Charles' Law Example Problem
Real-life applications for the ideal gas law at constant pressure
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Charles' law is a special case of the ideal gas law in which the pressure of a gas is constant. Charles' law states that volume is proportional to the absolute temperature of a gas at constant pressure. Doubling the temperature of gas doubles its volume, so long as the pressure and quantity of the gas are unchanged.
This example problem shows how to use Charles' law to solve a gas law problem: A 600 mL sample of nitrogen is heated from 27 °C to 77 °C at constant pressure. What is the final volume?
The first step to solving gas law problems should be converting all temperatures to absolute temperatures . In other words, if the temperature is given in Celsius or Fahrenheit, convert it to Kelvin. (This is where the most commonplace mistakes are made in this type of homework problem.)
T K = 273 + °C T i = initial temperature = 27 °C T i K = 273 + 27 T i K = 300 K T f = final temperature = 77 °C T f K = 273 + 77 T f K = 350 K
The next step is to use Charles' law to find the final volume. Charles' law is expressed as:
V i /T i = V f /T f where V i and T i is the initial volume and temperature V f and T f is the final volume and temperature Solve the equation for V f : V f = V i T f /T i Enter the known values and solve for V f . V f = (600 mL)(350 K)/(300 K) V f = 700 mL Answer: The final volume after heating will be 700 mL.

