:00:58.15 rotovirus, which we'll be talking about largely today. 00:01:02.21 Rotoviruses are the cause of childhood diarrhea, they're a 00:01:08.04 virus that grows in the small intestine, and is the major 00:01:12.05 source of infantile dehydrating diarrhea, which is 00:01:17.12 particularly serious in developing countries. There's a 00:01:20.29 recent introduction of a vaccine that may ameliorate the 00:01:26.09 spread of this virus, which has been, in recent years, 00:01:29.09 responsible for as many as half a million childhood deaths 00:01:34.24 each year. The virus particle is shown here in an electron 00:01:43.27 micrograph. You'll notice that the contrast appears to be 00:01:50.19 much less than in the micrograph that I show of influenza 00:01:55.18 virus because this is a micrograph taken with a 00:02:02.05 cryopreserved specimen with no stain, rather than a 00:02:05.19 micrograph contrasted with negative stain. We're going to 00:02:09.23 be talking a little bit in the course of looking at the 00:02:13.15 structure of rotovirus about how one uses electron 00:02:18.13 cryomicroscopy to get three-dimensional structures of 00:02:23.24 large macromolecular assemblies such as this one. I also 00:02:28.17 point out on this slide that enveloped viruses enter cells, 00:02:36.11 penetrate cells, by membrane fusion, as we discussed in 00:02:39.22 great detail in the last part, and non-enveloped viruses 00:02:46.01 need to get in by some sort of perforation process, since 00:02:50.07 they don't have a membrane of their own. We want to 00:02:53.29 talk a little bit about what we know about the mechanism 00:02:56.26 of this process in the second half of today's talk. So 00:03:02.15 here's an introduction to the rotovirus particle. It's 00:03:05.06 sometimes called a triple-layered particle because it has 00:03:08.14 three protein layers, an inner blue layer, an intermediate 00:03:13.10 green layer, and an outer yellow and red layer, composed 00:03:18.29 of proteins known as viral protein 2, rather unimaginative 00:03:23.02 nomenclature, VP2, viral protein 6 (the green one), and 00:03:28.18 viral protein 7 and 4 (the yellow and red). The red protein 00:03:33.20 is cleaved in a step that we're going to talk about a little 00:03:36.28 bit, to two fragments known as VP8 and VP5, and those 00:03:43.09 of you who have followed the previous part may begin to 00:03:48.22 see similarities as we go forward between this sort of 00:03:52.20 cleavage, and the kind of cleavage that activates the 00:03:56.06 viral fusion proteins, such as flu hemagglutinin. The 00:04:00.27 particle, by the way, is about 800 angstroms in diameter, 00:04:06.24 so it's almost as large as the 1000 angstrom diameter 00:04:11.25 influenza virus particle. It packages a double-strand RNA 00:04:16.10 genome. In this animation, I show you that the virus 00:04:22.04 particle, as it originally leaves a cell, does not have these 00:04:29.13 extended spikes, but a cleavage, that cleavage from VP4 00:04:35.10 to VP5 and 8, erects the spikes. The spike protein is quite 00:04:40.26 an unusual structure, we're going to talk about it. It's 00:04:43.26 anchored in the inner layer by the yellow outer layer 00:04:50.07 protein. It's the job of the outer layer to deliver the inner 00:05:01.02 particle into the cytoplasm. The inner particle never 00:05:05.15 uncoats, it has a polymerase and a capping enzyme. 00:05:11.11 There are 11 segments of double-strand RNA wound 00:05:15.15 inside, and the polymerase can transcribe that RNA, the 00:05:22.20 capping enzyme can cap it, and the message is extruded 00:05:26.15 from this so-called double-layer particle, or "DLP." Now 00:05:33.00 this animation is not pure fantasy, it's based on detailed 00:05:36.28 structural data: x-ray crystal structures of various proteins 00:05:42.05 and their fragments; an x-ray crystal structure of the intact 00:05:46.00 DLP; and, as we'll talk about in a little bit more detail, a 00:05:53.01 three-dimensional image reconstruction, or several 00:05:55.05 different three-dimensional image reconstructions, from 00:05:58.00 electron cryomicroscopy, or "cryoEM" for short. This slide 00:06:08.24 shows you that recent advances in electron 00:06:13.12 cryomicroscopy mean that we can now obtain density maps 00:06:20.12 representing the structure with essentially the same detail 00:06:25.20 that we've been able to get from x-ray crystallography of 00:06:28.16 large assemblies hitherto. And so, here's a comparison of 00:06:37.01 the x-ray crystal structure, or the density map obtained 00:06:41.07 from that analysis for the double-layered particle, one 00:06:45.02 particular little bit of it, and the similar density map from 00:06:51.27 cryoEM. This was done in collaboration with a colleague 00:06:56.12 at Brandeis named Niko Grigorieff, and these two 00:07:01.08 members, Xing Zhang and Ethan Settembre, of our 00:07:05.20 laboratories. Now, the process by which this sort of 00:07:13.10 analysis is carried out depends on being able to suspend 00:07:20.13 the particles you wish to analyze in a very thin film of 00:07:25.21 vitreous ice, ice or a solution suspension frozen so rapidly 00:07:32.10 that the ice doesn't form crystalline ice, and hence 00:07:34.29 doesn't expand in volume and distort the solute. In the 00:07:42.05 electron microscope, one is seeing a projection of each 00:07:46.14 particle, and by combining data from literally thousands of 00:07:53.09 such images, it's possible to obtain the sort of 00:07:57.29 reconstructed view that I showed you. The single 00:08:01.05 particles are randomly oriented in the vitreous ice, so one 00:08:04.19 has ever conceivable view, and mathematical algorithms 00:08:07.24 had been worked out (you might be familiar with some of 00:08:11.05 them from CAT scans) to determine the relative orientation 00:08:17.28 of all those views, and to combine the data into a three- 00:08:26.27 dimensional picture of the object in question. This 00:08:29.18 obviously depends on the fact that all of the particles are 00:08:33.13 identical. And so cryoEM images of biological structures 00:08:38.12 are possible when those particles are very uniform. But as 00:08:42.16 I've emphasized, the images themselves are very noisy 00:08:47.03 because of the very low electron dose that's required to 00:08:50.12 avoid specimen damage. If you were to try to get a less 00:08:55.02 noisy image, one with higher signal to noise, then you'd fry 00:08:59.24 the specimen. And so, you depend on the fact that the 00:09:04.03 particles are very uniform, and in the case of virus 00:09:07.08 particles, the additional huge advantage of their high 00:09:10.05 symmetry, to make it possible to reach the molecular 00:09:13.05 resolution that I've suggested. So what we're going to 00:09:18.29 focus on for today are the outer layer proteins and their 00:09:24.15 role in delivering the double-layered particle into the cell. 00:09:29.19 Remember that the outer layer proteins are VP4 (which 00:09:33.12 gets cleaved when this erected conformation is 00:09:38.25 established, to VP8 and VP5) and the yellow protein, as I 00:09:43.29 called it, VP7, which locks everything in place. Now VP4, 00:09:51.11 the spike protein, is actually, as you probably noticed, a 00:09:55.06 very curious structure indeed. I've colored the VP8 part in 00:10:04.25 magenta and the three different (it's a trimer, as you'll see 00:10:08.29 in a minute) VP5 parts in various shades of sort of red and 00:10:15.01 orange. And of course the first thing you probably noticed, 00:10:19.22 even with the first image I showed you, was this thing 00:10:23.07 looks like a dimer. There are two lobes sticking up and 00:10:27.27 two ears sticking off of them, and yet I've just said to you 00:10:31.11 it's a trimer, what's going on? Well, turns out that this is an 00:10:35.01 extremely unusual sort of asymmetric arrangement of three 00:10:41.09 proteins. The bottom part, we call it the foot, as you'll see, 00:10:47.06 is perfectly trimeric. The outer projecting spike has a very 00:10:56.08 nice twofold axis. And one is adapted to the other, by this 00:11:03.26 sort of diagonal cantilever. So if you now look, you'll see 00:11:09.14 that the beginning of each protein subunit (remember that 00:11:16.04 VP8 is magenta, and I'm about to show you it's the N- 00:11:20.24 terminal part) is down here, and there are three of them. 00:11:26.21 Polypeptide chain comes up, one of them "quits," and the 00:11:30.10 other two come on up and form these ears. Polypeptide 00:11:34.17 chain then resumes (the cleavage is between VP8 and 00:11:39.13 VP5) as this bean-shaped domain of VP5, and continues 00:11:45.18 on back down. The third bean-shaped domain is here, 00:11:50.27 and so we've lost one ear, it's almost certainly been 00:11:57.02 cleaved by a cleavage here and a cleavage here, as I'll 00:12:00.13 explain in a minute. And that third bean-shaped domain 00:12:05.04 forms this diagonal cantilever that supports the twofold 00:12:10.14 clustered spike of the remaining two. Now already this is a 00:12:16.05 pretty unusual conformation, but I'm about to show you 00:12:21.15 some other gyrations that this protein appears to be able 00:12:25.18 to go through. So as I said, the tryptic cleavage that 00:12:31.09 separates VP8 and VP5... and that probably occurs in the 00:12:37.25 gut as the virus emerges by lysis of the intestinal cells that 00:12:43.17 it has infected, or emerges by some other secretory 00:12:51.27 method, the actual emergence is a bit unclear. The tryptic 00:13:02.00 cleavage then allows the rearrangement of this spike. 00:13:07.27 The three protein subunits on the outer parts of them are 00:13:12.26 probably more disordered if the protein has not been 00:13:20.22 cleaved, and if you look in the electron microscope, you 00:13:23.13 don't see any ordered parts of the outer assembly. And 00:13:28.01 since I've said you have to average many images in order 00:13:31.17 to get a decent three-dimensional representation, then if 00:13:38.23 the outer part is disordered, the averaging will basically 00:13:42.05 blur out all of those elements, and all you'll see is the 00:13:47.23 ordered foot. So if we compare now these spike regions, 00:13:55.08 which in the electron micrographs, or in the three- 00:13:57.15 dimensional reconstruction from electron micrographs, are 00:14:00.21 not quite as well ordered as the parts I showed you, 00:14:03.23 because of some slight flexibility as these structures stick 00:14:10.01 out from the virus. But we can sit them very well with 00:14:13.29 known x-ray structures. If we compare them, see that an 00:14:17.05 x-ray structure of the beam-shaped domain shows a nice 00:14:24.00 dimer like this. It has some hydrophobic loops at the tips 00:14:28.15 that we're going to talk about, and then a separate x-ray 00:14:31.12 structure of this region yields a lectin-like domain that 00:14:39.16 binds sialic acid, which is a receptor for rhesus rotovirus, 00:14:45.07 from which these proteins in our experiments were 00:14:49.00 derived. Now, we therefore believe that the protein, 00:14:58.08 which is synthesized as a monomer, combines with the 00:15:03.18 double-layered particle, three of them for each of the 00:15:07.21 positions on which it sits (you may have noticed that there 00:15:10.04 were 60 spikes sticking out, corresponding to the 00:15:15.20 icosahedral symmetry), and inserts and is locked in by 00:15:21.28 VP7, but is flexible until tryptic cleavage occurs, in which 00:15:28.12 case, one of the VP8s is excised, and the rest of the 00:15:35.15 structure reorganizes to the unusual-looking spike that 00:15:43.05 I've shown you. Now, as if this weren't odd enough, if you 00:15:50.14 make a piece of the protein that's a bit longer than the 00:15:54.24 one that gave that dimer I showed you, you get this 00:15:59.20 trimeric structure, in which a segment of the protein that 00:16:06.09 was missing from the dimeric construct is present, and it 00:16:10.07 has formed a beautiful, trimeric, coiled coil, and the 00:16:15.28 hydrophobic loops of the bean-shaped domain are now 00:16:18.25 pointing, if you wish, down rather than up. In other words, 00:16:23.19 it's as if the structure has gone from this sort of 00:16:28.17 arrangement to this one, and those of you who were 00:16:34.08 following some of the conformational changes of 00:16:39.04 enveloped virus fusion proteins may find that familiar, 00:16:43.22 since it's essentially the same kind of conformational 00:16:48.04 change we've seen there. And just anticipating, we 00:16:51.29 believe that indeed it is that conformational change that, 00:16:55.22 in this case, drives not fusion (there's no membrane on 00:16:58.19 the virus) but drives the membrane disruption that will get 00:17:04.29 the double-layer particle into the cytoplasm. And so, our 00:17:11.24 scheme based on this information so far is that this spike- 00:17:17.27 like structure (and the tryptic cleavage is essential for viral 00:17:22.06 infectivity)... this spike-like structure attaches through 00:17:28.06 sialic acid, some suitable trigger (and we actually don't yet 00:17:34.17 know what that is) allows the two VP8s that are attached 00:17:44.06 to separate enough that these bean-shaped domains can 00:17:50.14 insert or interact with the target cell membrane through 00:17:56.00 those hydrophobic loops, and then this umbrella-like 00:18:01.18 folding back leads, in a way that we hope to understand 00:18:05.19 but don't fully yet, to a disruption event that can allow the 00:18:11.02 double-layer particle to translocate into the cytosol. Now, 00:18:17.27 this list basically tells you what we're thinking. An 00:18:23.21 extended intermediate forms, hydrophobic loops contact 00:18:27.08 the membrane, the protein folds back to the umbrella-like 00:18:30.10 conformation, and there's a coupling of the fold back and 00:18:34.08 the membrane, to perforate the bilayer. To test these 00:18:40.05 notions, we can take advantage of an extremely 00:18:43.22 interesting property of double-strand RNA viruses like 00:18:47.08 rotovirus, which I like to call functional recoating. If you'd 00:18:52.19 like to study the entry of a virus by genetic methods, by 00:18:59.11 trying to making mutations that would impair entry, you 00:19:03.03 have a problem on your hands, because how are you 00:19:07.09 going to get enough of an entry-incompetent virus to 00:19:10.25 study in the first place? You can get around that problem 00:19:15.22 with these viruses in quite a clever way. If you take 00:19:20.16 double-layered particles prepared by removing the outer 00:19:25.12 shell, VP7 and VP4 (or VP8 plus VP5), from infectious 00:19:34.26 virus particles, the double-layer particle's no longer 00:19:38.21 infectious because it can't get in. But if you recoat it with 00:19:45.02 recombinant proteins, recombinant VP4 and recombinant 00:19:50.04 VP7, and then treat with trypsin to cleave and activate 00:19:54.27 the VP4, you get perfectly infectious particles. This is a 00:20:00.07 process worked out (mimicking some experiments 00:20:06.26 originally done by Kartik Chandran and Max Nibert on 00:20:10.10 rheovirus) by Shane Trask and Phil Dormitzer. As a result, 00:20:16.05 we can use this trick to do two things: First, we can 00:20:19.13 mutate the hydrophobic loops and ask does the virus get 00:20:23.08 in or infect, and if it doesn't, what sort of properties does it 00:20:28.04 have? And second, as you'll see, we can use this 00:20:33.28 scheme actually to watch virus particles getting into a 00:20:37.07 cell. And so, some experiments carried out by Irene Kim, a 00:20:42.11 graduate student, showed that if you mutate the 00:20:45.12 hydrophobic residues, you lose infectivity, the virus 00:20:50.28 becomes engulfed in the cell, it's taken up by a process 00:20:55.07 I'm about to show you, but never infects. And you can 00:20:59.22 also show that is never disrupts a membrane, because 00:21:03.06 the virus can allow a toxin called α-sarcin to get into the 00:21:09.00 cell, sort of sweeps it in, so to speak, if it is actually 00:21:13.26 infectious, if it actually perforate some sort of endosomal 00:21:18.21 membrane, but the hydrophobic loop mutations not only 00:21:26.21 block infectivity, but block the capacity of the virus to 00:21:30.17 sweep this toxin into the cell along with it. And so, those 00:21:36.11 experiments are strongly consistent with the view that the 00:21:47.16 hydrophobic loops matter, but in order to understand more 00:21:52.12 of this process, we've really got to understand more about 00:21:56.02 the compartment that the virus arrives in when taken up 00:22:00.28 in a cell, about the kinetics of those events, and about 00:22:06.00 other characteristics, so that we can try to figure out how 00:22:10.03 to design experiments to look at these subsequent steps. 00:22:15.27 In order to describe the experiments that we've devised to 00:22:23.24 try to do this, I should mention the outer shell protein VP7, 00:22:28.03 the other partner here, which, as you see from these 00:22:32.01 images and from the animation early in this lecture, locks 00:22:40.08 the spike protein, which we believe to be the membrane 00:22:44.12 perforator, so to speak, into the particle. VP7 is a trimeric 00:22:50.11 protein, and it's held together by calcium, there are two 00:22:53.15 calcium ions that the x-ray crystal structure showed us 00:22:59.23 hold the protein subunits together. There are negatively 00:23:06.15 charged residues interacting with those calcium ions so 00:23:11.08 that the ligation of calcium is critical for the stability of this 00:23:15.20 trimer. As a result, we believe that some sort of calcium 00:23:20.27 withdrawal may be part of a triggering signal, but we have 00:23:26.10 as yet to define that more precisely. So, in order to try to 00:23:34.24 understand what's going on, we've taken advantage of 00:23:39.01 the recoating experiment in a different way. We found, or 00:23:45.23 Aliaa Abdelhakim in the laboratory has found, that one 00:23:50.27 can label with a fluorescent dye each of the components 00:23:58.15 of the particle before recoating, that is, the double-layered 00:24:03.17 particle, VP7, and VP4, with distinct fluorescent dyes, and 00:24:09.17 therefore follow not only the binding and entry of the 00:24:17.04 particle into the cell, but can detect the point at which the 00:24:21.11 double-layer particle is released into the cytosol, as I'll 00:24:25.02 show you. So here's an experiment with VP7 pseudo- 00:24:31.10 colored in red, the fluorophore on VP7, and the double- 00:24:35.14 layer particle in green. The experiment is done by looking 00:24:40.10 at the very thin edge of an epithelial cell in culture, so that 00:24:45.06 you can look at the top surface without much aberration, 00:24:48.12 and as you'll see, the particle in the circle will suddenly 00:24:53.27 release and fly around in the cytoplasm. If you look 00:24:59.27 closely, you can probably see a trace of red remaining in 00:25:04.01 the circle. This experiment in which the three components 00:25:12.11 had been labeled separately is a little easier to follow. I'll 00:25:16.23 go back and show you that this particle suddenly 00:25:22.29 releases, and if you look at the circles, you'll see that 00:25:26.13 there's still some purple, that is, VP4 and VP7 (or rather, 00:25:32.02 VP5/VP8 plus VP7), at the site of entry, and we stopped 00:25:41.01 the frame as the double-layer particle was diffusing 00:25:44.04 around rapidly in the cell after release. A summary of what 00:25:49.25 we think these images are telling us might be roughly as 00:25:53.23 follows. One has the particle that is activated by trypsin, 00:25:58.11 that is probably even before it emerges from one host and 00:26:04.13 enters another. This is a fecal-oral transmission virus and 00:26:09.01 probably is already exposed to trypsin in the gut of the 00:26:14.02 initial infected individual. It binds to the surface of the cell 00:26:20.08 it's going to infect, in the case of the virus infecting a 00:26:27.00 person or a monkey, since this is a rhesus rotovirus, then 00:26:32.13 it will bind to a small intestinal epithelial cell. It's taken up 00:26:38.15 by a process I'm going to tell you a little bit more about, 00:26:43.09 that does not depend on familiar endocytic routes like 00:26:47.12 clathrin-mediated uptake. Something triggers the 00:26:52.26 conformational changes we've seen, so the membrane 00:26:55.19 perforates, the particle escapes and can start to make 00:27:00.10 RNA. And so, what can we learn from this sort of 00:27:08.04 experiment about this sequence of events? We're just in 00:27:13.21 the process of being able to try to do that, these 00:27:16.09 experiments aren't even yet published, but I think that 00:27:22.29 what I'd like to end on then is a series of questions that I 00:27:27.27 think this kind of experiment can answer. And I hope that 00:27:32.21 they will illustrate to you that, by combining structural data 00:27:37.09 with the kinds of, in this case, single virus particle imaging 00:27:41.27 on living cells that takes advantage of contemporary 00:27:46.20 fluorescence microscopy, we can begin to answer these 00:27:51.05 sorts of questions. So the first question is about the 00:27:55.03 engulfment. Is it simply what I like to call an 00:27:58.14 "autoendosome," that is, does the virus just wrap itself up 00:28:01.22 in membrane? There are various reasons why we think 00:28:04.02 that's at least a reasonable possibility. One is that the 00:28:07.22 process is quite rapid and that it doesn't depend on any of 00:28:11.06 the known cellular pathways. Within a minute or two, the 00:28:15.15 virus particle is sequestered from agents like EDTA, 00:28:23.15 which would take it apart by pulling calcium off, or 00:28:27.11 antibodies that might bind to it. A second question is 00:28:33.14 about the membrane disruption that then occurs. Is the 00:28:38.04 membrane disruption by VP5, this folding back that I 00:28:43.25 showed you, purely mechanochemical, if you wish, as in 00:28:47.26 fusion, or are there other more, in you wish, detergent-like 00:28:54.18 qualities of that interaction? Does it indeed do what I 00:29:02.02 pictured in the previous slide, is there an extended 00:29:05.02 intermediate? Does it remain anchored in the particle 00:29:08.22 when it undergoes this whole process? What triggers it, 00:29:13.09 and when does VP7 come off? But I think you can 00:29:16.12 imagine we can begin by combining the powers of 00:29:22.14 structure-based mutation and design of constrained or 00:29:29.21 altered proteins with recoating and with the direct 00:29:34.14 visualization, particle by particle, of this entry process, to 00:29:38.15 answer some of these questions. And so in conclusion, let 00:29:43.01 me simply acknowledge the large number of people who 00:29:46.09 have participated in this work. I should acknowledge both 00:29:52.17 people in my own laboratory, as well as in collaborating 00:29:55.14 laboratories. Let me just name the specific collaborators 00:30:00.02 with whom the work has been carried out: Phil Dormitzer, 00:30:04.22 who began as a member of our laboratory, and I then 00:30:07.16 have collaborated with him extensively since he 00:30:12.10 established an independent research program; Niko 00:30:16.05 Grigorieff at Brandeis, one of the pioneers of the sorts of 00:30:21.01 cryoEM analysis that I described briefly to you; Tom 00:30:26.00 Kirchhausen, whose work on live-cell imaging and whose 00:30:30.17 development of technologies has allowed us to do the 00:30:34.07 experiments I just described; and Dick Bellamy at the 00:30:37.18 University of Auckland, who first got me interested in 00:30:40.23 rotovirus in the first place. And thank you.