More Examples of Charles' Law
If you think Charles' Law seems irrelevant to real-life situations, think again! By understanding the basics of the law, you'll know what to expect in a variety of real-world situations and once you know how to solve a problem using Charles' Law, you can make predictions and even start to plan new inventions. Here are several examples of situations in which Charles' Law is at play:
- If you take a basketball outside on a cold day, the ball shrinks a bit as the temperature is decreased. This is also the case with any inflated object and explains why it's a good idea to check your car's tire pressure when the temperature drops.
- If you over-inflate a pool float on a hot day, it can swell in the sun and burst.
- Pop-up turkey thermometers work based on Charles' law. As the turkey cooks, the gas inside the thermometer expands until it can "pop" the plunger.
Examples of Other Gas Laws
Charles' law is only one of the special cases of the ideal gas law that you may encounter. Each of the laws is named for the person who formulated it . It's good to know how to tell the gas laws apart and be able to cite examples of each one.
- Amonton's Law: Doubling temperature doubles pressure at constant volume and mass. Example: As automobile tires heat up when you drive, their pressure increases.
- Boyle's Law: Doubling pressure halves volume, at constant temperature and mass. Example: When you blow bubbles underwater, they expand as they rise to the surface.
- Avogadro's Law: Doubling the mass or number of moles of a gas doubles the volume at constant temperature and pressure. Example: Inhaling fills the lungs with air, expanding their volume.
- What Is the Formula for Charles' Law?
- Gay-Lussac's Gas Law Examples
- The Formula for Boyle's Law
- Avogadro's Law Example Problem
- Charles's Law Definition in Chemistry
- Gay-Lussac's Law Definition
- The Combined Gas Law in Chemistry
- Boyle's Law: Worked Chemistry Problems
- Gases - General Properties of Gases
- Boyle's Law Explained With Example Problem
- How to Calculate Density of a Gas
- Ideal Gas Law Example Problem
- The Formula for the Combined Gas Law
- Ideal Gas Law Test Questions
- Gases Study Guide
- Ideal Gas Example Problem: Partial Pressure
- Chemistry Concept Questions and Answers
- Charles law Questions
Charles Law Questions
Charle’s law is also referred to as the law of volumes. It tells us about the behaviour of gases. Charle’s law states that the volume is directly proportional to the temperature of the gas at constant pressure.
Or, V / T = k
Charles Law Chemistry Questions with Solutions
Q1. Suppose P, V, and T denote the gas’s pressure, volume, and temperature. In that case, the correct representation of Chale’s law is
- V is directly proportional to T (at constant P)
- V inversely proportional to T (at constant P)
- None of the above
Answer: (a), If P, V, and T denote the gas’s pressure, volume, and temperature, then the correct representation of Chale’s law is V is directly proportional to T (at constant P).
Q2. How can we convert a Celsius temperature to Kelvin temperature?
- By adding 37
- By subtracting 37
- By subtracting 273
- By adding 273
Answer: (d), We can convert a Celsius temperature to Kelvin temperature by adding 273 to it.
Q3. Which element should remain constant if Charle’s law is applied to a gas sample?
- Temperature and the number of moles of a gas
- Pressure and the number of moles of a gas
- Volume and the number of moles of a gas
- Pressure only
Answer: (d), If Charle’s law is applied than the pressure of the gas sample should remain constant.
Q4. What is the value of the gas constant R?
- 8.314 J mol -1 K -1
- 0.082 J litre atm
- 0.987 cal mol -1 K -1
- 83 erg mol -1 K -1
Answer: (a), The value of gas constant R is 8.314 J mol -1 K -1 .
Q5. According to Charle’s law, if the temperature of a gas at constant pressure is increased, the volume will also
- Remains the same
- Can’t be determined
Answer: (a), Charle’s law states that volume is directly proportional to the temperature at constant pressure.
Or, V = kT.
Thus, if the system’s temperature increases, its volume will increase.
Q6. What is Charle’s law?
Answer: Charle’s law states that the volume of the gas is directly proportional to the absolute temperature of the gas at constant pressure.
Thus, if the system’s temperature increases, its volume will increase or if the system’s temperature decreases, its volume will decrease.
Q7. Do you encounter any of the applications of Charle’s law in everyday life? If yes, Where?
Answer: Yes, we encounter applications of Charle’s law in everyday life. When we take a volleyball outside on a hot day, the ball expands a bit. As the temperature increases, its volume also increases, leading to the expansion of volleyball. Similarly, the volleyball shrinks on a cold day as the temperature drops; its size also decreases.
Q8. Is Charles Law indirect or direct relation?
Answer: Charle’s law is a direct relation between the temperature and the volume of the gas. When the molecule’s temperature rises, molecules move faster thereby creating more pressure on the gas container. Hence, increasing the volume of the container. If the pressure of the gas container remains constant then the number of the molecules also remains constant.
Q9. Can we use quantities in °C in Charle’s law?
Answer: No, we can not use quantities in °C in Charle’s law. The relationship between volume and temperature will work when the temperature is taken in kelvin while we can use any quantity for the volume of the gas.
Q10. Match the following.
Q11. Calculate the decrease in temperature (in Celsius) when 2.00 L at 21.0 °C is compressed to 1.00 L.
Answer: Given
Initial Volume (V 1 ) = 2 L
Initial Temperature (T 1 ) = 21.0 °C = (21 + 273) K = 294 K
Final Volume (V 2 ) = 1 L
To Find: Final Temperature (T 2 ) = ?
We can calculate the final temperature of the gas using Charle’s law.
V 1 / T 1 = V 2 / T 2
2 / 294 = 1 / T 2
T 2 = 294 / 2
T 2 = 147 K
T 2 = (147 – 273) = – 126 °C
Hence the final temperature of the gas at volume 1 L is equivalent to – 126 °C.
Q12. A gas occupies a volume of 600.0 mL at a temperature of 20.0 °C. What will be its volume at 60.0 °C?
Initial Volume (V 1 ) = 600.0 mL
Initial Temperature (T 1 ) = 20.0 °C = (20 + 273) K = 293 K
Final Temperature (T 2 ) = 60.0 °C = (60 + 273) K = 333 K
To Find: Final Volume (V 2 ) = ?
We can calculate the final volume of the gas using Charle’s law.
600 / 293 = V 2 / 333
V 2 = (600 X 333) / 293
V 2 = 199800 / 293
V 2 = 681.91 ≈ 682 mL
Hence the final volume of the gas at 60.0 °C is equivalent to 681.91 ≈ 682 mL.
Q13. A gas occupies a volume of 900.0 mL at a temperature of 27.0 °C. What is the volume at 132.0 °C?
Initial Volume (V 1 ) = 900.0 mL
Initial Temperature (T 1 ) = 27.0 °C = (27 + 273) K = 300 K
Final Temperature (T 2 ) = 132.0 °C = (132 + 273) K = 405 K
900 / 300 = V 2 / 405
V 2 = (900 X 405) / 300
V 2 = 364500 / 300
V 2 = 1215 mL
Hence the final volume of the gas at 132.0 °C is equivalent to 1215 mL or 1.215 L.
Q14. What change in volume results if 60.0 mL of gas is cooled from 33.0 °C to 5.00 °C?
Initial Volume (V 1 ) = 60.0 mL
Initial Temperature (T 1 ) = 33.0 °C = ( 33 + 273) K = 306 K
Final Temperature (T 2 ) = 5.0 °C = ( 5 + 273) K = 278 K
60 / 306 = V 2 / 278
V 2 = ( 60 X 278) / 306
V 2 = 16680 / 306
V 2 = 54.50 mL
Change in the volume = 60.0 – 54.5 = 5.5 mL.
Hence the change in the volume of the gas at 5.0 °C is equivalent to 5.5 mL.
Q15. A gas occupies a volume of 300.0 mL at a temperature of 17.0 °C. What is the volume at 10.0 °C?
Initial Volume (V 1 ) = 300.0 mL
Initial Temperature (T 1 ) = 17.0 °C = (17 + 273) K = 290 K
Final Temperature (T 2 ) = 10.0 °C = ( 10 + 273) K = 283 K
300 / 290 = V 2 / 283
V 2 = (300 X 283) / 290
V 2 = 84900 / 290
V 2 = 292.75 mL
Hence the final volume of the gas at 10.0 °C is equivalent to 292.75 mL.
Practise Questions on Charles Law
Q1. Differentiate between Boyle’s law and Charle’s law.
Q2. A gas occupies a volume of 500.0 mL at a temperature of 10.0 °C. What will be its volume at 50.0 °C?
Q3. A gas occupies a volume of 100.0 mL at a temperature of 27.0 °C. What is the volume at 10.0 °C?
Q4. What change in volume results if 10.0 mL of gas is cooled from 33.0 °C to 15.0 °C?
Q5. A gas occupies a volume of 1 L at a temperature of 17.0 °C. What is the volume at 10.0 °C?
Click the PDF to check the answers for Practice Questions. Download PDF
Recommended Videos
Ideal gas equation definitions, derivation.