- Educators of H. School / Intro Undergrad
- Educators of Adv. Undergrad / Grad
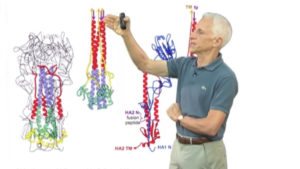
Talk Overview
Harrison begins his talk by asking why most non-enveloped viruses and some enveloped viruses are symmetrical in shape. He proceeds to show us lovely images of virus structures obtained by x-ray crystallography of numerous viral coat proteins. Deciphering these structures allowed scientists to understand that viral coat proteins form multimers, such as dimers and pentamers, which in turn interact with a scaffold that ensures that the coat proteins are correctly placed. This arrangement results in symmetrically shaped viruses.
In Part 1, Harrison also explains that enveloped viruses infect cells by inducing the fusion of the viral and host cell membranes. He delves deeper into the molecular mechanism of membrane fusion driven by the hemagglutinin or HA protein of the influenza virus in Part 2 of his talk. Non-enveloped viruses, on the other hand, must enter cells by a mechanism other than membrane fusion. This is the focus of Part 3. Using rotavirus as a model, Harrison and his colleagues have used a combination of Xray crystallography and electron cryomicroscopy to decipher how the spike protein on the viral surface changes its conformation and perforates the cell membrane allowing the virus to enter the cell.
Speaker Bio
Stephen harrison.