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Charles’s Law – Definition, Formula, Examples

Charles’s law or the law of volumes is an ideal gas law that states that the volume and temperature of a fixed amount of gas are proportional at constant pressure . Doubling the temperature of a gas doubles its volume. Halving the temperature of a gas halves its volume. The law takes its name from French scientist Jacques Charles, who formulated the law in the 1780s.
Charles’s law states that increasing the temperature of a gas at constant pressure increases its volume.
Charles’s Law Formula
There are a few ways to state Charles law as a formula:
V ∝ T V/T = k V = kT V 1 /T 1 = V 2 /T 2 V 2 /V 1 = T 2 /T 1 V 1 T 2 = V 2 T 1
Here, T is absolute temperature , V is volume, and k is a non-zero constant. Note that absolute temperature means Celsius and Fahrenheit temperature must be converted to Kelvin. The graph of volume versus pressure shows the linear relationship. Also, the line points toward the origin, although a gas could never reach it because it would change into a liquid or solid first.
Examples of Charles’s Law in Everyday Life
It’s easy to find examples of Charles’s law in everyday life.
- Hot air balloons fly based on Charles’s law. Heating the air in the balloon increases the balloon’s volume. This decreases its density, so the balloon rises in the air. To come down, chilling the air (not-heating-it) allows the balloon to deflate. The gas becomes more dense and the balloon sinks.
- If you take a filled balloon outside on a hot day, it expands (and may pop!). If you take it outdoors on a winter day, it deflates but returns to its normal volume when you take it indoors again. You can even use a balloon as a poor sort of thermometer, using Charles’s law.
Charles’s Law Example Calculation
A gas occupies 221 cm 3 at a temperature of 0 °C and pressure of 760 mm Hg. Find its volume at 100 °C.
First, don’t worry about the pressure. The number doesn’t enter into the calculation. All that matters is that it’s a constant.
Use the equation:
V 1 /T 1 = V 2 /T 2
Convert 0 °C and 100 °C to Kelvin:
V 1 = 221cm 3 ; T 1 = 273K (0 + 273); T 2 = 373K (100 + 273)
Plug the values into the equation and solve for V 2 :
V 1 /T 1 = V 2 /T 2 221cm 3 / 273K = V 2 / 373K V 2 = (221 cm 3 )(373K) / 273K V 2 = 302 cm 3
Find the final temperature of a sample of nitrogen gas at constant pressure if it starts at 27 °C and changes volume from 600 mL to 700 mL.
First convert the temperature to Kelvin.
T 1 = 273 + 27 T 1 = 300 K
Next, plug in the numbers.
V 1 /T 1 = V 2 /T 2 600 mL/300 K = 700 mL/T 2 (T 2 )(600 mL/300 K) = 700 mL T 2 = (700 mL)/(600 mL/300 K) T 2 = (700 mL)/(2mL/K) T 2 = 350 K
Why Temperature Must Be in Kelvin
Charles’s law calculations require temperature on an absolute scale, such as the Kelvin scale. So, using the formula requires converting from Celsius or Fahrenheit to Kelvin. There are two reasons for this. First, the negative temperatures on the Celsius and Fahrenheit scales could lead to impossible negative volume calculations. Second, the energy doesn’t scale properly using relative scales. So, a gas at 20 K has twice the energy of a gas at 10K, but the same is not true of as gas at 20 °C compared to 10 °C or 20 °F compared to 10 °F.
What Happens at Absolute Zero?
Like the other ideal gas laws, Charles’s law doesn’t apply under extreme conditions. It doesn’t make sense at absolute zero. First, matter can’t have zero volume. Second, a gas at constant pressure eventually changes into a liquid or solid as temperature drops.
- Fullick, P. (1994). Physics . Heinemann. ISBN 978-0-435-57078-1.
- Gay-Lussac, J. L. (1802). “Recherches sur la dilatation des gaz et des vapeurs” [ Research on the expansion of gases and vapors ]. Annales de Chimie . 43: 137–75.
- Krönig, A. (1856). “ Grundzüge einer Theorie der Gase “. Annalen der Physik . 99 (10): 315–22. doi:10.1002/andp.18561751008
Related Posts

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.2.1: Practice Problems- The Gas Laws
- Last updated
- Save as PDF
- Page ID 217291
PROBLEM \(\PageIndex{1}\)
Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. Why?
As temperature of a gas increases, pressure will also increase based on the ideal gas law. The volume of the tire can only expand so much before the rubber gives and releases the build up of pressure.
PROBLEM \(\PageIndex{2}\)
Explain how the volume of the bubbles exhausted by a scuba diver change as they rise to the surface, assuming that they remain intact.
As the bubbles rise, the pressure decreases, so their volume increases as suggested by Boyle’s law.
PROBLEM \(\PageIndex{3}\)
One way to state Boyle’s law is “All other things being equal, the pressure of a gas is inversely proportional to its volume.”
(a) What is the meaning of the term “inversely proportional?”
(b) What are the “other things” that must be equal?
The pressure of the gas increases as the volume decreases
amount of gas, temperature
PROBLEM \(\PageIndex{4}\)
An alternate way to state Avogadro’s law is “All other things being equal, the number of molecules in a gas is directly proportional to the volume of the gas.”
- What is the meaning of the term “directly proportional?”
- What are the “other things” that must be equal?
The number of particles in the gas increases as the volume increases
temperature, pressure
PROBLEM \(\PageIndex{5}\)
A spray can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot can?
3.40 × 10 3 torr
PROBLEM \(\PageIndex{6}\)
What is the temperature of an 11.2-L sample of carbon monoxide, CO, at 744 torr if it occupies 13.3 L at 55 °C and 744 torr?
PROBLEM \(\PageIndex{7}\)
A 2.50-L volume of hydrogen measured at –196 °C is warmed to 100 °C. Calculate the volume of the gas at the higher temperature, assuming no change in pressure.
PROBLEM \(\PageIndex{8}\)
A balloon inflated with three breaths of air has a volume of 1.7 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
PROBLEM \(\PageIndex{9}\)
A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level. What is the volume of the balloon under these conditions?
PROBLEM \(\PageIndex{10}\)
How many grams of gas are present in each of the following cases?
- 0.100 L of CO 2 at 307 torr and 26 °C
- 8.75 L of C 2 H 4 , at 378.3 kPa and 483 K
- 221 mL of Ar at 0.23 torr and –54 °C
7.24 × 10 –2 g
1.5 × 10 –4 g
PROBLEM \(\PageIndex{11}\)
A high altitude balloon is filled with 1.41 × 10 4 L of hydrogen at a temperature of 21 °C and a pressure of 745 torr. What is the volume of the balloon at a height of 20 km, where the temperature is –48 °C and the pressure is 63.1 torr?
1.2741 × 10 5 L or more correctly to 3 significant figures 1.27 × 10 5 L
PROBLEM \(\PageIndex{12}\)
While resting, the average 70-kg human male consumes 14 L of pure O 2 per hour at 25 °C and 100 kPa. How many moles of O 2 are consumed by a 70 kg man while resting for 1.0 h?
PROBLEM \(\PageIndex{13}\)
A balloon that is 100.21 L at 21 °C and 0.981 atm is released and just barely clears the top of Mount Crumpet in British Columbia. If the final volume of the balloon is 144.53 L at a temperature of 5.24 °C, what is the pressure experienced by the balloon as it clears Mount Crumpet?
PROBLEM \(\PageIndex{14}\)
If the temperature of a fixed amount of a gas is doubled at constant volume, what happens to the pressure?
Temperature and Pressure are directly proportional. Pressure will also have to increase (doubling).
PROBLEM \(\PageIndex{15}\)
If the volume of a fixed amount of a gas is tripled at constant temperature, what happens to the pressure?
Volume and pressure are inversely proportional. The pressure decreases by a factor of 3.
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology

HIGH SCHOOL
- ACT Tutoring
- SAT Tutoring
- PSAT Tutoring
- ASPIRE Tutoring
- SHSAT Tutoring
- STAAR Tutoring
GRADUATE SCHOOL
- MCAT Tutoring
- GRE Tutoring
- LSAT Tutoring
- GMAT Tutoring
- AIMS Tutoring
- HSPT Tutoring
- ISAT Tutoring
- SSAT Tutoring
Search 50+ Tests
Loading Page
math tutoring
- Elementary Math
- Pre-Calculus
- Trigonometry
science tutoring
Foreign languages.
- Mandarin Chinese
elementary tutoring
- Computer Science
Search 350+ Subjects
- Video Overview
- Tutor Selection Process
- Online Tutoring
- Mobile Tutoring
- Instant Tutoring
- How We Operate
- Our Guarantee
- Impact of Tutoring
- Reviews & Testimonials
- Media Coverage
- About Varsity Tutors
High School Chemistry : Using Charles's Law
Study concepts, example questions & explanations for high school chemistry, all high school chemistry resources, example questions, example question #8 : gases and gas laws.

Since pressure is kept constant, the only variable that is manipulated is temperature. This means that we can use Charles's law in order to compare volume and temperature. Since volume and temperature are on opposite sides of the ideal gas law, they are directly proportional to one another. As one variable increases, the other will increase as well.
Charles's law is written as follows:

To use this law, we must first convert the temperatures to Kelvin.

Use these temperatures and the initial volume to solve for the final volume.

Example Question #9 : Gases And Gas Laws
Which law is the following formula?

Charles's law
Ideal gas law
Boyle's law
Gay-Lussac's law
Combined gas law
Charles's law defines the direct relationship between temperature and volume. When the parameters of a system change, Charles's law helps us anticipate the effect the changes have on volume and temperature.

Example Question #1 : Using Charles's Law

Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation:

Our first step to solving this equation will be to convert the given temperatures to Kelvin.

Using these temperatures and the initial volume, we can solve for the final volume of the gas.

Our first step to solving this equation will be to convert the given temperature to Kelvin.

Using this temperature and the given volumes, we can solve for the final temperature of the gas.

Example Question #2 : Using Charles's Law
The graph depicted below represents which of the gas laws?
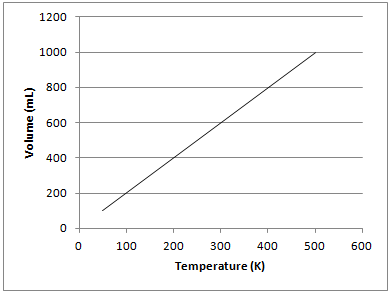
Newton's third law
The graph shows that there is a directly proportional relationship between the volume of a gas and temperature in Kelvin when kept at a constant pressure. This is known as Charles’s law and can be represented mathematically as follows:

Gay-Lussac's law shows the relationship between pressure and temperature. Boyle's law shows the relationship between pressure and volume. Newton's third law is not related to gas principles and states that for every force on an object, there is an equal and opposite force of the object on the source of force.

We expect the volume to increase since volume and temperature are directly proportional. We know that if we heat something the material will expand so we shouldn't get a value that is smaller than our initial volume. Charles Law says that

where the stuff on the left is the initial volume and temperature and the stuff on the right is the final volume and temperature. First off, we MUST convert the temperatures to Kelvin to use Charles Law. This gives

Solving for the final volume,

Report an issue with this question
If you've found an issue with this question, please let us know. With the help of the community we can continue to improve our educational resources.
DMCA Complaint
If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing the information described below to the designated agent listed below. If Varsity Tutors takes action in response to an Infringement Notice, it will make a good faith attempt to contact the party that made such content available by means of the most recent email address, if any, provided by such party to Varsity Tutors.
Your Infringement Notice may be forwarded to the party that made the content available or to third parties such as ChillingEffects.org.
Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially misrepresent that a product or activity is infringing your copyrights. Thus, if you are not sure content located on or linked-to by the Website infringes your copyright, you should consider first contacting an attorney.
Please follow these steps to file a notice:
You must include the following:
A physical or electronic signature of the copyright owner or a person authorized to act on their behalf; An identification of the copyright claimed to have been infringed; A description of the nature and exact location of the content that you claim to infringe your copyright, in \ sufficient detail to permit Varsity Tutors to find and positively identify that content; for example we require a link to the specific question (not just the name of the question) that contains the content and a description of which specific portion of the question – an image, a link, the text, etc – your complaint refers to; Your name, address, telephone number and email address; and A statement by you: (a) that you believe in good faith that the use of the content that you claim to infringe your copyright is not authorized by law, or by the copyright owner or such owner’s agent; (b) that all of the information contained in your Infringement Notice is accurate, and (c) under penalty of perjury, that you are either the copyright owner or a person authorized to act on their behalf.
Send your complaint to our designated agent at:
Charles Cohn Varsity Tutors LLC 101 S. Hanley Rd, Suite 300 St. Louis, MO 63105
Or fill out the form below:
Contact Information
Complaint details.