Stephen Harrison received his A.B. and Ph.D. from Harvard University. He became interested in molecular structures and began his studies using X-ray crystallography as a graduate student. After receiving his Ph.D., he remained at Harvard and continued his work to improve the resolution of the tomato bushy stunt virus structure. In 1977, after 7 years… Continue Reading
Playlist: Viruses

Related Resources
Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008 Jul;15(7):690-8.
Harrison SC. Looking inside adenovirus. Science. 2010 Aug 27;329(5995):1026-7. A Comment on the two papers below..
Liu H, Jin L, Koh SB, Atanasov I, Schein S, Wu L, Zhou ZH. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010 Aug 27;329(5995):1038-43..
Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 A resolution. Science. 2010 Aug 27;329(5995):1071-5..
Settembre EC, Chen JZ, Dormitzer PR, Grigorieff N, Harrison SC. Atomic model of an infectious rotavirus particle. EMBO J. 2011 Jan 19;30(2):408-16.
Reader Interactions
Sourav says
September 5, 2020 at 9:21 pm
Is virus living?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Find a Video
- Search All Videos
- Search YouTube
- Short Films
- All Playlists
- All Speakers
- Videos in Other Languages
- Biochemistry
- Bioengineering
- Cell Biology
- CRISPR-Cas Technology
- Development and Stem Cells
- Genetics and Gene Regulation
- Human Disease
- Microbiology
- Neuroscience
- Plant Biology
- Professional Development
For Educators
- Educator Newsletter
- Educator Resources
- Flipped Courses
- Online Courses Resources
- All Educator Resources
Educators Recommend
- Cells of the Immune System
- Discovering Cell Cycle Regulators
- Genome Engineering with CRISPR-Cas9
- Hardy-Weinberg Equilibrium
- Next Generation Sequencing
- Semi-Conservative Replication of DNA
- Toll-Like Receptors in Adaptive Immunity
Free Courses
- Free Online Courses
- Take a Free Course
- Learn Microscopy
- Intro to Microscopy
Career Development
- Professional Development Courses
- Professional Development Talks
- Career Exploration
- Biomedical Workforce
- NRMN Resources
Best of iBiology
- Short Science Films
- Meselson and Stahl
- Doudna & Charpentier’s Nobel Prize
- Decoding Ancestral Knowledge
- Microscopy Series
- Short Microscopy Series
- Famous Discoveries
- Bench to Bedside
- Share Your Research
- Our Mission
- Science Communication Lab
- Financial Conflict of Interest Policy


Virtual Viruses Reveal Complex Genomic Dynamics
Researchers used new simulations to obtain the first structures of elusive viruses..

Aparna is a freelance science writer with a PhD in bioinformatics and genomics at Harvard University. Her writing has also appeared in The Philadelphia Inquirer, Popular Science, PBS NOVA, and more.
View full profile.
Learn about our editorial policies.
ABOVE: Using sophisticated computer simulations, researchers predicted the structure of a complete bacteriophage, including its DNA. © iStock, Yabusaka Design
A virus may be microscopic, but it contains thousands of nucleic acid bases strategically packaged into a protein shell. Knowing how the virus organizes these vast information stores in a compact space is the key to understanding viral structure and designing better defenses against pathogenic viruses.
Peering into the viral protein shell, or capsid, is challenging. Typical structure discerning techniques such as cryo-electron microscopy can’t capture the varying configurations of genetic material in each virus. Back in 2010, Aleksei Aksimentiev , a biophysicist at the University of Illinois Urbana-Champaign, had an idea for computationally simulating a virus’s structure. However, computational methods were simply not sophisticated enough at the time.
“It was always at the back of our minds, and then, we made a breakthrough in terms of methodology,” Aksimentiev said.
Now, 14 years later, in a study published in Nature , his team reported using a new computational approach to simulate the individual atoms of a virus that is packed with nucleic acids. 1 They used this method to study the HK97 bacteriophage and proposed the first structure for the virus.
A few years ago, Aksimentiev’s team developed a method for mapping out complex DNA configurations by computationally simulating them at multiple resolutions. 2 They start at a coarse resolution, like a fuzzy image, and then on each iteration, they increase the level of detail in the simulated DNA structure.
In their new study, the researchers used this approach to computationally model the virus and its DNA during viral assembly. With prior experimental data such as the structure of the capsid and the force of the motor that loads DNA into the virus as a starting point, they simulated the behavior of each of the 26 million atoms during the chaotic process of loading DNA into the capsid. This was no small task; each simulation took anywhere between three months and one year to run, even on very powerful computers.
According to Eric May , a structural biologist at the University of Connecticut who was not involved in this study, the simulations provided unprecedented insights into the dynamics between the genome, the capsid, and other molecules in the virus that may be missed by experimental methods that can only obtain the average structure across many particles. “This computational approach doesn't have that kind of limitation,” he said. “We know the protein components already, but now seeing the genomic information in full atomic detail is very exciting.”
For example, the researchers predicted that DNA is packaged into the capsid through a method called loop extrusion , where proteins force the DNA into hairpin configurations. 3 Aksimentiev was surprised to see the diversity of genomic configurations produced by the simulations.
“We intuitively would think each configuration could be different, but what was surprising to us was the scale at which the structures were different,” Aksimentiev said. “If you look at the individual viral particles, they are different by the global configuration, which was introduced by the varied packaging process.”
Matthias Wolf , a structural biologist at the Okinawa Institute of Science and Technology who was not involved in this study, said that this addresses a long standing question about how viruses organize their genomes. However, he noted that the study lacked experimental validation of the predicted structures.
Aksimentiev thinks that they can improve the simulation to account for more physical forces and be less reliant on experimental input data. His group is also running simulations of other viruses that are more complex. May thinks that it will be critical to apply this model to pathogenic viruses such as HIV and SARS-CoV-2, even though they are harder to model because of their RNA genomes. “It would be interesting to see [the researchers] try to move in the direction of systems of great public health importance,” he said. “Also understanding the stages of viral infection: how is the structure of a virus different when it enters the cell? How is the genome getting released from a virus?”
Aksimentiev is optimistic that the simulations will extend to more complex viruses, including RNA viruses, by incorporating more targeted experimental data. His eye is also on an even loftier goal: simulating an entire cell. “It's probably not coming soon, but that's kind of the Holy Grail,” he said.
Explainable AI for Rational Antibiotic Discovery

Where Books Meet Bacteria

New Strategies in the Battle Against Infectious Diseases

Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

frankenstein
22 templates

el salvador
32 templates

summer vacation
19 templates

44 templates

17 templates

pediatrician
27 templates
Biology Presentation templates
Give interesting presentations about biology with these free ppt templates and google slides themes. all life forms should enjoy a nice slide deck, related collections.

18 templates
Middle School
26 templates

High School
68 templates

66 templates

DNA Lesson for High School
Download the "DNA Lesson for High School" presentation for PowerPoint or Google Slides. High school students are approaching adulthood, and therefore, this template’s design reflects the mature nature of their education. Customize the well-defined sections, integrate multimedia and interactive elements and allow space for research or group projects—the possibilities of...

Premium template
Unlock this template and gain unlimited access
Biology Thesis
Aristotle said that “by ‘life,’ we mean a thing that can nourish itself and grow and decay.” Biology is, indeed, the study of life as such. If you have just finished your thesis on biology and your viva is approaching, use our Biology Thesis template!

Biology Subject for High School: Birds Beaks & Adaptations
Download the "Biology Subject for High School: Birds Beaks & Adaptations" presentation for PowerPoint or Google Slides. High school students are approaching adulthood, and therefore, this template’s design reflects the mature nature of their education. Customize the well-defined sections, integrate multimedia and interactive elements and allow space for research or...

Anatomy and Physiology Major for College: Cell Biology
One of the most intriguing subjects of a major in Anatomy and Physiology is Cell Biology, and this template provides an introduction to its fascinating universe. From the basics of cellular structure and function, to specialized topics such as organelles and DNA replication, this template will provide an overview that...

Science Subject for High School - 9th Grade: The Building Blocks of Life
When you stack lots of building blocks, you end up having a nice figure or building, but when you have lots of "building blocks of life", you get organisms! Use this template for educational purposes and teach your students what are cells. We have added illustrations that are funny so...
Science Subject for High School - 9th Grade: The Building Blocks of Life Infographics
Download the "Science Subject for High School - 9th Grade: The Building Blocks of Life Infographics" template for PowerPoint or Google Slides and discover this set of editable infographics for education presentations. These resources, from graphs to tables, can be combined with other presentations or used independently. The most important...

Biochemistry Lesson for High School
How does the world around us work? That is a question too broad to be answered in a single class, but you can explain to your students in your next class a tiny and important part of its functioning and composition: what is a molecule and how they are made...

Science Subject for Elementary: Reproductive System
This presentation template is perfect for teaching elementary school students about the reproductive system. It includes engaging and informative illustrations to help reinforce understanding of the topic. Students will learn about the anatomy of reproduction, such as components like sperm and ovum, and they will understand how these components work...


Cell Behavior Thesis Defense
You're going to need a good number of zeros in order to write how many cells there are in the human body. How do they work and what's their function? People like you, who've studied cells, can help us know a bit more about them! We've got something in return:...

Ovarian Cancer Day
Download the "Ovarian Cancer Day" presentation for PowerPoint or Google Slides. Healthcare goes beyond curing patients and combating illnesses. Raising awareness about diseases, informing people about prevention methods, discussing some good practices, or even talking about a balanced diet—there are many topics related to medicine that you could be sharing...

Microbiology Breakthrough
Advances in medicine are always important events, and it’s great if the new findings can be presented in a clear manner. If you want to make a contribution to the medical community and share what you found out in your research, this new breakthrough presentation template is for you.
Machine Learning in Biostatistics - Master of Science in Biostatistics
Download the "Machine Learning in Biostatistics - Master of Science in Biostatistics" presentation for PowerPoint or Google Slides. As university curricula increasingly incorporate digital tools and platforms, this template has been designed to integrate with presentation software, online learning management systems, or referencing software, enhancing the overall efficiency and effectiveness...

Molecular Biology - Bachelor of Science in Human Biology
Download the "Molecular Biology - Bachelor of Science in Human Biology" presentation for PowerPoint or Google Slides. As university curricula increasingly incorporate digital tools and platforms, this template has been designed to integrate with presentation software, online learning management systems, or referencing software, enhancing the overall efficiency and effectiveness of...
Biology Infographics
Download the "Biology Infographics" template for PowerPoint or Google Slides and discover this set of editable infographics for education presentations. These resources, from graphs to tables, can be combined with other presentations or used independently. The most important thing is what you will achieve: transmit information in an orderly and...

Science Subject for Middle School - 9th Grade: Biology
If you encourage your students to get interested in science since middle school, who knows, some of them could even become renowned scientists! Let's go step by step: first, download this template and customize it so that you have a slideshow for your biology class. Define concepts in its slides,...

Marine Biology Science Lesson for College
Here’s a beautiful marine biology template if there ever was one! True to form, it’s all dreamy watercolors in ocean shades and an abundance of marine animal and plant life. From freshmen to grad students, everyone will be happy to listen to a lesson on this template! Download it today...

Science Subject for High School - 9th Grade: Molecular Genetics
DNA is fascinating! The Slidesgo team marvels at how within something so microscopic, there can be so much information about not only whether we have green or blue eyes, but even about our personality. So, genetics defines us and, therefore, studying it is very important. In any high school science...

Cellular Morphology
Download the "Cellular Morphology" presentation for PowerPoint or Google Slides. The education sector constantly demands dynamic and effective ways to present information. This template is created with that very purpose in mind. Offering the best resources, it allows educators or students to efficiently manage their presentations and engage audiences. With...
- Page 1 of 21
New! Make quick presentations with AI
Slidesgo AI presentation maker puts the power of design and creativity in your hands, so you can effortlessly craft stunning slideshows in minutes.

Register for free and start editing online
How immune cells communicate to fight viruses
Chemokines are signalling proteins that orchestrate the interaction of immune cells against pathogens and tumours. To understand this complex network, various techniques have been developed to identify chemokine-producing cells. However, it has not yet been possible to determine which cells react to these chemokines. Researchers at the University Hospital Bonn (UKB) and the University of Bonn have developed a new class of genetically modified mice that enables the simultaneous identification of chemokine producers and sensors. Using the chemokine Ccl3 as a "proof of principle," they discovered that its function in the immune defence against viruses is different than had been previously assumed. Their results have now been published in the Journal of Experimental Medicine.
Our immune response to infections is critically controlled by chemokines. In order to understand how these signalling proteins coordinate immune cells, researchers from Bonn took a closer look at the chemokine Ccl3. Using a novel technology known as Ccl3-EASER mice, they investigated its role in coordinating the immune response to cytomegalovirus (CMV) infection, which can lead to severe diseases in immunocompromised individuals. "Until now, it was thought that certain macrophages, cells which colonize all organs as immune guardians, produce Ccl3 to attract antiviral immune cells," says co-senior author Prof. Dr. Christian Kurts, Director of the Institute of Molecular Medicine and Experimental Immunology (IMMEI) at the UKB. He is also a member of the Transdisciplinary Research Area 3 (TRA 3) "Life & Health" and the Cluster of Excellence Immunosensation 2 at the University of Bonn.
NK cells are both chemokine producers and sensors
"However, we actually found that the natural killer cells -- NK cells for short -- are the most important Ccl3 producers during CMV infection," says co-senior author Prof. Dr. Natalio Garbi, research group leader from IMMEI at the UKB. He is also a member of the Cluster of Excellence Immunosensation 2 at the University of Bonn. NK cells are white blood cells that can directly destroy virus-infected body cells. The scientists found that NK cells are in a permanent alarm mode to be ready for rapid Ccl3 production. As soon as a viral infection occurs, the body releases type I interferon as an alarm signal. This triggers the NK cells to rapidly produce the chemokine Ccl3. "However, NK cells are not only the cellular source, i.e. the producers of Ccl3, but also the main sensors for the chemokine in this context," says co-senior author Prof. Dr. Niels A. Lemmermann, research group leader from the Institute of Virology at the UKB and member of the Cluster of Excellence Immunosensation 2 at the University of Bonn. This means that Ccl3 acts as an auto/paracrine signal through which NK cells communicate directly with each other and coordinate their antiviral response.
"The experimental strategy used here is completely new. It can also be used for messenger substances other than Ccl3, which are released during various infections, diverse forms of inflammation or cancers," explains Dr. Maria Belen Rodrigo, first author and scientist at the IMMEI of the UKB. With this work, the Bonn researchers have succeeded in gaining a better understanding of the complex choreography of immune cells in the defence against viruses.
- Immune System
- Medical Topics
- Biotechnology and Bioengineering
- Biotechnology
- Molecular Biology
- Brain tumor
- Immune system
- White blood cell
- Natural killer cell
- Cells of the stomach
- Transgenic plants
Story Source:
Materials provided by Universitatsklinikum Bonn . Note: Content may be edited for style and length.
Journal Reference :
- Maria Belen Rodrigo, Anna De Min, Selina Kathleen Jorch, Cristina Martin-Higueras, Ann-Kathrin Baumgart, Beata Goldyn, Sara Becker, Natalio Garbi, Niels A. Lemmermann, Christian Kurts. Dual fluorescence reporter mice for Ccl3 transcription, translation, and intercellular communication . Journal of Experimental Medicine , 2024; 221 (7) DOI: 10.1084/jem.20231814
Cite This Page :
Explore More
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
- Mice Given Mouse-Rat Brains Can Smell Again
- How Do Birds Flock? New Aerodynamics
- Cancer: Epigenetic Origin Without DNA Mutation
Trending Topics
Strange & offbeat.
share this!
April 26, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
CRISPR is promising to tackle antimicrobial resistance, but bacteria can fight back
by European Society of Clinical Microbiology and Infectious Diseases
In his presentation "How to use CRISPR-Cas to combat AMR" at the ESCMID Global Congress , Assistant Prof. Ibrahim Bitar, Department of Microbiology, Faculty of Medicine and University Hospital in Plzen, Charles University in Prague, Plzen, Czech Republic, will give an overview of the molecular biology of CRISPR technology in explaining how it can used to tackle antimicrobial resistance.
Clustered regularly interspaced short palindromic repeats (CRISPRs) and CRISPR associated genes (cas) are widespread in the genome of many bacteria and are a defense mechanism against foreign invaders such as plasmids and viruses. The CRISPR arrays are composed of a repeated array of short sequences, each originating from and exactly matching a nucleic acid sequence that once invaded the host.
Accompanying CRISPR sequences, there are 4-10 CRISPR-associated genes (cas), which are highly conserved and encode the Cas proteins. Cas proteins conduct adaptive immunity in prokaryotes (bacteria) based on immunological memories stored in the CRISPR array.
The CRISPR/Cas system integrates a small piece of foreign DNA from invaders such as plasmids and viruses into their direct repeat sequences and will recognize and degrade the same external DNA elements during future invasions.
As the CRISPR/Cas systems integrate DNA from invading pathogens in chronical order, genotyping can be used to trace the clonality and the origin of the isolates and define them as a population of strains that were subjected to the same environmental conditions including geographic location (region) and community/hospital settings and eventually further extended to track pathogenic bacteria around human society.
CRISPR/Cas systems can also be employed for developing antimicrobial agents: introduction of self-targeting crRNAs will effectively and selectively kill target bacterial populations. Due to the shortage of available effective antimicrobial agents in treating multidrug-resistant (MDR) infections, researchers started to search for alternative methods to fight MDR infections rather than going through the process of developing new antimicrobial agents which can go on for decades.
As a result, the concept of CRISPR/Cas-based selective antimicrobials was first developed and demonstrated in 2014. Vectors coding Cas9 and guide RNAs targeting genomic loci of a specific bacterial strain/species can be delivered to the target strain via bacteriophages or conjugative bacterial strains.
In theory, delivery of the engineered CRISPR/Cas systems specifically eliminates target strains from the bacterial population, yet it is not that simple.
While these systems can seem a target for manipulation/intervention, all bacteria are regulated by multiple pathways to ensure the bacteria retains control over the process. Therefore, there remain several major challenges in using this system as an antimicrobial agent.
Most methods require delivery of the re-sensitized system by conjugation; the vector is carried by a non-virulent lab strain bacteria that is supposed to go and share the vector/plasmid through conjugation. The conjugation process is a natural process that the bacteria do which results in sharing plasmids among each other (even with other species).
The percentage of conjugated (successfully delivered) bacteria in the total bacterial population is critical to the re-sensitized efficiency. This process is governed by several complicated pathways.
Bacteria also possess built-in anti-CRISPR systems, that can repair any damage caused by CRISPR-Cas systems.
Defense systems that the bacteria uses to protect itself from foreign DNA often co-localize within defense islands (genomic segments that contain genes with similar function in protecting the host from invaders) in bacterial genomes; for example: acr (a gene that acts, with other similar variants, as a repressor of plasmid conjugative systems) often cluster with antagonists of other host defense functions (e.g., anti-restriction modification systems) and experts hypothesize that MGEs (mobile genetic elements) organize their counter defense strategies in "anti-defense" islands.
Assistant Professor Bitar concludes, "In summary, this method seems very promising as an alternative way of fighting antimicrobial resistance. The method uses the concept of re-sensitizing the bacteria in order to make use of already available antibiotics—in other words, removing their resistance and making them vulnerable again to first-line antibiotics.
"Nevertheless, the bacterial pathways are always complicated and such systems are always heavily regulated by multiple pathways. These regulated pathways must be studied in depth in order to avoid selective pressure favoring anti-CRISPR systems activation, hence prevalence of resistance in a more aggressive manner."
Provided by European Society of Clinical Microbiology and Infectious Diseases
Explore further
Feedback to editors

Optical barcodes expand range of high-resolution sensor
13 hours ago

Ridesourcing platforms thrive on socio-economic inequality, say researchers
14 hours ago

Did Vesuvius bury the home of the first Roman emperor?

Florida dolphin found with highly pathogenic avian flu: Report

A new way to study and help prevent landslides

New algorithm cuts through 'noisy' data to better predict tipping points

Researchers reconstruct landscapes that greeted the first humans in Australia around 65,000 years ago
15 hours ago

High-precision blood glucose level prediction achieved by few-molecule reservoir computing

Enhancing memory technology: Multiferroic nanodots for low-power magnetic storage

Researchers advance detection of gravitational waves to study collisions of neutron stars and black holes
Relevant physicsforums posts, the cass report (uk).
Apr 24, 2024
Major Evolution in Action
Apr 22, 2024
If theres a 15% probability each month of getting a woman pregnant...
Apr 19, 2024
Can four legged animals drink from beneath their feet?
Apr 15, 2024
Mold in Plastic Water Bottles? What does it eat?
Apr 14, 2024
Dolphins don't breathe through their esophagus
More from Biology and Medical
Related Stories
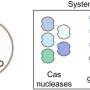
Researchers systematically investigate efficacy of CRISPR antimicrobial agents
Apr 25, 2024
Scientists uncover new way viruses fight back against bacteria
Oct 18, 2023
Genetically engineered plasmid can be used to fight antimicrobial resistance
Sep 16, 2019
Gene editing tool could help reduce spread of antimicrobial resistance
May 25, 2023
New bacterial defense mechanism of the CRISPR-Cas system uncovered
Jul 18, 2017
Recommended for you

Study suggests host response needs to be studied along with other bacteriophage research
17 hours ago

Automated machine learning robot unlocks new potential for genetics research

Study details a common bacterial defense against viral infection
16 hours ago

AI deciphers new gene regulatory code in plants and makes accurate predictions for newly sequenced genomes

Scientists discover higher levels of CO₂ increase survival of viruses in the air and transmission risk

Researchers decipher how an enzyme modifies the genetic material in the cell nucleus
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Admissions Visit Opportunities
The Charger Blog
Biology Major Hopes to Help Combat Viruses Through Research
Whether he was gaining hands-on experience on campus at an international company, Brendan Straut '24 has built his laboratory skills and his confidence throughout his time as a Charger. He's excited to begin his master's degree at the University this fall.
April 23, 2024
By Renee Chmiel, Office of Marketing and Communications

Throughout his time as a Charger, Brendan Straut '24 has had exciting experiences across campus. He's made connections and cultivated a network that includes classmates, mentors, teammates, and friends while also becoming a researcher.
A biology major , Straut is a member of the University's Honors program . He's been working on his thesis under the mentorship of Anna Kloc, Ph.D. , examining human heart inflammation and how it could be related to viral infections.
"The laboratory experience I gained from the many labs I've attended along with my research and lab assistant job have helped me gain confidence," said Straut, a member of the TriBeta, a biology honor society. "I can bring this to any lab in the future."
A leader on campus, Straut serves as a resident assistant in the University's Office of Residential Life . A student ambassador, he has led new and prospective students and their families on campus tours at Open Houses and Accepted Students Days . He enjoyed sharing with them what he has loved the most about his own experience as a Charger.

Whether he is attending a game or movie night on campus or going on a trip hosted by the Student Committee of Programming Events (SCOPE) , Straut has taken every opportunity to connect with his fellow Chargers – and to enjoy all that the University has to offer.
An active member of the University's esports community, Straut particularly enjoyed making connections while gaming in The Stable, the University's state-of-the-art esports and gaming center. Whether he was competing against teams from other schools or gaming with his friends on campus, he had fun sharing his passion for esports.
"I was a part of the Esports and Gaming club at the University, which enabled me to meet some of my best friends," he recalls. "I have attended a multitude of gaming tournaments during my time with my esports team, and I even spent this current semester being a part of a Varsity Esports team."
Straut is now looking forward to accepting his bachelor's degree as part of the University's 2024 Commencement , a three-day event that will be held at the University's West Haven campus. As he completed his degree, he gained laboratory experience both on and off campus. While interning for Takasago International Corporation , a fragrance company, he explored new laboratory techniques and cultivated his networking skills.
Straut is excited to continue to build those skills as he continues his education at the University of New Haven. He will begin his master's degree in cellular and molecular biology this fall. He's particularly interested in exploring viruses and the immune system.
"I learned so much more about the world of biology, and the University helped me narrow down my field of interest from general biology to cell and molecular biology," he explains. "In the future, I hope to be able to help researchers learn more about viruses, and perhaps contribute to combatting them."
Recent News

Graduate Students Present Mental Health Research at Prestigious Conference
Sanmit Jindal ’24 MPH and Krupa Ann Mathew ’24 MPH collaborated with each other and with their faculty mentors as they gathered data at one academic conference and presented their findings at another. It was an exciting opportunity to explore research, to network, and to examine adolescent mental health.

University Hosts Immersive Satellite Imagery Workshop
Satellite imagery is becoming an increasingly valuable tool in many fields, and several Chargers recently had the opportunity to learn how to apply it to do everything from tracking the conflict in Ukraine to determining whether images had been tampered with.

Senior Earns Prestigious Boren Fellowship Award
After she graduates with her bachelor’s degree in international affairs in May, Allison Mahr ’24 will head to the Republic of Georgia with the support of a Boren National Security Fellowship. She’s excited to use the award to build her skills and, ultimately, make an impact in the post-conflict reconstruction of the region.
- MBA/MPA Program - University of New Haven
- Arts & Sciences: Art & Design | University of...
- Make Your Gift
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Maggie Chen
Unruly Gut Fungi Can Make Your Covid Worse
Fungi are an indispensable part of your microbiome , keeping the body’s host of microorganisms healthy as part of a system of checks and balances. But when you’re hit by an infection , fungi can be thrown out of equilibrium with other organisms inside you, leading to a more severe infection and other symptoms of illness.
For this reason, the pandemic immediately set off alarms for Iliyan Iliev, an immunologist at Weill Cornell Medical School. “We were thinking, the first thing that’s going to happen is people will start getting fungal co-infections,” he says. With the microbiome unbalanced, fungi might start running riot inside Covid patients, Iliev reasoned. His fears were soon realized.
In research published in Nature Immunology , he and his team discovered that in patients with severe Covid, certain strains of gut fungi—knocked off-kilter by the virus—set off a prolonged immune response that could last long after the initial infection. This response potentially led to some of the respiratory symptoms experienced by these patients. These results, Iliev says, point to the critical role of the gut microbiome in the human immune response and could lead to better disease treatments down the line.
Imbalance of the gut microbiome has long been linked to disease. Ken Cadwell, an immunologist at the Perelman School of Medicine at the University of Pennsylvania, thinks of the microbiome as a metaphorical rainforest. “It’s a nice ecosystem—but if you cut down too many trees or bring in invasive species, you could make things go out of whack,” he says.
To see how the body’s internal fungi were affected during Covid and how this triggered the immune system, Iliev and his team started by looking at patients’ blood. After collecting samples from 91 people with Covid, they measured levels of antibodies against several fungi, to figure out if the body’s immune system was reacting against these. Significantly more anti-fungal antibodies, for instance, would indicate fungal overgrowth or invasion.
Takato Kusakabe, a postdoctoral fellow in Iliev’s lab and study author, ran plate after plate of experiments—a painstaking process—to quantify these antibody levels. The team found in patients with severe Covid, several fungi commonly found in the gut had increased antibodies against them (in comparison to uninfected people). Notably, these included Candida albicans , which is a common culprit of yeast infections. When the team then ran tests on fecal samples from 10 of the hospitalized Covid patients, these confirmed that the fungi being targeted by the antibodies were present in the patients’ guts—and at seemingly at higher levels than in uninfected controls, suggesting an imbalance in their microbiome.
Iliev recalls excitedly peering into the laboratory incubator to see the first round of fungal isolations. “The plate was full , with a lawn full of fungal organisms,” he says.
Now that they knew severe Covid was associated with an increase in certain gut fungi, the team wanted to figure out exactly how this would influence the body’s immune cells. It wasn’t long before they found something out of the ordinary—certain neutrophils (a subtype of white blood cell that fights off infection) were present in greater quantities in severe Covid patients compared to those with mild or no disease.

Matt Jancer

Boone Ashworth
David Nield

Scott Gilbertson
When the team infected mice with Candida albicans strains taken from the severely affected Covid patients, they discovered that the mice generated increased fungal antibodies and neutrophils. And when they then treated these infected mice with the common antifungal drug fluconazole, numbers of these fungal-induced neutrophils decreased—as did the quantity of fungal antibodies. This indicated that overgrowth of those fungi helped cause the number of neutrophils to rise, with the coronavirus itself kickstarting the process.
Neutrophils are an important part of the immune system, says Iliev, but excessive activity can lead to prolonged inflammation that is characteristic of Covid. “Neutrophils will keep coming because they think there is inflammation and infection,” he adds. “They basically start exploding to make these structures called neutrophil extracellular traps—which, instead of helping you, actually makes the disease worse.”
And the impact of this fungal overgrowth didn’t end once the patients’ Covid had subsided. By looking again at blood samples from the severe Covid patients, and comparing these to samples from healthy controls, the scientists found that the stem cells that created these neutrophils had become specifically adapted to target fungi. These stem cells were active long after the initial infection, even after levels of fungal antibodies and neutrophils had died down—essentially priming the body to respond dramatically to a future fungal threat. At this stage, it’s not clear if this would be helpful or problematic for patients—it’s plausible that the patients’ bodies might be primed to overreact to other fungal infections in future.
There was one final question puzzling Iliev and his colleagues. How, then, did the fungi nestled in the gut cause such drastic changes in the immune system located elsewhere—all the way down to the stem cells? To answer that question, the scientists looked for signaling molecules, known as cytokines. One of these, called IL6, they noticed was elevated in the infected mice, alongside the elevated neutrophils and fungal antibodies. When the team blocked IL6, both the neutrophils and fungal antibodies decreased in quantity. “Maybe the mediator here is a cytokine that the fungi induce,” Iliev says, suggesting that these are potentially the sign of some communication across the body that sets all of these processes in motion.
This complex crosstalk between the gut microbiome and the immune system is an example of how most things in the body are intertwined, says Alessio Fasano, a gastroenterologist at Massachusetts General Hospital, who was unaffiliated with the study. “The gut is not like Las Vegas,” he says. “What happens in the gut does not stay in the gut.”
Fasano can envision this kind of work pointing to a future of more personalized medicine. Measuring for increased levels of fungal antibodies in Covid patients, he says, could potentially uncover a subset of people who might benefit from taking antifungal drugs like fluconazole.
All the scientists note, though, that it is unfair to assign the blame of upending the immune system to one single strain of fungi. Because the microbiome is always in flux, reestablishing balance after infection is key—throwing lots of antibiotics or antifungals at the problem can result in a never-ending game of biological whack-a-mole where one imbalance leads to another.
Now, Iliev and Kusakabe are interested in exploring how fungal overgrowth may appear in long Covid—and how immunity is affected. “What’s the impact of this reprogramming of the immune system by the fungus and the virus?” Iliev asks. “What happens long-term if you have suffered from that?”
You Might Also Like …
Navigate election season with our Politics Lab newsletter and podcast
Think Google’s “Incognito mode” protects your privacy? Think again
Blowing the whistle on sexual harassment and assault in Antarctica
The earth will feast on dead cicadas
Upgrading your Mac? Here’s what you should spend your money on
Emily Mullin

R Douglas Fields

Lee Hedgepeth
Elise Cutts
Charlie Wood

Frankie Adkins
UH Hilo Stories
‘A‘ohe pau ka ‘ike i ka hālau ho‘okahi │ One learns from many sources │ A publication from the Office of the Chancellor, University of Hawai‘i at Hilo
- UH Hilo on Facebook
- UH Hilo on Instagram
- UH Hilo on X (Twitter)
- UH Hilo on YouTube
Students present their research at UH Hilo’s 14th Annual Tropical Conservation Biology and Environmental Science Symposium
This year’s symposium theme was “Hoʻomau: Sustaining Communities and Ecosystems in our Changing Climate.” Five students won awards for their outstanding presentations.

By Susan Enright .

University of Hawaiʻi at Hilo hosted the 14th Annual Tropical Conservation Biology and Environmental Science Symposium , April 11-12, 2024, at the Campus Center.
The event is organized each year by the Kaiameaola Club , which is made up of graduate students in the program .
This years theme was “Hoʻomau: Sustaining Communities and Ecosystems in our Changing Climate,” focusing on the changes occurring to ecosystems, endemic species, and Indigenous communities as anthropogenic climate change continues to exacerbate social and environmental concerns.
In light of the wild fire tragedy that recently occurred on Maui, student organizers emphasized the importance of building up community and ecological resilience.

Keynote speakers were Pelika Andrade , founder and executive director of Na Maka Onaona, a Hawaiʻi-based nonprofit, and an extension agent for the UH Sea Grant College Program, and Drew Kapp , assistant professor in geography at Hawaiʻi Community College who is active in supporting Native Hawaiian students in the sciences and is a member of the Unukupukupu hula hālau (dance group).
Award winners from this year’s symposium:
Graduate Student, 10-Minute Presentation
Emma Stierhoff , “Assessing the long-term impacts of chronic infection with avian malaria in Hawaiʻi ʻamakihi.”

“The introduction of avian malaria ( Plasmodium relictum ) to Hawaiʻi has decimated native forest bird populations, driving many species to extinction, and threatening those that remain. However, the Hawaiʻi ʻamakihi, a native honeycreeper, has shown resilience against acute infection with avian malaria. Hawaiʻi ʻmakihi who survive the acute stage remain chronically infected with low parasitemia levels. Although immediate costs of acute malarial infection have been closely studied, the costs of chronic infection are poorly understood in this species. We assessed the impact of chronic infection on physiological condition of Hawaiʻi ʻamakihi near ʻᾹinahou Ranch in Hawaiʻi Volcanoes National Park. During biweekly banding sessions from May 2022 to August 2023, Hawaiʻi ʻamakihi were banded, measured, and weighed, and a blood sample was collected for each bird. Blood samples were used to measure hematocrit, triglycerides, and reactive oxygen metabolites (ROMs) and to determine disease status using qPCR. There were no detectable differences in size, hematocrit, triglycerides, or ROMs based on disease status. These results further demonstrate the resilience Hawaiʻi ʻamakihi have against avian malaria and set the foundation for future research studying the factors facilitating this resilience and how that might be applied to protect more threatened honeycreeper populations.”
Graduate Student, 5-Minute Presentation
Lauren Smith , “Using bioacoustics to assess the success of incompatible insect technique.”

In Hawaiʻi, more than two-thirds of native forest bird species have gone extinct since the arrival of humans, largely driven by introduced avian diseases vectored by non-native southern house mosquitoes ( Culex quinquefasciatus ). On the island of Maui, the critically endangered kiwikiu ( Pseudonestor xanthrophrys ) and ʻākohekohe ( Palmeria dolei ) are likely facing extinction in as few as five years. To prevent extinctions and persistent declines in numerous species, conservation partners are employing the Incompatible Insect Technique (IIT) on a landscape scale to suppress mosquito populations and, in turn, decrease disease transmission. To monitor the response of the bird populations to IIT, we are deploying 80 autonomous recording units (ARUs) along transects inside and outside IIT treatment areas in The Nature Conservancy’s Waikamoi Preserve and Haleakalā National Park’s Kīpahulu Valley. We are processing these acoustic data using the machine learning classifier Perch, a new analytical method co-developed by the UH Hilo Listening Observatory for Hawaiian Ecosystems (LOHE) lab and Google Research. Perch identifies bird calls and calculates call densities, from which we estimated relative abundance and occupancy for six native species. We are also using begging call densities to estimate breeding success of each species. By comparing these data across breeding seasons, we expect to detect any changes in relative abundance, occupancy, productivity, and elevational shifts in range that may result from the suppression of mosquitoes and disease.
Undergraduate Student, 10-Minute Presentation
Manuela Cortes , “Exploring the impact of micro-fragmentation size on coral growth rates.”

Coral microfragmentation is commonly used to propagate corals in nurseries. The process involves breaking small pieces off of a parent colony and encouraging them to grow into independent colonies. This method has been shown to increase the rate of coral growth, potentially due to the difference in fragment size which affects the surface area available for the generation of new daughter polyps. I will be analyzing the relationship between coral fragment size and growth rate at the MOP Coral Nursery at the Pacific Aquaculture and Coastal Resource Center. My goal is to determine if there is an optimal size of coral fragment associated with higher growth and survivorship rates.
Undergraduate Student, 5-Minute Presentation
Krista Golgotiu , “Uniting Indigenous knowledge and scientific inquiry: A cultural approach to understanding the Hawaiian environment through the use of ʻŌlelo Noʻeau.”

This research proposal aims to bridge indigenous knowledge from the Hawaiian people focusing on ʻŌlelo Noʻeau (traditional Hawaiian proverbs and sayings), with scientific methods to prove the observations of ancient Hawaiians and enhance conservation efforts in modern-day Hawaiʻi. Using a collaborative approach involving members of the community, cultural practitioners, and scientists alike, this project aims to identify and validate specific ʻŌlelo Noʻeau that reference the ocean, animals, plants, and weather patterns. By first identifying these specific ʻŌlelo Noʻeau, through the use of Hawaiian community engagement the accuracy of the interpretation and understanding of these traditional sayings will be ensured. After choosing the specific ʻŌlelo Noʻeau, selecting key areas around Hawaiʻi to conduct field studies is essential. Field studies will be conducted in the areas associated with the specific sayings, through observation and data collection. The use of any past recorded data in regards to weather, ocean, or plant and animal growth patterns will also be utilized. With the inclusion of the Hawaiian community through participation in field studies, traditional knowledge and new information regarding these patterns will be combined with any observations and data collected in the field. Then by utilizing statistical methods and ecological modeling an assessment on the alignment between what is observed and the knowledge embedded in the ʻŌlelo Noʻeau can be made. This process will aim to confirm the ecological accuracy of traditional wisdom passed down. Proving ʻŌlelo Noʻeau can provide insights on sustainable resource management for local communities, and inform modern practices on how to use limited natural resources more sustainably by tracking natural indicators. Scientifically affirming ʻŌlelo Noʻeau through this project, from the research and data collected, will validate the scientific relevance of traditional knowledge and the important need to include indigenous communities in science.
Poster Presentation
Amy Durham , “He ‘Io Au: A community project about ʻIo (the Hawks on your block).

Do you have a hawk on your block? ʻIo is our Hawaiʻi island’s endemic hawk. Their story is linked to many aspects of our cultural and natural legacies. Since they are present in many of our daily lives, they are an ideal conduit for connecting to our native species – which is especially important in altered landscapes devoid of such interactions. Community-led conservation projects are invaluable resources for learning about and managing data-deficient species. Despite ʻio’s importance, minimal research has been conducted on their utilization of our urban and agricultural neighborhoods. We want to change that by collaborating with you! Our group of ʻio enthusiasts aims to spotlight ʻio by introducing the upcoming “He ʻIo Au” community platform. Our objective is to keep all eyes on ʻio by (1) Utilizing the volunteer He ʻIo Au platform to gather data on ʻio’s behavior in our neighborhoods, including details on population size, diet preferences, and breeding behavior (2) Highlighting the significance of ‘io by sharing our ʻio stories through interviews and an online blog and (3) Engaging in community outreach to educate about ‘io, raise awareness regarding intentional harm, and actively seek to share knowledge and collaborate on solutions.
Story by Susan Enright, a public information specialist for the Office of the Chancellor and editor of UH Hilo Stories. She received her bachelor of arts in English and certificate in women’s studies from UH Hilo.

Featured Events


IMAGES
VIDEO
COMMENTS
A virus is a tiny, infectious particle that can reproduce only by infecting a host cell. Viruses "commandeer" the host cell and use its resources to make more viruses, basically reprogramming it to become a virus factory. Because they can't reproduce by themselves (without a host), viruses are not considered living.Nor do viruses have cells: they're very small, much smaller than the cells of ...
4. Introduction to viruses A virus consists of two or three parts: genes, made from either DNA or RNA, long molecules that carry genetic information protein coat that protects the genes; and in some viruses, an envelope of fat Viruses vary in shape from the simple helical and icosahedral to more complex structures. Viruses range in size from 20 to 300 nanometres; it would take 30,000 to ...
Help. 1 Ch. 17 Warm-up 1. Why do many scientists classify viruses as non-living? 2. Draw the basic structure of a virus. Label and define capsid, viral envelope and nucleic acid. 2 Ch. 17 Warm-up 1. Draw the lytic/lysogenic cycle.
Concept 17.3: Viruses, viroids, and prions are formidable pathogens in animals and plants. Diseases caused by viral infections affect humans, agricultural crops, and livestock worldwide. Smaller, less complex entities called viroids and prions also cause disease in plants and animals, respectively.
Virus, infectious agent of small size and simple composition that can multiply only in living cells of animals, plants, or bacteria. Viruses possess unique infective properties and thus often cause disease in host organisms. ... Advancements that have been made in chemistry, physics, and molecular biology since the 1960s have revolutionized the ...
How Viruses Multiply. (Active Viruses) Virus attaches to the host cell Virus injects genetic material Viral DNA or RNA control production of viral protein and DNA or RNA Protein and genetic material are assembled Host cell bursts and virus is released. Active Viruses. Active Virus.
A virus is a chain of nucleic acids (DNA or RNA) which lives in a host cell, uses parts of the cellular machinery to reproduce, and releases the replicated nucleic acid chains to infect more cells. A virus is often housed in a protein coat or protein envelope, a protective covering which allows the virus to survive between hosts. Virus Structure.
Six coronaviruses (CoVs) are known to infect humans: 229E, OC43, SARS-CoV, NL63, HKU1, and MERS-CoV. Many CoVs are simultaneously maintained in nature, allowing for genetic recombination, resulting in novel viruses. SARS-CoV-2 is the third pathogenic novel coronavirus to emerge over the past two decades.
Biology library 37 units · 127 skills. Unit 1 Intro to biology. Unit 2 Chemistry of life. Unit 3 Water, acids, and bases. Unit 4 Properties of carbon. Unit 5 Macromolecules. Unit 6 Elements of life. Unit 7 Energy and enzymes. Unit 8 Structure of a cell.
Viruses that infect bacteria are known as bacteriophage or phage. A virulent phage is one that always lyses the host cell at the end of replication, after following the five steps of replication described above. This is called the lytic cycle of replication. There are also temperate phage, viruses that have two options regarding their ...
21. • The first step in viral infection is attachment, interaction of a virion with a specific receptor site on the surface of a cell. • Receptor molecules differ for different viruses but are generally glycoproteins. • human immunodeficiency virus binds to the CD4 receptor on cells of the immune system, • Rhinoviruses bind intercellular adhesion molecule 1 (ICAM-1), • Epstein-Barr ...
Virus Presentation templates Medical science is moving ahead at an immense speed! Share the most recent scientific researches, medical advances, and treatments of viruses that have marked the history of humanity, such as the Spanish flu or COVID-19. ... Download the "Immunology - Bachelor of Science in Human Biology" presentation for PowerPoint ...
15. Virus classification Before, viruses are classified according to the type of host that they infected. The current system reflect phenotypic characteristics. The Baltimore classification system distinguish viruses based on their - Method of replication - Genome type (DNA or RNA) The International Committee on Taxonomy of Viruses devised and implemented several rules on the naming and ...
Virus-host interactions are often described in terms of pathogenesis and virulence. Pathogenesis is the process whereby a virus interacts with its host in a discrete series of stages to produce disease ( Table 134-3 ). Virulence is the capacity of a virus to produce disease in a susceptible host.
Steps of Virus Infections. A virus must use its host-cell processes to replicate. The viral replication cycle can produce dramatic biochemical and structural changes in the host cell, which may cause cell damage. These changes, called cytopathic effects, can change cell functions or even destroy the cell.
To initiate infection, the virus must be able to bind to target cell. Binding occurs between: - Ligands on the virus surface. (viral attachment proteins) - Receptors on the plasma membrane of cell. Although there is a degree of specificity, quite different viruses may utilize the same receptor and, conversely, viruses in the same family or ...
General Biology 17. Viruses Viruses. Video duration: 14m. Play a video: Introduction to Viruses and Viral Replication. Craig Savage. 712. views. Was this helpful? 0. Bookmarked. Hide transcripts. ... Meet Tobacco Mosaic Virus (TMV). TMV has a family called Virgaviridae. simpleshow video maker. 857.
00:06:09.12 virus, which was studied historically as one of the very 00:06:13.01 first viruses for which detailed biochemistry and detailed 00:06:17.13 structure became available, is a helical array in which the 00:06:22.23 nucleic acid, the RNA, is wound into a groove on the 00:06:27.17 protein subunit and winds up with the protein, which forms
Now, 14 years later, in a study published in Nature, his team reported using a new computational approach to simulate the individual atoms of a virus that is packed with nucleic acids. 1 They used this method to study the HK97 bacteriophage and proposed the first structure for the virus.. A few years ago, Aksimentiev's team developed a method for mapping out complex DNA configurations by ...
Unit i discovery of viruses ppt. ... In Nayak DP (ed): Molecular Biology of Animal Viruses. Marcel Dekker, New York, 1977 . Morse SS (ed): The Evolutionary Biology of Viruses. Raven Press, New York, 1994 . Palmer EL, Martin ML: An Atlas of Mammalian Viruses. CRC Press, Boca Raton, 1988 . Nermut MV, Stevens AC (eds): Animal Virus Structure.
Download the "Biology Subject for High School: Birds Beaks & Adaptations" presentation for PowerPoint or Google Slides. High school students are approaching adulthood, and therefore, this template's design reflects the mature nature of their education. Customize the well-defined sections, integrate multimedia and interactive elements and ...
NK cells are white blood cells that can directly destroy virus-infected body cells. The scientists found that NK cells are in a permanent alarm mode to be ready for rapid Ccl3 production.
In his presentation "How to use CRISPR-Cas to combat AMR" at the ESCMID Global Congress, Assistant Prof. Ibrahim Bitar, Department of Microbiology, Faculty of Medicine and University Hospital in ...
Materi biologi - Virus .ppt presentation Ismail Lathiif ... Biology 11 Federal.pdf. Biology 11 Federal.pdf Unidad de Emprendimiento ambulante ...
Biology Major Hopes to Help Combat Viruses Through Research. Whether he was gaining hands-on experience on campus at an international company, Brendan Straut '24 has built his laboratory skills and his confidence throughout his time as a Charger. He's excited to begin his master's degree at the University this fall.
In research published in Nature Immunology, he and his team discovered that in patients with severe Covid, certain strains of gut fungi—knocked off-kilter by the virus—set off a prolonged ...
Graduate Student, 5-Minute Presentation. Lauren Smith, "Using bioacoustics to assess the success of incompatible insect technique." Lauren Smith delivers her presentation at UH Hilo's 14th Annual Tropical Conservation Biology and Environmental Science Symposium held April 11-12, 2014. (Courtesy photo: TCBES/UH Hilo, click to enlarge ...
Lytic Cycle - the virus enters the cell, replicates itself hundreds of times, and then bursts out of the cell, destroying it. 2. Lysogenic Cycle - the virus DNA integrates with the host DNA and the host's cell helps create more virus DNA. An environmental change may cause the virus to enter the Lytic Cycle.