PhysicsTeacher.in
High School Physics
Numerical Problems based on Charles’ Law with solution
In this post, we will solve numerical problems using Charles ’ Law . The formulas to be used are as follows:
If V1 is the volume of a certain mass of a gas at temperature T1 and V2 is the volume of the same mass of the same gas at temperature T2 at constant pressure, then according to Charles’ law ,
V 1 /T 1 = V 2 /T 2 (mass and pressure constant) …… (1)
Alternate presentation of Charles’ law gives us this formula:
∴ Volume at t °C, V t = Initial volume at 0°C + Increase in volume = V 0 + V 0 x (1/273) x t
V t = V 0 ( 1 + t/273) ………………. (2)
[ Find detailed discussion on Charles’ law here ]
Solving Numerical Problems using Charles’ Law
A sample of gas occupies 1.50 L at 25°C. If the temperature is raised to 60°C, what is the new volume of the gas if the pressure remains constant?
Solution: V1 = 1.50 L
T1 = 273 + 25 = 298 K
T2 = 60 + 273 = 333 K
Since pressure remains constant, therefore, by applying Charles’ law
V1/T1 = V2/T2
or V2 = (V1/ T1) x T2
= [(1.50 L) /(298 K)] x (333 K)
A sample of helium has a volume of 520 mL at 100°C. Calculate the temperature at which the volume will become 260 mL. Assume that pressure is constant.
V1 = 520 mL
V2 = 260 mL
T1 = 100 + 273 = 373 K
Since pressure remains constant, therefore, by applying Charles’ law :
or T2= (T1/ V1) xV2 = (373/520) (260)= 186.5 K
or t = 186.5 – 273 = –86.5°C
At what centigrade temperature will a given volume of a gas at 0°C become double its volume, pressure remaining constant?
Solution: Let the volume of the gas at 0°C be V.
T1 = 273 + 0 = 273 K
Since pressure remains constant, therefore, by applying
Charles’ law, V 1/T1 = V2/T2
T 2 = (T1/ V1) xV2 = (273 x 2V) / V = 546 K
Changing the temperature to centigrade scale,
Temperature = 546 – 273 = 273°C.
On a ship sailing in a pacific ocean where the temperature is 23.4°C, a balloon is filled with 2L air. What will be the volume of the balloon when the ship reaches the Indian ocean, where the temperature is 26.1°C ?
Solution: According to Charles’ law
T1 = 273 + 23.4 = 296.4 K
T2 = 273 + 26.1 = 299.1
V2 = (V1/T1). T2 = (V1 x T2 )/ T1 = 2L x 299.1K / 296.4K = 2.018 L
What is the increase in volume when the temperature of 800 mL of air increases from 27°C to 47°C under constant pressure of 1 bar ?
Solution: Since the amount of gas and the pressure remains constant, Charles’ law is applicable. i.e.
V 1/T1 = V2/T2
V 1 = 800 mL V2 = ?
T1 = 273 + 27 = 300 K
T2 = 273 + 47 = 320 K
V2 = (V1/T1). T2 = (V1 x T2 )/ T1
V 2 = (800x 320) /300 = 853.3 mL
∴ Increase in volume of air = 853.3 – 800 = 53.3 mL
Related Posts:
- Numerical problems based on Gay Lussac’s law or pressure law
- Harder Numerical problems based on SNELL’S LAW - in Light chapter physics
- Numerical Problems based on combined Gas law or Gas equation & ideal gas equation
- Numerical problems based on the efficiency of machines
- Numerical Problems Based on Lever's mechanical advantage
- Numerical problems based on the inclined plane physics - solved
P 1 P 2 ––– = ––– T 1 T 2 3.00 x ––– = ––– 293 323 Solution technique: cross-multiply and divide. x = 3.31 atm (to three sig figs)
P 1 / T 1 = P 2 / T 2 1.00 atm / 20.0 = x / 30.0 x = 1.50 atm
1.00 atm / 293 = x / 303 x = 1.03 atm Makes bit of a difference, doesn't it?
0.370 atm x –––––––– = ––––– 323 K 273 K x = 0.313 atm (to three sig figs)
699.0 mmHg 760.0 mmHg –––––––– = –––––––––– 313 K x x = 340. K (or 67.0 °C. to three sig figs)
P 1 P 2 ––– = ––– T 1 T 2 750.0 mmHg x ––––––––––– = –––––––– 323.0 K 273.15 K (750.0 mmHg) (273.15 K) = (323.0 K) (x) x = 634.2 mmHg (to four sig figs)
15.0 atm 16.0 atm –––––––– = –––––––––– 298 K x x = 44.9 °C. to three sig figs)
P 1 / T 1 = P 2 / T 2 3.00 atm / 293.0 K = x / 323.0 K x = 3.31 atm (to three sig figs) Notice the inclusion of a volume in the problem. It was put there as a deliberate distraction. You get all concerned about what happens to the volume and you miss that it's a metal container, which we can assume has a fixed volume.
P 1 / T 1 = P 2 / T 2 P 1 T 2 = P 2 T 1 P 2 = (P 1 T 2 ) / T 1 P 2 = [(3000 mmHg) (273 K)] / 773 K The answer should be determined to three significant figures.
P 2 = (P 1 T 2 ) / T 1 P 2 = [(30.0 kPa) (1273 K)] / 173 K The answer should be determined to three significant figures.
P 2 = [(130.0 atm) (298 K)] / 1273 K The answer should be determined to three significant figures.
P 1 / T 1 = P 2 / T 2 We want the pressure to double, so set P 1 = 1 and P 2 = 2. The units don't matter because they are the same: atm, kPa, mmHg, torr. It does not matter which one you use. We want to see what the temperature does, so set T 1 to 1 K and T 2 to x. The temperature does matter, it MUST be in Kelvins. Therefore: 1 / 1 = 2 / x x = 2 K The answer is that T 2 would have to be double the value of T 1 .

IMAGES
VIDEO
COMMENTS
Solution: Write Charles Law and substitute values in: V 1 / T 1 = V 2 / T 2. x / 588 K = 852 mL / 725 K. (x) (725 K) = (852 mL) (588 K) x = 691 mL. Note the large °C values, trying to get you to forget to add 273. Remember, only Kelvin temperatures are allowed in the calculations. Bonus Problem: An open "empty" 2 L plastic pop container, which ...
Charles' Law Problems Name_____ Don't forget to use the Kelvin Temp.!!!! 1) A 50.0 ml soap bubble is blown in a 27.0°C room. It drifts out an open window and lands in a snow bank at -3.0°C. What is its new volume? 2) A balloon was inflated to a volume of 5.0 liters at a temperature of 7.0°C. It landed in an oven and was heated to 147°C.
Charles's Law Problems. 1. A gas sample at 40.0 C occupies a volume of 2.32 L. If the temperature is raised to 75.0 C, what will the volume be, assuming the pressure remains constant? 2. A gas at 89 C occupies a volume of 0.67 L. At what Celsius temperature will the volume increase to 1.12 L? 3.
Charles' Law Worksheet 1) The temperature inside my refrigerator is about 40 Celsius. If I place a balloon in my fridge that initially has a temperature of 220 C and a volume of 0.5 liters, what will be the volume of the balloon when it is fully cooled by my refrigerator? 2) A man heats a balloon in the oven. If the balloon initially has a ...
Plan the problem. First, rearrange the equation algebraically to solve for V2 V 2. V2 = V1 ×T2 T1 V 2 = V 1 × T 2 T 1. Cancel units and calculate. Now substitute the known quantities into the equation and solve. V2 = 2.20L × 344 K 295 K = 2.57L V 2 = 2.20 L × 344 K 295 K = 2.57 L. Think about your result.
Scanned by CamScanner. Charles' Law Worksheet Name per Jacques Charles made the observation the volume of a gas is directly proportional to the Kelvin temperature of the gas. If the Kelvin temperature is doubled, the volume also doubles. The equation for this relationship is VA _ , where Vrepresents volume and T represents temperature.
Boyle's Law. Relationship between volume and pressure of a gas. Volume can be in mL or L but V1 and V2 must be same unit. Pressures must match units. Pressure can be measured in multiple units (atm, mmHg, Pa, kPa etc) We will not worry about converting them.
3-2 Charles Law Sol.doc 3-2 HW Charles Law SOLUTIONS V/T = k or V 1 /T 1 = V 2 /T 2 For the following problems calculate the value which the question asks for. Be sure to follow the proper steps and show all your work (1) Convert values (temp) to correct units if necessary and list variables (2) write the
Charles' Law. Chem Worksheet 14-2. Name ________________. Jacques Charles made the observation the volume of a gas is directly proportional to the Kelvin temperature of the gas. If the Kelvin temperature is doubled, the volume also doubles. The equation for this relationship is V V.
Here's the set up to solve the problem: x / 308 K = 0.432 mL/K. x = 133 mL. It is unusual to see a question that uses V / T = k. The much, much more common equation for Charles' Law problem solving is V 1 / T 1 = V 2 / T 2. Bonus Problem: In an air-conditioned room at 19.0 #&176;C, a spherical balloon had the diameter of 50.0 cm.
T f K = 350 K. The next step is to use Charles' law to find the final volume. Charles' law is expressed as: Vi/Ti = Vf/Tf. where. V i and T i is the initial volume and temperature. V f and T f is the final volume and temperature. Solve the equation for V f: V f = V i T f /T i.
Charles Law Chemistry Questions with Solutions. Q1. Suppose P, V, and T denote the gas's pressure, volume, and temperature. In that case, the correct representation of Chale's law is. V is directly proportional to T (at constant P) V inversely proportional to T (at constant P) PV = nRT. None of the above. Answer: (a), If P, V, and T denote ...
In gas laws, temperatures must always be expressed in kelvins. 6.3 Gas Laws - Boyle's and Charles' Laws is shared under a license and was authored, remixed, and/or curated by LibreTexts. The behavior of gases can be modeled with gas laws. Boyle's law relates a gas's pressure and volume at constant temperature and amount.
Gas Law Worksheet #2 (Dalton's Law and Ideal Gas Law) Dalton's Law: PT = P1 + P2 + P3 + . . . 1. Determine the total pressure of a gas mixture that contains oxygen at a pressure of 150.mmHg, nitrogen at 350.mmHg pressure, and helium at a pressure of 200.mmHg. 2. A gas mixture containing oxygen, nitrogen, and carbon dioxide has a pressure of ...
Example #1. A gas occupies 221 cm 3 at a temperature of 0 °C and pressure of 760 mm Hg. Find its volume at 100 °C. First, don't worry about the pressure. The number doesn't enter into the calculation. All that matters is that it's a constant. Use the equation: V 1 /T 1 = V 2 /T 2. Convert 0 °C and 100 °C to Kelvin:
PROBLEM 7.2.1.11 7.2.1. 11. A high altitude balloon is filled with 1.41 × 10 4 L of hydrogen at a temperature of 21 °C and a pressure of 745 torr. What is the volume of the balloon at a height of 20 km, where the temperature is -48 °C and the pressure is 63.1 torr? Answer. Click here to see a video solution.
Correct answer: Explanation: Charles's law of gases indicates that, at a constant pressure, the volume of a gas is proportional to the temperature. This is calculated by the following equation: Our first step to solving this equation will be to convert the given temperatures to Kelvin. Using these temperatures and the initial volume, we can ...
6. Charles's Law: When _____ is held constant, the volume and temperature of a gas are _____ proportional. 7. Mathematically, Charles's Law is stated: T V = _____ or 1 1 T V =_____. 8. The _____ temperature scale must be used in all gas law problems. 9. At 189 K, a sample of gas has a volume of 32.0 cm3. What volume does the gas
The form of the Combined Gas Law most often used is this: (P 1 V 1) / T 1 = (P 2 V 2) / T 2. Most commonly V 2 is being solved for. The rearrangement looks like this: V 2 = (P 1 V 1 T 2) / (T 1 P 2) A reminder: all these problems use Kelvin for the temperature. I will not usually comment on the change from °C to K.
T2 = (T1/ V1) xV2 = (273 x 2V) / V = 546 K. Changing the temperature to centigrade scale, Temperature = 546 - 273 = 273°C. Example 4. On a ship sailing in a pacific ocean where the temperature is 23.4°C, a balloon is filled with 2L air. What will be the volume of the balloon when the ship reaches the Indian ocean, where the temperature is 26.1°C
Solution: P 2 = (P 1 T 2) / T 1. P 2 = [ (30.0 kPa) (1273 K)] / 173 K. The answer should be determined to three significant figures. Problem #10: Calculate the final pressure inside a scuba tank after it cools from 1.00 x 10 3 °C to 25.0 °C. The initial pressure in the tank is 130.0 atm. Solution: